Summary
The corpus callosum is the most prominent commissural connection between the cortical hemispheres, and numerous neurodevelopmental disorders are associated with callosal agenesis. Using mice with either meningeal overgrowth or selective loss of meninges, we’ve identified a cascade of morphogenic signals initiated by the meninges that regulates corpus callosum development. The meninges produce BMP7, an inhibitor of callosal axon outgrowth. This activity is overcome by the induction of expression of Wnt3 by the callosal pathfinding neurons, which antagonizes the inhibitory effects of BMP7. Wnt3 expression in the cingulate callosal pathfinding axons is developmentally regulated by another BMP family member, GDF5, produced by the adjacent Cajal-Retzius neurons and turns on before outgrowth of the callosal axons. The effects of GDF5 are in turn under the control of a soluble GDF5 inhibitor, Dan, made by the meninges. Thus, the meninges and medial neocortex use a cascade of signals to regulate corpus callosum development.
Introduction
The corpus callosum coordinates inter-hemispheric functions critical for cognition by providing axonal connectivity across the midline between cortical areas required for a variety of sensory, motor and emotional processing. In addition, callosal agenesis is associated with a wide variety of neurodevelopmental and psychiatric diseases (Paul et al., 2007). The corpus callosum develops late in gestation and is evolutionarily young, having developed in importance as neocortical size and function increased (Mihrshahi, 2006). In mice, medially projecting callosal axons reach the midline at E15 and the first cingulate pioneer axons cross the midline at E16 (Koester and O’Leary, 1994; Ozaki and Wahlsten, 1998; Rash and Richards, 2001). If cortical axons approach the midline but the pathfinding cells don’t cross the midline, the callosum fails to form and Probst bundles form, consisting of cortical axons projecting anterior-posterior instead of crossing the midline (Paul et al., 2007).
The paired cerebral hemispheres develop by producing excitatory projection neurons in the neurogenic niche adjacent to the ventricles. These cells migrate radially away from the ventricles to generate laminae in the more superficial cortex. Maturation of the cortical neurons occurs near the pial/meningeal surface and many neurons send dendrites toward the pial surface while axons generally project in the opposite direction toward the ventricle eventually turning laterally to project caudally out of the cortex or medially to project across the callosum. The midline meninges, across which the callosum forms, is the only site in the cortex where axons reach and project across the pial surface.
The three cortical meningeal layers are derived from the cranial neural crest, which generates a variety of cellular derivatives important for face and head development and evolution (Serbedzija et al., 1992). Recently we reported that the meninges are a key regulator of embryonic cortical neurogenesis by secreting an instructive cue (retinoic acid) regulating the onset of neuron production (Siegenthaler et al., 2009). These data along with previous work from our lab and others (Borrell and Marin, 2006; Li et al., 2008; Li et al., 2009; Lopez-Bendito et al., 2008; Paredes et al., 2006) indicate that the meninges are an instructive signaling source during cortical development. This led us to consider the idea that the meninges may also play a role in axon guidance during callosum formation since these axons appear to directly interact with the meninges (Alcolado et al., 1988).
There are still major unanswered questions about how the corpus callosum forms. Previous studies showed that Slit ligands, presented from a localized group of midline radial glial cells called the glial wedge, help to funnel callosal axons toward the appropriate midline location for future crossing (Bagri et al., 2002; Shu and Richards, 2001). Cingulate cortex pioneer neurons are generated from radial glial progenitors in the medial cortex, and these progenitors also form the glial wedge. These pioneer neurons are the first cells to project across the midline and serve as a critical scaffold for the remainder of the callosal axons to successfully cross (Rash and Richards, 2001; Shu and Richards, 2001). Thus, the interaction of cingulate neurons and the apposed meningeal tissues could be important for formation of the callosum. Importantly, unveiling further mechanisms regulating corpus callosum formation may provide important insights into callosal agenesis in humans.
We had the opportunity to develop and test this hypothesis in the course of examining a mouse model we generated which expresses more meningeal secretory molecules due to meningeal overgrowth. We used Msx2-Cre to generate excess meninges around the cortex and cortical midline and discovered that this leads to defects in callosal formation. This suggests that the meninges produce factors that prohibit midline crossing. In support of this idea, ablation of midline meningeal cells at mid-corticogenesis leads to an expanded corpus callosum. We then directly tested the functions of meningeal-secreted factors on corpus callosal crossing and have identified a novel cascade of signals that regulates callosum development. Specifically, we identified a complex interplay between BMP7 secreted by the meninges and Wnt3 produced by the callosal pioneer neurons that coordinates the timing of corpus callosum formation.
Results
Expression of stabilized β-Catenin in the skin leads to inappropriate Wnt6 expression
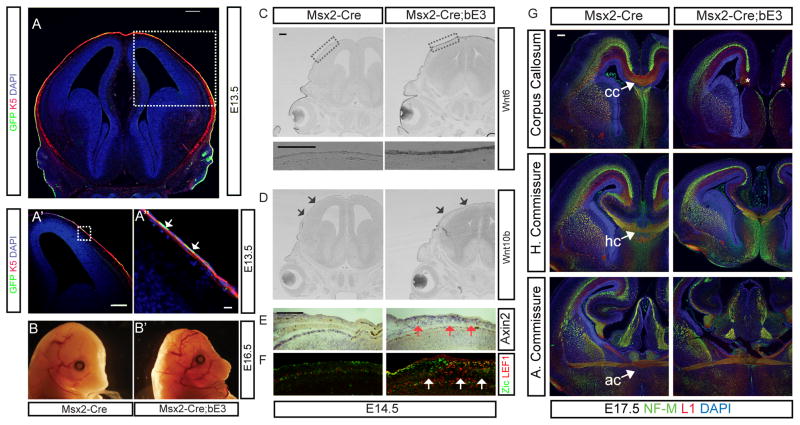
To investigate the effect of meningeal secretory molecules on embryonic brain development, we set out to generate a transgenic mouse line with expanded meninges using a meninges-specific cre mouse line. We decided to try using an Msx2-Cre mouse line which uses the 439bp 5′ flanking region of the mouse Msx2 gene and crossed the line with exon3 floxed β-Catenin (Ctnnb1lox(ex3), shortened as bE3 in the figures) to express stabilized constitutively active β-catenin in the meninges (Harada et al., 1999; Sun et al., 2000). The native Msx2 gene is normally expressed in the meninges (Rice et al., 2003), however, the transgenic Msx2-Cre line drives recombination not in the meninges but instead in the skin, revealed by crossing with the ROSA-YFP CRE reporter line (Figures 1A′-A″ and low magnification in Suppl. Fig. 1B) and the Rosa-LacZ CRE reporter line (Suppl. Fig. 1C). There was no YFP or LacZ expression in the meninges or any regions of the brain. Msx2-Cre drove recombination starting from E13.5 and the YFP expression persisted postnatally (Suppl. Fig. 1 and data not shown). At E16.5, the Msx2-Cre; Ctnnb1lox(ex3) mutant embryos were smaller and the skin over the head was thinned and the skull malformed (Figure 1B-B′). Persistent activation of the Wnt pathway in the skin of the Msx2-Cre; Ctnnb1lox(ex3) mutants led to increased expression of Axin2, a well-established Wnt target gene (Jho et al., 2002), in the skin (Fig. 1E). Interestingly, there was also increased expression of the Wnt responsive transcription factor Lef1 in a non-autonomous manner in the underlying mesenchymal tissue, including the meninges (Figure 1F). This made us wonder if there was Wnt signaling dependent expression of a Wnt ligand in the skin that was then signaling to the underlying tissues. Wnt6 is normally expressed high laterally and low dorsally in the skin of the head (Figure 1C) and is absent from the midline. However, in the Msx2-Cre; Ctnnb1lox(ex3) mice Wnt6 expression covered the entire dorsal surface of the head. We also found that Wnt6 was elevated about 1.5 fold compared to control using quantitative real time PCR (qPCR) on mRNA isolated from whole head (Suppl. Fig. 2C). Expression of Wnt10b, another skin-specific Wnt, was not altered (Figure 1D) indicating that persistent activation of the Wnt signaling pathway in the skin leads to specific upregulation of Wnt6 expression.
Figure 1.
Msx2-Cre drives recombination of Ctnnb1lox(ex3) in the skin and leads to ectopic Wnt6 expression and callosal agenesis. A-A″) The ROSA-YFP reporter reveals the recombination pattern for Msx2-Cre in the outermost layer of the head, the skin, which was double immunostained with anti-K5 cytokeratin (red) and anti-GFP (green) antibodies. Nuclei were counterstained with DAPI (blue). B-B′) Comparison of E16.5 mutant and control embryos shows that mutants have skin and skull defects. C) Representative coronal sections of the mouse brains at E14.5 show Wnt6 and D) Wnt10b expression – arrows indicate the low level, unchanged expression in the skin. E) Ectopic Axin2 expression in the corresponding region (red arrows). F) Lef1 (red) expression in anti-Zic labeled meningeal cells (green) at E14.5 and expansion of Lef1 and Zic-double positive meningeal cells in the Msx2-Cre; Ctnnb1lox(ex3) brains (white arrows). G) Mutant mice are acallosal Immunostaining of NF-M (green) and L1 (red) in the WT and Msx2-Cre; Ctnnb1lox(ex3) E17.5 brains at the level of corpus callosum (CC), hippocampal commissure (HC) and anterior commissure (AC). All embryos were obtained from different litters and different pairs of parents. To minimize the staining variation, control and mutant sections were placed side by side on the same slide and stained in a same procedure. All images in the same comparison were acquired on the same microscopic setting. >6 embryos were examined for control and experimental and these were from multiple litters. The immunohistochemistry was performed a minimum of 6 times on different embryos and the in situ hybridization performed 3 times on different embryos. All scale bars = 200 μm except A″ = 16 μm.
Mutants with persistent activation of the Wnt pathway in the skin are acallosal
Beyond the skin and calvarial defects, we found that in the Msx2-Cre;Ctnnb1lox(ex3) mice the main cortical commissural pathway, the corpus callosum, failed to form (Figure 1G). However, in the same mutants other commissural pathways including the anterior commissure and hippocampal commissures were still formed (although the hippocampal commissure is slightly smaller in size than normal) (Figure 1G). At E17.5, a day before the mutant embryos die, it was apparent that the callosal axons stopped at the cortical midline and rather than crossing formed Probst bundles (* in Figure 1G), which are aberrant axonal tracts made up of callosal axons that fail to cross the midline (Paul et al., 2007). These callosal defects were also observed in horizontal sections of mutant animals (Suppl. Figure 1A) and showed full penetrance from fourteen mutant embryos analyzed. Since the failure of corpus callosum formation is a dramatic midline structural defect, we wondered how excess Wnt signaling in the dorsal skin might cause this phenotype. There have also been numerous studies showing strain differences in the appearance of corpus callosum defects in mice with some strains (eg 129 and Balb/c), however, our colonies of Msx2-Cre and Ctnnb1lox(ex3) mice have been extensively crossed into the CD-1 background, not noted for defects in the corpus callosum.
One possible cause of agenesis of the corpus callosum could be defects in the development of the cortical projection neurons. This phenotype has been often observed in mutant animals for the transcription factors governing maturation of the cortical callosal neurons comprising layer II/III (Alcamo et al., 2008; Armentano et al., 2006; Britanova et al., 2008; Molyneaux et al., 2007; Paul et al., 2007; Piper et al., 2009; Shu et al., 2003). We examined the expression of several of these factors, such as Satb2, NFia and NFib, and found that their expression was intact in the Msx2-Cre;Ctnnb1lox(ex3) brains at E15.5, a day before the formation of the corpus callosum (Suppl. Figure 1D). Some of the earlier born neurons that make up layer V/VI also contribute axons to the corpus callosum, so we also examined Ctip2 and Tbr1, two markers of these early born neurons. We found that the laminar organization of the mutant cortex was similar to wild type littermates. We also did not see any changes of the proliferative zone using an M-phase cell cycle marker (phospho-histone H3 - pH3), a ventricular zone progenitor markers (Nestin and Pax6) or a marker for the basal intermediate progenitors in the subventricular zone (Tbr2) (Suppl. Fig. 2A). Another potential cause of callosal agenesis in these mice may be alterations in expression of guidance molecules, such as semaphorins, slits, Wnt5a, Draxin and ephrins, expressed in the cortical midline and previously shown to regulate callosal axonal crossing (Bagri et al., 2002; Islam et al., 2009; Keeble et al., 2006; O’Donnell et al., 2009; Paul et al., 2007). To address this we examined expression of a panel of these ligands and their receptors in our mutant mice but did not observe any obvious differences in the pattern of expression between mutant and control brains (Suppl. Fig. 2B).
Wnt6 induces expansion of neural crest derived meningeal tissues
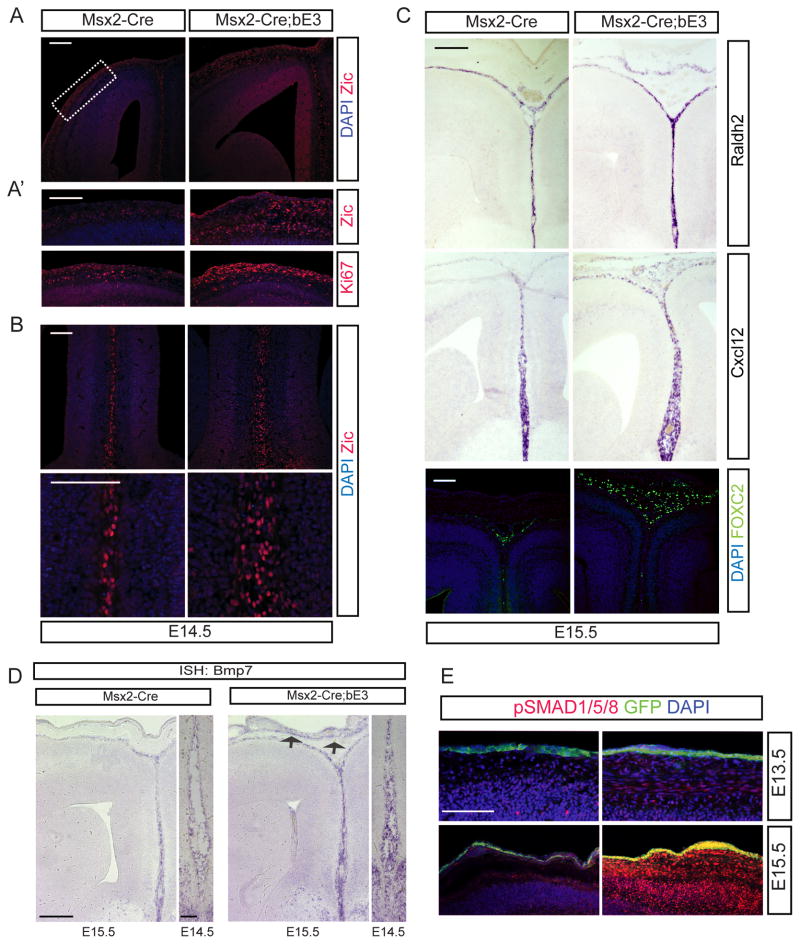
We wondered whether the excess Wnt6 in the head itself might be an inhibitor of corpus callosum formation, so we electroporated Wnt6 into the cortical midline prior to callosum formation and found that the corpus callosum still formed normally (data not shown). We reasoned that another possible mechanism for callosal agenesis might be via the known role of Wnts as a growth factor for neural crest cells. Since the meninges overlying the cortex originate from the cranial neural crest (Serbedzija et al., 1992) and Wnt6 induces expansion of cranial neural crest cells in avian species (Garcia-Castro et al., 2002; Schmidt et al., 2007), we looked for meningeal abnormalities in the Msx2-Cre;Ctnnb1lox(ex3) mutants. We examined meningeal development at E14.5-E15.5, before the formation of the corpus callosum in control and mutant mice. Using Ki-67, a cell proliferation marker, we found that meningeal cell proliferation was elevated in Msx2-Cre;Ctnnb1lox(ex3) mutants (Figure 2A′) and this is consistent with our findings of ectopic Axin2 and Lef1 expression (Figure 1E–F). Furthermore, using an anti-Zic1 antibody, which labels meningeal cells (Inoue et al., 2008), we found expanded meninges both over the surface of the cortex, and, even more interestingly, in the interhemispheric fissure where the corpus callosal axons will eventually form (Figure 2A, A′ and B). To more carefully examine the three meningeal layers, we used markers specific for each layer expressed during embryonic development (Siegenthaler et al., 2009; Zarbalis et al., 2007). Raldh2 normally is expressed in the developing arachnoid meninges, but in the mutants it was ectopically expressed under the skin implying that Wnt6-induced meningeal progenitors to produce extra arachnoid cells (Figures 2C). We also used anti-Foxc2 antibody which labels predominantly the dural meningeal precursors (Zarbalis et al., 2007); Foxc2 expressing dural cells are also expanded in the mutant embryos (Figure 2C). Cxcl12, which labels the inner pial layer, was likewise expanded and ectopically expressed in the mutants (Figure 2C). These results suggest that ectodermal Wnt6 induces pia, arachnoid and dural meningeal cells to overgrow both over the surface of the cortex but also between the hemispheres. We suspected that the non-cell autonomously expanded meninges may be the cause of the corpus callosal agenesis and that perhaps we could use the Msx2-Cre;Ctnnb1lox(ex3) mutants to identify a normal role for the meninges in corpus callosum development.
Figure 2.
Meningeal expansion by activation of Wnt6 in the skin and increased expression of BMP7. Low-magnification (A) and high-magnification (A′ and B) of Zic immunostaining in WT and Msx2-Cre; Ctnnb1lox(ex3) E14.5 heads. Lateral (A′) and medial (B) regions of brains are presented. Staining of Ki67 shows excess meningeal fibroblast proliferation (A′ bottom). C) In situ hybridization of meninges-specific markers such as Raldh2 and Cxcl12 and immunostaining of FOXC2 in the WT and Msx2-Cre; Ctnnb1lox(ex3) E15.5 forebrains. D) In situ hybridization of BMP7 expression in the medial meninges of E14.5 and the lateral and medial cortical regions of E15.5 WT and Msx2-Cre; Ctnnb1lox(ex3) brains. Expression of BMP7 is increased in the meninges, but also ectopically in the meninges (arrows). E) Immunostaining of phosho-SMAD1/5/8 at E13.5 and E15.5 shows increased BMP signaling in the cortex of the Msx2-Cre; Ctnnb1lox(ex3) embryos at E15.5. GFP staining reveals the skin where Msx2-Cre is expressed. All embryos were obtained from different litters and different pairs of parents. To minimize the staining variation, control and mutant sections were placed side by side on the same slide and stained in a same procedure. All images in the same comparison were acquired on the same microscopic setting. >6 embryos were examined for control and experimental and these were from multiple litters. The immunohistochemistry was performed a minimum of 6 times on different embryos and the in situ hybridization performed 3 times on different embryos. Scale bars = 200 μm; D (E14.5) = 100 μm.
Elevated Bmp signaling in the meninges and cortex in mutant mice
The Wnt6 expression zone marks the areas of epithelial-mesenchymal transition and Bmp signaling is known to be involved in this transition (Kalluri and Weinberg, 2009; Lavery et al., 2008), and in the mutant embryos, the development of the skull vault appeared compromised (Figure 1B′). Since the normal source of BMPs for much of skull development is the meninges (Kim et al., 1998; Rice et al., 2005), we hypothesized that the ectopic meningeal tissue may express BMP signaling components affecting the skeletogenic mesenchyme for the mouse skull vault. This also raised the possibility that excess Bmps produced by the meninges might interfere with corpus callosum development. We examined the expression of several Bmp ligands and found that BMP7 was ectopically expressed in the excess meningeal tissue at E14.5-15.5 and that the overall expression level in the meninges was increased (Figures 2D; Suppl. Figure 2C). To determine whether the increased expression of BMP7 alters the underlying Bmp signaling levels within the cortex, we stained the brain with an anti-pSMAD1/5/8 antibody. This showed that the mutant brains had increased phosphorylation of SMAD1/5/8, confirming that the ectopic BMP7 expression in the meninges is biologically active and increases cortical Bmp signaling (Figure 2E). Interestingly, the increased phospho-SMAD staining was barely present at E13.5 but dramatically increased by E15.5 (Figure 2E), in parallel with the onset of recombination in the skin. Phosphorylation of SMAD2, which is a downstream target of canonical TGF-β ligands rather than Bmp ligands, was not induced (data not shown). An interesting supporting point for the increased Bmp signaling in the developing cortex that we see with these markers is increased Zic1 expression in the cortical ventricular zone (Figure 2A). Zics are known to be induced by Bmp ligands in the developing CNS (Aruga et al., 2002).
Mice with excess meninges have generally normal midline glial development and appropriate production of cingulate pioneer neurons
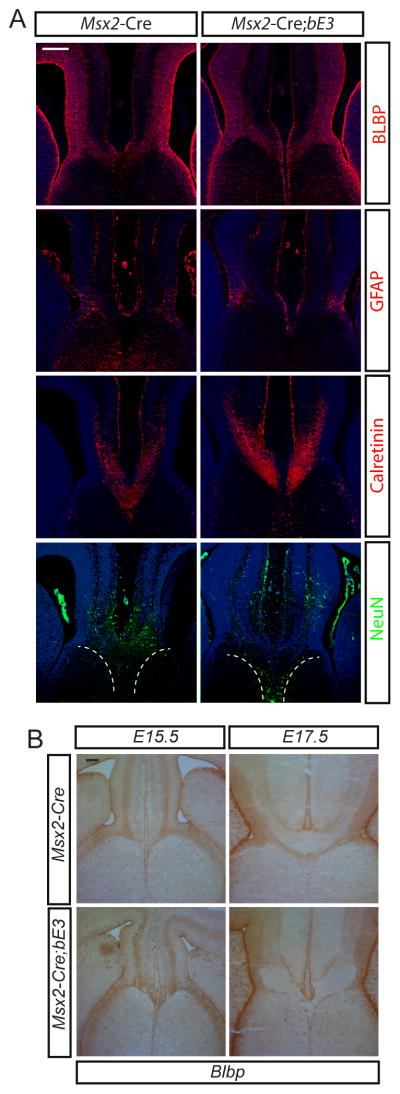
There has been extensive study of the development of the corpus callosum and it’s clear that some of the causes of callosal agenesis are due to structural defects at the cortical midline leading to failure of the fusion of the midline (Paul et al., 2007). We wanted to determine whether the excess meningeal tissue or the increased BMP signaling leads to defects in the structural elements required for corpus callosum formation. We examined whether the midline glial structures critical for development of the corpus callosum (Shu and Richards, 2001; Smith et al., 2006) were affected in the mutants. Staining the E16.5 cortex with BLBP and GFAP, markers for glial wedge cells, did not show alterations (Figure 3A). We also used BLBP to examine the glial wedge both before (E15.5) and after (E17.5) the callosum forms and found that before the callosum forms there is no clear defect in the organization of this structure (Figure 3B). The glial wedge is made up of radial glial cells that function both as progenitor cells for the Calretinin-expressing medial cingulate neurons that produce the pioneer axons crossing the callosum and as part of the scaffolding for these crossing axons (Rash and Richards, 2001). Anti-Calretinin antibody labeled these cingulate neurons and their axons (Figure 3A) and the mutants did not show any difference in the numbers of these cingulate cortical neurons although there were apparent defects in their axon projections across the midline (Figure 3A). We also dated the birth of Calretinin+ neurons in the cingulate cortex with BrdU injections and found that Calretinin+ neurons were born around E12.5 (data not shown). Staining for BrdU at E14.5 also did not show any apparent difference in the number of neurons born at E12.5. In addition, the subcallosal sling is made up of a group of migratory neurons, frequently visualized with NeuN staining (Shu and Richards, 2001), that make up the ventral limit of callosal axon projection. In both control and experimental acallosal mice, NeuN clearly labeled this group of glial sling neurons (Figure 3A). All of these results suggest that neither a mechanical effect of the excess meninges nor the ectopic BMP7 expressed by the meninges affected the cingulate pathfinding neuron generation, the midline glial wedge or the glial sling neurons in the mutant medial cortex but that nevertheless the axons failed to cross the midline.
Figure 3.
A) Midline glial structures are not affected in the mutant but pathfinding axons fail to cross the midline. Immunostaining of BLBP and GFAP shows midline glial structures and NeuN shows subsling migratory neurons in the WT and Msx2-Cre; Ctnnb1lox(ex3) E16.5 brains (dashed white line outlines the general distribution of these neurons). Immunostaining of Calretinin shows guidance neurons and their axons. B) BLBP staining of glial wedge before callosum formation (E15.5) and after the callosum forms (E17.5). All embryos were obtained from different litters and different pairs of parents. To minimize the staining variation, control and mutant sections were placed side by side on the same slide and stained in a same procedure. All images in the same comparison were acquired on the same microscopic setting. >6 embryos were examined for control and experimental and these were from multiple litters. The immunohistochemistry was performed a minimum of 6 times on different embryos. Scale bars A = 200 μm; B= 100μm.
Midline BMP7 inhibits callosal axon outgrowth
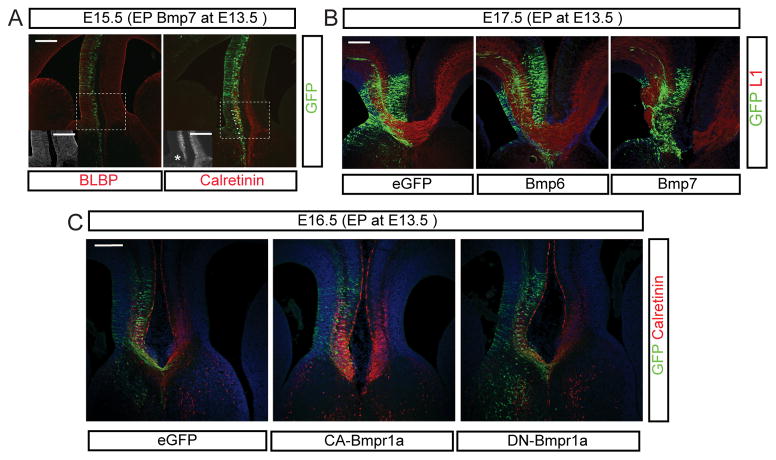
Although the Calretinin+ cingulate neurons were generated, their axons failed to cross the midline in the mutant cortex (Figure 3A). Given that the glial wedge and sling are apparently minimally affected, we hypothesized that increased BMP7 released by the meninges might affect axon outgrowth of cingulate cortical neurons. To test this we introduced a BMP7 expression construct into the E13.5 embryonic cortical midline by in utero electroporation. We examined the electroporated brains at E15.5, a day before the initial pioneer axons should cross the midline. BLBP+ midline glial structures appeared normal, consistent with the previous results obtained from mutant brains, however, Calretinin+ cingulate cortical axons were disorganized in the electroporated hemisphere (Figure 4A). By E17.5, when the corpus callosum should have formed, we found that BMP7 had potently inhibited formation of the corpus callosum (Figure 4B). This effect was specific for BMP7 since BMP6 expression in the same region did not affect callosum formation (Figure 4B). Considering the disorganization of pioneer axons at E15.5 in the midline by BMP7 overexpression, this suggests that BMP7 protein acts as an inhibitor of pioneer callosal axon outgrowth, although another possibility is that excess BMP7 in the cortex leads to abnormalities in the meninges at the midline.
Figure 4.
Failure of callosal axon growth caused by BMP7 overexpression. Electroporation of Bmps (A and B) and Bmp receptors (C) into the E13.5 medial cortex. A) Immunostaining of BLBP and Calretinin in BMP7 electroporated cortex. The BLBP and Calretinin staining are also shown in the insets in black and white for increased clarity. * indicates the area of disorganized Calretinin axons in the electroporated hemisphere. B) Immunostaining of GFP (green) and L1 (red) using eGFP, Bmp6 and BMP7 electroporated E17.5 forebrains at the level of the corpus callosum. C) Immunostaining of GFP and Calretinin using E16.5 brains electroporated with mutant forms of type I Bmp receptors such as constitutively active (CA) and dominant negative (DN) Bmp receptors. For these experiments N=6 for both experimental and control electroporations in each case. Three embryos were used for in situ hybridization and six embryos were used for immunostaining. All immunostaining was performed on at least 6 embryos for each condition obtained from at least two litters, and staining was performed multiple times with control and experimental samples placed on the same slides to minimize differences in staining conditions. Scale bars = 200 μm.
To address this latter question we used a cell autonomous means to mimic the activation of Bmp signaling in the cingulate cortical neurons by expressing a constitutively active form of type I Bmp receptor (CA-Bmpr1a) in the medial cortex from E13.5 to E16.5, when the first cingulate callosal axons cross the midline. We compared this to eGFP controls as well as overexpression of dominant negative forms (DN-Bmpr1a) (Figure 4C). This experiment showed that cell-autonomous activation of BMP signaling in the cingulate cortical neurons inhibited the growth of corpus callosal axons in the electroporated hemisphere, however, the dominant-negative form of type I Bmp receptors had no apparent effect on callosum formation (Figure 4C). This result supports the idea that BMP7 in the midline meninges acts as an inhibitor for corpus callosal axon crossing the midline and rules out the possibility that BMP7 expressed within the cortex is non-autonomously acting on meningeal cells and reciprocally inhibiting callosal outgrowth. One of the important features of these experiments is that manipulation of one side of the cortex apparently is sufficient to block the formation of this commissure with bilateral contributions. This suggests that the initial formation of the callosum by Calretinin+ cingulate pioneer neurons involves interaction of these axons from both sides at the midline, perhaps via a mutual handshake.
Mice with mid-corticogenesis meningeal defects have expansion of the corpus callosum
Our initial observations are consistent with the idea that BMP7, expressed by the meninges, is a potent negative regulator of corpus callosum formation. Our data mostly rests on the generation of a novel mouse mutant that has meningeal overgrowth, although the direct ectopic expression of BMP7 within the cortex also blocks callosum formation. To strengthen our arguments we wanted to develop comparable loss-of-meningeal function mouse mutants that might allow us to confirm the negative role of the meninges in the formation of the callosum. We wished to undertake two approaches toward this goal but first needed to identify a meninges selective Cre line. Preferably, one that begins expression in the meninges at a later developmental timepoint thus allowing us to generate mice with a more limited meningeal phenotype. To this end, we tested the Pdgfrβ-Cre line (Foo et al., 2006), and found that it is active in some meningeal derivatives in addition to blood vessel associated pericytes (also neural crest-derived cells) beginning at about E13.5 (Figure 5A).
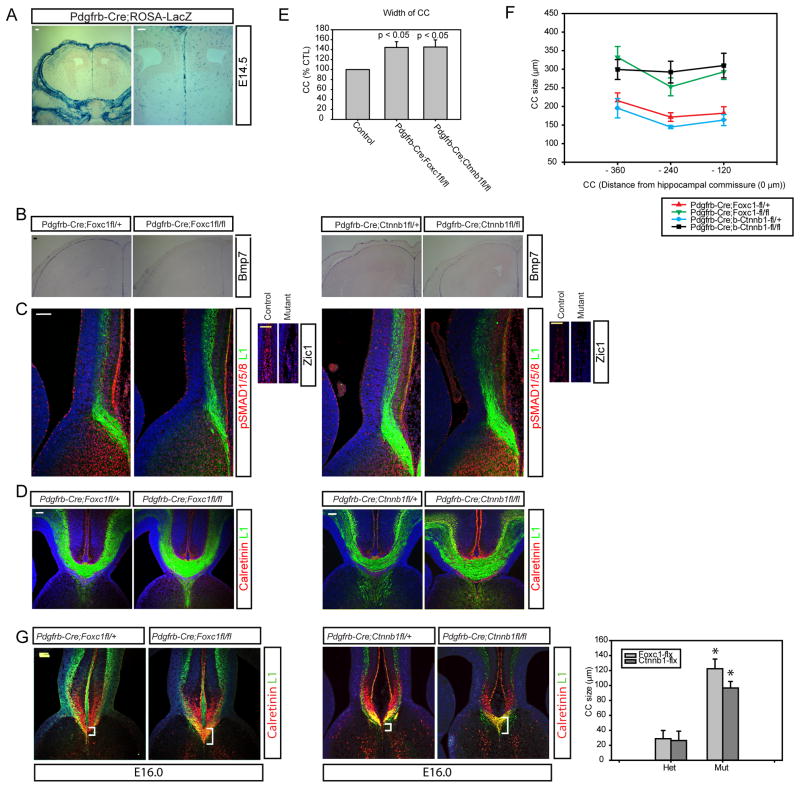
Figure 5.
Enlarged corpus callosum in the loss of function meninges mutants. A) ROSA-LacZ CRE reporter line shows Pdgfrβ-Cre expression in the midline meninges at E14.5. LacZ is also expressed in the pericytes associated with blood vessels widely in the brain. B) In situ hybridization of Bmp7 using E15.5 brain tissues from Pdgfrβ-Cre; Foxc1lox/+, Pdgfrβ-Cre; Foxc1lox/lox, Pdgfrβ-Cre; Ctnnb1lox(lof)/+, and Pdgfrβ-Cre; Ctnnb1lox(lof)/(lof). C) Immunostaining of phospho-SMAD1/5/8 (red) at E15.5 and L1 (green) is counterstained. Anti-Zic staining reveals midline meninges in the A′ (heterozygote control) and B′ (mutant). D) Immunostaining of L1 and Calretinin to show corpus callosum at E17. E) Graph depicts the size of L1-stained corpus callosum. F) Graph shows the size of the corpus callosum at multiple anatomic levels reflecting the distance in the rostral-caudal plane from the hippocampal commissure. N=7 of each genotype for this measurement. G) Development and anatomy of the corpus callosum in Pdgfrβ-Cre; Foxc1lox/+, Pdgfrβ-Cre; Foxc1lox/lox, Pdgfrβ-Cre; Ctnnb1lox(lof)/+, and Pdgfrβ-Cre; Ctnnb1lox(lof)/(lof) mice at an early stage of callosum development (E16.0) and the graph shows the size of the corpus callosum at E16.0. The * indicates that the mutants are significantly different than their genotype control, P<0.05 using Student’s T-test and N = 4 for these callosal measurements. Scale bars = 100 μm.
We previously showed that Foxc1 mutant mice have major defects in meningeal development (Siegenthaler et al., 2009; Zarbalis et al., 2007) and that these mice largely lack meningeal cells over much of their cortex, including the medial cortex. Failure to form normal meninges leads to detachment of the radial glial cells from the basement membrane and also major neurogenic defects (thus lacking most callosal neurons) (Siegenthaler et al., 2009; Zarbalis et al., 2007). However, using the Pdgfrβ-Cre line and a conditional Foxc1lox line we generated mice with a later deletion of Foxc1 that have a relatively intact brain organization. Analysis of these late meningeal Foxc1 mutants at E15.5 shows that there is reduced meningeal BMP7 expression in these mice both over the cortex and in the interhemispheric fissure (Figure 5B). Zic1+ meningeal cells are diminished in the interhemispheric fissure of Pdgfrβ-Cre; Foxc1lox/lox mice, indicating that decrease in BMP7 is likely due to a reduction in BMP7-expressing meningeal cells (Figure 5C, A′ & B′, we used ‘fl’ for floxed allele in the figures).
Msx2-Cre;Ctnnb1lox(ex3) mutant mice have excess meninges due to increased production of Wnt6 by the overlying skin. Expansion of the meninges is accompanied by increased expression of a target of the Wnt signaling pathway (Axin2) as well as a Wnt signaling mediator (Lef1). This suggests that canonical Wnt signaling may be an important component of meningeal development. Indeed, previous studies using the Wnt1-Cre line crossed with the Ctnnb1lox(lof) allele had shown a failure of formation of many cranial neural crest components (Brault et al., 2001), however, this phenotype is developmentally too early to evaluate callosal crossing. Instead, we crossed the Ctnnb1lox(lof) with the Pdgfrβ-Cre line and found that, similar to the Pdgfrβ-Cre; Foxc1lox/lox mutants, there was a notable decrease in meningeal BMP7 and a reduction in interhemispheric meningeal cell numbers (Figure 5B, C).
We next used our two novel meningeal mutants to determine how loss of midline BMP7 affects callosal crossing. In addition to the reduced expression of BMP7 in the meninges (Figure 5B) there were markedly decreased levels of phospho-SMAD1/5/8 activity in the medial cortex of both mutants (Figure 5C). Thus, these mice apparently have the opposite phenotype of the Msx2-Cre;Ctnnb1lox(ex3) mice in that they have less interhemispheric meninges, and, consequently, reduced BMP7 and BMP signaling. Next, we examined the development of the corpus callosum in these mice and found that remarkably, the Pdgfrβ-Cre;Ctnnb1lox(lof) and Pdgfrβ-Cre;Foxc1lox mice had larger corpus callosums than their littermate controls with more axonal fibers crossing (Figure 5D). We quantified this difference by measuring the corpus callosum in cohorts of mutants and control mice and found that the callosum was significantly increased in size at E17.5 throughout the rostro-caudal anatomic levels of the corpus callosum (Figure 5E, E′).
One important question to address is why the callosal size is increased in these mice. One possibility is that the callosum is larger because it begins to be formed earlier (due to loss of Bmp7 from the meninges) and thus is larger at the stages we examined. To address this, we examined the size of the callosum in these two mutant lines at an earlier stage when the callosum has just started forming, E16. At this time the mutant mice still have a marked increase in callosal size (Figure 5F), consistent with the idea that the callosum begins to form early in these mutants and is thus at a more advanced stage of development at E17.5.
Another potential mechanism for increased callosal size is that there could be an increased number of neuron types that contribute to the callosum in these mutant lines due to potential effects of the meninges on cortical development. To address this we examined the expression of layer specific markers in the developing cortex of both lines as well as the Msx2-Cre; Ctnnb1lox(ex3) mice. Interestingly, we found that the Pdgfrβ-Cre;Foxc1lox but not the Pdgfrβ-Cre;Ctnnb1lox(lof) mice have an alteration in the numbers and distribution of superficial neurons that would contribute to the callosum (Supplemental Figure 3). Since we observed this phenotype in only one of the lines, we suspect that this is not the cause of the increased callosal size, rather it is accelerated formation of the callosum due to early crossing in mice lacking Bmp7 at the midline.
Wnts are positive regulators of callosal axon growth
Our data thus far is consistent with the idea that the meninges normally limit the formation of the corpus callosum and that one of the important mediators of this function is BMP7 expressed by the meninges acting on the medial cortex and cingulate pathfinding axons. One puzzling aspect of these observations is the fact that normally BMP7 is expressed in the midline meninges, albeit at lower levels, yet these axons still do manage to cross the midline in the face of the normal presence of BMP7. Why does the callosum ever form if BMP7 is always present in the meninges? The corpus callosum is the only cortical structure where axons make trajectories across meningeal tissues. In this sense, it seems possible that there is a BMP7-counteracting molecule in the cortical midline that is induced prior to formation of the corpus callosum and that the action of BMP7 produced by the meninges is in part to prevent premature formation of the corpus callosum until this positive influence is produced. Since Frizzled-3 mutant mice, which fail to transduce much Wnt signaling in the cortical projection neurons, also fail to form the corpus callosum (Wang et al., 2002) and Wnt signaling is critical for axon guidance in other areas of the nervous system (Agalliu et al., 2009; Bovolenta et al., 2006; Ciani and Salinas, 2005; Dickson, 2005; Krylova et al., 2002; Lyuksyutova et al., 2003; Maro et al., 2009; Schmitt et al., 2006), we wondered whether a Wnt signal might be the positive cue allowing pioneer axons to cross the midline. To address this, we inhibited endogenous Wnt signaling in the medial cortex of normal mice by electroporating the soluble Wnt inhibitor Dkk1 into the cortical midline. This resulted in the failure of cingulate axons to cross the midline and led to callosal agenesis (Figure 6A), indicating a probable role for Wnt signaling in this process and making a Wnt ligand a possible key regulator of the formation of the callosum.
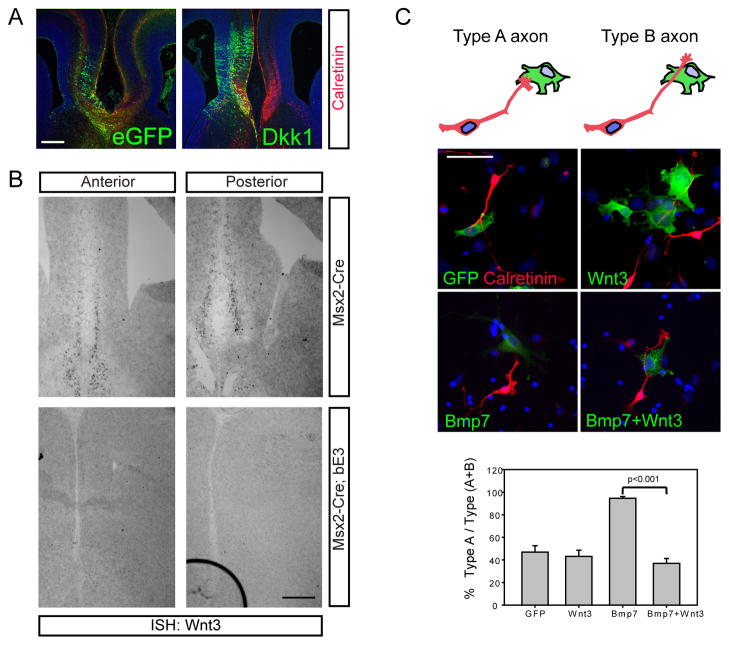
Figure 6.
Wnt3 antagonizes the negative effects of BMP7. A) Wnt signaling is required for corpus callosum formation. Immunostaining of GFP and Calretinin in E16.5 brains electroporated with eGFP or Dkk1 at E13.5 shows that inhibiting Wnt signaling at the midline blocks corpus callosum formation. Electroporations were performed 6 times. B) In situ hybridization of Wnt3 expression in the WT and Msx2-Cre; Ctnnb1lox(ex3) E14.5 heads shows that Wnt3 expression is missing from the midline of mutant mice. The in situ hybridization was repeated 3 times on different samples from different litters. C) Inhibition of BMP7 effect on Calretinin+ axon growth by Wnt3 in vitro. BMP7 or Wnt3 expressing COS7 cells were co-cultured with midline cortical neurons obtained at E14.5. Quantification of Type A and Type B axons (see diagram in C). Error bars represent standard error of the mean. Significance value considered by Student’s t test, p<0.001. The actual numbers of cells assayed were GFP (96/180), Wnt3 (90/138), Bmp7 (372/396), Bmp7+Wnt3 (72/192) and the experiment was repeated three times. Scale bars, A, B = 200 μm, C = 100 μm.
In order to determine the likely endogenous Wnt ligand responsible for this function, we screened expression of Wnt genes by in situ hybridization to see which Wnt is expressed in a manner supporting a role in cingulate cortical axon projection toward the contralateral cortex. We found that Wnt3 is expressed in a subpopulation of the midline cingulate cortical neurons in control mice at E14.5, just before the callosum is formed (Figure 6B). Interestingly, in the Msx2-Cre;Ctnnb1lox(ex3) embryos at E14.5, Wnt3 expression was absent where the corpus callosum should appear in 1.5 days (Figure 6B). Thus, Wnt3 is apparently expressed in the right location to be the Wnt ligand regulating callosum formation and strikingly, is also missing from acallosal Msx2-Cre;Ctnnb1lox(ex3) mutant mice.
Wnt3 antagonizes the negative effects of BMP7 on callosal axon outgrowth and rescues the callosum of mutant mice
Our results suggest a model that Wnt3 is expressed in cingulate neurons at the cortical midline in order to act locally to oppose the negative influence of BMP7 from the meninges and that the appearance of Wnt3 at E14.5 is a crucial step toward corpus callosum formation. We designed both in vivo and in vitro experiments to test whether Wnt3 interacts with BMP7 in regulating callosal axon growth. Initially we tested whether the meninges produce a secreted chemorepellent using collagen explant assays. We embedded explants of cingulate cortex in collagen and confronted them with either meningeal explants from control and mutant mice with excess meninges (the Msx2-Cre;Ctnnb1lox(ex3) mice) or COS7 aggregates expressing BMP7. These experiments revealed no evidence of any repellent effect at a distance by the meninges (Supplemental Figure 4). Next, in order to determine if there is a more short-range or contact dependent effect, we collected midline cortical neurons from E14.5 embryonic brains and co-cultured cortical neurons with COS7 cells expressing BMP7, Wnt3 or both ligands. In these cultures, calretinin+ axons frequently grew out but failed to grow across BMP7 expressing COS7 cells, similar to the effects of many axonal growth inhibitors in other systems (Law et al., 2008; Niederkofler et al., 2010). To quantify this effect we counted the number of axons that approached BMP7-expressing COS7 cells but didn’t cross them (Type A axons) and axons that grew across the BMP7-expressing COS7 cells (Type B axons) and found that BMP7 is a potent inhibitor in this assay, essentially causing 100% of the cells to be Type A, compared to GFP transfected COS7 cells (Figure 6C). Wnt3 expression alone had no clear effect on axon growth compared to control. In both the Wnt3 and GFP conditions many axons (about 60%) freely grew across the COS7 cells (Figure 6C). To test the ability of Wnt3 to antagonize the negative effects of BMP7 in this assay we co-expressed the ligands and found that Calretinin+ axons now quite readily crossed BMP7+Wnt3-expressing COS7 cells (Figure 6C, p<0.001). Thus, Wnt3 apparently has minimal if any stimulatory effect on axon growth in this assay, unless BMP7 is present, in which case it apparently counteracts the negative effects of BMP7.
To examine this interaction in vivo, we introduced BMP7 along with Wnt3 in utero. Strikingly, we observed formation of the corpus callosum when we expressed Wnt3 expression along with BMP7 (Figure 7A). Thus, it appears that Wnt3 is able to counteract the negative effects of BMP7 on callosal pathfinding axon outgrowth. This is consistent with the onset and spatial distribution of Wnt3 at E14.5 being a critical regulator of callosum formation by allowing the pioneer axons to cross the BMP7 expressing midline meninges.
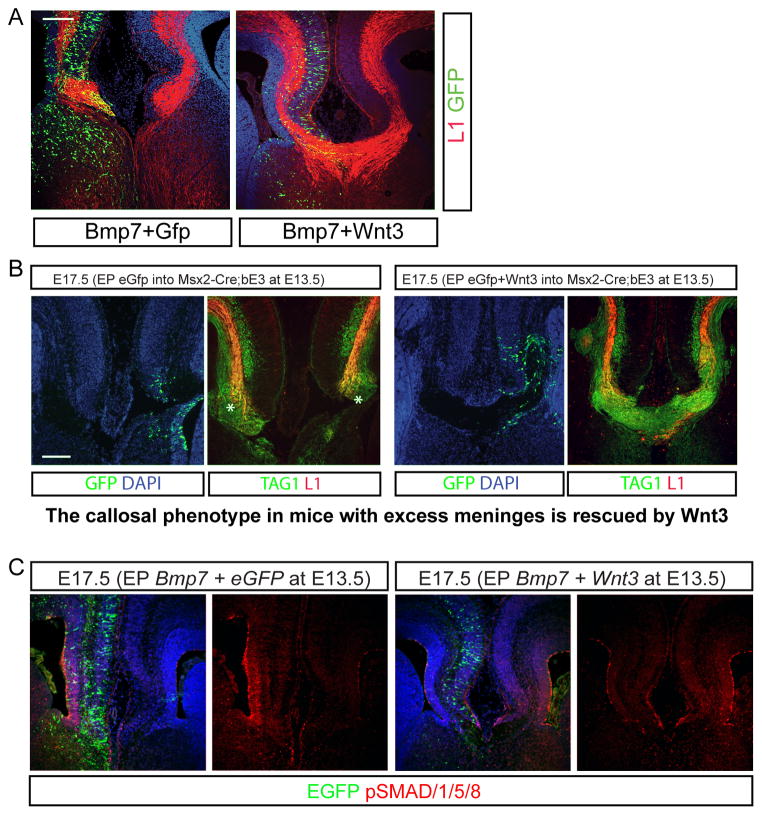
Figure 7.
Co-expression of Wnt3 blocks the negative effects of BMP7 in vivo. A) Co-electroporation was conducted with the combination of BMP7 with eGFP or Wnt3 at E13.5 and the brains were analyzed at E17.5 for callosal agenesis. B) Wnt3 overexpression rescues corpus callosum formation in Msx2-Cre; Ctnnb1lox(ex3) mutant mice. Mutant mice were electroporated in utero at E13.5 with eGFP or Wnt3 plasmids and then stained for GFP, Tag1 or L1 expression. C) Expression of phospho-SMAD1/5/8 after electroporation of BMP7 with eGFP or Wnt3. For all of the experiments in this figure the N=6 for experimental and control. Scale bars = 200 μm.
Since the mutant cortex lost Wnt3 expression before the initial pioneer axons crossed the midline, we wondered whether adding back Wnt3 would rescue the failure of the pioneer axons crossing the midline in the mutants with excess meninges (the Msx2-Cre;Ctnnb1lox(ex3) mice). To test this, we electroporated a Wnt3 expression construct into the midline cortex of Msx2-Cre;Ctnnb1lox(ex3) mice at E13.5 and examined E17.5 embryos and found that TAG1 and L1 positive corpus callosal axons are obvious in the Wnt3 electroporated brain but GFP-electroporated brains failed to form the midline callosal trajectories (Figure 7B).
To further address our hypothesis that Wnt3 signaling antagonizes BMP7 signaling allowing the corpus callosal axons to cross the midline, we examined staining for pSMAD1/5/8 in the medial cortex of BMP7 electroporated mice either with GFP or Wnt3 co-electroporation. In mice that were electroporated with BMP7 and GFP, as expected, the level of pSMAD1/5/8 immunoreactivity was markedly increased in the BMP7 electroporated medial cortex (Figure 7C). However, when Wnt3 was co-electroporated with BMP7 and the brains examined three days later, the pSMAD1/5/8/levels were blunted and perhaps even lower than seen in the opposite unelectroporated hemisphere (Figure 7C). To quantify these effects we performed western blotting for pSMAD1/5/8 and compared it to the signal using an antibody that sees all SMAD1 or GAPDH to normalize. In these experiments we found that BMP7+eGFP electroporated cortex had a 40% higher level of pSMAD1/5/8 compared to cortex electroporated with Wnt3+BMP7 (Supplemental Figure 5A–B). We also examined the fluorescence intensity ratio of the tissues labeled with pSMAD1/5/8 antibody on the electroporated side compared to the opposite hemisphere in BMP7+eGFP electroporated mice vs Wnt3+BMP7 electroporated mice (essentially quantitating the signal in Figure 7C but in five new electroporated embryos in each condition) and found a dramatic difference in the pSMAD1/5/8 ratio between the two conditions (Supplemental Figure 5C).
Gdf5 from Cajal-Retzius Cells and its inhibitor Dan from the meninges control Wnt3 expression and regulate corpus callosum formation
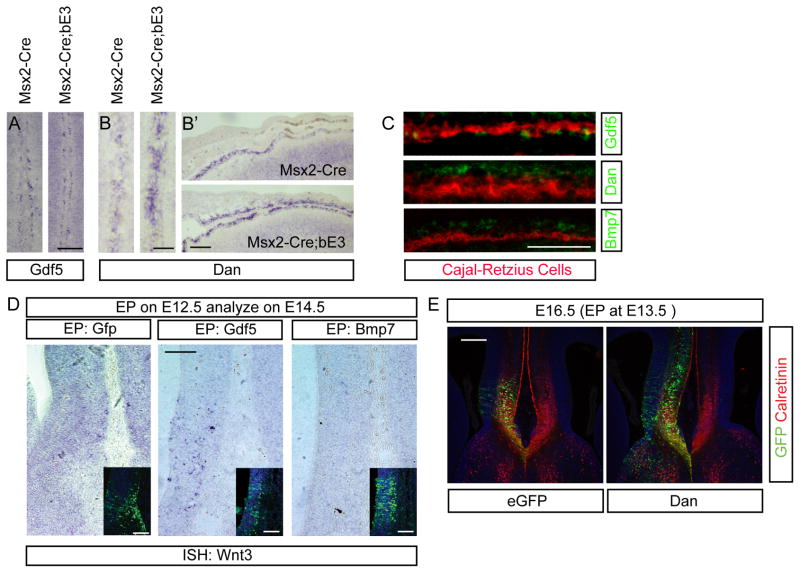
Our analysis so far has led us to propose that BMP7 helps to block the formation of the corpus callosum by inhibiting callosal pioneer axon outgrowth, thereby inhibiting formation of the corpus callosum until such time as Wnt3 expression begins in the pioneer neurons and antagonizes the effects of BMP7, allowing initial callosal axon outgrowth. This left us with one significant puzzle – why were the Msx2-Cre;Ctnnb1lox(ex3) mice missing the expression of Wnt3? We hypothesized that another secreted factor normally produced by the meninges was also overexpressed in the mutants and that this factor helps to regulate Wnt3 expression in the cingulate cortex. Our thoughts immediately turned to the Gdf5/6/7 inhibitory molecule Dan (Dionne et al., 2001), which some time ago we showed is expressed by the meninges (Kim and Pleasure, 2003). In that same study we also showed that one of the ligands that Dan inhibits, Gdf5, is expressed by the Cajal-Retzius cells, but we were unable at that time to identify any functional significance for this pattern of expression (Kim and Pleasure, 2003). Interestingly, the Cajal-Retzius cells are the most superficial cortical neurons, in Layer 1, and lie immediately adjacent to the cingulate pioneer neurons in the medial cortex.
To test the role of these factors, we examined the expression of Gdf5 and Dan in the mutant mice and found that Gdf5 is expressed in Cajal-Retzius cells in both control and mutant mice (Figure 8A, C) and the expanded meninges in the mutant express abundant Dan (Figure 8B-B′), implying that the levels of this inhibitor were increased in the vicinity. We also performed double labeling in the dorsal neocortex using Calretinin as a Cajal-Retzius cell marker (in the cingulate Calretinin stains both pathfinding neurons and Cajal-Retzius cells, but in the rest of the cortex Calretinin is a selective marker for Cajal-Retzius cells) and confirmed that Gdf5 was coexpressed by Cajal-Retzius cells, while Dan was expressed in the overlying meninges (Figure 8C). We then examined whether electroporation of Gdf5 in the midline of the cortex at E12.5 is sufficient to induce early Wnt3 expression by E14.5 and found that indeed Gdf5 electroporation induces low levels of Wnt3 expression in the medial cortex (Figure 8D). Thus, our model is that Wnt3 expression in the cingulate pioneer neurons is normally positively controlled by GDF5 activity from the adjacent Cajal-Retzius neurons but that the excess meningeally produced Dan in the mutant leads to decreased expression of Wnt3 in the mutant embryos. If our observations and model are correct, then overexpression of Dan in the cortical midline should also inhibit corpus callosum formation by antagonizing the actions of Gdf5. Indeed, expression of Dan led to delay of Calretinin+ pathfinding axons to cross the midline and failure of corpus callosum formation compared to control at E16.5 (Figure 8E), although this effect was apparently transient, because by E17.5, the callosum was formed in these mice (data not shown). This result implies that Wnt3 expression is finely controlled by neighboring cell types, which control the timing of corpus callosum formation by inducing the expression of Wnt3, allowing these axons to overcome the inhibitory effects of BMP7 from the meninges.
Figure 8.
Control of Wnt3 expression by the interaction of Cajal-Retzius cells and meningeal cells. A – B′) In situ hybridization of Gdf5 (A) and Dan (B, B′) expression in the WT and Msx2-Cre; Ctnnb1lox(ex3) E15.5 heads. Gene expression in the medial regions (A and B) and lateral regions (B′) are presented. C) Double in situ hybridization and Calretinin immunostaining (Cajal-Retzius cells) in the lateral cortex to reveal cell-type specific expression of Gdf5 (Cajal-Retzius cells), Dan (meningeal fibroblast) and BMP7 (meningeal fibroblast). D) In situ hybridization of Wnt3 using E14.5 cortex electroporated with Gdf5 or BMP7 at E12.5. Insets represent the GFP immunostaining of the adjacent section to reveal the electroporated side. N=3 for all of the above in situ hybridization experiments. E) Electroporation of eGFP and Dan into the E13.5 medial cortex with immunostaining of GFP (green) and Calretinin (red) at E16.5. Note that corpus callosum formation and crossing of Calretinin+ axons is blocked by excess Dan (N=6 experimental and 6 control for this experiment). Comparison of the electroporated samples was conducted by using embryos obtained from the same litters to minimize the individual variation. The embryos are electroporated at E13.5 with experimental electroporations performed in one half of the uterine horns and the control in the other half. Since the experiment is terminated at E16.5, it is easy to return to the same embryos for analysis. Scale bars for A–D = 100 mm; for E = 200 μm.
Discussion
BMP7 from the interhemispheric meninges prevents crossing of callosal axons
One of the early events in corticogenesis is the elaboration of the cranial neural crest derived mesenchymal cell layers that make up the meninges (Alcolado et al., 1988; Etchevers et al., 1999; Mack et al., 2009; Siegenthaler et al., 2009; Vivatbutsiri et al., 2008; Zarbalis et al., 2007). Generally the meninges have been neglected as a significant source of developmental signals regulating cortical development, but in recent years, several labs, including our own, have shown that the meninges control aspects of cortical neurogenesis and neuronal migration (Borrell and Marin, 2006; Li et al., 2008; Lopez-Bendito et al., 2008; Paredes et al., 2006; Siegenthaler et al., 2009). Our experiments show that BMP7, produced either by overexpression in the medial cortical wall or by hyperplastic meninges, is sufficient to cause callosal agenesis. In addition, we’ve shown that mice with limited mid-corticogenesis defects in the meninges and reduced BMP7 expression have increased callosal thickness. Thus, we believe that one important function of the meninges may be to prevent early formation of the corpus callosum. Our conclusions are somewhat different than those of another group that also found that loss of BMP7 blocks callosum formation (Sanchez-Camacho et al.). In this study the authors found that mutants lacking BMP7 also were acallosal and concluded that this was due to abnormal development of the midline glial structures. They thus concluded that BMP7 acts primarily to control glial development at the midline. However, since their conclusions were based in part on mice with genetic disruption of BMP7, it is quite possible that these mice have additional midline defects that contribute to their findings. The generally subtle findings that they showed on the development of the midline glia and our additional studies showing the interactions of Wnt3 and BMP7 (including the rescue of BMP7 effects by Wnt3, the actions of dominant active BMP1a on callosum formation and our direct in vitro effects of BMP7 on pathfinding axons) indicate a more specific and direct role of BMP7 on formation of the callosum.
Why is it important that the corpus callosum be prevented from forming early? One likely reason is the role of the midline glial specializations and guidance cues from the septum underlying the callosum. These aren’t apparently affected in our mutant mice, but they don’t normally develop until just before the callosal pathfinding axons project across the midline. It seems likely that these regulate the connectivity of the two hemispheres as well as the formation of other ascending and descending tracts that form in the area. If these midline specializations are not present as callosum formation proceeds, this may lead to disorganization. Secondly, one of the primary sources of callosal axons (along with the callosal projection neurons of layer 5) is the superficial Satb2 expressing neurons of layers 2–4, born between E14.5-17.5. It is possible that it is crucial that these later born neurons be present at the time the callosum forms for proper initial organization of the callosal projections; perhaps these cells play some additional intermediate regulatory roles as the axons cross and enter the opposite hemisphere. Future studies will be needed to address this. Thus, an important function of the meninges and BMP7 is apparently to serve as a barrier to early projection of axons across the midline.
Induction of Wnt3 in callosal pioneer neurons overcomes BMP7 inhibition
Given that the meninges and BMP7 provide a barrier to callosal development, why does the callosum form at all? Previous anatomic studies have shown that a group of cingulate neurons extend axons that serve as pioneer axons to form the initial callosal connection (Koester and O’Leary, 1994; Ozaki and Wahlsten, 1998; Rash and Richards, 2001). These cingulate neurons are adjacent to the Cajal-Retzius cells as well as meningeal tissues. It is at this time that Wnt3 expression commences in these cells and allows them to overcome the negative effects of BMP7. In our mutant mice this interaction is apparently ineffective, since Wnt3 expression isn’t induced in these neurons.
The induction of Wnt3 expression in the cingulate neurons thus presents a key step in the development of the corpus callosum, and perhaps also was an important evolutionary development accompanying the appearance of the corpus callosum as a relatively late specialization coincident with the massive expansion of the cortex in mammals. Amazingly, the induction of Wnt3 expression is also, albeit indirectly, under the control of the meninges. Our mice, expressing stabilized β-Catenin using Msx2-Cre in the skin leading to meningeal hyperplasia, allowed us insight into this critical time of corpus callosum formation. Expression of β-Catenin in the skin induces Wnt6 in the skin leading to expansion of the neural crest derived meningeal cells leading to excess Zic+/Sdf1+/Dan+/BMP7+ meningeal tissue.
Throughout the neuraxis of vertebrates the dorsal neural neural tube develops under the influence of both Bmps and Wnts (Lee and Jessell, 1999; Lee et al., 1998; Liem et al., 1995; Megason and McMahon, 2002). Both families of morphogens have also been described to have axon guidance roles. The interaction of Wnt3 and BMP7 functions in corpus callosum formation raises the possibility that antagonism between Wnts and BMPs is more generally involved in development of the CNS.
Callosal agenesis in humans
Agenesis of the corpus callosum is found in 1 in 4000 individuals and is associated with a wide range of developmental defects and syndromes (Paul et al., 2007). Many affected patients have mental retardation, seizures or autism spectrum disorders. There are many causes of callosal agenesis in humans and it is commonly associated with cortical malformations or other midline defects, although it can also be an isolated finding in otherwise quite normal individuals. There are many identified genetic syndromes associated with callosal agenesis, some with identified genes and many others with still unidentified genes (Paul et al., 2007) One interesting recently identified callosal agenesis gene is the Zfhx1b (also called SMAD-interacting-protein 1 - Sip1) transcription factor that has been identified as the etiology of Mowat-Wilson syndrome (Mowat et al., 2003). In mice this transcription factor has been shown to be downstream of Bmp signaling in the cortex and to also integrate this pathway with Wnt signaling in cortical midline development (Miquelajauregui et al., 2007). This is particularly interesting considering our finding in this study that there are potent roles for both Bmp and Wnt signaling in regulating the development of the corpus callosum. It seems likely that other genes regulating these pathways will turn out to be causative for callosal agenesis as some of the syndromes with unknown genes are identified. In addition, our discovery that an inherited form of callosal agenesis (the mutant phenotype in our mice) could be rationally targeted and successfully treated by replacing a signaling molecule missing in the mutant mice is of potential significance for the future application of translational approaches to developmental disorders. It is possible that future application of reagents regulating signaling at the appropriate developmental time could prove specific interventions allowing treatment of patients with developmental phenotypes associated with specific defects in axon outgrowth.
Experimental procedures
Animals
The individual alleles used in the study are detailed in the supplemental material. All mice were bred using standard mouse husbandry approaches to obtain the genotypes described in the text and according to UCSF IACUC standards and protocols. In addition, all procedures were performed with approval of the UCSF IACUC.
In utero electroporation
This procedure was performed using a standard procedure with plasmids as described in the text. For all electroporation experiments multiple litters (at least three) were obtained for each construct and high-expressing, properly targeted electroporations were collected to get a minimum of N=6 embryos were examined and the results were uniform.
Immunostaining, in situ hybridization, western blots and quantitative RT-PCR
Standard techniques were used for all of this analysis and are described in detail in the supplemental material, as are the sources for antibodies and materials. All staining experiments were performed by comparing control and mutant sections stained on the same slides to minimize variation.
Co-culture of COS7 cells with cortical neurons and explant cultures
Cos7 cells were transfected with plasmids as described in the text and co-cultured with dissociated cingulate cortical neurons. Cingulate explants were embedded in collagen and cocultured with meningeal explants as described in the text.
Statistics
For the pair-wise analysis of cell counting from co-culture of cingulate neurons and COS7 cells, Student’s t-test was used to evaluate the significance. Error bars depict +/− SEM.
Supplementary Material
Acknowledgments
The authors thank Guangnan Li, Roeben Munji and John Rubenstein for helpful discussions, Trung Huynh for technical assistance and Kurt Thorn and the Nikon Imaging Center at UCSF for use of the confocal microscope. This work was supported by R01 DA017627 and R01 MH077694. S.J.P. is also supported by funds from the family of Glenn W. Johnson, Jr. J.S. is supported by a K99-R00 Pathway to Independence award from NINDS (NS070920). The authors also thank Dr. Gail Martin, Dr. Makoto Taketo, and Dr. Tom Kume for sharing mouse reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agalliu D, Takada S, Agalliu I, McMahon AP, Jessell TM. Motor neurons with axial muscle projections specified by Wnt4/5 signaling. Neuron. 2009;61:708–720. doi: 10.1016/j.neuron.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Alcolado R, Weller RO, Parrish EP, Garrod D. The cranial arachnoid and pia mater in man: anatomical and ultrastructural observations. Neuropathol Appl Neurobiol. 1988;14:1–17. doi: 10.1111/j.1365-2990.1988.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Armentano M, Filosa A, Andolfi G, Studer M. COUP-TFI is required for the formation of commissural projections in the forebrain by regulating axonal growth. Development. 2006;133:4151–4162. doi: 10.1242/dev.02600. [DOI] [PubMed] [Google Scholar]
- Aruga J, Tohmonda T, Homma S, Mikoshiba K. Zic1 promotes the expansion of dorsal neural progenitors in spinal cord by inhibiting neuronal differentiation. Dev Biol. 2002;244:329–341. doi: 10.1006/dbio.2002.0598. [DOI] [PubMed] [Google Scholar]
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Borrell V, Marin O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci. 2006;9:1284–1293. doi: 10.1038/nn1764. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Rodriguez J, Esteve P. Frizzled/RYK mediated signalling in axon guidance. Development. 2006;133:4399–4408. doi: 10.1242/dev.02592. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Wnts send axons up and down the spinal cord. Nat Neurosci. 2005;8:1130–1132. doi: 10.1038/nn0905-1130. [DOI] [PubMed] [Google Scholar]
- Dionne MS, Skarnes WC, Harland RM. Mutation and analysis of Dan, the founding member of the Dan family of transforming growth factor beta antagonists. Mol Cell Biol. 2001;21:636–643. doi: 10.1128/MCB.21.2.636-643.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchevers HC, Couly G, Vincent C, Le Douarin NM. Anterior cephalic neural crest is required for forebrain viability. Development. 1999;126:3533–3543. doi: 10.1242/dev.126.16.3533. [DOI] [PubMed] [Google Scholar]
- Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Garcia-Castro MI, Marcelle C, Bronner-Fraser M. Ectodermal Wnt function as a neural crest inducer. Science. 2002;297:848–851. doi: 10.1126/science.1070824. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Ogawa M, Mikoshiba K, Aruga J. Zic deficiency in the cortical marginal zone and meninges results in cortical lamination defects resembling those in type II lissencephaly. J Neurosci. 2008;28:4712–4725. doi: 10.1523/JNEUROSCI.5735-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam SM, Shinmyo Y, Okafuji T, Su Y, Naser IB, Ahmed G, Zhang S, Chen S, Ohta K, Kiyonari H, et al. Draxin, a repulsive guidance protein for spinal cord and forebrain commissures. Science. 2009;323:388–393. doi: 10.1126/science.1165187. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeble TR, Halford MM, Seaman C, Kee N, Macheda M, Anderson RB, Stacker SA, Cooper HM. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J Neurosci. 2006;26:5840–5848. doi: 10.1523/JNEUROSCI.1175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AS, Pleasure SJ. Expression of the BMP antagonist Dan during murine forebrain development. Brain Res Dev Brain Res. 2003;145:159–162. doi: 10.1016/s0165-3806(03)00213-x. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Rice DP, Kettunen PJ, Thesleff I. FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development. 1998;125:1241–1251. doi: 10.1242/dev.125.7.1241. [DOI] [PubMed] [Google Scholar]
- Koester SE, O’Leary DD. Axons of early generated neurons in cingulate cortex pioneer the corpus callosum. J Neurosci. 1994;14:6608–6620. doi: 10.1523/JNEUROSCI.14-11-06608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylova O, Herreros J, Cleverley KE, Ehler E, Henriquez JP, Hughes SM, Salinas PC. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- Lavery DL, Davenport IR, Turnbull YD, Wheeler GN, Hoppler S. Wnt6 expression in epidermis and epithelial tissues during Xenopus organogenesis. Dev Dyn. 2008;237:768–779. doi: 10.1002/dvdy.21440. [DOI] [PubMed] [Google Scholar]
- Law CO, Kirby RJ, Aghamohammadzadeh S, Furley AJ. The neural adhesion molecule TAG-1 modulates responses of sensory axons to diffusible guidance signals. Development. 2008;135:2361–2371. doi: 10.1242/dev.009019. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1999;22:261–294. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Mendelsohn M, Jessell TM. Neuronal patterning by BMPs: a requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev. 1998;12:3394–3407. doi: 10.1101/gad.12.21.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Adesnik H, Li J, Long J, Nicoll RA, Rubenstein JL, Pleasure SJ. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J Neurosci. 2008;28:1085–1098. doi: 10.1523/JNEUROSCI.4602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Kataoka H, Coughlin SR, Pleasure SJ. Identification of a transient subpial neurogenic zone in the developing dentate gyrus and its regulation by Cxcl12 and reelin signaling. Development. 2009;136:327–335. doi: 10.1242/dev.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Sanchez-Alcaniz JA, Pla R, Borrell V, Pico E, Valdeolmillos M, Marin O. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- Mack J, Squier W, Eastman JT. Anatomy and development of the meninges: implications for subdural collections and CSF circulation. Pediatr Radiol. 2009;39:200–210. doi: 10.1007/s00247-008-1084-6. [DOI] [PubMed] [Google Scholar]
- Maro GS, Klassen MP, Shen K. A beta-catenin-dependent Wnt pathway mediates anteroposterior axon guidance in C. elegans motor neurons. PLoS One. 2009;4:e4690. doi: 10.1371/journal.pone.0004690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Mihrshahi R. The corpus callosum as an evolutionary innovation. J Exp Zool B Mol Dev Evol. 2006;306:8–17. doi: 10.1002/jez.b.21067. [DOI] [PubMed] [Google Scholar]
- Miquelajauregui A, Van de Putte T, Polyakov A, Nityanandam A, Boppana S, Seuntjens E, Karabinos A, Higashi Y, Huylebroeck D, Tarabykin V. Smad-interacting protein-1 (Zfhx1b) acts upstream of Wnt signaling in the mouse hippocampus and controls its formation. Proc Natl Acad Sci U S A. 2007;104:12919–12924. doi: 10.1073/pnas.0609863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Mowat DR, Wilson MJ, Goossens M. Mowat-Wilson syndrome. J Med Genet. 2003;40:305–310. doi: 10.1136/jmg.40.5.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkofler V, Baeriswyl T, Ott R, Stoeckli ET. Nectin-like molecules/SynCAMs are required for post-crossing commissural axon guidance. Development. 2010;137:427–435. doi: 10.1242/dev.042515. [DOI] [PubMed] [Google Scholar]
- O’Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki HS, Wahlsten D. Timing and origin of the first cortical axons to project through the corpus callosum and the subsequent emergence of callosal projection cells in mouse. J Comp Neurol. 1998;400:197–206. doi: 10.1002/(sici)1096-9861(19981019)400:2<197::aid-cne3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Paredes MF, Li G, Berger O, Baraban SC, Pleasure SJ. Stromal-derived factor-1 (CXCL12) regulates laminar position of Cajal-Retzius cells in normal and dysplastic brains. J Neurosci. 2006;26:9404–9412. doi: 10.1523/JNEUROSCI.2575-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Piper M, Moldrich RX, Lindwall C, Little E, Barry G, Mason S, Sunn N, Kurniawan ND, Gronostajski RM, Richards LJ. Multiple non-cell-autonomous defects underlie neocortical callosal dysgenesis in Nfib-deficient mice. Neural Dev. 2009;4:43. doi: 10.1186/1749-8104-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash BG, Richards LJ. A role for cingulate pioneering axons in the development of the corpus callosum. J Comp Neurol. 2001;434:147–157. doi: 10.1002/cne.1170. [DOI] [PubMed] [Google Scholar]
- Rice R, Rice DP, Olsen BR, Thesleff I. Progression of calvarial bone development requires Foxc1 regulation of Msx2 and Alx4. Dev Biol. 2003;262:75–87. doi: 10.1016/s0012-1606(03)00355-5. [DOI] [PubMed] [Google Scholar]
- Rice R, Rice DP, Thesleff I. Foxc1 integrates Fgf and Bmp signalling independently of twist or noggin during calvarial bone development. Dev Dyn. 2005;233:847–852. doi: 10.1002/dvdy.20430. [DOI] [PubMed] [Google Scholar]
- Sanchez-Camacho C, Ortega JA, Ocana I, Alcantara S, Bovolenta P. Appropriate Bmp7 levels are required for the differentiation of midline guidepost cells involved in corpus callosum formation. Dev Neurobiol. 71:337–350. doi: 10.1002/dneu.20865. [DOI] [PubMed] [Google Scholar]
- Schmidt C, McGonnell IM, Allen S, Otto A, Patel K. Wnt6 controls amniote neural crest induction through the non-canonical signaling pathway. Dev Dyn. 2007;236:2502–2511. doi: 10.1002/dvdy.21260. [DOI] [PubMed] [Google Scholar]
- Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Shu T, Butz KG, Plachez C, Gronostajski RM, Richards LJ. Abnormal development of forebrain midline glia and commissural projections in Nfia knock-out mice. J Neurosci. 2003;23:203–212. doi: 10.1523/JNEUROSCI.23-01-00203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Richards LJ. Cortical axon guidance by the glial wedge during the development of the corpus callosum. J Neurosci. 2001;21:2749–2758. doi: 10.1523/JNEUROSCI.21-08-02749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Ohkubo Y, Maragnoli ME, Rasin MR, Schwartz ML, Sestan N, Vaccarino FM. Midline radial glia translocation and corpus callosum formation require FGF signaling. Nat Neurosci. 2006;9:787–797. doi: 10.1038/nn1705. [DOI] [PubMed] [Google Scholar]
- Sun X, Lewandoski M, Meyers EN, Liu YH, Maxson RE, Jr, Martin GR. Conditional inactivation of Fgf4 reveals complexity of signalling during limb bud development. Nat Genet. 2000;25:83–86. doi: 10.1038/75644. [DOI] [PubMed] [Google Scholar]
- Vivatbutsiri P, Ichinose S, Hytonen M, Sainio K, Eto K, Iseki S. Impaired meningeal development in association with apical expansion of calvarial bone osteogenesis in the Foxc1 mutant. J Anat. 2008;212:603–611. doi: 10.1111/j.1469-7580.2008.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Thekdi N, Smallwood PM, Macke JP, Nathans J. Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. J Neurosci. 2002;22:8563–8573. doi: 10.1523/JNEUROSCI.22-19-08563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbalis K, Siegenthaler JA, Choe Y, May SR, Peterson AS, Pleasure SJ. Cortical dysplasia and skull defects in mice with a Foxc1 allele reveal the role of meningeal differentiation in regulating cortical development. Proc Natl Acad Sci U S A. 2007;104:14002–14007. doi: 10.1073/pnas.0702618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.