SUMMARY
The effect of attention on firing rates varies considerably within a single cortical area. The firing rate of some neurons is greatly modulated by attention while others are hardly affected. The reason for this variability across neurons is unknown. We found that the variability in attention modulation across neurons in area MT of macaques can be well explained by variability in the strength of tuned normalization across neurons. The presence of tuned normalization also explains a striking asymmetry in attention effects within neurons: when two stimuli are in a neuron’s receptive field, directing attention to the preferred stimulus modulates firing rates more than directing attention to the non-preferred stimulus. These findings show that much of the neuron-to-neuron variability in modulation of responses by attention depends on variability in the way the neurons process multiple stimuli, rather than differences in the influence of top-down signals related to attention.
Attention improves perception of visual stimuli (Posner, 1980; Carrasco, 2011; Chun et al., 2011) and enhances the firing rate of cortical sensory neurons that respond to attended stimuli (Maunsell and Cook, 2002; Yantis and Serences, 2003; Reynolds and Chelazzi, 2004). Modulations of firing rate are thought to depend on top-down feedback of attention-related signals from higher cortical areas (Corbetta and Shulman, 2002; Knudsen, 2007; Bisley and Goldberg, 2010; Noudoost et al., 2010; Baluch and Itti, 2011).
It has long been recognized that the amount that attention modulates neuronal responses tends to be greater in later stages of cortical processing (see Maunsell and Cook, 2002). Even within a single cortical area there is considerable variability in modulation by attention across neurons (Moran and Desimone, 1985; Treue and Maunsell, 1996; Reynolds et al., 1999; Recanzone and Wurtz, 2000; Martinez-Trujillo and Treue, 2002; Ghose and Maunsell, 2008). This variance is seen even when neurons are recorded simultaneously (Cohen and Maunsell, 2010), indicating that it does not arise from varying levels of behavioral effort. The source of this variability in modulation by attention is unknown.
Recent models of electrophysiological and fMRI data have suggested that modulation by attention depends on normalization (Boynton, 2009; Lee and Maunsell, 2009; Reynolds and Heeger, 2009), an idea that has also been proposed using psychophysical data (Lee et al., 1999). Normalization is a form of gain control that limits the dynamic range of the responses of a neuron, particularly when more than one stimulus is present in the receptive field (Barlow, 1953; Kuffler, 1953; Baccus and Meister, 2002; Heimel et al., 2010; Olsen et al., 2010; Ohshiro et al., 2011; Papadopoulou et al., 2011). An influential divisive normalization model hypothesizes that the response of a neuron is reduced in proportion to the pooled activity of other neurons in the neighborhood (Heeger, 1992; Carandini and Heeger, 1994; Carandini et al., 1997). This model explains a broad range of response properties, in particular why the response of a neuron to an optimal stimulus is suppressed by the addition of a non-optimal, yet excitatory, stimulus to the receptive field (Morrone et al., 1982; Bonds, 1989; DeAngelis et al., 1992; Britten and Heuer, 1999; Heuer and Britten, 2002). Models of attention that incorporate divisive normalization explain the effects of attention across a broad range of behavioral and stimulus conditions (Boynton, 2009; Lee and Maunsell, 2009; Reynolds and Heeger, 2009; Lee and Maunsell, 2010).
A relationship between normalization and modulation by attention suggests an explanation for the variability in modulation by attention across neurons. Lee and Maunsell (2009) reported that the strength of the normalization mechanism can vary between neurons in the middle temporal area (MT) of macaque monkeys, and that this variance is associated with differences in attention modulation: the more potent the normalization mechanism, the greater the attention modulation. They showed that this correlation could be explained by a normalization model in which attention modulates the contrast at which neuronal responses saturate. Neurons with the most saturated responses were the least affected by normalization and attention. However, in the current study we extended the range of conditions tested and obtained new electrophysiological data that could not be accounted for using the prior model. Instead, we show that the covariance between the strength of the normalization and modulation by attention across all conditions is well explained by variance in the amount of tuned normalization. Tuned normalization (Rust et al., 2006, Carandini et al., 1997) is a variant of divisive normalization that does not weight all stimuli equally. Instead, non-preferred stimuli are given less weight in normalization. Prior studies describing normalization have not addressed how tuned normalization affects modulation by attention (Boynton, 2009; Lee and Maunsell, 2009; Reynolds and Heeger, 2009).
We found that the strength of tuned normalization varies considerably across MT neurons, and that modulation by attention depends greatly on the extent to which the normalization of a neuron is tuned. Tuned normalization also explains a pronounced asymmetry in attention modulation that occurs when attention is directed to a preferred or non-preferred stimulus in the receptive field. These results suggest that much of the variance in attention modulation between neurons may arise from differences in the amount of tuned normalization they express, rather than differences in the strength of the top-down attention signals that they receive.
RESULTS
We studied how well tuned divisive normalization can explain variation in attention modulation across neurons by recording the activity of isolated neurons in the middle temporal area (MT) of two rhesus monkeys (Macaca mulatta). We measured separately the strength of modulation by attention and strength of normalization for 117 isolated neurons (68 from monkey 1, 49 from monkey 2).
Measuring normalization and attention modulation strengths
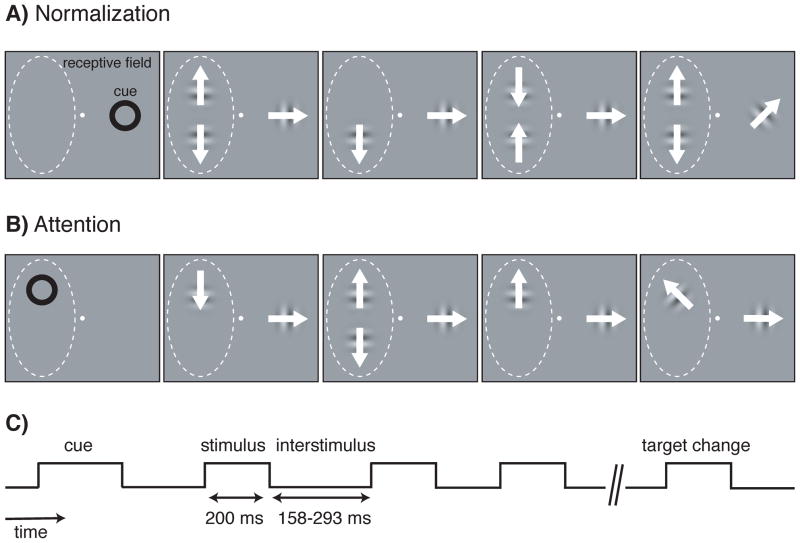
We trained each monkey to do a direction change-detection task (Figure 1). The animal fixated a spot at the center of a video monitor, then was cued by an annulus to attend to one of three locations on the monitor. Two locations were within the receptive field of the neuron being recorded. The third location was on the opposite side of the fixation point. All three stimulus locations were equidistant from the fixation point. Following the extinction of the cue, a series of drifting Gabors was presented at each of the three locations simultaneously. Each set of Gabors (one drifting Gabor per location) was presented for 200 ms with successive sets simultaneously separated by interstimulus periods that varied randomly between 158–293 ms (Figure 1C). The Gabors presented at the two locations within the receptive field drifted in either the preferred or null (180° from preferred) direction of the neuron, and the Gabors presented at the location outside of the receptive field drifted in the intermediate direction. The monkey was rewarded for detecting when a Gabor appeared at the cued location with a slightly different (< 90°) drift direction than the preceding stimulus at that location. Slight changes in the direction of motion occurred at all three locations, but the trial ended without reward if the animal responded to a slight change at an uncued location.
Figure 1.
Experimental design to measure normalization and attention modulations of firing rates. During each trial, the monkey was cued to attend to one of three locations (two within and one outside the receptive field of the MT neuron being recorded) while series of drifting Gabor stimuli (each having 0%, 50%, or 100% contrast) were presented simultaneously at the three locations. The Gabors presented within the receptive field drifted in either the preferred or null (180° from preferred) direction of the neuron, and the Gabors presented outside the receptive field drifted in the intermediate direction. The monkey was rewarded for detecting when a Gabor appeared at the cued location with a slightly different (< 90°) drift direction. A) To measure the normalization modulation strength of the neuron, attention was directed outside of the receptive field. B) To measure the attention modulation strength of a neuron, attention was directed to a location within the receptive field. C) Following the cue, stimuli were briefly presented multiple times in a trial, with blank interstimulus periods of random duration separating the presentations.
To measure the effect of normalization for each neuron (Figure 1A), we collected data while the animal was cued to attend to the location outside of the receptive field, so that spatial attention did not modulate the neuron’s rate of firing. To prevent feature attention from modulating the response, the Gabors presented at the cued location always drifted in the same direction, which was intermediate between the preferred and null directions of the neuron. While attention was directed outside the receptive field, series of Gabors were presented at the two locations within the receptive field. Whenever a pair of Gabors appeared in the receptive field, one drifted in the preferred direction for the neuron and the other drifted in the null direction, but the locations for the preferred and null stimuli were pseudorandomly selected on each presentation. Additionally, each receptive field stimulus had a pseudorandomly selected contrast of 0, 50, or 100%. Using 0% contrast meant that stimuli sometimes briefly appeared alone in the receptive field. The stimulus presentations were short (200 ms; Figure 1C) so that the animal did not have time to adjust its attention based on the contrast or number of Gabors that appeared (Williford and Maunsell, 2006; Lee and Maunsell, 2009; Lee and Maunsell, 2010).
To measure the effect of spatial attention for each neuron (Figure 1B), the animal’s attention was directed to one of the two locations within the receptive field. The drifting Gabors within the receptive field were independently and pseudorandomly set to a contrast of 0 or 100% on each presentation. One Gabor within the receptive field drifted in the preferred direction and the other drifted in the null direction. For most neurons (72 of 117) drift direction was pseudorandomly assigned to the receptive field locations for each short stimulus presentation so that the animal did not have time to adjust its attention based on the direction at the attended location during the short stimulus presentation. If the animal responded to a direction change from preferred to null or vice versa (i.e., 180° direction change) the trial was terminated without reward. For the remaining neurons (45/117) the locations of the preferred and null directions were fixed, but results from those neurons were not significantly different. By presenting the Gabors at 0 or 100% contrast, we could measure attention with one or two stimuli in the receptive field.
Tuned normalization
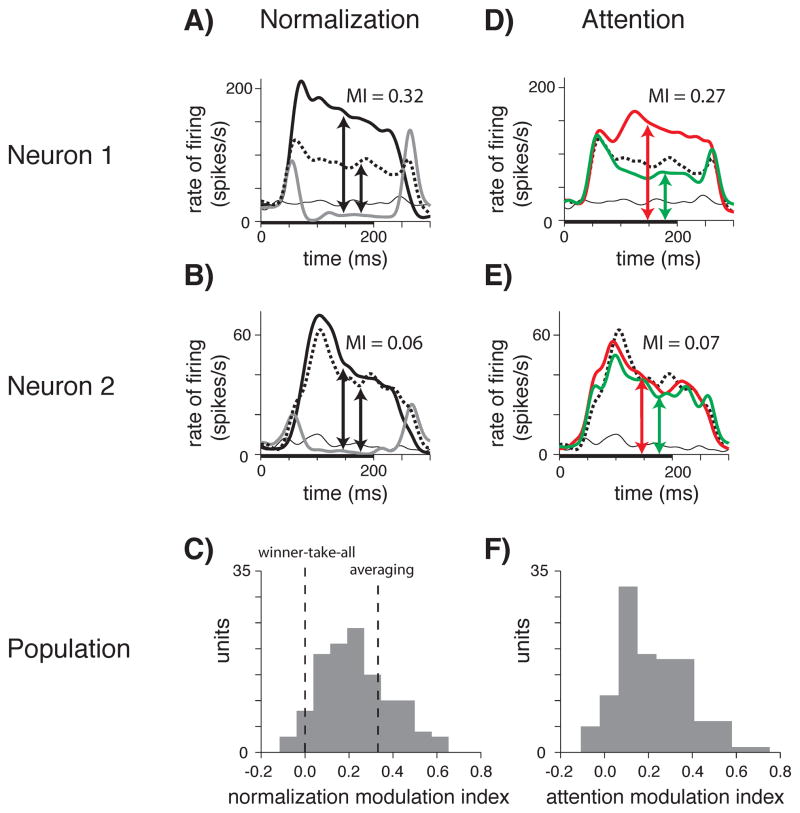
Different MT neurons showed different degrees of normalization. Figure 2A shows responses from a neuron with pronounced normalization. The average response to a preferred direction alone (in either receptive field location; thick black line) was substantially reduced when a null stimulus was added to the other receptive field location (dashed line). The response to preferred and null stimuli together was approximately the average of the responses to the preferred stimulus alone (thick black line) and the null stimulus alone (gray line).
Figure 2.
Different MT neurons show different degrees of normalization and attention modulation. A) “Averaging” neuron: for neuron 1, peristimulus time histograms (PSTH) show that the average response to the preferred and null stimuli together (dashed line) was approximately the average of the responses to the preferred stimulus alone (thick black line) and null alone (gray line). B) “Winner-take-all” neuron: for neuron 2, the response to the preferred stimulus alone (thick black line) was only slightly reduced when a null stimulus was added to the receptive field (dashed line), though the neuron hardly responded to the null stimulus alone (gray line). C) A histogram displaying the normalization modulation indices (MI) of the population illustrates that MT neurons span a range of normalization strengths from “winner-take-all” to “averaging” (dashed lines indicate respective ideal MI). D) Attending the preferred (red line) versus the null (green line) of two stimuli in the receptive field greatly modulates the firing rates of neuron 1 (A). E) Attention does not strongly modulate firing rates of neuron 2 (B). F) A histogram displaying attention MI of the population illustrates that MT neurons span a range of attention modulation strengths. A, B, D, E) For all PSTH: arrows indicate the two measurements (X, Y) taken to calculate an MI, (X − Y)/(X + Y); thick bars along the x-axis indicate the timing of the stimulus presentation; each PSTH was smoothed by a Gaussian window (SD 10 ms); both dotted and thin black lines are plotted in each PSTH, dotted lines indicate the response to the preferred and null stimuli together with attention outside of the receptive field, thin black lines indicate the spontaneous firing rate.
An intermediate response of this sort is expected from normalization and can be described by the equation (modified from Carandini et al., 1997):
| (1) |
where cP and cN are the contrasts of the two Gabors, LP and LN are the responses of the linear receptive field to the individual Gabors at unit contrast, and σ is a positive term that represents the semisaturation constant for the contrast response function of the neuron. The divisive normalization of the neuron’s firing rate is mediated by the denominator, with cP and cN representing the normalization activity associated with the preferred and the null stimuli. In this equation, the neuron’s preference for one direction of motion over the other is captured by LP and LN in the numerator, but the stimulus-related terms in the denominator depend only on the contrast of the stimuli, irrespective of the direction of motion, and are therefore “untuned” in terms of the direction of stimulus motion. This equation does an excellent job of capturing the inhibition of the firing rate due to the null stimulus for neurons such as the one shown in Figure 2A, which effectively averages the responses to preferred alone and null alone when they appear together.
Other MT neurons were less affected by the addition of a null stimulus to a preferred stimulus. For another neuron (Figure 2B), the average response to the preferred stimulus alone (thick black line) was only slightly reduced when a null stimulus was added to the receptive field (dashed line), although the neuron responded hardly at all to the null stimulus alone (gray line). For this neuron, the response to preferred and null together was much closer to the response to the preferred stimulus alone than it was to the average of the responses to preferred alone and null alone. The response of this neuron was therefore more like a “winner-take-all” response, with the stronger individual response determining the response to the pair.
For most MT neurons, the effect of adding a null stimulus to a preferred stimulus fell between “averaging” (neuron 1, Figure 2A) and “winner-take-all” (neuron 2, Figure 2B). To quantify the strength of normalization for each neuron, we calculated a modulation index based on responses to different stimuli, [(Preferred − Null) − (Both − Null)]/[(Preferred − Null) + (Both − Null)]. When stimuli have contrasts that are well into the upper saturation of the contrast response function (cP = cN ≫ σ), as is generally the case for contrasts of 50% and 100% in MT (Sclar et al., 1990), this index is 0.33 for “averaging” neurons that respond to preferred and null together with a response that is the average of the responses to preferred and null presented individually, and 0 for “winner-take-all” neurons that give the same response to the preferred and null together as they do to the preferred alone. Correspondingly, the normalization modulation indices for the neurons in Figures 2A and B were 0.32 and 0.06. The histogram in Figure 2C plots the distribution of normalization modulation indices for all 117 MT neurons, and shows that MT neurons spanned the full range of normalization from averaging to winner-take-all, and some distance on either side.
This range of behaviors from MT neurons cannot be explained by differences in selectivity for preferred over null stimuli. Neurons with winner-take-all behavior are usually highly direction selective (e.g., Figure 2B, see below), as are most MT neurons. We found no correlation between normalization modulation index and direction selectivity modulation index [(Preferred − Null)/(Preferred + Null)] across the population of MT neurons (R = 0.11, p = 0.25).
Equation 1 dictates that adding a null stimulus at 100% contrast (cN = 1 ≫ σ) to a receptive field containing a preferred stimulus also at 100% contrast (cP = 1 ≫ σ) should always produce a response to the two stimuli together that is approximately the average of the responses to the two stimuli separately (i.e., normalization modulation index of 0.33). Consequently, Equation 1 cannot account for the range of normalization modulation indices seen among MT neurons (Figure 2C). The differences between MT neurons can be readily explained by tuned normalization, in which different stimuli contribute differentially to normalization. Tuned normalization has been described for MT before (Rust et al., 2006), and can be captured by adding a term that adjusts the contributions of different stimuli to normalization (modified from “anisotropic normalization” of Carandini et al., 1997):
| (2) |
Here α scales how much the null stimulus contributes to normalization relative to the preferred stimulus. When α is 1 an average response results, and when α is 0 the response is winner-take-all. We will take this approach to explain the variability in the normalization of MT neurons, and show that this variability in tuned normalization accounts for much of the variability in the attention modulation of MT neurons.
Normalization and attention modulation strengths are correlated
Differences in normalization between neurons were correlated with differences in the strength of modulation by attention. Figures 2D and E plot the effects of spatial attention on the responses of neurons 1 and 2 (Figures 2A and B). These neurons differed greatly in the extent to which they were modulated by attention. When both the preferred and the null stimuli were presented in the receptive field of neuron 1 (Figure 2D), responses were much stronger when attention was directed to the location containing the preferred stimulus (red) than when attention was directed to the location containing the null stimulus (green). Strong modulation from shifting spatial attention between preferred and null stimuli in the receptive field has been described many times in a variety of cortical areas (Moran and Desimone, 1985; Treue and Maunsell, 1996; Reynolds et al., 1999; Recanzone and Wurtz, 2000; Martinez-Trujillo and Treue, 2002; Ghose and Maunsell, 2008). In contrast, Figure 2E shows that attention had much less effect on the responses of neuron 2 (Figure 2B). For each neuron, we calculated an attention index, (Attend Preferred − Attend Null)/(Attend Preferred + Attend Null). The attention indices for the neurons in Figures 2D and E are 0.27 and 0.07. As shown in Figure 2F, the responses of some MT neurons were virtually unmodulated by attention (0) while the responses of others were modulated by a factor of three (0.5) or more.
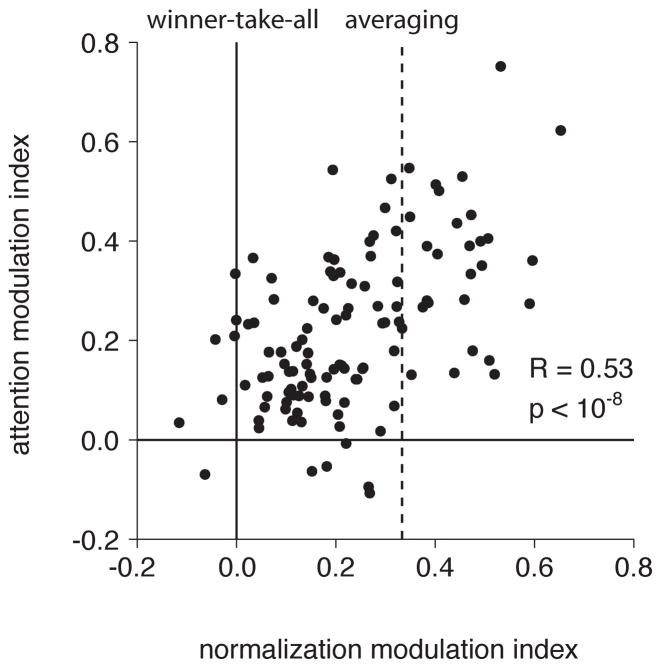
Modeling studies have suggested that modulation by attention may depend on normalization mechanisms (Boynton, 2009; Lee and Maunsell, 2009; Reynolds and Heeger, 2009) and one neurophysiological study showed that there is a neuron-to-neuron correlation between the strength of normalization of MT neurons and the strength of their modulation by spatial attention (Lee and Maunsell, 2009). The current data confirm that neurons with pronounced normalization modulation also show pronounced modulation by attention. Figure 3 shows the relationship between normalization and attention modulations across neurons in our sample (R = 0.53, p < 10−8). As normalization approaches zero, modulation by attention approaches zero.
Figure 3.
The strength of normalization modulation is correlated with the strength of attention modulation across the population of MT neurons. The normalization modulation indices of ideal “winner-take-all” and “averaging” neurons are indicated by solid and dashed lines, respectively.
It is important to recognize that a correlation between modulation by normalization and modulation by attention could depend in part on differences in direction selectivity: a neuron that did not discriminate between preferred and null directions and therefore responded equally to both would not be expected to show any normalization or any attention modulation. However, the direction selectivities (preferred:null) of the MT neurons are high (average of 9:1 in our sample), and we found no significant correlation between the normalization modulation indices for the neurons we recorded and their direction selectivity (R = 0.11, p = 0.25). Furthermore, the partial correlation between normalization and attention modulation controlling for variance in direction selectivity across neurons remains highly significant (R = 0.52, p < 10−8).
Because tuned normalization affects how a neuron weights two different stimuli that drive that neuron with different efficacy, we hypothesize that the variance in tuned normalization is the source for the variance in attention modulation. For example, because a winner-take-all neuron largely disregards the presence of a non-preferred stimulus, attention to a non-preferred stimulus may have little effect on the response of that neuron. In contrast, an averaging neuron that gives equal weight to preferred and null stimuli may show much wider swings in response when attention modulates inputs associated with one or the other.
Asymmetry of attention modulation strengths within neurons
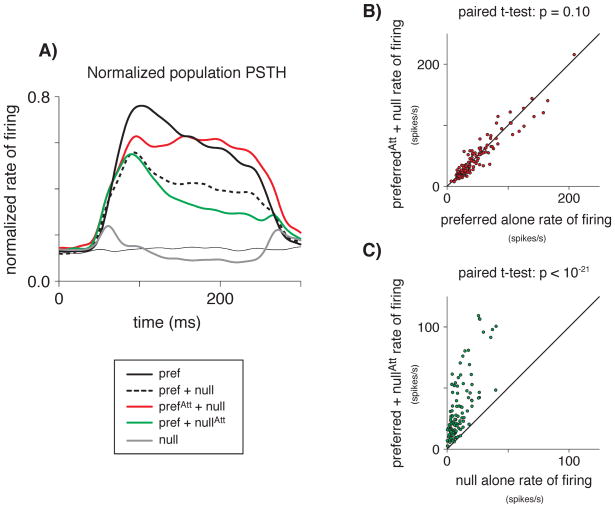
Tuned normalization might also account for a striking asymmetry in attention effects that we observed in our data. With two stimuli in the receptive field, modulation by attention is greater with attention to the preferred versus attention to the null stimulus in the receptive field. Figure 4A shows the average population responses to different stimulus and attention conditions. As described for individual neurons above (Figure 2), when the animal’s attention is directed outside the receptive field the response to the preferred and null stimuli in the receptive field (dashed line) is intermediate between the response to preferred alone (thick black line) or null alone (gray line). Attention to the preferred stimulus in the presence of the null stimulus increases the response (red), bringing it close to the response to the preferred stimulus alone (thick black line). This effective elimination of the non-preferred stimulus by attention has been described previously (Reynolds and Desimone, 1999; Reynolds et al., 1999; Recanzone and Wurtz, 1999). However, attention to the null stimulus in the presence of the preferred stimulus decreases the response relatively little (green), leaving it well above the response to the null stimulus alone (gray line).
Figure 4.
Tuned normalization can account for an asymmetry in attention effects between attending-preferred and attending-null. A) Normalized population PSTH. Compared to the response to two stimuli in the receptive field when attention is directed outside of the receptive field (dashed line), the modulation of neuronal responses due to attending the preferred stimulus (red line) is greater than the modulation due to attending the null stimulus (green line) for the population. B) When both the preferred and the null stimuli are in the receptive field, attention to the preferred stimulus (y-axis) makes the firing rate of a neuron indistinguishable from the firing rate for the preferred stimulus presented alone (x-axis). C) Attending to the null of the two stimuli (y-axis) does not return the firing rate of a neuron to the firing rate for the null stimulus presented alone (x-axis).
With two stimuli in the receptive field, the average attention index for attention to the preferred stimulus, (Attend Preferred − Attend Out)/(Attend Preferred + Attend Out), is 0.15. The average attention index for attention to the null stimulus, (Attend Out − Attend Null)/(Attend Out + Attend Null), is 0.08. Attention modulation with attention to the preferred stimulus is greater across the population of MT neurons (paired t-test: p < 0.01).
Though attention to one of two stimuli in a receptive field has been hypothesized to almost completely eliminate the influence of the unattended stimulus, regardless of whether the attended stimulus is preferred or null (Reynolds and Desimone, 1999; Reynolds et al., 1999), the asymmetry in attention effects in MT is further illustrated in Figures 4B and C. The scatterplots show the effects of attention to the preferred and null stimuli for each MT neuron recorded. When the preferred and null stimuli are both in the receptive field, attention to the preferred stimulus makes the firing rate of the neuron indistinguishable from the firing rate for the preferred stimulus presented alone (paired t-test: p = 0.10, Figure 4B). However, attending to the null stimulus does not decrease the firing rate of the neuron to the level of the firing rate for the null stimulus presented alone (paired t-test: p < 10−21, Figure 4C). Because the preferred and the null stimuli were presented pseudorandomly and very briefly at the attended location within trials, this difference cannot be attributed to different levels of attention to the two types of stimuli. We found, however, that tuned normalization predicts a strong asymmetry in attention modulation.
A tuned normalization model of attention
To explore the extent to which tuned normalization can explain the range and asymmetry of attention modulations in MT, we extended Equation 2 to include modulation by attention:
| (3a) |
| (3b) |
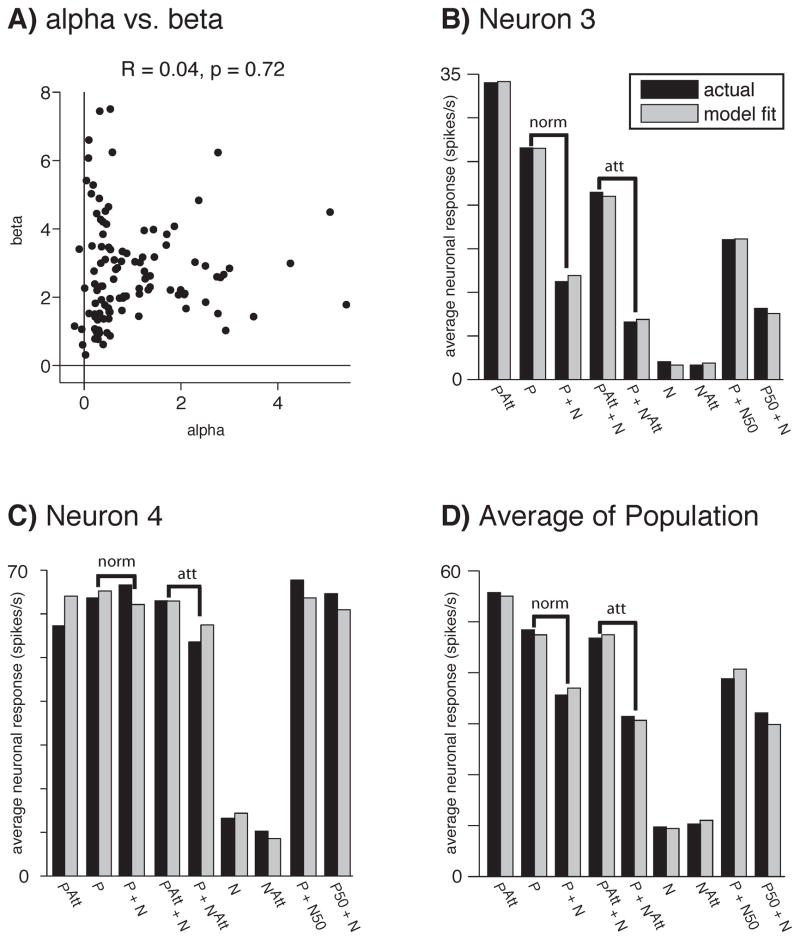
In these equations β is a factor that increases the weight of the attended stimulus (the preferred stimulus in the case of 3a and the null stimulus in the case of 3b). To determine how well the model fit the neuronal data, average firing rates per neuron for nine stimulus conditions (plotted along the x-axes in Figures 5B–D) were fit to Equations 3a and 3b.
Figure 5.
Model fits of the data. A) When the top-down attention signal parameter (β) is fit as a free parameter of the model, β determinations are not correlated with determinations of α, the tuned normalization parameter (seven neurons were excluded due to extreme parameter fits (α or β > 10), but for the remaining 110 neurons plotted here, β was still not correlated with α). B) Even with β fixed at 2.75, the model (gray) provided an excellent fit for the average firing rates (black) of an example “averaging” neuron, C) as well as for an example “winner-take-all” neuron. D) The model also provided an excellent fit of the population, fitting the asymmetrical attention effects of attending the preferred (PAtt + N) versus the null stimulus (P + NAtt) in the receptive field, as compared to attention out of the receptive field (P + N). The indicated stimuli presented (preferred: P, null: N) were presented at 100% contrast unless otherwise noted (e.g., preferred at 100% and null at 50% contrast: P + N50), with attention (Att) or without attention directed to a stimulus in the receptive field. The modulations in firing rates due to normalization and attention are indicated by “norm” and “att” above the bar plots (B–D).
Variations in the parameter β correspond to neuron-to-neuron differences in the top-down attention signal. There are two hypothetical mechanisms by which attention modulations of firing rates could become correlated with the strength of normalization of the MT neurons: 1) the top-down attention signal per sensory neuron could co-vary with the normalization strength of each sensory neuron, or 2) variance in the tuned normalization mechanism alone could result in attention modulation variance across the neurons.
To test the first hypothesis, we determined whether or not the top-down attention signal parameter (β) is correlated with the tuned normalization parameter (α) across neurons. When β and α are fit as free parameters in Equation 3 (along with free parameters LP, LN, and σ) the value of β is not significantly correlated with α (Figure 5A). The attention signal (β) did not co-vary with the normalization strength (normalization modulation index) of each sensory neuron (R = 0.06, p = 0.55). Therefore, in subsequent analyses we fixed β at 2.75 (its mean when estimated as a free parameter) for all neurons (see Methods), to determine whether variance in the tuned normalization parameter alone could result in attention modulation variance across the neurons.
Even with β fixed, Equation 3 provided an excellent fit of the data based on the four remaining free parameters (α, LP, LN, σ). Using this approach Equation 3 explained > 99% of the variance in the mean responses for a particularly well-fit “averaging” neuron (neuron 3, Figure 5B), which demonstrated a strong normalization (P versus P+N) and a large attention modulation (PAtt+N versus P+NAtt). Similarly, Equation 3 explained 97% of the variance in the mean responses of the particularly well-fit “winner-take-all” neuron (neuron 4, Figure 5C) that demonstrated minimal normalization and attention modulation. Across the entire sample of MT neurons, the average explained variance was 95% (Figure 5D).
Equation 3 not only accommodates broad ranges of normalization and modulation by attention, but also accounts for the asymmetric effects of attending the preferred versus the null stimulus in the receptive field (Figure 4). Figure 5D shows that across the sample of MT neurons, attending to the preferred stimulus (PAtt+N) elevated responses substantially above the responses to the same stimuli with attention directed outside the receptive field (P+N), but attending to the null stimulus (P + NAtt) caused less modulation of responses.
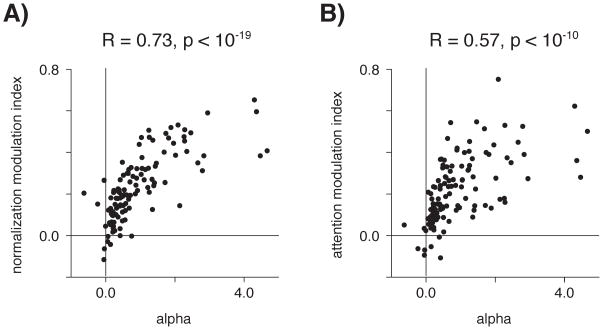
Because the attention term (β) was fixed for these fits, it cannot explain the difference in attention modulation between the “averaging” and “winner-take-all” neurons shown in Figures 5B & 5C, nor the asymmetric effect of attending to preferred and null stimuli. Instead, these effects can be attributed to the tuned normalization. When neuronal responses were fit using Equation 3 (with β fixed at 2.75), only the parameter associated with tuned normalization (α) had a significant partial correlation with normalization modulation indices while controlling for the variability in attention modulation indices (Spearman’s ρ = 0.73, p < 10−19, Figure 6A) and also with attention modulation indices while controlling for the variability in normalization modulation indices (Spearman’s ρ = 0.57, p < 10−10, Figure 6B, Bonferroni correction for multiple comparisons). None of the three remaining free parameters were significantly correlated with attention modulation while controlling for the variability in normalization modulation indices (LP: R = 0.16, p = 0.10; LN: R = −0.05, p = 0.57; σ: R = 0.19, p = 0.04; Bonferroni corrected), nor was direction selectivity (calculated as the ratio of LP:LN, R = −0.10, p = 0.31).
Figure 6.
Only the parameter describing the strength of the tuned normalization of the neuron (α) had a significant correlation with: A) normalization modulation indices (controlling for the variance in attention modulation indices), and (B) attention modulation indices (controlling for the variance in normalization modulation indices).
Correspondingly, no significant partial correlation exists between normalization and attention modulation indices when controlling for the variance in α (R = 0.15, p = 0.10). The partial correlation remains significant when controlling for the variance in any other parameter (LP: R = 0.54, p < 10−9; LN: R = 0.50, p < 10−8; σ: R = 0.50, p < 10−8; LP:LN: R = 0.51, p < 10−8).
Superficially, it might appear that attention and normalization are symmetric, and that one might equally well fix the tuned normalization term (α) and explain variance in normalization by differences in the feedback attention signal (β). This is not possible, however, because measurements of the strength of normalization were made in a single attention state with attention directed outside the receptive field. In that condition attention acts equally on both stimuli in the receptive field (Equation 2) and cannot modulate normalization. That is, attention always occurs on a background of some amount of tuned normalization, but normalization occurs in the absence of differential attention.
To further ensure that the α term for each neuron described tuned normalization, and not variations in the attention gain factor (β), we also fit the firing rates for eight stimulus conditions that were recorded with attention fixed to the stimulus location outside of the receptive field (see Methods). The average explained variance for the population of neurons using these eight single and paired stimulus conditions was 97%. The α terms from these fits were highly correlated with those from the fit to the normalization conditions plus the four attention conditions illustrated in Figure 4 (R = 0.81, p < 10−27). Therefore, directing attention to the receptive field of each neuron did not strongly modulate the value of α. Furthermore, when we applied β = 2.75 in Equation 3 to the parameters obtained by fitting the eight normalization conditions (attention directed away from the receptive field), 94% of the variance in average responses was explained for the four attention conditions (attention directed to the receptive field). Therefore, fitting the free parameters of the model to the normalization conditions alone, then applying β = 2.75 according to Equation 3, was enough to predict the firing rate effects of attention per neuron.
DISCUSSION
Relationship between attention and normalization
Our results show that a significant portion of the variance in attention modulation across neurons in MT can be attributed to variance in normalization strengths across neurons. Importantly, this correlation is not dependent on the tuning of the neurons to the individual stimuli presented. Even when neurons strongly differentiate between preferred and null stimuli, different neurons respond differently when a null stimulus is added to a preferred stimulus. This variation can be attributed to differences in tuned normalization. For neurons with normalization that is not tuned (α = 1) a null stimulus that does not drive a response will nevertheless be factored into normalization, causing them to respond much less when a null stimulus is paired with preferred stimulus. For neurons with highly tuned normalization (α = 0), a null stimulus not only fails to produce a response but also is effectively prevented from contributing to normalization, such that the response to the preferred stimulus is unaffected by the addition of a null stimulus to the receptive field. While many studies have investigated the biophysical mechanisms underlying the normalization mechanism in general (Abbott et al., 1997; Carandini et al., 1997; Shadlen and Newsome, 1998; Carandini et al., 2002; Chance et al., 2002; Mitchell and Silver, 2003; Prescott and De Koninck, 2003; Carandini and Heeger, 1994; Finn et al., 2007; Buia and Tiesinga, 2008, Kouh and Poggio, 2008; Priebe and Ferster, 2008, Chaisanguanthum and Lisberger, 2011), the biophysical mechanisms underlying tuned normalization are not known.
Several reports have shown how normalization can explain the large modulations that are seen when attention is shifted between preferred and null stimuli in the receptive field of a neuron (Boynton, 2009; Lee and Maunsell, 2009; Reynolds and Heeger, 2009). Because responses to the preferred and null stimuli contributed both to the excitatory drive and also to divisive normalization, relatively modest modulations of the inputs associated with each stimulus are effectively amplified by the normalization mechanism. Strongly tuned normalization effectively removes a null stimulus from normalization, and therefore removes the basis for the strong modulations by attention that can occur from shifting attention between preferred and null stimuli. When tuned normalization completely negates the null stimulus, modulation by attention is reduced to the modest level seen when shifting attention between an isolated preferred stimulus and a stimulus far outside the receptive field. The wide range of modulation by attention across our neurons could be explained based on the amount of tuned normalization (α) even when we held the signal from attention (β) fixed across neurons, simulating the unrealistic scenario in which attention allocation remained constant despite differences in stimulus size, location, direction, and separation.
Although it has been suggested that attention might modulate responses by specifically adjusting suppressive mechanisms associated with normalization (Lee and Maunsell, 2009; Sundberg et al., 2009), our analysis shows that this might not be the case. The correlation between attention and normalization strengths across neurons can arise from attention modifying the inputs associated with the attended stimulus (β of Equation 3; see also Ghose and Maunsell, 2008). Attention did not act selectively on normalization in our model, and fitting different attention conditions did not significantly change the tuned normalization parameter (α).
Other studies
Previous reports have described relationships between stimulus interactions and modulation by attention based on stimulus selectivity (Reynolds, et al., 1999; Reynolds and Desimone, 2003) or stimulus location compared to the vertical meridian (Chelazzi et al., 1998), which are distinct from the relationship we describe here. The current study describes a relationship based on tuned normalization: when a neuron’s normalization is highly tuned, adding a null stimulus to a preferred stimulus has little effect on that neuron’s response, and shifting attention between the preferred and null stimuli modulates the response very little. There is an alternative way in which a second stimulus may fail to affect a neuron’s response, regardless of whether normalization is tuned. If a second preferred stimulus is added to a first preferred stimulus, normalization models predict no change in response, whether that normalization is tuned or not. Correspondingly, when attention is shifted between two preferred stimuli in a neuron’s receptive field, the shift will cause little modulation (Lee and Maunsell, 2010). This alternative form of correlation between stimulus interactions and modulation by attention described by prior studies (Reynolds, et al., 1999; Reynolds and Desimone, 2003) depends on presenting neurons with stimuli that evoked the same response when presented individually. Neither normalization nor attention is expected to function with two equivalent stimuli. Tuned normalization is needed to explain the failure of normalization and attention modulations in the current results, where stimuli evoked markedly different responses (an average response ratio of 9:1 for preferred versus null).
Several recent reports have shown that divisive normalization models can explain a variety of attention effects (Boynton, 2009; Lee and Maunsell, 2009; Reynolds and Heeger, 2009); however, none addresses the importance of tuned normalization in determining the strength of attention modulation. A previous report from our lab (Lee and Maunsell, 2009) described the same correlation between the strength of normalization and the strength of modulation by attention across neurons reported here. However, that report did not identify tuned normalization as the source of this difference. Instead it suggested that for some neurons the normalization mechanism could saturate at low to moderate contrasts, so that manipulating contrasts or attention when using moderate to high contrast stimuli would have no effect on the responses of those neurons. That explanation, however, cannot explain why the responses of some neurons are unaffected by adding a null stimulus to a preferred stimulus (a condition that was not examined for the neuronal responses in the prior report). Nor can it account easily for the asymmetric effects of attending to preferred and null stimuli (Figure 4, also not examined in the earlier report). For these reasons we believe that tuned normalization provides a better explanation than saturated normalization for the range of effects from normalization and attention described in this study.
While the effect of tuned normalization on the modulation of responses by attention has not been previously treated, tuned normalization has been described before. Carandini and colleagues (1997) address the possibility of tuned normalization in macaque V1. They found little evidence for tuned normalization when testing neurons with superimposed gratings that had different orientations, although they noted that their study was not designed to provide a strong test of the extent of tuned normalization.
Rust and colleagues (2006) used a model that included tuned normalization to account for the responses of MT neurons to plaid stimuli. They found that tuned normalization was needed to model the MT responses, and more pronounced tuned normalization was needed for pattern cells than for component cells. Their results suggest that the neurons we recorded with strong tuned normalization and little attention modulation may tend to be pattern selective cells.
Hints of tuned normalization have also been seen in the responses of V4 neurons. While the responses of most V4 neurons to a preferred stimulus are reduced by the addition of a less preferred stimulus to the receptive field, for some neurons the addition of a less preferred stimulus has little or no effect (Figure 4 of Reynolds et al., 1999). Tuned normalization might be widespread in sensory cortex and perhaps throughout cortical processing.
Asymmetry of attention modulation
When the effects of attention with two stimuli inside a receptive field were first described by Moran and Desimone (1985), they suggested that attention gates visual processing by filtering out irrelevant stimuli from within the receptive field. Consistent with this idea, Reynolds and Desimone (1999) reported that attention almost precisely eliminates the contribution of an unattended stimulus, whether it is preferred or non-preferred. However, we found a pronounced asymmetry in the effects of attending to preferred and null stimuli in the receptive fields of MT neurons (Figures 4B & C).
Although this asymmetric effect of attention can be seen in previously reported data from MT (Lee and Maunsell, 2010), we are unaware of any treatment of its origins. However, some existing models of the effects of attention can account for this asymmetry (Ghose and Maunsell, 2008; Lee and Maunsell, 2009). Tuned normalization provides a ready explanation for this asymmetric effect of attention. In Equation 3B attention to a null stimulus can be largely discounted with tuned normalization. Its effect on direct excitatory drive is small because the stimulus is not preferred (LN ~ 0), and its effect on normalization is small because it is weighted by the tuning of the normalization (α < 1). The ability of tuned normalization to account for both the range of modulation of neuronal responses when shifting attention between a preferred and null stimulus in the receptive field and for the asymmetry of this modulation gives strong support to its importance in both sensory processing and modulation by attention.
While attention to the preferred stimulus when it was paired with a null stimulus brought responses close to those seen when the preferred stimulus was presented alone, this should not be viewed as an invariant outcome from attention to a preferred stimulus. The amount by which attention modulates neuronal responses depends greatly on the effort that the subject puts into the task (Spitzer et al., 1988, Boudreau et al., 2006). It is likely that if the direction change-detection task had been easier (e.g., the changes were much larger), the monkeys would have directed less attention to the cued location. We expect that the asymmetry in the modulations from attention to the preferred stimulus versus attention to the null stimulus would persist as the absolute magnitude of the modulations varied, but that will need to be tested experimentally.
EXPERIMENTAL PROCEDURES
All experiments followed the protocols approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Animal preparation and behavioral task
Two male rhesus monkeys (Macaca mulatta) weighing 8 and 12 kg were each implanted with a head post and a scleral search coil under general anesthesia. Following recovery, each animal was trained on a motion direction change-detection task. Throughout each trial, the animal maintained fixation within ±1° of a small white spot presented at the center of a monitor (44° × 34°, 1024×768 pixels, 75 Hz refresh rate, gamma-corrected) on a gray background (42 cd/m2) until the change detection. On each trial, the fixation point was presented for 250 ms, and then an annulus was presented for 250 ms to cue the animal to attend to one of three locations on the monitor. Two of the locations were within the receptive field of the neuron being recorded, and the third location was at a symmetric location on the opposite side of the fixation point. All three locations were the same eccentricity from the fixation point. Next, a series of drifting Gabors was presented at each of the three locations simultaneously, each set of Gabors presented for 200 ms with successive sets separated by interstimulus periods that varied randomly between 158–293 ms. The two Gabors presented inside of the receptive field were presented at locations separated by at least 5 times the SD of the Gabors (mean Gabor SD 0.45°, SD of Gabor SD 0.04°, Gabor SD range 0.42–0.50°, mean separation of Gabor centers 4.2°, SD 0.86°, range 2.2–6.9°). Receptive fields in MT are large (Desimone and Ungerleider, 1986) and thus could readily accommodate two stimuli within them.
The goal of the animal was to detect when a Gabor appeared at the cued location with a slightly different (< 90°) drift direction (target). The animal indicated this detection by making a saccade directly to the Gabor with the different drift direction within 100–600 ms of its presentation. The animal was rewarded for correct change detections with drops of juice. Changes in direction occurred at the two uncued locations (distractors) with the same probability as changes in drift direction at the cued location, but the trial ended without reward if the animal responded to the distractors. The timing of the appearance of the target stimulus followed an exponential distribution (a flat hazard function for direction change) to encourage the animal to maintain an attention level that was constant with time. If a trial reached 6 s without a direction change occurring at the cued location (about 20% of trials), the trial was terminated and the animal was rewarded for maintaining fixation.
For each recorded neuron, normalization and attention modulations on firing rates were measured (see Results) independently in blocks, and at least two complete blocks of each data type were collected for each neuron. The degree of direction change of the target was adjusted independently for each of the three stimulus locations for each neuron using an adaptive staircase procedure (QUEST, Watson and Pelli, 1983) to maintain the behavioral performance at 82% correct across all target locations.
Electrophysiological recordings
After the animals were trained on the behavioral task, a recording chamber was implanted on each animal to allow a posterior approach to MT (axis ~22–40° from horizontal in a parasagittal plane). Recordings were made with glass-insulated Platinum-Iridium microelectrodes (~1 MΩ at 1 kHz). The dura was penetrated using a guide tube and grid system (Crist et al., 1988). Extracellular signals were filtered between 250 Hz-8 kHz, amplified, and digitized at 40 kHz. Action potentials from individual neurons were isolated using a window discriminator, and spike times were recorded with 1 ms resolution.
Once a single neuron was isolated, the receptive field location was estimated using a hand-controlled visual stimulus. Computer-controlled presentations of Gabor stimuli were then used to measure tuning for direction (8 directions) and temporal frequency (5 frequencies) while the animal performed a fixation task. The direction that produced the strongest response was used as the preferred direction, the opposite direction was used as the null direction, and a direction 90° from the preferred direction was used as the intermediate direction. The temporal frequency that produced the strongest response was used for all of the Gabors. The temporal frequency was rounded to a value that produced an integral number of cycles of drift during each stimulus presentation, so that the Gabors started and ended with odd spatial symmetry, such that the spatiotemporal integral of the luminance of each stimulus was the same as the background. Spatial frequency was set to 1 cycle per degree for all of the Gabors. The preferred Gabor was used to quantitatively map the receptive field (3 eccentricities and 5 polar angles) while the animal performed a fixation task. The two stimulus locations within the receptive field were chosen to be at equal eccentricities from the fixation point and to give approximately equal responses, and the third location was 180° from the center point between the two receptive field locations, at an equal eccentricity from the fixation point as the other locations.
Data analysis
Neurons were included in the analysis if they were held for at least two blocks each of both the normalization and attention data collection, presented in alternating blocks. Approximately 13 repetitions of each stimulus condition were collected per block. Data analysis was performed on the response period of 50–250 ms after the stimulus onset. Firing rates for each stimulus condition of each neuron were determined by taking the average firing rate during this analysis period across all stimulus repetitions. Stimuli presented at the same time as a target or distractor stimulus were excluded from analysis, as were stimuli that appeared after the target, and the first one or two stimulus presentations (within 400 ms) of each stimulus series to reduce variance that could arise from stronger responses to the start of a stimulus series.
Modulation indices for the modulations of firing rates reported in this study were calculated using a normalization modulation index, [(Preferred − Null) − (Both − Null)]/[(Preferred − Null) + (Both − Null)], or an attention modulation index, (Attend Preferred − Attend Null)/(Attend Preferred + Attend Null). The asymmetry in attention modulation with two stimuli in the receptive field comparing attention to the preferred versus attention to the null stimulus was determined by calculating an attention index for attention to the preferred stimulus, (Attend Preferred − Attend Out)/(Attend Preferred + Attend Out), and an attention index for attention to the null stimulus, (Attend Out − Attend Null)/(Attend Out + Attend Null). The modulation index for neuronal direction tuning was calculated using a tuning index, (Preferred − Null)/(Preferred + Null). All indices were determined using the average firing rate responses to the indicated stimulus conditions with the indicated stimuli at 100% contrast.
Equation 3 was fit using four free parameters (LP, LN, σ, α, see Results for definitions). A fifth parameter (β) was fixed at 2.75, the average β determination when β was allowed to be a free parameter, for all of the neurons. The model parameters were fit via unconstrained nonlinear optimizing that minimized the sum-of-squares error. The model parameters were constrained in the fit to be greater than 0, but there were no other constraints on the model fits. The goodness of fit of the model was calculated for each neuron as the total explained variance, which was determined by taking the square of the correlation coefficient between the estimated firing rates from the model and the firing rates of the neuron across the stimulus conditions fit by the model.
For the main experiment, nine stimulus conditions were fit by the model to determine the free parameter estimations: five conditions with spatial attention directed outside of the receptive field, four conditions with spatial attention directed inside of the receptive field. As a control to ensure that the α term estimations were not biased by the four stimulus conditions with spatial attention directed to the receptive field (see Results), eight stimulus conditions with spatial attention directed outside of the receptive field and to the intermediate direction of motion were fit to the model to determine α term estimations without the influence of attention. These conditions were: Preferred 50% contrast, Preferred 100% contrast, Null 50% contrast, Null 100% contrast, Preferred 50% contrast + Null 50% contrast, Preferred 100% contrast + Null 50% contrast, Preferred 50% contrast + Null 100% contrast, Preferred 100% contrast + Null 100% contrast. A value of β = 2.75 was applied according to Equation 3 to the α, LP, LN, and σ determinations from these eight sensory interaction conditions, to determine how well the free parameters determined by the eight stimulus interaction conditions alone fit the data collected during the attention conditions. The model provided an excellent fit of the attention conditions using a value of β = 2.75 and the predetermined α, LP, LN, and σ estimations.
P-values were computed for Pearson’s linear correlation coefficients using a Student’s t distribution, unless it was noted that a Spearman’s rho was determined instead, in which case the p-values were computed using large-sample approximations. A Bonferroni correction was applied in the case of multiple comparisons.
Acknowledgments
We thank Marlene Cohen, Incheol Kang, and Patrick Mayo for helpful comments and discussions. We thank Anna Chambers, Steven Sleboda, Jon Hendry, and Vivian Imamura for technical assistance. This work was supported by National Institutes of Health R01EY005911 and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron. 2002;36:909–919. doi: 10.1016/s0896-6273(02)01050-4. [DOI] [PubMed] [Google Scholar]
- Baluch F, Itti L. Mechanisms of top-down attention. Trends Neurosci. 2011;34:210–224. doi: 10.1016/j.tins.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Summation and inhibition in the frog’s retina. J Physiol. 1953;119:69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds AB. Role of inhibition in the specification of orientation selectivity of cells in the cat striate cortex. Vis Neurosci. 1989;2:41–55. doi: 10.1017/s0952523800004314. [DOI] [PubMed] [Google Scholar]
- Boudreau CE, Williford TH, Maunsell JHR. Effects of task difficulty and target likelihood in area V4 of macaque monkeys. J Neurophysiol. 2006;96:2377–2387. doi: 10.1152/jn.01072.2005. [DOI] [PubMed] [Google Scholar]
- Boynton GM. A framework for describing the effects of attention on visual responses. Vision Res. 2009;49:1129–1143. doi: 10.1016/j.visres.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten KH, Heuer HW. Spatial summation in the receptive fields of MT neurons. J Neurosci. 1999;19:5074–5084. doi: 10.1523/JNEUROSCI.19-12-05074.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buia CI, Tiesinga PH. Role of interneuron diversity in the cortical microcircuit for attention. J Neurophysiol. 2008;99:2158–2182. doi: 10.1152/jn.01004.2007. [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Summation and division by neurons in primate visual cortex. Science. 1994;264:1333–1336. doi: 10.1126/science.8191289. [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ, Movshon JA. Linearity and normalization in simple cells of the macaque primary visual cortex. J Neurosci. 1997;17:8621–8644. doi: 10.1523/JNEUROSCI.17-21-08621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ, Senn W. A synaptic explanation of suppression in visual cortex. J Neurosci. 2002;22:10053–10065. doi: 10.1523/JNEUROSCI.22-22-10053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. Visual attention: The past 25 years. Vision Res. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisanguanthum KS, Lisberger SG. A neurally efficient implementation of sensory population decoding. J Neurosci. 2011;31:4868–4877. doi: 10.1523/JNEUROSCI.6776-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Duncan J, Miller EK, Desimone R. Responses of neurons in inferior temporal cortex during memory-guided visual search. J Neurophysiol. 1998;80:2918–2940. doi: 10.1152/jn.1998.80.6.2918. [DOI] [PubMed] [Google Scholar]
- Chun MM, Golomb JD, Turk-Browne NB. A taxonomy of external and internal attention. Annu Rev Psychol. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR. A neuronal population measure of attention predicts behavioral performance on individual trials. J Neurosci. 2010;30:15241–15253. doi: 10.1523/JNEUROSCI.2171-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crist CF, Yamasaki DS, Komatsu H, Wurtz RH. A grid system and a microsyringe for single cell recording. J Neurosci Methods. 1988;26:117–122. doi: 10.1016/0165-0270(88)90160-4. [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Robson JG, Ohzawa I, Freeman RD. Organization of suppression in receptive fields of neurons in cat visual cortex. J Neurophysiol. 1992;68:144–163. doi: 10.1152/jn.1992.68.1.144. [DOI] [PubMed] [Google Scholar]
- Desimone R, Ungerleider LG. Multiple visual areas in the caudal superior temporal sulcus of the macaque. J Comp Neurol. 1986;248:164–189. doi: 10.1002/cne.902480203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn IM, Priebe NJ, Ferster D. The emergence of contrast-invariant orientation tuning in simple cells of cat visual cortex. Neuron. 2007;54:137–152. doi: 10.1016/j.neuron.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose GM, Maunsell JHR. Spatial summation can explain the attentional modulation of neuronal responses to multiple stimuli in area V4. J Neurosci. 2008;28:5115–5126. doi: 10.1523/JNEUROSCI.0138-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger DJ. Normalization of cell responses in cat striate cortex. Vis Neurosci. 1992;9:181–197. doi: 10.1017/s0952523800009640. [DOI] [PubMed] [Google Scholar]
- Heimel JA, Saiepour MH, Chakravarthy S, Hermans JM, Levelt CN. Contrast gain control and cortical TrkB signaling shape visual acuity. Nat Neurosci. 2010;13:642–648. doi: 10.1038/nn.2534. [DOI] [PubMed] [Google Scholar]
- Heuer HW, Britten KH. Contrast dependence of response normalization in area MT of the rhesus macaque. J Neurophysiol. 2002;88:3398–3408. doi: 10.1152/jn.00255.2002. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Kouh M, Poggio T. A canonical neural circuit for cortical nonlinear operations. Neural Comput. 2008;20:1427–1451. doi: 10.1162/neco.2008.02-07-466. [DOI] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Lee DK, Itti L, Koch C, Braun J. Attention activates winner-take-all competition among visual filters. Nat Neurosci. 1999;2:375–381. doi: 10.1038/7286. [DOI] [PubMed] [Google Scholar]
- Lee J, Maunsell JHR. A normalization model of attentional modulation of single unit responses. PLoS ONE. 2009;4:e4651. doi: 10.1371/journal.pone.0004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Maunsell JHR. Attentional modulation of MT neurons with single or multiple stimuli in their receptive fields. J Neurosci. 2010;30:3058–3066. doi: 10.1523/JNEUROSCI.3766-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Trujillo J, Treue S. Attentional modulation strength in cortical area MT depends on stimulus contrast. Neuron. 2002;35:365–370. doi: 10.1016/s0896-6273(02)00778-x. [DOI] [PubMed] [Google Scholar]
- Maunsell JHR, Cook EP. The role of attention in visual processing. Philos Trans R Soc Lond B Biol Sci. 2002;357:1063–1072. doi: 10.1098/rstb.2002.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Burr DC, Maffei L. Functional implications of cross-orientation inhibition of cortical visual cells. I Neurophysiological evidence. Proc R Soc Lond B Biol Sci. 1982;216:335–354. doi: 10.1098/rspb.1982.0078. [DOI] [PubMed] [Google Scholar]
- Noudoost B, Chang MH, Steinmetz NA, Moore T. Top-down control of visual attention. Curr Opin Neurobiol. 2010;20:183–190. doi: 10.1016/j.conb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshiro T, Angelaki DE, DeAngelis GC. A normalization model of multisensory integration. Nat Neurosci. 2011;14:775–782. doi: 10.1038/nn.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66:287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou M, Cassenaer S, Nowotny T, Laurent G. Normalization for sparse encoding of odors by a wide-field interneuron. Science. 2011;332:721–725. doi: 10.1126/science.1201835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Gain control of firing rate by shunting inhibition: roles of synaptic noise and dendritic saturation. Proc Natl Acad Sci USA. 2003;100:2076–2081. doi: 10.1073/pnas.0337591100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. Inhibition, spike threshold, and stimulus selectivity in primary visual cortex. Neuron. 2008;57:482–497. doi: 10.1016/j.neuron.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Wurtz RH. Shift in smooth pursuit initiation and MT and MST neuronal activity under different stimulus conditions. J Neurophysiol. 1999;82:1710–1727. doi: 10.1152/jn.1999.82.4.1710. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Wurtz RH. Effects of attention on MT and MST neuronal activity during pursuit initiation. J Neurophysiol. 2000;83:777–790. doi: 10.1152/jn.2000.83.2.777. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Desimone R. The role of neural mechanisms of attention in solving the binding problem. Neuron. 1999;24:19–29. 111–125. doi: 10.1016/s0896-6273(00)80819-3. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Desimone R. Interacting roles of attention and visual salience in V4. Neuron. 2003;37:853–863. doi: 10.1016/s0896-6273(03)00097-7. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust NC, Mante V, Simoncelli EP, Movshon JA. How MT cells analyze the motion of visual patterns. Nat Neurosci. 2006;9:1421–1431. doi: 10.1038/nn1786. [DOI] [PubMed] [Google Scholar]
- Sclar G, Maunsell JH, Lennie P. Coding of image contrast in central visual pathways of the macaque monkey. Vision Res. 1990;30:1–10. doi: 10.1016/0042-6989(90)90123-3. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci. 1998;18:3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer H, Desimone R, Moran J. Increased attention enhances both behavioral and neuronal performance. Science. 1988;240:338–340. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- Sundberg KA, Mitchell JF, Reynolds JH. Spatial attention modulates center-surround interactions in macaque visual area v4. Neuron. 2009;61:952–963. doi: 10.1016/j.neuron.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Maunsell JHR. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Williford T, Maunsell JHR. Effects of spatial attention on contrast response functions in macaque area V4. J Neurophysiol. 2006;96:40–54. doi: 10.1152/jn.01207.2005. [DOI] [PubMed] [Google Scholar]
- Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Curr Opin Neurobiol. 2003;13:187–193. doi: 10.1016/s0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]