SUMMARY
Two non-ribosomal peptide synthetases (NRPS), NocA and NocB, together comprising 5 modules, are essential for the biosynthesis of the D,L,D configured tripeptide backbone of the monocyclic β-lactam nocardicin A. We report a double replacement gene strategy in which point mutations were engineered in the two encoding NRPS genes without disruption of the nocABC operon by placing selective markers in adjacent genes. A series of mutants was constructed to inactivate the thiolation (T) domain of each module, evaluate an HHxxxDR catalytic motif in NocA and an atypical extended histidine motif in NocB. The loss of nocardicin A production in each of the T domain mutants indicates all 5 modules are essential for its biosynthesis. Conversely, production of nocardicin A production was not affected by mutation of the NocB histidine motif or the R828G mutation in NocA.
INTRODUCTION
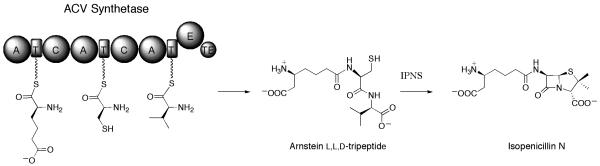
The biosynthesis of nocardicin A, isolated from the fermentation of Nocardia uniformis subsp. tsuyamanensis, has been of interest as the paradigm of a monocyclic β-lactam antibiotic and for its activity against gram-negative bacteria and relative stability to β-lactamases [1-3]. Before discovery of the nocardicin A gene cluster (Figure 1A), precursor incorporation studies have shown the D,L,D tripeptide backbone of this antibiotic family originates from 2 units of L-p-hydroxyphenylglycine (L-pHPG ) and one unit of L-Ser [4]. A double label experiment demonstrated that nocardicin G, a D,L,D tripeptide β-lactam, to be the earliest isolable biosynthetic intermediate of this pathway [4, 5]. Based on these early experiments and the known structure of nocardicin G, the nocardicin A gene cluster had been expected to encode a three module nonribosomal peptide synthetase (NRPS) whose initiation and termination modules installed D-pHPG into the peptide core by activation and epimerization of L-pHPG and a central module that activated and incorporated L-serine to form the tripeptide core of the nocardicins. This expectation was founded on δ-(L-α-aminoadipyl-L-cysteinyl-D-valine (ACV) synthetase, the NRPS responsible for biosynthesis of the immediate tripeptide precursor of isopenicillin N, a fused bicyclic β-lactam, and one of the few comparatively well-characterized NRPSs at the time. The 400 - 425 kDa ACV synthetase had been isolated from both bacteria and fungi and exemplified the classical “linear” NRPS architecture depicted in figure 2 [6]. The order of adenylation (A), thiolation (T) or peptidyl carrier, condensation (C) domains and a C-terminal epimerase (E) and thioesterase (TE) fully accounted for synthesis of the Arnstein L,L,D-tripeptide. Also prior to characterization of the nocardicin gene cluster, ~200 kDa and ~150 kDa proteins that demonstrated ATP/PPi exchange in the presence of L-pHPG were isolated from wild-type N.uniformis. Fortuitously, the higher molecular weight protein also exhibited ATP/PPi exchange in the presence of L-serine [7] but reconstitution experiments failed to produce nocardicin G. Against expectation, when the nocardicin A gene cluster was discovered, it revealed two overlapping genes encoding a pair of NRPSs, nocA (11.1 kbp) and nocB (5.8 kbp), which were predicted to translate a five module NRPS system and contained only a single epimerization domain (Figure 1C) [7]. The ~200 kDa and 150 kDa proteins isolated from wild-type N. uniformis were identified as NocB and a proteolytic fragment of NocB, respectively – not of NocA, in keeping with earlier failed reconstitution attempts.
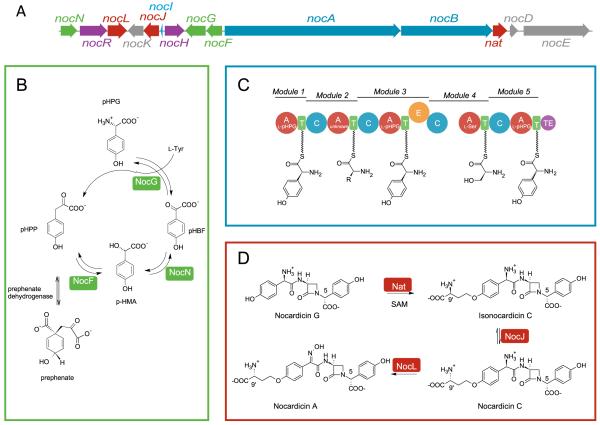
Figure 1.
Biosynthesis of nocardicin A in N. uniformis. A. Gene cluster for the biosynthetic pathway which includes genes encoding two NRPSs (blue), tailoring enzymes (red), proteins involved in regulation and transport (violet), proteins involved in biosynthesis of pHPG (green) and proteins shown to be non-essential for nocardicin A biosynthesis (gray) . B. p-HPG biosynthetic pathway. C. Predicted domain and module organization of Noc A and NocB; Substrates predicted by bioinformatic analysis of the A domain active sites are shown. D. Late stage biosynthetic steps; conversion of nocardicin G to nocardicin A.
Figure 2.
Biosynthesis of the Arnstein L,L,D-tripeptide, the precursor to isopenicillin N by ACV synthetase. IPNS denotes isopenicillin N synthase.
A combination of in vitro, gene inactivation, and bioinformatic experiments has been successfully applied to define the role of each protein encoded in the nocardicin A gene cluster: nocF, nocG, and nocN encode proteins responsible for the biosynthesis of the non-proteinogenic amino acid precursor L-pHPG (Figure 1B) [7]; nat, nocJ, and nocL encode proteins responsible for the late stage biosynthetic steps - the addition and epimerization of the homoseryl side chain and oxime formation (Figure 1D) [8-10]. NocR has been demonstrated to be a transcriptional activator for the nocABC operon [11], NocI belongs to the MbtH family of proteins, recently shown to be involved in activating A domains in some NRPSs [12-14], and NocH is homologous to membrane transport proteins of the major facilitator family (MFS) [7]. Proteins encoded by nocK, nocD, nocE, and nocO have been shown by in vivo gene knockout experiments to be non-essential for nocardicin A biosynthesis [11, 15].
The NRPSs of the gene cluster, NocA and NocB, are likely responsible for the synthesis of the nonribosomal D,L,D-tripeptide core of nocardicin G based on adenylation domain substrate prediction algorithms consistent with the activation of L-pHPG by modules 1, 3 and 5 and L-Ser by module 4 [7, 16]. The substrate of the module 2 A domain is less defined, but has a signature suggesting L-N5-hydroxyornithine [7]. Because the gap in biosynthetic logic between the five module NRPS encoded in the gene cluster and the requirement for a three module NRPS suggested by the structures of the isolated nocardicins, several experiments were undertaken to further characterize the nocardicin NPRSs. Unfortunately, attempts to heterologously express NocA and NocB separately or partially as individual modules in E.coli and Streptomyces for further in vitro analysis were unsuccessful. Because of the high titer of nocardicin A produced from wild-type N. uniformis coupled with the successful isolation of NocB and the expectation that NocA and NocB would be translated in equal amounts from co-transcription of the nocABC operon, the isolation of NocA from the native bacterium was again pursued. However, despite assiduous attempts and the re-isolation of NocB and its 150 kDa fragment, the ~345 kDa NocA protein was never observed.
In the face of these setbacks, we returned to in vivo mutagenesis experiments to determine if NocA and or NocB were essential for nocardicin A biosynthesis, and if so, to determine if they function in a non-linear manner either by employing module skipping or iterative logic. Because the genes encoding NocA, NocB, and NocC (Nat) appear in a single operon, however, insertional mutagenesis of nocA or nocB would likely affect transcription of the downstream genes automatically altering the production of nocardicin A. Seeking precedents where the nocABC operon would not be disrupted, it was found that in Streptomyces and Bacillus subtilis double gene replacement strategies to introduce markerless targeted mutations into PKS or NRPS systems have been successfully developed [17-21]. Of particular relevance are module deletion, module substitution methods and module exchange experiments in NRPS systems [17-25].
In an extension of the genetic system previously developed for insertional mutagenesis studies of N. uniformis [15] described here are double replacement experiments in which a dual function selection marker was used in the second, ‘knock-in’ step. A new strategy was devised that can be particularly amenable to probing natural product biosynthetic gene clusters and takes advantage of the methods developed for insertional mutagenesis experiments successfully completed on genes nocF and nocE located adjacent to the nocABC operon. The preparation of 12 point mutants is presented, which can be divided into three key areas of interest. First, is the preparation of S to A point mutants for each T domain (total = 5) of NocA and NocB. These experiments primarily test the hypothesis that one or more modules of the NRPS is inactive. A second experiment addresses the unusual HHxxxDR catalytic motif of C2 of which the R residue is unexpected [26]. An R828G point mutant was prepared to determine if this unusual feature is critical for antibiotic production. Finally, an atypical ‘extended His-motif’, reminiscent of that critical for catalysis in condensation and epimerization domains in NRPS systems, was noted in the A5 domain of NocB. The third set of point mutants addressed whether the residues of this motif play a role in the biosynthesis of nocardicin A.
RESULTS and DISCUSSION
Considerations for 2-Step Gene Replacement Experiment
Our goal was to develop an in vivo mutagenesis system for N. uniformis for the preparation of markerless point mutants in NocA and NocB, as was done for the Streptomyces and Bacillus systems described earlier. Several blocked mutants of genes in the nocardicin A cluster have been prepared in previous studies in which an antibiotic cassette, tsr or apr, was inserted into the gene of interest [15]. The preparation of an analogous N. uniformis insertional mutant is the first step of the double replacement experiment. Protoplasts of wild-type N.uniformis were prepared, transformed with a pULVK2 Nocardia-E.coli replicating shuttle vector, grown for several rounds without selection, and successfully screened for homologous recombination mutants. However, the second step of the double gene replacement protocol proved more problematic given the unavailability of a suicide or temperature sensitive plasmid compatible with N. uniformis of the type that were successfully employed for gene replacements in Streptomyces [18]. Initial studies in which a second pUVLK2 vector, engineered with a replacement sequence, transformed into the knock-out or deletion mutant, failed to undergo homologous recombination and produce the desired engineered mutant. Further experimentation revealed the vector could not be maintained without antibiotic selection.
Given the scarcity of plasmids available for Nocardia systems, a gene replacement strategy was developed to incorporate antibiotic selection markers in genes adjacent to the nocABC operon. Previous studies had shown that the downstream genes encoding NocD and NocE are not essential in nocardicin A biosynthesis [11]. Upstream of the NRPS operon, the nocA-nocF intergenic region is essential for the binding of the pathway specific transcriptional activator NocR. The immediate upstream target, nocF, encodes a p-hydroxymandelate synthetase involved in the biosynthesis of the pHPG precursor. Incorporation experiments in which isotopically labeled pHPG was added to fermentation cultures of wild-type N. uniformis showed efficient incorporation into nocardicin A [4]. Thus, it was anticipated that insertional inactivation of nocF should be chemically complemented by addition of pHPG to the fermentation medium.
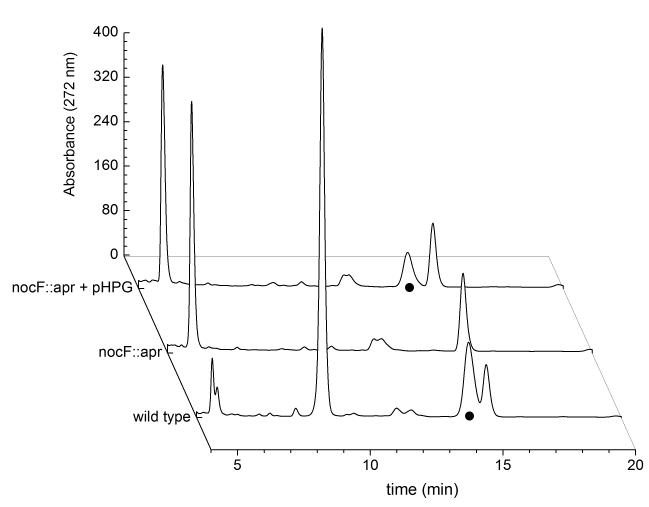
A nocF::apr insertional inactivation mutant was prepared and evaluated for antibiotic production. The culture supernatants of nocF::apr mutants confirmed by Southern analysis were bioassayed against E.coli ESS and characterized by HPLC analysis. Figure 3 compares chromatograms of the nocF::apr mutant with wild-type N. uniformis. Nocardicin A [retention time = 14.6 min (●)] was not detected in the nocF::apr mutant but was substantially restored by the addition of 0.5 mM L-pHPG to the fermentation culture, as anticipated. Another notable difference between the chromatograms of the wild-type and mutant is the minimal depletion of tyrosine (retention time = 5 min) and absence of p-hydroxybenzoyl formate (pHBF) accumulation (retention time = 8.6 min) observed in the nocF::apr mutant growths. This observation is consistent with previous studies of the pHPG biosynthetic cycle (Figure 1B) [27, 28] However, the ability to restore nocardicin A production in the nocF::apr N. uniformis mutant confirmed that this insertional mutant could be efficiently chemically complemented by fortification of the culture medium with L-pHPG.
Figure 3.
HPLC chromatograms of the culture supernatents of nocF::apr N. uniformis with and without chemical complementation with L-pHPG compared to wild-type N. uniformis. Key components are tyrosine (retention time = 5 min), p-hydroxybenzoyl formate (retention time = 8.6 min) and nocardicin A (retention time = 14.6 min), indicated by a dot (●).
The first step, therefore, involved the preparation of deletion mutants T2KO and T3KO for targeted gene replacements in nocA (Figure 4A) and T45KO for targeted gene replacements in nocB (Figure 4B) in which the region between the location of the apr cassette to be inserted in the second replacement step and the NRPS domain of interest was deleted and replaced with the tsr gene. As expected based on previous studies [7], nocardicin A production was absent in each of these deletion mutants. In preparation for the second step, pULVK2 vectors were cloned containing nocF::apr - nocA (Figure 4A) for nocA gene replacement studies or nocBC - apr - nocE (Figure 4B) for nocB gene replacement studies. In both cases, the pULVK2 ‘knock-in’ vector encoded the gene, containing the apr resistance cassette, and the NRPS sequence to at least 1000 bp past the desired mutation. The step one deletion mutants and the encoding of pULVK2 vectors for the step two transformations were designed such that homologous recombination would result in only one type of double cross-over mutant. The resulting mutant has the Aprr Tssphenotype and encodes the engineered nocABC operon. This protocol allows for the construction of NRPS mutants without disrupting the regulatory elements of the gene cluster [11].
Figure 4.

In vivo 2-Step gene replacement strategy. The first step required the preparation of a deletion mutant. Transformation of the deletion mutant with a vector containing the native or engineered sequence followed by homologous recombination gives the desired mutant. A. Strategy for mutagenesis of nocA in which an apr gene cassette is placed in the adjacent gene nocF. The nocF::apr mutant is chemically complemented by the addition of L-pHPG to the culture medium. B. Strategy for mutagenesis of nocB in which an apr gene cassette is placed in the non-essential gene nocE.
For each step one deletion mutant, a control ‘knock-in’ mutant was prepared encoding the native sequence. Thus, successful control experiments should result in the restoration of nocardicin A production. The restoration of antibiotic production in all three deletion mutants, T2KO, T3KO and T45KO was observed by replacement of the tsr cassette with their respective native sequences of nocA or nocB, validating the strategy of the overall experiment and providing additional evidence for accuracy in the cloning of the pULVK2 vectors. In addition, because an apr resistance marker disrupts nocF in T2KO and T3KO knock-in experiments, antibiotic production should only be observable in native knock-in mutants with addition of pHPG to the fermentation medium, a result that was observed in all the control (native sequence) ‘knock-in’ experiments. Although this two step-gene replacement strategy requires a proximal non-essential or chemically complemented gene for placement of a selection marker, it was attractive for several reasons, but particularly because this method proved robust in producing the desired engineered mutants and selecting for them using antibiotic sensitivity for the phenotype. Following antibiotic selection, 1-4 putative mutants of each type were screened by Southern analysis followed by sequence analysis. Mutants were identified and confirmed from only one round of screening. This strategy should be generally applicable to the investigation of other natural product gene clusters.
Characterization of Mutants
To test the hypothesis that one or more modules of the NocA+NocB NRPS system may be non-essential or skipped, a serine to alanine mutation was engineered at the conserved phosphopantethienylation site of the T domain for each module and mutants were prepared using the double gene replacement strategy outlined in Figure 4. Each of the resulting mutants was characterized for antibiotic production, as assessed by the E.coli ESS bioassay and HPLC analysis for nocardicin A production. A summary of results is shown in Table 1.
Table 1.
Summary of N. uniformis Mutants
|
N. uniformis Strain |
Mutation/Description | mg nocardicin A/ L culture supernatent |
Bioassay |
|---|---|---|---|
| Wild-type | None | 280 | + |
| T2KO | ΔnocF-ΔnocA (M1-2)::tsr | 0 | − |
| Control/T2KI | nocF::apr | 50 | + |
| T1PM | nocF::apr; nocA S626A | 0 | − |
| T2PM | nocF::apr; nocA S1671A | 0 | − |
| C2PM | nocF::apr; nocA R828G | 37 | + |
| Control/T3KI | nocF::apr | 33 | + |
| T3KO | ΔnocF-ΔnocA (M1-3)::tsr | 0 | − |
| T3PM | nocF::apr; nocA S2782A | 0 | − |
|
N. uniformis Strain |
Mutation/Description | mg nocardicin A/ L culture supernatent |
Bioassay |
|---|---|---|---|
| Wild-type | None | 280 | + |
| T45KO | ΔnocBCDE::tsr | 0 | − |
| Control/T45KI | nocE, apr | 106 | + |
| T4PM | nocB S571A | 0 | − |
| T5PM | nocB S1648A | 0 | − |
| M3-4 | nocB H1411A | 81 | + |
| M4-8 | nocB H1412A | 65 | + |
| M6-2 | nocB E1417A | 104 | + |
| M7-2 | nocB D1418A | 54 | + |
See also Figure S1.
Although most NPRS systems are linear, i.e. the primary sequence of the peptide product formed is co-linear with the ordered modules of its corresponding synthetase, there are now a number of known ‘non-linear’, Type C, NRPS systems in which module skipping, iteration, or a combination thereof have been shown to occur in the formation of their peptide products [29]. Based on the hypothesis that the D,L,D-configured tripeptide backbone of nocardicin G originates from NocA + NocB, the nocardicin NRPS system was classified as a non-linear Type C NRPS [30]. Furthermore, within modules 1 and 2 were noted several atypical regions in which additional sequences of short amino acid repeats were found, leading to the hypothesis that perhaps modules 1 and 2 were skipped or inactive and thus the three remaining modules were responsible for the biosynthesis of D-pHPG-L-Ser-D-pHPG [7]. Evidence for module skipping due to a defective T domain in which the conserved active site serine is not located in the expected position was reported in myxochromide biosynthesis in Stigmatella aurantica. As a consequence, proline is not incorporated into the peptide chain despite an active A domain [31, 32].
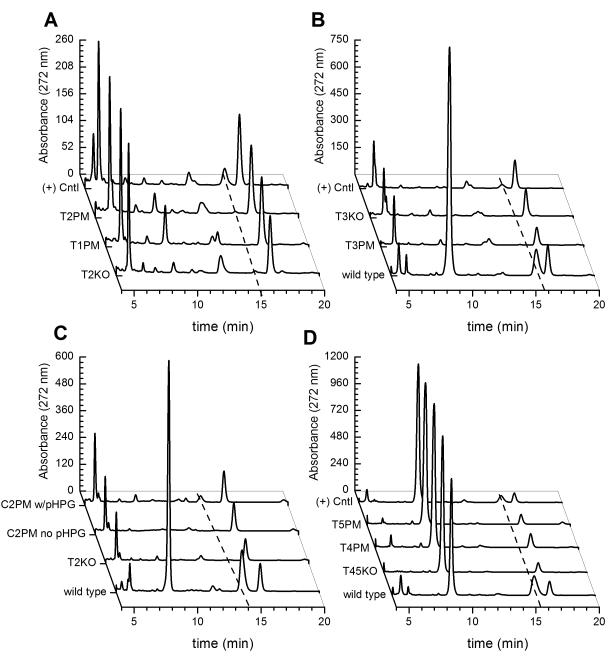
However, contrary to this hypothesis, antibiotic production was not detected in any of the T domain point mutants. Figure 5 (A-C) shows the chromatograms of the culture supernatants for each mutant, comparing them to the chromatograms of their respective first step deletion mutant, positive control, and wild-type N. unformis. Figure 5A and 5B plot chromatograms for the nocA T1PM (S626A), T2PM (S1671A) and T3PM (S2782A) mutants and reveal few distinctions between the T1PM, T2PM, T3PM point mutants and their parent T2KO and T3KO deletion mutants, each grown in culture medium supplemented with 0.5 mM L-pHPG. Chromatograms for nocB T4PM (S571A) and T5PM (S1648A) are plotted in Figure 5D. Again, the chromatograms of T4PM and T5PM lack a peak for nocardicin A and resemble the chromatograms of their parent T45KO deletion mutant. Because the apr resistance marker in the second step of the nocB gene replacement is located at the end of the gene cluster and thus does not perturb pHPG biosynthesis, the depletion of tyrosine and the accumulation of HBF are observed in these mutants. In addition, no new peaks that might correspond to NRPS derailment products were seen in the HPLC chromatograms of the T-domain mutants. In all cases, control experiments in which native sequences were restored to the T2KO, T3KO, and T45KO deletion mutants demonstrated significant restoration of nocardicin A production, unlike the T domain mutants, indicating that each of the 5 modules of this NRPS is essential for nocardicin A biosynthesis.
Figure 5.
HPLC chromatograms of the culture supernatents from each set of N. uniformis mutants prepared in this study compared to wild-type, analyzed following fermentation for 5 days. In these studies, the culture medium was supplemented with 0.5 mM L-pHPG, except where noted otherwise. The peak for nocardicin A is indicated by a dashed line. A. Chromatograms for deletion mutant T2KO, T domain mutants nocA S626A (T1PM) and nocA S1671A (T2PM), and the native ‘knock-in’ experiment ((+) Cntl), in which the native nocA sequence was restored. B. Chromatograms for deletion mutant T3KO, T domain mutant nocA S2782A (T3PM) and a positive control ((+) Cntl), in which the native nocA sequence was restored compared to wild-type N. unformis. C. Chromatograms for mutant nocA R828G, located in the C2 domain are compared to the deletion mutant T2KO and wild-type N. unformis . For comparison, the chromatogram of the C2PM mutant, grown without pHPG supplementation is shown. D. Chromatograms for deletion mutant T45KO, T domain mutants nocB S571A (T4PM) and nocB S1648A (T5PM), and a positive control ((+) Cntl), in which the native nocB sequence was restored are plotted.
Mutagenesis of the nocA C2 domain was prepared to determine whether replacement of the arginine from the unusual catalytic motif HHxxxDR to the consensus HHxxxDG would impact nocardicin A production. The canonical catalytic motif of C domains is HHxxxDG, also called the ‘His motif’ [26]. Mutagenic studies on the N-terminal C domain of TycB (tyrocidine) showed that the second histidine and aspartate residues of this signature are essential for C domain activity [33, 34] but there is conflicting evidence as to whether the terminal glycine of this motif is also essential. Although the HHxxxDR motif is also found in the C13 domain of daptomycin synthetase, catalyzing the addition of L-kynurenine to the nascent peptide in S. roseosporus [35], studies on the C domain of EntF (enterobactin) found the terminal G residue of the HHxxxDG motif to be essential. When a G to L mutation was introduced, it abolished activity, which was thought to result from steric occlusion of the substrate channel [36]. This observation posed the question whether the arginine residue of the HHxxxDR motif in C2 might render this domain inactive and block transfer of the growing peptide from the first two modules to the third. A precedent for the presence of an inactive C domain that is compensated for by the next downstream active C domain is ovserved in bleomycin biosynthesis [37]. The nocA R828G mutant was found to retain antibiotic production (Table 1). Based on HPLC analyses of the culture supernatent of this mutant (Figure 5C), this mutation also did not result in a difference in nocardicin A production compared to the native control and no new peaks were observed. This result is consistent with the structural analysis of the TycC6 condensation domain [38], in which the second H residue is catalytic and the D residue is essential for providing interactions that stabilize the active site. In the crystal structure, a sulfate ion sits in the active site and is ‘presumed’ to be situated to mimic the thiol terminus of the pantethienyl moiety. This sulfate is hydrogen bonded to the peptide backbone at the G residue, suggesting that the side chain of the amino acid residue at this position is not critical and thus less conserved.
The observation that each of the five T domains is required for nocardicin A biosynthesis allows us to draw several conclusions. First, this study demonstrates that both NocA and NocB are essential for nocardicin biosynthesis and there is no evidence that module skipping occurs in this NRPSs system. This was unexpected. The absence of wild-type NocA, but the comparatively ready isolation of NocB led us to suspect that the former was swiftly proteolyzed, as was clearly observed for NocB, to smaller fragments that might act in trans . It was hypothesized that the unusual repeated sequences inserted after A1 and before module 3, for example, presented vulnerable sites where just such events could take place. Our observations make it less likely that a proteolytic product of NocA or NocB is acting in trans with NocB to produce a tri-peptide. The other possibility previously entertained was that NocA was not essential and an alternate starting module existed, as in anabaenopeptin biosynthesis in Anabaena [39], encoded in a yet unknown gene cluster but capable of ‘crosstalking’ with the nocardicin gene cluster. Cross talk between gene clusters has been observed in the biosynthesis of erythrochelin [40, 41] and rhodochelin [42].
There are several known examples of NRPSs with “too many modules”. The exochelin gene cluster in Mycobacterium smegmatis encodes a six module NRPS – however, exochelin is a pentapeptide. Whether all six modules are essential for exochelin biosynthesis is not known [43]. Thiocoraline biosynthesis in Micromonospora is another system that appears to involve extra modules. The thiocoraline gene cluster encodes four NRPSs, TioR, TioS, TioY, and TioZ, of which, the roles of TioY and TioZ are unclear. Although it appears that only TioR and TioS should be required for the biosynthesis of the peptide precursor, in vivo mutagenesis experiments conclude TioY and TioZ are also essential for thiocoraline biosynthesis [44]. A third example is the tandem TE domains that terminate the NRPSs involved in lysobactin, arthrofactin, massetolide, and syringopeptin biosyntheses. In vivo studies of the arthrofactin system showed both TE domains to be involved in efficient product formation [45]. Recent in vitro studies of the terminal module of the lysobactin NRPS demonstrated proteolytic cleavage of the terminal TE domain, leading the authors to propose that the terminal TE domain is also proteolyzed from the NRPS in the native Lysobacter to then function as an editing stand-alone type II TE domain [46].
The combination of bioinformatic analysis of the nocardicin A gene cluster, particularly the NRPS proteins NocA and NocB as well as characterization of the in vivo NocA and NocB point mutants indicate the likelihood that NocA+NocB is forming a pentapeptide or a pentapeptide β-lactam. If the NocA+NocB product is a pentapeptide, how and at what point in the pathway is it trimmed to a tripeptide? There are natural products with a closely related, inactive biosynthetic precursor that can be accumulated in the host until an appropriate time for release. In lantibiotic biosynthesis, the leader peptide has been proposed to act as an in-cis chaperone for post-translational modification enzymes or a provider of stabilizing interactions that prevent degradation and assist in folding of the precursor peptide [47]. In Pseudomonas syringae, the di-peptide precursor of tabtoxin, tabtoxinine-β-lactam, is trimmed by a periplasmic aminopeptidase encoded in the gene cluster, to activate the β-lactam product [48, 49]. Due to the absence of a specifically encoded protease in the nocardicin cluster, the possibility that M2 might append a moiety such as N5-hydroxyornithine or ornithine and that cleavage of the first 2 residues might be autocatalytic has been considered [7].
Another anomaly of nocardicin A biosynthesis considered in this study is the conversion of L-pHPG to its D antipode at two centers in nocardicin A when only one epimerase is found in its synthetase. D-Amino acids are usually incorporated into nonribosomal products by the action of an epimerization domain, located immediately downstream of the T domain in a typical NRPS module, as seen in module 3 of NocA [50]. Only rarely are D-amino acids substrates for direct activation by A domains in NRPSs, although cyclosporin, fusaricidin and leinamycin synthetases have been shown to activate D-alanine for direct incorporation into the product peptide [51-54]. An alanine racemase, located in the cyclosporin gene cluster, has been characterized, whereas the fusaricidin and leinamycin gene clusters do not appear to contain a similar racemase [51, 52]. A third strategy for the addition of D-amino acids into nonribosomal products involves action of C domains that also catalyze epimerase activity. These dual C/E domains are observed in arthrofactin, syringomycin, syringopeptin and ramoplanin synthetases [55], and are characterized by an extended histidine sequence at the N-terminus, HH(I/L)xxxxDG, in addition to the conventional ‘His motif’ (HHxxxDG) known to be essential for condensation of the peptide bond. A fourth, so far unique example for epimerization has been found in the PchE protein of pyochelin synthetase. Between the A8 and A9 motifs of the cysteine activating A domain exists an approximately 300 amino acid insert resembling a methyl transferase domain. However, mutagenesis of histidine 1204 to alanine was shown to eliminate racemization of the tethered benzoylcysteine intermediate, suggesting that this insert functions as an epimerization domain [56].
Because of the absence of a second epimerization domain, a dual C/E domain or a racemase in the gene cluster and the pyochelin precedent, the discovery of an extended histidine motif, HHTCAPEDG, between motifs A5 and A6 of the A5 domain posed the question of whether this could be an epimerization motif. In addition, the lack of epimerization domains in the initiation modules of several glycopeptides was of interest [57]. Alignment of the A1 domains of chloroeremomycin, balhimycin, A47934, complestatin and teicoplanin synthetases shows conservation at the second histidine and aspartate residues, which correspond to the catalytic residues of epimerization motifs. (Figure 6) All the mutants prepared in the A5 domain, H1411A, H1412A, E1417A and D1418A produced nocardicin A at levels virtually unchanged compared to that of the native control (Table 1 and Figure S1), indicating that this motif is not essential for formation of the peptide precursor and thus is unlikely to be involved in an epimerization reaction.
Figure 6.
Comparison of extended His motif observed in the NocB A5 domain to the initiation A domains of NRPSs for chloroeremomycin (CepA), balhimycin (BpsA), complestatin (ComA), A47934 (StaA) and teicoplanin (TcpA). Identical residues are shaded. The conserved histidine and acidic residues of the extended His motif are noted with an asterisk (*). Numbers indicate amino acid residues from the N-terminus of the protein. Gene Bank accession numbers are as follows: CepA, AJ223999; BpsA, Y16952; ComA, AF386507; StaA, U82965; TcpA, AJ605139.
Several hypotheses can account for conversion from the L- to D-antipode of the C-terminal pHPG in nocardicin A biosynthesis. The possibility that this activity is cryptically embedded in nocardicin synthetase has not been eliminated. Second, a racemase encoded outside the known gene cluster may have a role. Other than D-Ala and D-Glu, known components of bacterial peptidoglycan, other D-amino acids have been found in a variety of prokaryotes along with the discovery of a broad spectrum racemase [58]. Finally, synthetic studies have shown the C5 position to be base-labile, suggesting that epimerization at C5 might occur in tandem with amide deprotonation and β-lactam formation [59].
SIGNIFICANCE
Two NRPS enzymes, NocA and NocB, act centrally in the biosynthesis of the nocardicin monocyclic β-lactam antibiotics. Bioinformatic comparisons of these proteins reveal several anomalies as, for example, extended repeat sequences and atypical histidine motifs that call into question whether these multidomain enzymes are conventionally functional, particularly modules 1 and 2 of NocA, and what their roles are in the biosynthesis. While NocA and NocB comprise 5 modules, the last three would appear sufficient to generate the tripeptide core of this antibiotic family. Expression of these peptide synthetases in E. coli and Streptomyces hosts to begin to answer these questions proved intractable. A two-step, in vivo gene replacement strategy reported here enabled the preparation of a series of point mutants to evaluate the roles of individual residues and modules in NocA+NocB without disruption of promoter or regulatory elements. The use of chemical complementation of an ancillary precursor biosynthetic gene in the critical second ‘knock-in’ step proved to be robust and reliable and may be more widely useful in analogous studies of natural product biosynthetic pathways. Using this method the phosphopantethiene attachment site in each of the five T-domains was specifically mutated to alanine and in each instance proved essential to antibiotic production. An uncommon HHxxxDR catalytic motif in C2 was mutated to the canonical HHxxxDG, but both were equivalently active in nocardicin A biosynthesis. A similar extended His motif in A5, potentially responsible for the cryptic C-terminal epimerization characteristic of all known nocardicins, was unaffected by mutation. These data suggest that all modules of NocA and NocB are required for nocardicin G synthesis and these unusually modified proteins, in fact, appear to function normally. Their interplay is now more sharply defined but more complex questions remain in the full orchestration of precursor peptide assembly, editing, C-terminal epimerization and β-lactam formation.
EXPERIMENTAL PROCEDURES
Bacterial strains and plasmid construction
Strains and plasmids and oligonucleotide primers used in PCR amplification and Quick ChangeTM reactions are included in the Supplemental Information. PCR amplification reactions, employing previously prepared cosmid or plasmid templates, were performed using Pfu (Stratagene, LaJolla, CA) DNA polymerase, using primers purchased from IDT-DNA (Coralville, IA). Amplified products were typically subcloned into pT7B3 (EMD Biosciences, Gibbstown, NJ) or pUC19 using standard protocols. Further manipulation of DNA for plasmid preparation was performed using standard procedures [60].
Point mutations were engineered into plasmids of interest using QuickChangeTM (Stratagene) [61]. DNA polymerases Pfu Ultra or Pfu Turbo (Stratagene) were employed combined with a manual ‘hot start’ PCR amplification protocol. Following PCR, products were treated with Dpn I to remove template DNA. DNA was concentrated using Pellet Paint (EMD Biosciences) prior to transformation into E.coli XL1-Blue by electroporation. The construction of all plasmids was confirmed by DNA sequencing at the Biosynthesis and Sequencing Facility, Johns Hopkins Medical School, Baltimore MD.
Culture and assay conditions
Nocardia uniformis subsp. tsuyamanesis ATCC 21806 (wild-type strain), producer of the antibiotic nocardicin A, was maintained on ISP2 solid medium (Difco Laboratories, Detroit, MI) at 28 °C. Seed cultures were prepared in trypic soy broth (TSB) medium (Difco Laboratories) and incubated at 28 °C, with shaking, for inoculation of fermentation medium (containing, per liter, 10 g peptone, 4 g yeast extract, 10 g KH2PO4, 4 g NaH2PO4, 2.4 g MgSO4, 2 g glycine, 2 mL trace minerals, 20 g soluble starch, 1 g tyrosine, 75 mg L-methionine) as previously described [8]. During the 5 – 7 day growth period for N. uniformis, aliquots of the culture supernatents were sampled and assayed for the production of nocardicin A and related precursors by bioassay versus E.coli ESS and quantitative HPLC. Culture aliquots were centrifuged to separate the cell mass from the supernatent, and both were stored at −20 °C. A paper disc bioassay analysis to detect antibiotic production was prepared by the application of 200 μL of culture supernatant to paper discs placed upon solid LB medium inoculated with E. coli ESS. Plates were analyzed for developed zones of antibiosis following incubation at 37 °C overnight. Quantitative analyses of nocardicin A production were performed using an Agilent 1100 HPLC system equipped with a diode array detector. Filtered supernatents (nylon 0.45um) were injected directly onto a Luna C18(2) 250 × 46 mm column (Phenomenex, Torrance, CA), using an isocratic mobile phase: 90:10 water:acetonitrile with 0.08% trifluoroacetic acid (TFA) at a flow rate of 1mL/min. Analytes were detected by absorbance at 272 nm and were quantified by comparison to a standard curve.
Protoplast transformation of N. uniformis and preparation of mutants
Prior to protoplast transformation into N. uniformis, the constructed pULVK2 (Nocardia – E.coli) shuttle vectors were transformed into E. coli JM110 cells to provide non-methylated DNA. The protocol for PEG-mediated transformation of N. uniformis with pULVK2 vectors has been described previously [15]. N. uniformis pULVK2 vector transformants were selected by a 200 μg/mL kanamycin and 100 μg/mL apramycin or 25 μg/mL thiostreptone overlay. The Aprr Kanr or TSr Kanr phenotype was confirmed by plating on ISP2 solid medium containing 200 μg/mL kanamycin and 100 μg/mL apramycin or 25 μg/mL thiostreptone. Transformants were usually subjected to three rounds of non-selective plating on ISP2 to obtain double cross-over mutants. However, selective propagation on ISP2, using 100 μg/mL apramycin, was employed following the transformation of the T2KO, T3KO and T45KO mutant N. uniformis strains. Putative double cross-over mutants were identified by their predicted phenotype and confirmed by Southern analysis and sequencing of genomic DNA.
Plasmid Construction for nocF::apr disruption mutant
The nocF gene (1035 bp) was amplified using primers nocF_For and nocF_Rev and sub-cloned into pT7B3 (EMD Biosciences). The resulting pT7B3/nocF plasmid was linearized by digestion with Bbs I and blunted with Klenow. The apr insert was prepared by digestion from plasmid pT7B3/apr and ligated into the linearized nocF plasmid. The disrupted gene cassette nocF::apr (3.1 kbp) was ligated into the EcoR I site of pULVK2 to generate pULVK2/nocF::apr.
Plasmid construction for 2-step gene replacement experiments
Detailed procedures for the cloning of plasmids for both the step one deletion mutants and the step two ‘knock in’ mutants are described in the Supplemental Information.
Supplementary Material
HIGHLIGHTS.
In vivo double replacement gene strategy for preparation of markerless mutants
Inactivation of T domain of each module and in vivo evaluation of atypical motifs
All five NRPS modules required for biosynthesis of tripeptide core of nocardicin A
ACKNOWLEDGEMENTS
This work was supported by NIH grant AI014937. Dr. D. M. Bartley is thanked for construction of plasmid pCRBlunt/T1PM and Ms. Anne Rigby for help constructing the figures in this manuscript. Drs. T. Bililign and R. F. Li are thanked for their technical expertise and the Greenberg group (JHU) for use of their phosphoimager and providing space and support for radiochemical experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hashimoto M, Komori T.-a., Kamiya T. Nocardicin A, A New Monocyclic ß-Lactam Antibiotic II. Structure Determination of Nocardicins A and B. J. Antibiot. 1976;29:890–901. doi: 10.7164/antibiotics.29.890. [DOI] [PubMed] [Google Scholar]
- 2.Aoki H, Sakai H-I, Kohsaka M, Konomi T, Hosoda J, Kubochi Y, Iguchi E, Imanaka H. Nocardicin A, A New Monocyclic ß-Lactam Antibiotic I. Discovery, Isolation and Characterization. J. Antibiot. 1976;29:492–500. doi: 10.7164/antibiotics.29.492. [DOI] [PubMed] [Google Scholar]
- 3.Kojo H, Mine Y, Nishida M, Goto S, Kuwahara S. Nature of Monocyclic ß-Lactam Antibiotic Nocardicin A to ß-Lactamases. Microbiol. Immunol. 1988;32:119–130. doi: 10.1111/j.1348-0421.1988.tb01371.x. [DOI] [PubMed] [Google Scholar]
- 4.Townsend CA, Brown AM. Nocardicin A: Biosynthetic Experiments with Amino Acid Precursors. J. Am. Chem. Soc. 1983;105:913–918. [Google Scholar]
- 5.Townsend CA, Wilson BA. The Role of Nocardicin G in Nocardicin A Biosynthesis. J. Am. Chem. Soc. 1988;110:3320–3321. [Google Scholar]
- 6.Martin JF. alpha-Aminoadipyl-cysteinyl-valine synthetases in beta-lactam producing organisms - From Abraham’s discoveries to novel concepts of non-ribosomal peptide synthesis. J. Antibiot. 2000;53:1008–1021. doi: 10.7164/antibiotics.53.1008. [DOI] [PubMed] [Google Scholar]
- 7.Gunsior M, Breazeale SD, Lind AJ, Ravel J, Janc JW, Townsend CA. The Biosynthetic Gene Cluster for a Monocyclic β-Lactam Antibiotic, Nocardicin A. Chem. Biol. 2004;11:927–938. doi: 10.1016/j.chembiol.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Reeve AM, Breazeale SD, Townsend CA. Purification, Characterization, and Cloning of an S-Adenosylmethionine-dependent 3-Amino-3-carboxypropyltransferase in Nocardicin Biosynthesis. J. Biol. Chem. 1998;273:30695–30703. doi: 10.1074/jbc.273.46.30695. [DOI] [PubMed] [Google Scholar]
- 9.Kelly WL, Townsend CA. Role of the Cyctochrome P450 NocL in Nocardicin A Biosynthesis. J. Am. Chem. Soc. 2002;124:8186–8187. doi: 10.1021/ja025926g. [DOI] [PubMed] [Google Scholar]
- 10.Kelly WL, Townsend CA. Mutational Analysis and Characterization of Nocardicin C-9′ Epimerase. J. Biol. Chem. 2004;279:38220–38227. doi: 10.1074/jbc.M405450200. [DOI] [PubMed] [Google Scholar]
- 11.Davidsen JM, Townsend CA. Identification and Characterization of NocR as a Positive Transcriptional Regulator of the β-Lactam Nocardicin A in Nocardia uniformis. J. Bacteriol. 2009;191:1066–1077. doi: 10.1128/JB.01833-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Heemstra JR, Walsh CT, Imker HJ. Activation of the Pacidamycin PacL Adenylation Domain by MbtH-like Proteins. Biochemistry. 2010;49:9946–9947. doi: 10.1021/bi101539b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felnagle EA, Barkei JJ, Park H, Podevels AM, McMahon MD, Drott DW, Thomas MG. MbtH-Like Proteins as Integral Components of Bacterial Nonribosomal Peptide Synthetases. Biochemistry. 2010;49:8815–8817. doi: 10.1021/bi1012854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heemstra JR, Walsh CT, Sattely ES. Enzymatic Tailoring of Ornithine in the Biosynthesis of the Rhizobium Cyclic Trihydroxamate Siderophore Vicibactin. J. Am. Chem. Soc. 2009;131:15317–15329. doi: 10.1021/ja9056008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly WL, Townsend CA. Mutational analysis of nocK and nocL in the nocardicin a producer Nocardia uniformis. J Bacteriol. 2005;187:739–746. doi: 10.1128/JB.187.2.739-746.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Challis GL, Ravel J, Townsend CA. Predictive, structure-based model of amino acid recognition by nonribosomal peptide synthetase adenylation domains. Chem. Biol. 2000;7:211–224. doi: 10.1016/s1074-5521(00)00091-0. [DOI] [PubMed] [Google Scholar]
- 17.Stachelhaus T, Schneider A, Marahiel MA. Rational Design of Peptide Antiobiotics by Targeted Replacement of Bacterial and Fungal Domains. Science. 1995;269:69–72. doi: 10.1126/science.7604280. [DOI] [PubMed] [Google Scholar]
- 18.Khosla C, Ebert-Khosla S, Hopwood DA. Targeted gene replacements in a Streptomyces polyketide synthase gene cluster: role for the acyl carrier protein. Mol. Microbiol. 1992;6:3237–3249. doi: 10.1111/j.1365-2958.1992.tb01778.x. [DOI] [PubMed] [Google Scholar]
- 19.Mootz HD, Kessler N, Linne U, Eppelmann K, Schwarzer D, Marahiel MA. Decreasing the Ring Size of a Cyclic Nonribosomal Peptide Antibiotic by In-Frame Module Deletion in the Biosynthetic Genes. J. Am. Chem. Soc. 2002;124:10980–10981. doi: 10.1021/ja027276m. [DOI] [PubMed] [Google Scholar]
- 20.Uguru GC, Milne C, Borg M, Flett F, Smith CP, Micklefield J. Active-Site Modifications of Adenylation Domains Lead to Hydrolysis of Upstream Nonribosomal Peptidyl Thioester Intermediates. J. Am. Chem. Soc. 2004;126:5032–5033. doi: 10.1021/ja048778y. [DOI] [PubMed] [Google Scholar]
- 21.Butz D, Schmiederer T, Hadatsch B, Wohlleben W, Weber T, Süssmuth R. Module Extention of a Non-Ribosomal Peptide Synthetase of the Glycopeptide Antibiotic Balhimycin Produced by Amycolatopsis balhimycina. ChemBioChem. 2008;9:1195–1200. doi: 10.1002/cbic.200800068. [DOI] [PubMed] [Google Scholar]
- 22.Powell A, Borg M, Amir-Heidari B, Neary JM, Thirlway J, Wilkinson B, Smith CP, Micklefield J. Engineered Biosynthesis of Nonribosomal Lipopeptides with Modified Fatty Acid Side Chains. J. Am. Chem. Soc. 2007;129:15182–15191. doi: 10.1021/ja074331o. [DOI] [PubMed] [Google Scholar]
- 23.Baltz RH. Chapter 20 Biosynthesis and Genetic Engineering of Lipopeptides in Streptomyces roseosporus Methods in Enzymology. In: Hopwood DA, editor. Complex Enzymes in Microbial Natural Product Biosynthesis, Part A: Overview Articles and Peptides. Volume 458. Academic Press; 2009. pp. 511–531. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen KT, He X, Alexander DC, Li C, Gu J-Q, Mascio C, Van Praagh A, Mortin L, Chu M, Silverman JA, Brian P, Baltz RH. Genetically Engineered Lipopeptide Antibiotics Related to A54145 and Daptomycin with Improved Properties. Antimicrob. Agents Chemother. 2010;54:1404–1413. doi: 10.1128/AAC.01307-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander DC, Rock J, He X, Brian P, Miao V, Baltz RH. Development of a Genetic System for Combinatorial Biosynthesis of Lipopeptides in Streptomyces fradiae and Heterologous Expression of the A54145 Biosynthesis Gene Cluster. Appl. Environ. Microbiol. 2010;76:6877–6887. doi: 10.1128/AEM.01248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rausch C, Hoof I, Weber T, Wohlleben W, Huson DH. Phylogenetic analysis of condensation domains in NRPS sheds light on their functional evolution. BMC Evol. Biol. 2007;7:78. doi: 10.1186/1471-2148-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubbard BK, Thomas MG, Walsh CT. Biosynthesis of p-hydroxyphenylglycine, a non-proteinogenic amino acid constituent of peptide antibiotics. Chem. Biol. 2000;7:931–942. doi: 10.1016/s1074-5521(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 28.Müller U, van Assema F, Gunsior M, Orf S, Kremer S, Schipper D, Wagemans A, Townsend CA, Sonke T, Bovenberg R, Wubbolts M. Metabolic engineering of the E. coli L-phenylalanine pathway for the production of D-phenylglycine (d-Phg) Metab. Eng. 2006;8:196–208. doi: 10.1016/j.ymben.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Haynes SW, Challis GL. Non-linear enzymatic logic in natural product modular mega-synthases and -synthetases. Curr. Opin. Drug. Disc. 2007;10:203–218. [PubMed] [Google Scholar]
- 30.Mootz HD, Schwarzer D, Marahiel MA. Ways of Assembling Complex Natural Products on Modular Nonribosomal Peptide Synthetases. ChemBioChem. 2002;3:490–504. doi: 10.1002/1439-7633(20020603)3:6<490::AID-CBIC490>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 31.Wenzel SC, Kunze B, Höfle G, Silakowski B, Scharfe M, Blöcker H, Müller R. Structure and Biosynthesis of Myxochromides S1-3 in Stigmatella aurantiaca: Evidence for an Iterative Bacterial Type I Polyketide Synthase and for Module Skipping in Nonribosomal Peptide Biosynthesis. ChemBioChem. 2005;6:375–385. doi: 10.1002/cbic.200400282. [DOI] [PubMed] [Google Scholar]
- 32.Wenzel SC, Meiser P, Binz TM, Mahmud T, Müller R. Nonribosomal Peptide Biosynthesis: Point Mutations and Module Skipping Lead to Chemical Diversity. Angew. Chem. Int. Edit. 2006;45:2296–2301. doi: 10.1002/anie.200503737. [DOI] [PubMed] [Google Scholar]
- 33.Stachelhaus T, Mootz HD, Bergendahl V, Marahiel MA. Peptide Bond Formation in Nonribosomal Peptide Biosynthesis. J. Biol. Chem. 1998;273:22773–22781. doi: 10.1074/jbc.273.35.22773. [DOI] [PubMed] [Google Scholar]
- 34.Bergendahl V, Linne U, Marahiel MA. Mutational analysis of the C-domain in nonribosomal peptide synthesis. Eur. J. Biochem. 2002;269:620–629. doi: 10.1046/j.0014-2956.2001.02691.x. [DOI] [PubMed] [Google Scholar]
- 35.Miao V, Coeffet-LeGal M-F, Brian P, Brost R, Penn J, Whiting A, Martin S, Ford R, Parr I, Bouchard M, Silva CJ, Wrigley SK, Baltz RH. Daptomycin biosynthesis in Streptomyces roseosporus: cloning and analysis of the gene cluster and revision of peptide stereochemistry. Microbiology. 2005;151:1507–1523. doi: 10.1099/mic.0.27757-0. [DOI] [PubMed] [Google Scholar]
- 36.Roche ED, Walsh CT. Dissection of the EntF Condensation Domain Boundary and Active Site Residues in Nonribosomal Peptide Synthesis. Biochemistry. 2003;42:1334–1344. doi: 10.1021/bi026867m. [DOI] [PubMed] [Google Scholar]
- 37.Du L, Chen M, Zhang Y, Shen B. BlmIII and BlmIV Nonribosomal Peptide Synthetase-Catalyzed Biosynthesis of the Bleomycin Bithiazole Moiety Involving Both in Cis and in Trans Aminoacylation. Biochemistry. 2003;42:9731–9740. doi: 10.1021/bi034817r. [DOI] [PubMed] [Google Scholar]
- 38.Samel SA, Schoenafinger G, Knappe TA, Marahiel MA, Essen L-O. Structural and Functional Insights into a Peptide Bond-Forming Bidomain from a Nonribosomal Peptide Synthetase. Structure. 2007;15:781–792. doi: 10.1016/j.str.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Rouhiainen L, Jokela J, Fewer DP, Urmann M, Sivonen K. Two Alternative Starter Modules for the Non-Ribosomal Biosynthesis of Specific Anabaenopeptin Variants in Anabaena (Cyanobacteria) Chem. Biol. 2010;17:265–273. doi: 10.1016/j.chembiol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Robbel L, Helmetag V, Knappe TA, Marahiel MA. Consecutive Enzymatic Modification of Ornithine Generates the Hydroxamate Moieties of the Siderophore Erythrochelin. Biochemistry. 2011;50:6073–6080. doi: 10.1021/bi200699x. [DOI] [PubMed] [Google Scholar]
- 41.Lazos O, Tosin M, Slusarczyk AL, Boakes S, Cortés J, Sidebottom PJ, Leadlay PF. Biosynthesis of the Putative Siderophore Erythrochelin Requires Unprecedented Crosstalk between Separate Nonribosomal Peptide Gene Clusters. Chem. Biol. 2010;17:160–173. doi: 10.1016/j.chembiol.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Bosello M, Robbel L, Linne U, Xie X, Marahiel MA. Biosynthesis of the Siderophore Rhodochelin Requires the Coordinated Expression of Three Independent Gene Clusters in Rhodococcus jostii RHA1. J. Am. Chem. Soc. 2011;133:4587–4595. doi: 10.1021/ja1109453. [DOI] [PubMed] [Google Scholar]
- 43.Yu S, Fiss E, Jacobs WRJ. Analysis of the Exochelin Locus in Mycobacterium smegmatis: Biosynthesis Genes Have Homology with Genes of the Peptide Synthetase Family. J. Bacteriol. 1998;180:4676–4685. doi: 10.1128/jb.180.17.4676-4685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lombó F, Velasco A, Castro A, de la Calle F, Braña AF, Sánchez-Puelles JM, Méndez C, Salas JA. Deciphering the Biosynthesis Pathway of the Antitumor Thiocoraline from a Marine Actinomycete and Its Expression in Two Streptomyces Species. ChemBioChem. 2006;7:366–376. doi: 10.1002/cbic.200500325. [DOI] [PubMed] [Google Scholar]
- 45.Roongsawang N, Washio K, Morikawa M. In Vivo Characterization of Tandem C-Terminal Thioesterase Domains in Arthrofactin Synthetase. ChemBioChem. 2007;8:501–512. doi: 10.1002/cbic.200600465. [DOI] [PubMed] [Google Scholar]
- 46.Hou J, Robbel L, Marahiel, Mohamed A. Identification and Characterization of the Lysobactin Biosynthetic Gene Cluster Reveals Mechanistic Insights into an Unusual Termination Module Architecture. Chem. Biol. 2011;18:655–664. doi: 10.1016/j.chembiol.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Oman TJ, van der Donk WA. Follow the leader: the use of leader peptides to guide natural product biosynthesis. Nat. Chem. Biol. 2010;6:9–18. doi: 10.1038/nchembio.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinscherf TG, Willis DK. The Biosynthetic Gene Cluster for the β-Lactam Antibiotic Tabtoxin in Pseudomonas syringae. J. Antibiot. 2005;58:817–821. doi: 10.1038/ja.2005.109. [DOI] [PubMed] [Google Scholar]
- 49.Levi C, Durbin RD. The isolation and properties of a tabtoxin-hydrolyzing aminopeptidase from the periplasm of Pseudomonas syringae pv. tabaci. Physiol. Mol. Plant Pathol. 1986;28:345–352. [Google Scholar]
- 50.Linne U, Marahiel MA. Control of Directionality in Nonribosomal Peptide Synthesis: Role of the Condensation Domain in Preventing Misinitiation and Timing of Epimerization. Biochemistry. 2000;39:10439–10447. doi: 10.1021/bi000768w. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann K, Schneider-Scherzer E, Kleinkauf H, Zocher R. Purification and characterization of eucaryotic alanine racemase acting as key enzyme in cyclosporin biosynthesis. J. Biol. Chem. 1994;269:12710–12714. [PubMed] [Google Scholar]
- 52.Li J, Jensen SE. Nonribosomal Biosynthesis of Fusaricidins by Paenibacillus polymyxa PKB1 Involves Direct Activation of a d-Amino Acid. Chem. Biol. 2008;15:118–127. doi: 10.1016/j.chembiol.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Zocher R, Nihira T, Paul E, Madry N, Peeters H, Kleinkauf H, Keller U. Biosynthesis of Cyclosporin A: Partial Purification and Properties of a Multifunctional Enzyme from Tolypocladium inflatum. Biochemistry. 1986;25:550–553. doi: 10.1021/bi00351a005. [DOI] [PubMed] [Google Scholar]
- 54.Tang G-L, Cheng Y-Q, Shen B. Chain Initiation in the Leinamycin-producing Hybrid Nonribosomal Peptide/Polyketide Synthetase from Streptomyces atroolivaceus S-140: Discrete, Monofunctional Adenylation Enzyme and Peptidyl Carrier Protein that Directly Load D-Alanine. J. Biol. Chem. 2007;282:20273–20282. doi: 10.1074/jbc.M702814200. [DOI] [PubMed] [Google Scholar]
- 55.Balibar CJ, Vaillancourt FH, Walsh CT. Generation of D Amino Acid Residues in Assembly of Arthrofactin by Dual Condensation/Epimerization Domains. Chem. Biol. 2005;12:1189–1200. doi: 10.1016/j.chembiol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Patel HM, Tao J, Walsh CT. Epimerization of an L-Cysteinyl to a D-Cysteinyl Residue during Thiazoline Ring Formation in Siderophore Chain Elongation by Pyochelin Synthetase from Pseudomonas aeruginosa. Biochemistry. 2003;42:10514–10527. doi: 10.1021/bi034840c. [DOI] [PubMed] [Google Scholar]
- 57.Sosio M, Donadio S. Understanding and manipulating glycopeptide pathways: the example of the dalbavancin precursor A40926. J. Ind. Microbiol. Biotechnol. 2006;33:569–576. doi: 10.1007/s10295-006-0124-1. [DOI] [PubMed] [Google Scholar]
- 58.Lam H, Oh D-C, Cava F, Takacs CN, Clardy J, de Pedro MA, Waldor MK. D-Amino Acids Govern Stationary Phase Cell Wall Remodeling in Bacteria. Science. 2009;325:1552–1555. doi: 10.1126/science.1178123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salituro GM, Townsend CA. Total Syntheses of (-)-Nocardicins A-G: A Biogenetic Approach. J. Am. Chem. Soc. 1990;112:760–770. [Google Scholar]
- 60.Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Publicatations; Cold Spring Harbor, New York: 1982. [Google Scholar]
- 61.Weiner MP, Costa GL, editors. Rapid PCR site-directed mutagenesis. CSH Laboratory Press; Cold Spring Harbor, NY: 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.