Abstract
Objectives
To conduct a direct head-to-head comparison of different stem cell types in vitro for various assays of potency, and in vivo for functional myocardial repair in the same mouse model of myocardial infarction.
Background
Adult stem cells of diverse origins (e.g., bone marrow, fat, heart) and antigenic identity have been studied for repair of the damaged heart, but the relative utility of the various cell types remains unclear.
Methods
Human cardiosphere-derived stem cells (CDCs), bone marrow-derived mesenchymal stem cells (BM-MSCs), adipose tissue-derived mesenchymal stem cells (AD-MSCs), and bone marrow mononuclear cells (BM-MNCs) were compared.
Results
CDCs revealed a distinctive phenotype with uniform expression of CD105, partial expression of c-kit and CD90, and negligible expression of hematopoietic markers. In vitro, CDCs showed the greatest myogenic differentiation potency, highest angiogenic potential, and relatively high production of various angiogenic and anti-apoptotic secreted factors. In vivo, injection of CDCs into the infarcted mouse hearts resulted in superior improvement of cardiac function, the highest cell engraftment and myogenic differentiation rates, and the least-abnormal heart morphology 3 weeks after treatment. CDC-treated hearts also exhibited the lowest number of apoptotic cells. The c-kit+ subpopulation purified from CDCs produced lower levels of paracrine factors and inferior functional benefit when compared to unsorted CDCs. To validate the comparison of cells from various human donors, selected results were confirmed in cells of different types derived from individual rats.
Conclusions
CDCs exhibit a balanced profile of paracrine factor production, and, among various comparator cell types/subpopulations, provide the greatest functional benefit in experimental myocardial infarction.
Keywords: cardiac stem cells, mesenchymal stem cells, myocardial regeneration, paracrine effects
Resident cardiac stem cells are now recognized to exist in the adult mammalian heart, including mouse, rat, and human.1-7 Several groups have expanded the small population of cardiac stem cells from adult human heart tissue with a view to clinical applications in regenerative therapy.7-9 We have developed a technique to expand tens of millions of cardiosphere-derived cells (CDCs), a mixture of cardiac stem cells and supporting cells, from percutaneous endomyocardial biopsies,8 and this technology is in phase I-II clinical study for the treatment of post-ischemic heart failure (the CADUCEUS trial, NCT00893360; www.clinicaltrials.gov). Nevertheless, heart-derived cells are relative newcomers to regenerative cardiology.
Multiple extra-cardiac cell sources, including bone marrow mononuclear cells (BM-MNCs), bone marrow-derived mesenchymal stem cells (BM-MSCs), adipose tissue-derived mesenchymal stem cells (AD-MSCs), endothelial progenitor cells, and myoblasts have been used clinically in attempts to regenerate the damaged heart.10-18 The implantation of these cells of extra-cardiac origin has been found to produce generally positive effects, mostly through paracrine mechanisms.19-22 Although resident cardiac stem cells can mediate direct cardiogenesis and angiogenesis,1-7, 23,24 recent studies have found that even these cells exert most of their benefits via indirect paracrine mechanisms.25,26 Therefore, no convincing evidence supports the superiority of heart-derived cells for myocardial repair. Even within heart-derived cells, two cell products are in clinical trials, but these have yet to be compared directly. The first, CDCs, are a natural mixture of stromal, mesenchymal and progenitor cells;8 the second cell product represents the c-kit+ subpopulation purified from mixed heart-derived cells.1,9
Here, we have performed a head-to-head comparison of different stem cell types/subpopulations for functional myocardial repair by assessing multiple in vitro parameters, including secretion of relevant growth factors, and in vivo cell implantation into an acute myocardial infarction model in severe combined immunodeficiency (SCID) mice.
Methods
Cell Sources
Human CDCs were expanded as previously described from minimally-invasive endomyocardial biopsies.27 Human BM-MSCs and BM-MNCs were purchased from Lonza (Walkersville, MD). Human AD-MSCs were purchased from Invitrogen (Carlsbad, CA). These cells were freshly-isolated from healthy donors. The c-kit+ stem cell subpopulation was purified from CDCs using a CELLection Pan Mouse IgG Kit and a Dynal Magnetic Particle Concentrator-15 (Invitrogen).
For confirmatory rat studies, four-month-old Wistar Kyoto rats were used to expand CDCs, BM-MSCs, and AD-MSCs as previously described.23,28,29 BM-MNCs were also collected from the same rats by gradient centrifugation.19 Freshly-collected BM-MNCs and twice-passaged CDCs, BM-MSCs, and AD-MSCs were used for rat experiments.
Unless otherwise noted, IMDM basic medium (Gibco) supplemented with 10% FBS (Hyclone) and 20 mg/ml gentamycin was used to culture all cell lines.
Flow cytometry
The characterization of CDCs, BM-MSCs, AD-MSCs, and BM-MNCs was investigated by flow cytometry as described.6,8 Briefly, cells were incubated with FITC or PE-conjugated antibodies against CD29, CD31, CD34, CD45, CD90, CD105, CD117 (c-kit), and CD133 (eBioscience) for 30 minutes. Isotype-identical antibodies served as negative control. Quantitative analysis was performed using a FACSCalibur flow cytometer with CellQuest software (BD Biosciences).6,8
ELISA
To compare the potency of the production of growth factors, cells were seeded in 24-well culture plates at densities of 1×106/ml (BM-MNCs) or 1×105/ml (all other cell types) in FBS-free IMDM media (all cell types) for 3 days. The supernatants were collected and the concentrations of angiopoietin-2, bFGF, HGF, IGF-1, PDGF, SDF-1, and VEGF were measured with human ELISA kits (R&D Systems Inc.), according to the manufacturer’s instructions. Given the limited number of rat-specific ELISA kits, we only measured the concentrations of HGF (B-Bridge International, Inc.), IGF-1, and VEGF in the supernatant with 3 days’ culture of rat cells (R&D Systems Inc.).
To compare the production of growth factors from the purified c-kit+ subpopulation and unsorted CDCs, we seeded cells (5×104/ml) on 24-well culture plates and culture for 2 days under 20% O2. Growth factors in conditioned media were measured by ELISA as described above.
Immunostaining
To determine myogenic differentiation in vitro, cells were seeded on fibronectin-coated 4-chamber culture slides. After 7 days of culture, cells were fixed, blocked with goat serum for 30 minutes, and then incubated with mouse anti-human troponin T antibody (R&D Systems Inc.) for human cells or with goat anti-rat troponin T antibody for rat cells. After 1 hour incubation at room temperature, culture slides were washed and then incubated with a PE-conjugated secondary antibody. Cell nuclei were stained with DAPI. Cardiomyogenic differentiation was quantified by counting positively-stained cells.
In vitro angiogenesis assay
Angiogenic potency was assayed by tube formation using a kit (Chemicon Int.), according to the manufacturer’s instructions. Briefly, cells were seeded on ECMatrix™-coated 96-well plates at a density of 2×105 cells (BM-MNCs) or 2×104 cells (all other cell types) per well. Human umbilical vein endothelial cells (HUVECs) were included as positive controls. After 6 hours, tube formation was imaged. The total tube length was then measured with Image-Pro Plus software (version 5.1.2, Media Cybernetics Inc., Carlsbad, CA).
TUNEL assay
To quantify the resistance to oxidative stress in vitro, cells were seeded on fibronectin-coated 4-chamber culture slides. After 24 hours of culture, cells were cultured with or without the addition of 100 μM H2O2 to the medium for another 24 hours. Cells were fixed, and apoptotic cells were detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using the In Situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany), according to the manufacturer’s instructions. Cell nuclei were stained with DAPI; apoptotic cells were counted by TUNEL-positive nuclei.
Myocardial infarction model and cell implantation
Acute myocardial infarction was created in male SCID-beige mice (10-12 weeks old), as described.6,8 Briefly, just after ligation of the left anterior descending artery with 9-0 prolene, hearts were injected at four points in the infarct border zone with a total of 40 μl of one of the following interventions: phosphate-buffered saline (Control, n=8), 1×105 CDCs (CDCs, n=20), 1×105 BM-MSCs (n=20), 1×105 AD-MSCs (n=20), 1×106 BM-MNCs (high BM-MNCs, n=11), or 1×105 BM-MNCs (low BM-MNCs, n=9). We studied two dosages in BM-MNCs, including one with 10-fold more cells than in the comparator groups, because MNCs are smaller than the other cell types,19 and we did not want to bias against them in terms of total transplanted cell mass.
To compare the c-kit+ stem cell subpopulation purified from CDCs to unsorted CDCs, we performed a separate study by injecting 1×105 purified c-kit+ cells (c-kit+, n=16) and 1×105 unsorted CDCs (unsorted, n=11) into the infarcted hearts of SCID mice, using the methods described above.
Echocardiography
Mice underwent echocardiography 3 hours (baseline) and 3 weeks after surgery using Vevo 770™ Imaging System (VISUALSONICS™, Toronto, Canada).8 After the induction of light general anesthesia, the hearts were imaged two-dimensionally in long-axis views at the level of the greatest left ventricular (LV) diameter. LV end diastolic volume, LV end systolic volume, and LV ejection fraction (LVEF) were measured with VisualSonics V1.3.8 software from 2D long-axis views taken through the infarcted area. Blinded reading of echos was conducted independently by two experienced echocardiographers (K.M. and J.T.). The results correlated well (Supplemental Figure 1; R2=0.66 and 0.73 for Baseline and 3 weeks measurement, respectively). A Bland-Altman plot indicates the limit of agreement to be from −14.07 to 13.56.30 Thus, the averages of the two readings for LVEF in each mouse were used for statistical analysis.
Histology
Mice were sacrificed 3 weeks after treatment. Hearts were sectioned in 5 μm sections and fixed with 4% paraformaldehyde. The engraftment of implanted human cells was identified by immunostaining for human nuclear antigen (HNA; Chemicon Int.). To measure cell engraftment, 10 images of the infarct and border zones were selected randomly from each animal. To quantify the apoptotic cells in the heart, slides were fixed and apoptotic cells were detected by TUNEL assay as described above. The differentiation of implanted human cells into cardiomyocytes in the infarcted hearts of SCID mice was identified by immunostaining with monoclonal antibodies against human specific α-sarcomeric actin (Sigma), as described previously.8,31 For morphometric analysis, animals in each group were euthanized at 3 weeks (after cardiac function assessment) and the hearts were harvested and frozen in OCT compound. Sections every 100 μm (5 μm thick) were prepared. Masson’s trichrome staining was performed as per manufacturer’s instructions (HT15 Trichrome Staining (Masson) Kit; Sigma). Images were acquired with a PathScan Enabler IV slide scanner (Advanced Imaging Concepts, Princeton, NJ). From the Masson’s trichrome-stained images, morphometric parameters including infarct wall thickness and infarct perimeter were measured in each section with NIH ImageJ software.8,31
Quantification of engraftment by real time PCR
Quantitative PCR was performed 3 weeks after cell injection to quantify cell engraftment. We injected male human cells to enable detection of the SRY gene located on the Y chromosome as a marker of engrafted cells.31 The whole mouse heart was harvested, weighed, and homogenized. The TaqMan® assay was used to quantify the number of transplanted cells with the human SRY gene as template (Applied Biosystems, CA). A standard curve was generated with multiple dilutions of genomic DNA isolated from the injected CDCs to quantify the absolute gene copy numbers. All samples were spiked with equal amounts of genomic DNA from non-injected mouse hearts as control. For each reaction, 50 ng of genomic DNA was used. Real time PCR was performed in triplicate with an Applied Biosystems 7900 HT Fast real-time PCR machine. The number of engrafted cells per heart was quantified from the standard curve.
Statistical analysis
Statistical analysis was performed independently (by H.K.). All results are presented as mean ± SD except as noted. Data sets were first tested for normality (Kolmogorov-Smirnov’s test) and variance (Levene’s test). If both were assured, statistical significance was determined by one-way ANOVA followed by post hoc test (Dr. SPSS II, Chicago, IL). If either normality or variance tests failed, nonparametric tests (Kruskal-Wallis followed by Dunns’s post test) were used. Differences were considered statistically significant when p<0.05.
Results
Characterization of cell phenotypes
Unlike BM-MNCs, which grow in suspension as small round cells, all other cell types studied (CDCs, BM-MSCs, and AD-MSCs) typically grow as adherent monolayers (Figure 1A). Flow cytometry distinguished BM-MNCs from other cell types by the predominant expression of pan-hematopoietic marker CD45 (74.7%), as compared to <1% in CDCs, BM-MSCs, and AD-MSCs (Figure 1B). Conversely, >99% of CDCs, BM-MSCs, and AD-MSCs expressed CD105, a TGF-β receptor subunit commonly associated with MSCs. However, these three cell types can be distinguished by CD90: >99% of BM-MSCs and 85% of AD-MSCs expressed CD90, but only 18% of CDCs did so. CD90 (well-known as Thy-1) was originally discovered as a thymocyte antigen.32 In humans, Thy-1 is also expressed by endothelial cells, smooth muscle cells, a subset of CD34+ bone marrow cells, and umbilical cord blood-, fibroblasts, and fetal liver-derived hematopoietic cells. CD90 is widely used as a marker of a variety of stem cells, e.g. MSCs, hepatic stem cells, keratinocyte stem cells, putative endometrial progenitor/stem cells, and hematopoietic stem cells.33,34 On the basis of these findings, CDCs appear to contain a minority of fibroblast and/or weakly-committed hematopoietic cells, while such populations dominate in the cells of bone marrow and adipose origins; alternatively, the CD90 in CDCs may simply mark the cardiac mesenchymal subpopulation.
Figure 1. Morphology and phenotype characterization.
A) Representative images of cardiosphere-derived stem cells (CDCs), bone marrow-derived mesenchymal stem cells (BM-MSCs), adipose tissue-derived mesenchymal stem cells (AD-MSCs), and bone marrow-derived mononuclear cells (BM-MNCs) after 3 days in culture under 20% O2. CDCs, BM-MSCs, and AD-MSCs demonstrate adherent growth and stromal (mesenchymal) cell-like morphology; BM-MNCs have smaller size and round shape. B) Expression profile of CD29, CD31, CD34, CD45, CD90, CD117 (c-kit), and CD133 in CDCs, BM-MSCs, AD-MSCs, and BM-MNCs. Bar = 50 μm.
In vitro secretion of growth factors
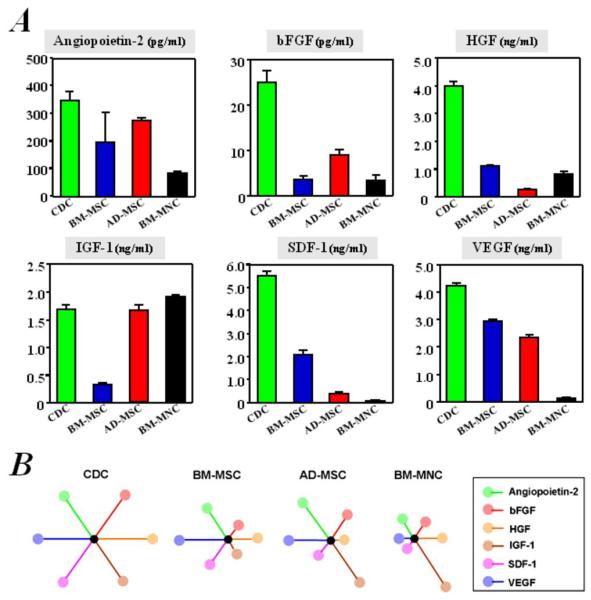
Increasing evidence supports the generalization that cell therapy boosts cardiac function largely via paracrine mechanisms.25 We thus compared the production of six growth factors (angiopoietin-2, bFGF, HGF, IGF-1, SDF-1, and VEGF) by the various cell types. CDCs were unique in their ability to secrete large amounts of all growth factors (Figure 2A). In contrast, the other cell types failed to express meaningful levels of one or more growth factors: BM-MNCs produced little VEGF and SDF-1; BM-MSCs secreted little IGF-1 and bFGF; and AD-MSCs were not rich sources of HGF and SDF-1 (Figure 2A). Figure 2B depicts schematically the secretion of each of the six cytokines in each given cell type, as wheel-and-spoke diagrams in which the length of each spoke is proportional to the growth factor concentration in conditioned media. The symmetrical starburst pattern highlights the relatively high and distributed paracrine factor production of CDCs.
Figure 2. Comparison of in vitro production of growth factors from cells cultured.
Concentrations of angiopoietin-2, bFGF, HGF, IGF-1, SDF-1, and VEGF, measured by ELISA (n=3), are depicted (A). B) Schematic depicting the secretion of each of the six cytokines in each given cell type, as wheel-and-spoke diagrams in which the length of each spoke is proportional to the growth factor concentration in conditioned media and is normalized to that from CDCs. The symmetrical starburst pattern highlights the uniquely well-balanced paracrine profile of CDCs.
One limitation of our comparison is that we used 10-fold higher concentrations of BM-MNCs in these experiments, with the rationale that these cells are much smaller than the other three cell types,24 and thus have a lower secretory biomass. Another limitation of our comparison is the fact that the various human cell types originated from different subjects. In order to ensure that the findings did not simply reflect donor-specific idiosyncrasies, we compared growth factor secretion by the various cell types derived from individual rats. In agreement with the findings in human stem cells, higher levels of HGF, IGF-1, and VEGF were also observed in media conditioned by rat CDCs than by rat BM-MSCs, AD-MSCs, and BM-MNCs, all collected from the same animals (Supplemental Figure 2). The well-balanced release of growth factors in vitro confirms the notion that CDCs are particularly rich biological factories,25,35 a feature which may favor enhanced myocardial repair through paracrine mechanisms after implantation into the heart.
Cardiomyogenic differentiation
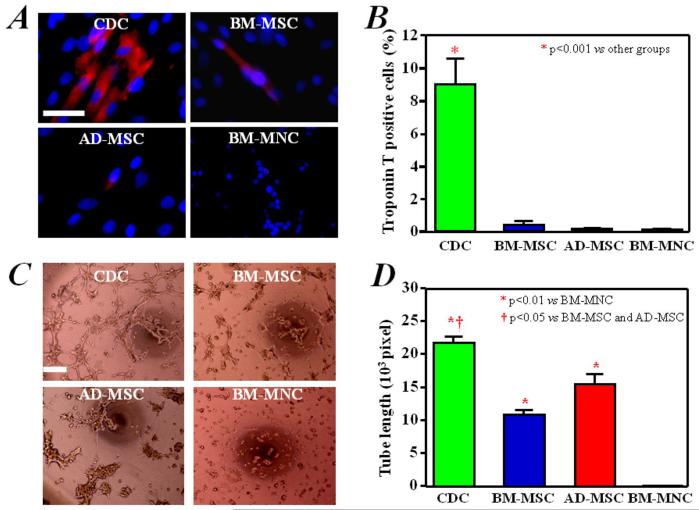
Another potential advantage of CDCs is their reported ability to differentiate into cardiomyocytes,8,24,25,31 although this propensity has not been compared quantitatively with other cell types. We therefore examined the ability to undergo spontaneous cardiomyogenic differentiation in vitro by immunostaining for cardiac-specific troponin T. Many human CDCs expressed troponin T (Figure 3A), in contrast to human BM-MSCs, AD-MSCs, or BM-MNCs, few of which were positive for troponin T. Quantitative analysis showed that ~9% of CDCs ended up expressing troponin T, while <1% did so in the other cell types (Figure 3B). Similar findings were observed using rat CDCs, BM-MSCs, AD-MSCs, and BM-MNCs, all collected from the same animals (Supplemental Figure 3).
Figure 3. In vitro myogenic differentiation and angiogenesis assay.
A) Troponin T, with distinct myocyte-like appearance, was expressed spontaneously in a fraction of CDCs cultured for 7 days. This cardiac-specific marker was rarely expressed in BM-MSCs, AD-MSCs, and BM-MNCs. B) Quantitative analysis of Troponin T expression (n=3) in CDCs (9% of the cells positive), BM-MSCs (0.4% positive) and AD-MSCs and BM-MNCs (approximately 0.1% positive). C) CDCs, BM-MSCs, and AD-MSCs produced capillary-like tube formations in extracellular matrix. BM-MNCs did not form similar structures under these conditions. D) Quantitation and comparison of tube formation capacity by the different cell types (n=3). Bars = 50 um.
Tube formation
We quantified the angiogenic ability of the various cell types using an in vitro tube-forming assay.36 All cell types could form capillary-like networks on matrigel within 6 hours, with the exception of BM-MNCs (Figure 3C). Quantitative analysis showed that the mean tube length of the capillary-like networks was greater in CDCs than in the other cell types (p<0.05, Figure 3D). Thus, CDCs, at least by this simple in vitro assay, are superior in mediating the formation of capillary-like structures.
Resistance to oxidative stress
Enhanced cell resilience to oxidative stress favors transplanted cell engraftment and functional benefit.35 We assayed sensitivity to oxidative stress by exposing cells to H2O2, a powerful oxidant. After 24 hours of exposure to 100μM H2O2, the number of apoptotic cells tended to be lower in human CDCs than in human BM-MNCs (p=0.067, Supplemental Figure 4), but there was no significant difference among CDCs, BM-MSCs, and AD-MSCs. In rat cells, higher apoptosis was observed in BM-MNCs compared to any of the other three cell types after H2O2 (p<0.05, Supplemental Figure 5). Taken together, these data highlight a relative deficiency of BM-MNCs in terms of resistance to oxidative stress.
Cell engraftment and in vivo differentiation
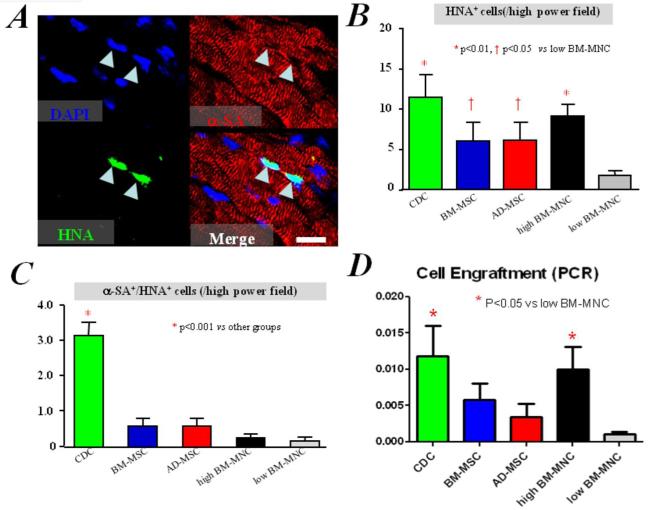
While most of the benefit of cell therapy is now recognized to be indirect, study after study has revealed a positive correlation between long-term cell engraftment and functional benefit.10,19-22,25 We therefore evaluated the engraftment and differentiation of human cells 3 weeks after direct intramyocardial injection into the infarcted hearts of SCID mice. Consistent with previous reports,7,8,23-25,31 histology revealed expression of α-sarcomeric actin (α-SA) in some of the surviving progeny of human CDCs (positive for human nuclear antigen [HNA]; Figure 4A), confirming the cardiomyogenic differentiation in vivo. In contrast, human cells positive for α-sarcomeric actin were observed rarely and inconsistently in mice injected with BM-MSCs, AD-MSCs, and BM-MNCs (data not shown). Quantitative image analysis confirmed that the engraftment (i.e. the numbers of HNA+ cells) was greater in mice implanted with human CDCs than with comparator cells (p<0.05, Figure 4B). In addition, the numbers of cardiomyocytes derived from the transplanted cells (HNA+/α-SA+) were greater in mice implanted with human CDCs than with any of the other cell types (p<0.01, Figure 4C). Quantitative PCR confirmed the histological analysis: more human cells were detected in the CDC group than in the low BM-MNC group (p<0.05, Figure 4D) 3 weeks after injection. Also, there was a trend indicating higher engraftment in the CDC group than in the BM-MSC and AD-MSC groups, by PCR analysis.
Figure 4. Cell engraftment and in vivo myogenic differentiation.
A) Immunostaining shows some human CDCs (green, HNA) expressing α-sarcomeric actin, indicating myogenic differentiation, 3 weeks after implantation into infarcted mice hearts. B) Quantitation of engraftment (HNA+ cells). C) Quantitation of cardiomyocytes differentiated from transplanted human cells (HNA+/α-SA+ cells). Bar = 20 um. D) Quantitation of engraftment by quantitative PCR (n=3 mice per group).
Cell apoptosis
In addition to tissue regeneration, tissue preservation may be a salutary component of cell therapy for acute myocardial infarction.25 To evaluate this possibility, we quantified apoptotic nuclei in infarcts of control mice and mice injected with each of the comparator cell types. TUNEL staining revealed apoptotic nuclei in the infarcted hearts 3 weeks after treatment (Figures 5A&B). We counted the total number of apoptotic cells in the infarct and peri-infarct area. The hearts of mice implanted with CDCs exhibited fewer TUNEL-positive cells, compared to all other cell-treated groups (p<0.05, Figure 5C). We speculate that this enhanced tissue preservation may be due to the larger amount of pro-angiogenic and anti-apoptotic factors produced by CDCs.19,25,35
Figure 5. Cell apoptosis.
A) & B) Representative images of TUNEL-positive cells in the infarcted hearts of mice 3 weeks after cell treatment with CDCs (A) and PBS (B). C) Quantitative assessment of TUNEL-positive cells in the myocardium of mice treated with different cell types and control, is shown (n=3 mice per group). Bar = 500 um.
Cardiac function
The most meaningful indicator of cell potency, in practice, is the ability to produce functional benefit after transplantation into the injured heart. We measured cardiac function by echocardiography, and all images were interpreted blindly and independently by two experienced sonographers. Figure 6 summarizes the results. The LVEF at baseline (i.e., two hours post-infarction) was comparable, indicating similar ischemic injury among groups. Among the various treatments, the implantation of CDCs resulted in the greatest LEVF at 3 weeks and it is the only group that was significantly better than Control (p<0.05 vs Control). The other cell types, although higher on average than controls, had no clear functional benefit statistically. Echocardiography also revealed smaller end-diastolic and -systolic LV volumes in the CDC-treated animals (Supplemental Figure 6).
Figure 6. Cardiac function.
LVEF at baseline (4 hrs post-MI) did not differ among groups, indicating a similar infarct size in animals of all groups. After 3 weeks, LVEF was higher in mice implanted with CDCs, compared to controls injected with saline only. Differences between any other two groups are not significant. Data are presented as mean ± SEM.
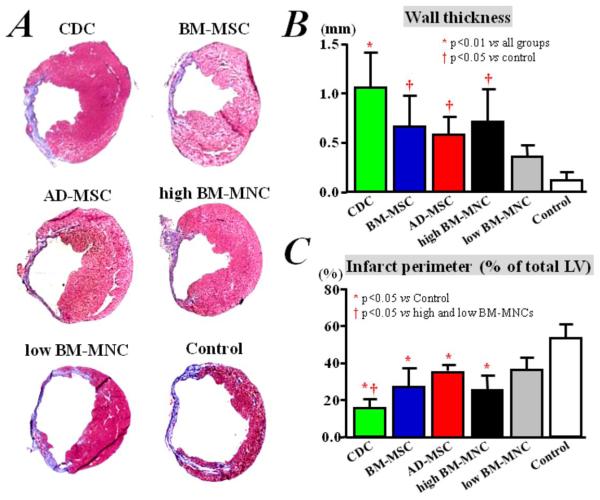
Ventricular remodeling
Potentiating the functional benefits of cell therapy is attenuation of adverse ventricular remodeling.8 To evaluate this process, we examined the morphological consequences of transplantation of the various cell types on myocardial infarct size and wall thinning. Heart morphometry at 3 weeks showed severe LV chamber dilatation and infarct wall thinning in the control hearts (Figure 7A; Supplemental Figure 7). In contrast, all the cell-treated groups exhibited attenuated LV remodeling. Compared to control, the implantation of any type of human cells decreases fractional infarct perimeter and, conversely, increases the minimal infarct wall thickness, 3 weeks after treatment (p<0.05 vs Control group, Figures 7B&C). The protective effect was greatest in the CDC-treated hearts, which had thicker infarcted walls (Figure 7B; p<0.01), but a smaller fractional infarct perimeter (Figure 7C; p<0.05) than any of the other cell-treated groups.
Figure 7. Ventricular remodeling.
A) Representative images of Masson’s staining of infarcted mice hearts, after implantation of different types of human cells or saline injection only. Quantitative analyses (n=3 mice per group) of LV wall thickness (B) and infarct perimeter (C) show that remodeling was attenuated more efficiently by CDC implantation, compared with BM-MSCs, AD-MSCs, and BM-MNC treatment, although implantation of BM-MSCs, AD-MSCs, and BM-MNCs resulted in less remodeling compared to control treatment with saline injection only.
Unsorted CDCs versus the c-kit+-purified cell subpopulation
Having established that CDCs are the most effective cell type among those studied, we moved on to consider how CDCs compare to purified c-kit+ cells. This is germane because c-kit has been argued to identify cardiac stem/progenitor cells,1,7-9 and a purified c-kit+ cardiac-derived cell product is currently being tested clinically (NCT00474461; www.clinicaltrials.gov). Therefore, in a separate study, we compared unsorted CDCs to equal numbers of c-kit+ stem cells purified from CDCs by magnetic cell sorting. Purified c-kit+ cells were inferior to unsorted CDCs in terms of functional benefit after transplantation into the infarcted heart, although they did outperform vehicle-injected controls (Figure 8A). The sorting procedure did not itself compromise cell functional efficacy, as CDCs sorted for CD105 (expressed by >99% of CDCs) exhibited an LVEF comparable to that of unsorted CDCs (data not shown). To investigate one potential mechanism for the functional superiority of CDCs, we measured a variety of paracrine factors in conditioned media from sorted and unsorted cells, Indeed, unsorted CDCs produced higher amounts of paracrine factors in vitro as compared to purified c-kit+ cells (Figure 8B), providing one potential rationale for their enhanced benefit.
Figure 8. Comparison of purified c-kit+ stem cells and unsorted CDCs.
A) 3 weeks after infarction, LVEF was higher in mice that received unsorted CDCs than those with c-kit+ cells purified from the same CDCs, although these purified c-kit+ stem cells also improved cardiac function when compared to controls injected with saline only. B) Although the same number of cells was used for culture, the purified c-kit+ stem cells released less VEGF, SDF, IGF-1, and HGF than the unsorted CDCs.
Discussion
By head-to-head direct comparison, we demonstrated a functional superiority of heart-derived cells as compared to three types of adult stem cells of extracardiac origin: bone marrow mononuclear cells, and mesenchymal stem cells from bone marrow or adipose tissue. Furthermore, the superiority of CDCs for myocardial repair was consistent with their well-balanced secretion of paracrine factors as well as their higher cardiomyogenic differentiation capacity and engraftment, although further in vivo intervention experiments (e.g., with blocking antibodies) will be required to identify the precise mechanisms of functional superiority.
We selected four cell types for comparison that have been used clinically and are considered among the most promising at present for myocardial repair/regeneration. Previous studies have shown that the implantation of BM-MNCs, BM-MSCs, and AD-MSCs into the damaged heart can improve cardiac function, likely through paracrine mechanisms, with rare events of myogenic differentiation.19-22 Despite the paucity of direct regeneration of new myocardium from these stem cells of extracardiac origin, the improvement of cardiac function reported in previous randomized clinical trials is encouraging.10-18
One distinctive feature of resident cardiac stem cells is their ability to undergo consistent cardiomyogenic and angiogenic differentiation,1-9,23-25 a finding confirmed here. Although almost 10% of CDCs expressed troponin T in vitro, human CDCs expressing α-sarcomeric actin were infrequently observed in the infarcted hearts of SCID mice 3 weeks after treatment. Furthermore, we did not examine whether these α-sarcomeric actin positive human CDCs were physiologically integrated with the resident cardiomyocytes of mice. Therefore, regeneration of new functional myocardium directly from the implanted human CDCs is a consistent finding, but one of questionable significance; the present evidence supports the notion that mechanism of functional benefit after CDC implantation, as with other cell types, is predominantly dependent on paracrine effects and recruitment of endogenous regeneration.25
We thus compared the secretion of growth factors that generally are recognized to play critical roles in angiogenesis, anti-apoptosis, or cardioprotection. PDGF was undetectable in media conditioned by any of the four cell types, but, beyond even our own expectations, CDCs robustly produced a variety of growth factors, including angiopoietin, bFGF, HGF, IGF-1, SDF-1, and VEGF. Although BM-MSCs, AD-MSCs, and BM-MNCs could produce some of these factors in comparable levels to CDCs, the paracrine profile was uniquely well-balanced in CDCs. The robust production of diverse paracrine factors by CDCs provides a potential mechanism underlying their superiority for functional myocardial repair, but further work will be required to understand the precise role of each paracrine factor, as well as the roles of others known to be secreted but not quantified here.37 The use of the same medium (IMDM) for all cell types avoids possible confounding effects due to different culture conditions; nevertheless, the fact that cells were cultured uniformly may minimize favorable features unique to each cell type that might be more apparent with media optimized for each cell type.
Tube formation assays are commonly used to measure the angiogenic potential of endothelial cells.36 The superiority of tube formation observed in CDCs indicates their angiogenic potential, which is in agreement with CDCs’ robust secretion of pro-angiogenic cytokines (Figure 2). The observation that BM-MNCs failed to form any tubes may due to the fact that MNCs need a longer time (24 hours) to form tubes,36 while we performed the assay at the 6 hour time point. We did not stain for endothelial markers (e.g. CD31), so it is unknown whether these tube structures actually represent endothelial cells differentiated from the various cell types; indeed, it has been reported that mesenchymal and fibroblast cells assist in blood vessel formation by aligning into tube networks.38
We also found CDCs were more resistant to oxidative stress-induced apoptosis in vitro (Supplemental Figures 4&5)) and MI-induced apoptosis in vivo (Figure 5). The superiority of CDCs to other cell types in enhancing host tissue preservation is striking. Therefore, it is conceivable that this action is responsible for the superior impact of CDCs in terms of preserving cardiac function and attenuating pathological remodeling, although the relative importance of the various favorable effects remains to be defined. It should be noted that we assayed apoptosis in vivo 3 weeks after MI/cell treatment. At that time point, the acute phase of cell death due to ischemia might have already resolved; therefore, the apoptosis we observed was a reflection of long-term remodeling and heart failure. One limitation of this experiment is that we did not perform double staining for TUNEL- and HNA-positive nuclei. Therefore we cannot distinguish whether apoptosis is reduced in endogenous cells, transplanted cells, or both. In addition, our results do not exclude the possibility that different levels of inflammatory infiltration among groups might contribute to observed differences in apoptotic rates. Nevertheless, the superior tissue preservation capacity of CDCs is consistent with their robust secretion of anti-apoptotic and pro-angiogenic factors.
We did not include a number of other stem cell types in our comparison, including myoblasts and peripheral blood-derived endothelial progenitors, although they have already been tested in clinical trials.17,18 The major reason is the relative lack of enthusiasm in the field for these cell types as candidates for further clinical development for cardiac regeneration (although endothelial progenitors do have positive effects on angina and critical limb ischemia17). We also did not select ES- or iPS-derived cardiomyocytes for comparison, because they are far from clinical applicability at present, although both may excel in the direct production of new functional myocardium.39,40
We did, however, compare the therapeutic potency of cardiac progenitor cells purified by virtue of their c-kit-positivity,35,41 relative to unselected CDCs.42 We confirmed the reported ability of c-kit+ cardiac cells to boost cardiac function post-infarction,1,9 but we discovered that c-kit+ cells are not as potent as the CDC mixture either in terms of functional benefit or in paracrine factor secretion. One trivial explanation for this finding would be antibody-related interference with cell potency, but we believe that is unlikely: The c-kit antibody used43 but has been shown to interfere minimally with ligand binding, receptor phosphorylation, and internalization in c-kit-expressing cell lines.44 Also, magnetic-activated cell sorting for mast cells using this c-kit antibody neither induced histamine release nor did it impair the ability of cells to release histamine when stimulated.45 We must acknowledge, however, that subtle differences in specific sorting and culture methods for c-kit+ cells may affect the efficacy of the cells. Nevertheless, the finding that the natural CDC mixture is superior to the purified c-kit+ subpopulation adds a new dimension to the emerging notion that mesenchymal cells favor endogenous cardiac regeneration,10,46 at least partly via the attraction of c-kit+ cells. We conjecture that the stromal and mesenchymal cells synergize with the c-kit+ cells in the natural CDC mixture to enhance overall paracrine potency and thereby to boost functional benefit; alternatively, c-kit+ cells may be tangential to the mechanism of benefit of CDCs. More experiments are required to flesh out these ideas.
Summary
In this first comprehensive head-to-head comparison of 4 different cell types in the same animal model in the same laboratory, with blinded analysis, CDCs emerge as superior in terms of paracrine factor secretion, angiogenesis, cardiomyogenic differentiation, ischemic tissue preservation, anti-remodeling effects and functional benefit. The CDC mixture is more potent than the c-kit+ subpopulation. Ongoing and future clinical studies will serve as the ultimate test of potency, but the present results give good reason for optimism regarding the therapeutic potential of CDCs.
Supplementary Material
Acknowledgments
Funding: This study was supported by the NIH (HL095203) to Capricor, Inc., by the NIH (U54 HL081028) to E.M. and by the Cedars-Sinai Board of Governors Heart Stem Cell Center. E.M. is the Mark S. Siegel Family Professor of the Cedars-Sinai Medical Center.
Abbreviations and Acronyms
- CDCs
cardiosphere-derived stem cells
- BM-MSCs
bone marrow-derived mesenchymal stem cells
- AD-MSCs
adipose tissue-derived mesenchymal stem cells
- BM-MNCs
bone marrow mononuclear cells
- bFGF
basic fibroblast growth factor
- HGF
Hepatocyte growth factor
- IGF-1
insulin-like growth factor 1
- PDGF
Platelet-Derived Growth Factor
- SDF-1
stromal cell-derived factor 1
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: Eduardo Marbán and Linda Marbán hold founders’ equity in Capricor, Inc. R.R. Smith and Linda Marbán are employed by Capricor, Inc. The remaining authors report no conflicts.
References
- 1.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 2.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuura K, Nagai T, Nishigaki N, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–91. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 4.Pfister O, Mouquet F, Jain M, et al. CD31-but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 5.Laugwitz KL, Moretti A, Lam J, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li TS, Suzuki R, Ueda K, Murata T, Hamano K. Analysis of the origin and population dynamics of cardiac progenitor cells in a donor heart model. Stem Cells. 2007;25:911–17. doi: 10.1634/stemcells.2006-0497. [DOI] [PubMed] [Google Scholar]
- 7.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 8.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 9.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valina C, Pinkernell K, Song YH, et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–77. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 12.Li TS, Murakami M, Kobayashi T, Shirasawa B, Mikamo A, Hamano K. Long-term efficacy and safety of the intramyocardial implantation of autologous bone marrow cells for the treatment of ischemic heart disease. J Thorac Cardiovasc Surg. 2007;134:1347–9. doi: 10.1016/j.jtcvs.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 14.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J. 2009;30:2978–84. doi: 10.1093/eurheartj/ehp374. [DOI] [PubMed] [Google Scholar]
- 15.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 16.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 17.Losordo DW, Schatz RA, White CJ, et al. Henry TD. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–72. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 18.Menasché P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi M, Li TS, Suzuki R, et al. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291:H886–93. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 20.Yoon CH, Koyanagi M, Iekushi K, et al. Mechanism of improved cardiac function after bone marrow mononuclear cell therapy: role of cardiovascular lineage commitment. Circulation. 2010;121:2001–11. doi: 10.1161/CIRCULATIONAHA.109.909291. [DOI] [PubMed] [Google Scholar]
- 21.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–10. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 23.Davis DR, Zhang Y, Smith RR, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston P, Sasano T, Mills K, et al. Engraftment, differentiation and functional benefit of autologous cardiosphere-derived cells in a porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–83. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang XL, Rokosh G, Sanganalmath SK, et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li TS, Marbán E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells. 2010;28:1178–85. doi: 10.1002/stem.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi M, Suzuki E, Oba S, et al. Adipose tissue-derived stem cells inhibit neointimal formation in a paracrine fashion in rat femoral artery. Am J Physiol Heart Circ Physiol. 2010;298:H415–23. doi: 10.1152/ajpheart.00391.2009. [DOI] [PubMed] [Google Scholar]
- 29.Hahn JY, Cho HJ, Kang HJ, et al. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–43. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 31.Cheng K, Li TS, Malliaras K, Davis DR, Zhang Y, Marbán E. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res. 2010;106:1570–81. doi: 10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ades EW, Zwerner RK, Acton RT, Balch CM. Isolation and partial characterization of the human homologue of Thy-1. J Exp Med. 1980;151:400–6. doi: 10.1084/jem.151.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saalbach A, Kraft R, Herrmann K, Haustein UF, Anderegg U. The monoclonal antibody AS02 recognizes a protein on human fibroblasts being highly homologous to Thy-1. Arch Dermatol Res. 1998;290:360–6. doi: 10.1007/s004030050318. [DOI] [PubMed] [Google Scholar]
- 34.Carlyle JR, Zúñiga-Pflücker JC. Lineage commitment and differentiation of T and natural killer lymphocytes in the fetal mouse. Immunol Rev. 1998;165:63–74. doi: 10.1111/j.1600-065x.1998.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 35.Li TS, Cheng K, Malliaras K, et al. Expansion of human cardiac stem cells in physiological oxygen improves cell production efficiency and potency for myocardial repair. Cardiovasc Res. 2010;89:157–65. doi: 10.1093/cvr/cvq251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12:267–74. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 37.Stastna M, Chimenti I, Marbán E, Van Eyk JE. Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics. 2010;10:245–53. doi: 10.1002/pmic.200900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghajar CM, Kachgal S, Kniazeva E, et al. Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Exp Cell Res. 2010;316:813–25. doi: 10.1016/j.yexcr.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada S, Nelson TJ, Crespo-Diaz RJ, et al. Embryonic stem cell therapy of heart failure in genetic cardiomyopathy. Stem Cells. 2008;26:2644–53. doi: 10.1634/stemcells.2008-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–9. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 41.Li TS, Cheng K, Lee ST, et al. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–98. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith RR, Chimenti I, Marbán E. Unselected human cardiosphere-derived cells are functionally superior to c-kit- or CD90-purified cardiosphere-derived cells. Circulation. 2008;118:S420. [Google Scholar]
- 43.Lerner NB, Nocka KH, Cole SR, et al. Monoclonal antibody YB5.B8 identifies the human c-kit protein product. Blood. 1991;77:1876–83. [PubMed] [Google Scholar]
- 44.Ashman LK, Buhring HJ, Aylett GW, Broudy VC, Muller C. Epitope mapping and functional studies with three monoclonal antibodies to the c-kit receptor tyrosine kinase, YB5.B8, 17F11, and SR-1. J Cell Physiol. 1994;158:545–54. doi: 10.1002/jcp.1041580321. [DOI] [PubMed] [Google Scholar]
- 45.Okayama Y, Hunt TC, Kassel O, Ashman LK, Church MK. Assessment of the anti-c-kit monoclonal antibody YB5.B8 in affinity magnetic enrichment of human lung mast cells. J Immunol Methods. 1994;169:153–61. doi: 10.1016/0022-1759(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 46.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–98. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.