Abstract
Background
Cerebral hypoxia/ischemia during infant congenital heart surgery is not uncommon, and may induce devastating neurologic disabilities persistent over the lifespan. Hypoxia/ischemia-induced cerebrovascular dysfunction is thought to be an important contributor to neurological damage. No pharmacological agents have been found to prevent this. Mitogen activated protein kinase (MAPK), including extracellular signal regulated kinase (ERK), c-Jun-N-terminal kinase (JNK) and p38, is thought to contribute to ischemic preconditioning. We investigated whether pretreatment with salvinorin A, the only natural non-opioid kappa receptor agonist, could preserve autoregulation of the pial artery via MAPK.
Methods
The response of the pial artery to hypotension and hypercapnia was monitored in piglets equipped with a closed cranial window before and after hypoxia and ischemia in the presence or absence of U0126, an inhibitor for the protein kinase upstream of ERK, sp600125, an inhibitor of c-JNK or sb203580, an inhibitor of p38. Salvinorin A (10 μg/kg IV) was administered 30 minutes before hypoxia/ischemia in salvinorin-treated animals. Cerebrospinal fluid samples were collected before and 30 minutes after salvinorin A administration for the measurement of MAPK. Data (n=5) were analyzed by repeated-measures analysis of variance.
Results
Pial artery dilation to hypercapnia and hypotension was blunted after hypoxia/ischemia, but preserved well by pretreatment with salvinorin A. U0126, but not sp600125 or sb203580, abolished the preservative effects of salvinorin A on cerebral vascular autoregulation to hypotension and hypercapnia. The ratio of pERK/ERK in cerebrospinal fluid increased significantly in salvinorin-treated animals, which was inhibited by U0126.
Conclusions
Salvinorin A pretreatment preserves autoregulation of the pial artery to hypotension and hypercapnia after hypoxia/ischemia via ERK in a piglet model.
Cerebral hypoxia/ischemia because of the interruption of cerebral blood flow during cardiopulmonary bypass with deep hypothermia circulation arrest (DHCA) surgery for congenital cardiac surgery is a significant clinical issue (1). Fifty percent of children with complex congenital heart disease undergoing cardiopulmonary bypass with DHCA have developmental deficits, such as disabilities in speech and attention deficit disorder by school age(2). Cerebral hypoxia /ischemia occurred during DHCA is predictable(1); thus, it is possible to minimize the brain injury induced by ischemia with pharmacologic approaches. Unfortunately, no pharmacological agent with proven clinical benefit has yet been identified.
Loss of cerebral vascular autoregulation is one of the key features of cerebral hypoxia/ischemia (3-5). The loss of autoregulation to hypotension could result in a pressure passive cerebral circulation, which may decrease cerebral blood flow and further aggravate brain ischemia(6). Loss of cerebrovascular regulation to hypercapnia also contributes to the development of the pressure passive circulation and periventricular leukomalacia(6). Thus, preservation of cerebral vascular autoregulation from ischemia is very important to reduce brain injury from ischemia. We recently demonstrated that salvinorin A, an active component of Salvia divinorum and a non-opioid kappa opioid receptor (KOR) agonist, is a potent cerebral vascular dilator in normal and pathological conditions (7). It is likely that salvinorin A could protect cerebral vasculature from ischemia. Unlike other KOR agonists, salvinorin A has long been used by different ethnic groups for various purposes, including spiritual experiences and “treating” illnesses (8,9), indicating its high potential as a clinically acceptable medication.
It has been demonstrated that systemic administration of KOR agonists has neuroprotective effects in animal models of cerebral ischemia (10,11). KOR agonists could activate mitogen-activated protein kinase (MAPK) (12,13). MAPK is a key intracellular signaling system, which includes extracellular signal regulated kinase (ERK), c-Jun-N-terminal kinase (JNK) and p38 (14). It was demonstrated that prolonged and persistent activation of the ERK cascade is an important contributory mechanism of cerebral ischemic preconditioning (15-17). This pathway is also involved in many other forms of pharmacological preconditioning, such as isoflurane and sevoflurane (18,19). Thus, it is likely that salvinorin A may generate cerebral protective effects via this pathway. Based on the above evidence, we hypothesized that salvinorin A pretreatment might preserve autoregulation of pial vessels to hypotension and hypercapnia from hypoxia/ischemia via activation of MAPK.
Methods
Salvinorin A (purity ≥98%) was from ChromaDex, Inc. (Irvine, CA, USA). Isoproterenol, U0126, sp600125 and sb203580 were obtained from Sigma-Aldrich (MO, St. Louis, MO, USA). All other chemicals were also obtained from Sigma and were of reagent grade.
Animals and Surgery
One to 5-day-old piglets were used. Protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania (Philadelphia). Isoflurane (1–2 minimum alveolar concentration) was initially used for induction, followed by alpha-chloralose for maintenance of anesthesia (30–100 mg/kg, supplemented with 5-30mg/kg every 20-30min IV). After tracheotomy, piglets’ lungs were mechanically ventilated with room air and kept warm with a heating pad, maintaining rectal temperature at 37 to 39°C. Femoral arteries were cannulated for continuous arterial blood pressure monitoring or intermittent blood gas monitoring, and the femoral vein was catheterized for medication administration. As described previously (20), a closed cranial window was placed for direct pial artery visualization and diameter measurement (20). Small pial arteries (120 to 160 μ m) and arterioles (50 to 70 μ m) were identified under a microscope, visualized on a monitor connected to the microscope, and measured via a video microscaler (model VPA 550, For-A-Corp., Los Angeles, CA). The cranial window was a steel ring with a glass coverslip, connecting to three ports for cerebrospinal fluid (CSF) sampling, washout and medicine administration. Cortical periarachnoid CSF was collected through one of the above ports at baseline and 30 minutes after administration of salvinorin A or U0126 plus salvinorin A for ERK/MAPK analysis.
Protocol
Hypoxia was induced for 10 minutes by switching room air to N2 for ventilation, followed by restoring ventilation to room air. Global cerebral ischemia was then induced by infusing saline through a hollow bolt in the cranium to maintain intracranial pressure higher than the mean arterial blood pressure for 20 min. Global ischemia was confirmed when the blood flow in the pial artery stopped, visualized on the monitor connected to the microscope over the cranial window. In order to avoid Cushing response (arterial blood pressure increasing dramatically because of high intracranial pressure), blood was withdrawn when necessary to maintain mean arterial blood pressure no higher than 100 mmHg. The blood was returned via the femoral vein at the end of ischemia.
Five sets of experiments were performed (n=5 in each set of experiments): (1) hypoxia/ischemia with vehicle of salvinorin A, dimethylsulfoxide (DMSO), 1μl/kg administered 30 minutes before hypoxia/ischemia; (2) hypoxia/ischemia with salvinorin A, 1μg/μl in DMSO, 10 μg/kg IV; (3) hypoxia/ischemia with salvinorin A (10 μg/kg IV) and U0126 (1mg/kg, IV), an inhibitor for the protein kinase upstream of ERK, (4) hypoxia/ischemia with salvinorin A and sp600125 (1μM, topically injected through one port of the cranial windows), an inhibitor of JNK, and (5) hypoxia/ischemia with salvinorin A and sb203580 (10μM, topically injected through one port of the cranial windows), an inhibitor of P38. U0126, sp600125 and sb203580 were administered 30 minutes before salvinorin A. Sp600125 and sb203580 were co-administered with the vasoactive stimulus so as to have continued exposure of the cerebral cortical surface after injury.
Hypercapnia (PaCO2 of 50 to 60 mmHg for low level, 70 to 80 mmHg for high level) was produced by inhalation of a high concentration CO2 mixture gas (10% CO2; 21% O2; 69%N2). Hypotension was produced by withdrawing blood from the femoral artery (25% decrease in mean arterial blood pressure as moderate and 45% as severe). Pial artery responses to hypotension, hypercapnia, and isoproterenol (10 nM, 1μM) were obtained before hypoxia/ischemia and 60 minutes after injury as described previously(21).
ERK and pERK Measurement
To test the role of ERK on the observed effects of salvinorin A on brain hypoxia/ischemia, CSF samples were collected for MAPK. MAPK isoforms were measured by commercially available ELISA kits (Enzo Life Sciences International, Inc., Plymouth Meeting, PA).
Statistical Analysis
Data obtained for the investigation of the effects of cerebral hypoxia/ischemia on pial artery responses to hypercapnia, hypotension, and isoproterenol on pial artery diameter were analyzed by repeated measures ANOVA with a Greenhouse Geisser correction. Bonferroni correction was used for all post hoc analyses (10 comparisons for each stimulation). Five different treatments (DMSO, salvinorin A, SB203580, U0126, and SP600125) were used as the factors for comparisons between groups, and four measurements for three stimulations before and after hypoxia/ischemia was used as the repeated measure factor (5 × 4 × 3 × 2). The baseline was not included in the repeated measure analysis. The repeated measures ANOVA with a Greenhouse Geisser correction was used for the pERK/ERK data to compare the ratio changes before and after administration of salvinorin A with three different treatments (DMSO, salvinorin A and salvinorin A +U0126) as the between group factor and time (before and 30min after salvinorin A) as the repeated measure factor. An alpha level of P<0.05 was considered significant in all statistical tests. All values are represented as means ± standard error. All P-values reported in the paper have been corrected for the effect of multiple comparisons. Although the sample size in this study is rather small, there was no apparent violation of the assumptions of lack of interaction, homogeneity of variance, and normal distribution.
Results
Salvinorin A preserved pial artery autoregulation to hypotension after hypoxia/ischemia
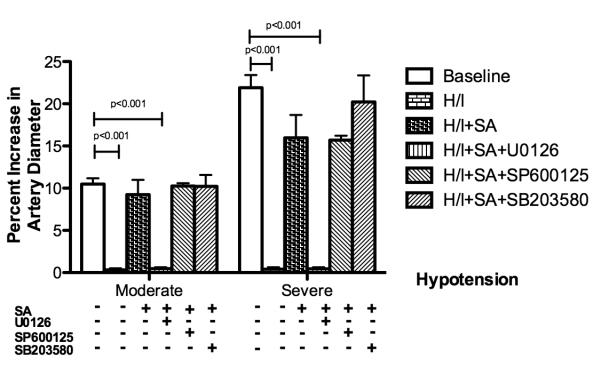
As indicated in Figure 1, small pial arteries dilated to two levels of hypotension at baseline before hypoxia/ischemia, but the dilatation response was decreased significantly after hypoxia/ischemia(p<0.001 compared with that before hypoxia/ischemia). Pretreatment with salvinorin A (10 μg/kg, IV.) preserved the dilation response of pial arteries to hypotension. This was abolished by U0126 (p<0.001 compared with salvinorin A group), the antagonist of ERK. However, there were no significant changes after treatment with SP600125 (antagonist of JNK, p>0.05 compared with salvinorin A group) and SB203580 (antagonist of P38 ,p>0.05 compared with salvinorin A group) which were administered 30 minutes before administration of salvinorin A. Similar observations were obtained in pial arterioles (data not shown).
Figure 1.
Effects of hypotension on pial artery diameter before (baseline), after hypoxia/ischemia (H/I; PO2 of 35 mm Hg for 10 minutes followed by global cerebral ischemia for 20 minutes), after H/I pretreated with salvinorin A (10μg/kg IV; H/I+SA) 30 minutes before H/I, and after H/I pretreated with U0126 (1mg/kg, IV; H/I+SA+U0126), the antagonist of extracellular signal regulated kinase (ERK), 30 minutes before salvinorin A, SP600125 (1μM, administered topically; H/I+SA+SP600125), the antagonist of c-Jun-N-terminal kinase (JNK), 30 minutes before salvinorin A, SB203580 (10μM, administered topically; H/I+SA+SB203580), the antagonist of P38, 30 minutes before salvinorin A. Pretreatment with salvinorin A preserved the dilation response of the pial artery to hypotension, which is abolished by U0126. SA: Salvinorin A; H/I: Hypoxia/ischemia; Moderate: moderate hypotension (25 % decrease of mean arterial blood pressure [MAP]); Severe: severe hypotension (45% decrease of MAP). N= 5 each group; baseline bar represents the data from all 25 animals. All nonlisted corrected P-values >0.405. All corrected 95% confidence interval width <10.32
Salvinorin A preserved pial artery autoregulation to hypercapnia after hypoxia/ischemia
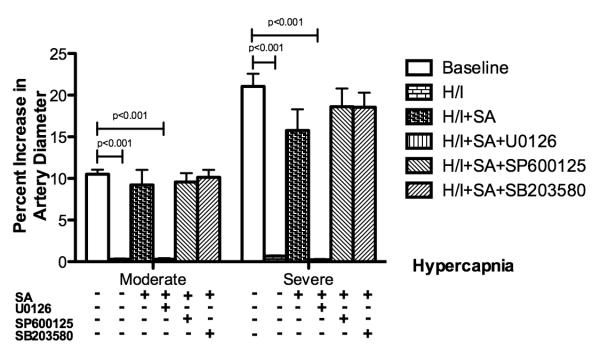
Similar to the response to hypotension, small pial arteries dilated to two levels of hypercapnia at baseline before hypoxia/ischemia (Figure 2). The dilatation response was blunted after hypoxia/ischemia. Pretreatment with salvinorin A (10 μg/kg, IV.) preserved the dilation response of pial arteries to hypercapnia. This was abolished by U0126, the antagonist of ERK. No significant change in the preservative effects was observed from SP600125 and SB203580 administered 30 minutes before administration of salvinorin A (P>0.05). Similar observations were obtained in pial arterioles (data not shown).
Figure 2.
Effects of hypercarbia on pial artery diameter before (baseline), after hypoxia/ischemia (H/I; PO2 of 35 mm Hg for 10 minutes followed by global cerebral ischemia for 20 minutes), after H/I pretreated with salvinorin A (10μg/kg IV; H/I+SA) 30 minutes before H/I, and after H/I pretreated with U0126 (1mg/kg, IV; H/I+SA+U0126), the antagonist of extracellular signal regulated kinase (ERK), 30 minutes before salvinorin A, SP600125 (1μM, administered topically; H/I+SA+SP600125), the antagonist of c-Jun-N-terminal kinase (JNK), 30 minutes before salvinorin A, SB203580 (10μM, administered topically; H/I+SA+SB203580), the antagonist of P38, 30 minutes before salvinorin A. Pretreatment with salvinorin A preserved the dilation response of the pial artery to hypercarbia, which is abolished by U0126. SA: Salvinorin A; H/I: Hypoxia/ischemia; Moderate: moderate hypercapnia with PaCO2 of 50 to 60 mmHg; Severe: severe hypercapnia with PaCO2 of 70 to 80 mmHg. N= 5 each group; baseline bar represents the data from all 25 animals. All non-listed corrected P-values >0.108. All corrected 95% confidence interval width <10.43.
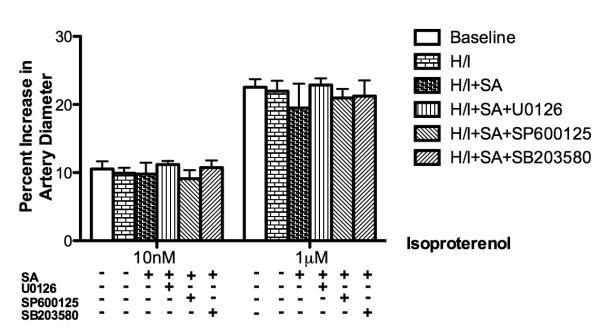
Pial artery response to isoproterenol unchanged in all sets of experiments
As a positive control and indicated in Figure 3, the pial artery response to isoproterenol was unchanged in all groups before and after hypoxia/ischemia in the presence or absence of the above interventions.
Figure 3.
Effects of isoproterenol (10nM, 1μM) on pial artery diameter before (baseline) and after hypoxia/ischemia did not change significantly in the presence and absence of various interventions. SA: Salvinorin A; H/I: Hypoxia/ischemia. N= 5 each group; baseline bar represents the data from all 25 animals. All nonlisted corrected P-values =1. All corrected 95% confidence interval width <10.13.
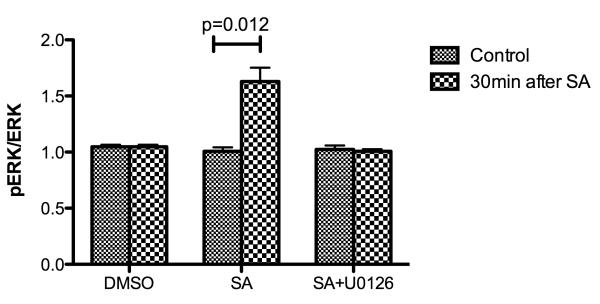
ERK involved in the preservation effects of salvinorin A
The ratio of pERK/ERK in CSF increased significantly 30 minutes after salvinorin A pretreatment (Fig.4). However, if U0126, the antagonist of ERK, was administered 30 minutes before salvinorin administration, the ratio of pERK/ERK was unchanged 30 minutes after salvinorin A pretreatment (Fig.4).
Figure 4.
The ratio of p extracellular signal regulated kinase (ERK)/ERK before administration of salvinorin A and 30 minutes after pretreatment of salvinorin A or U0126 plus salvinorin A. The ratio of pERK/ERK in cerebrospinal fluid (CSF) increased significantly 30 minutes in the salvinorin A pretreatment group; and such increase was abolished by the ERK antagonist (U0126) pretreatment SA: Salvinorin A. H/I: Hypoxia/ischemia. All nonlisted corrected P-values =1. All corrected 95% confidence interval width <0.33.
Discussion
There are two principal new findings in this study. First, pretreatment with salvinorin A preserved cerebrovascular autoregulatory ability after hypoxia/ischemia. Second, ERK/MAPK is involved in the ability of salvinorin A to preserve autoregulation. The present study also confirmed our earlier findings that global hypoxia/ischemia in newborn piglets blunts the autoregulatory ability of the cerebral vascular response to hypotension and hypercapnia (21,22).
Although the pathophysiological responses to cerebral hypoxia/ischemia in infants is not fully elucidated, cerebrovascular dysfunction plays a very important role in neurological insult after hypoxia/ischemia (6). There is no optimal pharmacological intervention that could be used to prevent or preserve cerebral vascular autoregulatory responses to hypotension and hypercapnia secondary to brain injury. The only medication approved by the Food and Drug Administration for stroke is recombinant tissue plasminogen activator (tPA)(23). However, despite its salutary role in reopening the clotted blood vessel, tPA increases stroke infarct volume in mice (24) and it enhances the impairment of autoregulation induced by hypoxia/ischemia (21). L-NNA, an inhibitor of nitric oxide synthesis, was proven to be able to restore cerebral vascular autoregulation administered after ischemia (25); however, its safety profile is unclear for clinical usage.
In the present study, we demonstrated that salvinorin A pretreatment preserved the autoregulatory responses to hypotension and hypercapnia after hypoxia/ischemia in a piglet model, which opens new possibilities for its clinical application to attenuate cerebral hypoxia/ischemia, especially for anticipated brain ischemia during DHCA in infants.
Unlike other KOR receptors that have no proven clinical values, salvinorin A is extracted from an abundant natural plant, Salvia Divinorum. Very similar to the history of opium, Salvia Divinorum has been used by humans for various purposes for several centuries. It has been proposed that salvinorin A, the active component of Salvia Divinorum, could be a potential new kappa agonist to be used in clinical practice (8,26,27).
Salvinorin A is a potent cerebral vascular dilator in normal and constricted conditions as we demonstrated recently (7). However, this dilatation effect is short lived unless it is continually administered; thus, the preservation of autoregulation is unlikely induced from the dilatation effects because salvinorin was administered 30 min before hypoxia/ischemia. MAPK is proven to be very important in signal transduction from the cell surface to the nucleus(14). An increase of ERK/MAPK before ischemia is related to neuronal survival after ischemia (14). Activation of ERK/MAPK in the hippocampal CA1 region after sublethal ischemia correlates with neuroprotection induced by preconditioning (15,28). Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating ERK/MAPK (29). In the present study, pERK/ERK in CSF increased significantly 30 minutes after administration of salvinorin A, indicating the activation of ERK and suggesting that salvinorin A might be vascularly or neuronally protective when administered before brain ischemia. However, other studies have observed that an increase of ERK/MAPK in the immediate postinjury reperfusion period is associated with impairment of responses to cerebrovasodilators as well as histopathology after hypoxia/ischemia in the piglet (30). The reason for the observed duality of ERK/MAPK function is uncertain but may relate to the cellular site of origin, signal coupling, or temporal pattern of release.
Newborn piglets used in the present study offer the unique advantage of a gyrencephalic brain containing substantial white matter, which is more sensitive to ischemic damage than rodent brain, and more similar to humans (31,32). One limitation of this study is that subtle differences might be missed because of the underpowered statistical analysis as evidenced by the 95% confidence interval width less than 11. Future studies will also be needed to fully characterize the duality of ERK/MAPK in the regulation of cerebral hemodynamics during physiologic and pathologic conditions in the perinatal period.
Conclusions
In conclusion, salvinorin A pretreatment preserved cerebrovascular autoregulation to hypotension and hypercapnia after brain hypoxia/ischemia via ERK/MAPK in a piglet model.
Acknowledgments
Funding: This research was supported by departmental funding from the department of anesthesiology and Critical Care at University of Pennsylvania, funding from Foundation for Anesthesia Education and Research, and K08-GM-093115-01and NS53410 from the National Institutes of Health.
Footnotes
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
DISCLOSURES: Name: Diansan Su, M.D., Ph.D
Contribution: This author helped design the study, conduct the study, and analyze the data.
Attestation: Diansan Su has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: John Riley, B.A.
Contribution: This author helped conduct the study and write the manuscript.
Attestation: John Riley has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: William M. Armstead, Ph.D.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: William M. Armstead has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Renyu Liu, M.D., Ph.D.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Renyu Liu has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Diansan Su, Visiting scholar from the Department of Anesthesiology, School of Medicine, Shanghai Jiaotong University, Shanghai, China.
John Riley, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA
William M. Armstead, Department of anesthesiology and critical care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA
Renyu Liu, Department of anesthesiology and critical care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA
References
- 1.Albers EL, Bichell DP, McLaughlin B. New approaches to neuroprotection in infant heart surgery. Pediatr Res. 2010;68:1–9. doi: 10.1203/PDR.0b013e3181df5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markowitz SD, Ichord RN, Wernovsky G, Gaynor JW, Nicolson SC. Surrogate markers for neurological outcome in children after deep hypothermic circulatory arrest. Semin Cardiothorac Vasc Anesth. 2007;11:59–65. doi: 10.1177/1089253206297481. [DOI] [PubMed] [Google Scholar]
- 3.Armstead WM. Altered release of prostaglandins contributes to hypoxic/ischemic impairment of NOC/oFQ cerebrovasodilation. Brain Res. 2000;859:104–12. doi: 10.1016/s0006-8993(00)01949-1. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Haim G, Armstead WM. Role of cAMP and K(+) channel-dependent mechanisms in piglet hypoxic/ischemic impaired nociceptin/orphanin FQ-induced cerebrovasodilation. Brain Res. 2000;884:51–8. doi: 10.1016/s0006-8993(00)02882-1. [DOI] [PubMed] [Google Scholar]
- 5.Armstead WM. Contribution of kca channel activation to hypoxic cerebrovasodilation does not involve NO. Brain Res. 1998;799:44–8. doi: 10.1016/s0006-8993(98)00462-4. [DOI] [PubMed] [Google Scholar]
- 6.Volpe JJ. Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol. 1998;5:135–51. doi: 10.1016/s1071-9091(98)80030-2. [DOI] [PubMed] [Google Scholar]
- 7.Su D, Riley J, Kiessling WJ, Armstead WM, Liu R. Salvinorin A Produces Cerebrovasodilation through Activation of Nitric Oxide Synthase, kappa Receptor, and Adenosine Triphosphate-sensitive Potassium Channel. Anesthesiology. 2011;114:374–9. doi: 10.1097/ALN.0b013e318204e029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vortherms TA, Roth BL. Salvinorin A: from natural product to human therapeutics. Mol Interv. 2006;6:257–65. doi: 10.1124/mi.6.5.7. [DOI] [PubMed] [Google Scholar]
- 9.Yan F, Roth BL. Salvinorin A: a novel and highly selective kappa-opioid receptor agonist. Life Sci. 2004;75:2615–9. doi: 10.1016/j.lfs.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Baskin DS, Hosobuchi Y, Loh HH, Lee NM. Dynorphin(1-13) improves survival in cats with focal cerebral ischaemia. Nature. 1984;312:551–2. doi: 10.1038/312551a0. [DOI] [PubMed] [Google Scholar]
- 11.Kusumoto K, Mackay KB, McCulloch J. The effect of the kappa-opioid receptor agonist CI-977 in a rat model of focal cerebral ischaemia. Brain Res. 1992;576:147–51. doi: 10.1016/0006-8993(92)90621-f. [DOI] [PubMed] [Google Scholar]
- 12.Lemos JC, Roth CA, Chavkin C. Signaling Events Initiated by Kappa Opioid Receptor Activation: Quantification and Immunocolocalization Using Phospho-Selective KOR, p38 MAPK, and K(IR) 3.1 Antibodies. Methods Mol Biol. 2011;717:197–219. doi: 10.1007/978-1-61779-024-9_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn JW, Jagwani S, Kim E, Rendell VR, He J, Ezerskiy LA, Wesselschmidt R, Coscia CJ, Belcheva MM. Mu and kappa opioids modulate mouse embryonic stem cell-derived neural progenitor differentiation via MAP kinases. J Neurochem. 2010;112:1431–41. doi: 10.1111/j.1471-4159.2009.06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol. 2001;23:1–19. doi: 10.1385/MN:23:1:01. [DOI] [PubMed] [Google Scholar]
- 15.Shamloo M, Rytter A, Wieloch T. Activation of the extracellular signal-regulated protein kinase cascade in the hippocampal CA1 region in a rat model of global cerebral ischemic preconditioning. Neuroscience. 1999;93:81–8. doi: 10.1016/s0306-4522(99)00137-2. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Zulueta M, Feldman AB, Klesse LJ, Kalb RG, Dillman JF, Parada LF, Dawson TM, Dawson VL. Requirement for nitric oxide activation of p21(ras)/extracellular regulated kinase in neuronal ischemic preconditioning. Proc Natl Acad Sci. 2000;97:436–41. doi: 10.1073/pnas.97.1.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu Z, Jiang Q, Zhang G. Extracellular signal-regulated kinase and c-Jun N-terminal protein kinase in ischemic tolerance. Neuroreport. 2001;12:3487–91. doi: 10.1097/00001756-200111160-00023. [DOI] [PubMed] [Google Scholar]
- 18.Bickler PE, Zhan X, Fahlman CS. Isoflurane preconditions hippocampal neurons against oxygen-glucose deprivation: role of intracellular Ca2+ and mitogen-activated protein kinase signaling. Anesthesiology. 2005;103:532–9. doi: 10.1097/00000542-200509000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Lee HT, Kim M, Jan M, Emala CW. Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol. 2006;291:F67–78. doi: 10.1152/ajprenal.00412.2005. [DOI] [PubMed] [Google Scholar]
- 20.Armstead WM. Vasopressin-induced protein kinase C-dependent superoxide generation contributes to atp-sensitive potassium channel but not calcium-sensitive potassium channel function impairment after brain injury. Stroke. 2001;32:1408–14. doi: 10.1161/01.str.32.6.1408. [DOI] [PubMed] [Google Scholar]
- 21.Armstead WM, Cines DB, Higazi AA. Plasminogen activators contribute to impairment of hypercapnic and hypotensive cerebrovasodilation after cerebral hypoxia/ischemia in the newborn pig. Stroke. 2005;36:2265–9. doi: 10.1161/01.STR.0000181078.74698.b0. [DOI] [PubMed] [Google Scholar]
- 22.Armstead WM. NOC/oFQ and NMDA contribute to piglet hypoxic ischemic hypotensive cerebrovasodilation impairment. Pediatr Res. 2002;51:586–91. doi: 10.1203/00006450-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Kim YH, Park JH, Hong SH, Koh JY. Nonproteolytic neuroprotection by human recombinant tissue plasminogen activator. Science. 1999;284:647–50. doi: 10.1126/science.284.5414.647. [DOI] [PubMed] [Google Scholar]
- 24.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–31. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 25.Dorrepaal CA, Steendijk P, Baan J, van Bel F. Inhibition of nitric oxide synthesis following severe hypoxia-ischemia restores autoregulation of cerebral blood flow in newborn lambs. Early Hum Dev. 2001;60:159–70. doi: 10.1016/s0378-3782(00)00104-3. [DOI] [PubMed] [Google Scholar]
- 26.Braida D, Capurro V, Zani A, Rubino T, Vigano D, Parolaro D, Sala M. Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br J Pharmacol. 2009;157:844–53. doi: 10.1111/j.1476-5381.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A. 2002;99:11934–9. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi JS, Kim HY, Cha JH, Lee MY. Ischemic preconditioning-induced activation of ERK1/2 in the rat hippocampus. Neurosci Lett. 2006;409:187–91. doi: 10.1016/j.neulet.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 29.Liebelt B, Papapetrou P, Ali A, Guo M, Ji X, Peng C, Rogers R, Curry A, Jimenez D, Ding Y. Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating heat shock protein-70 (heat shock protein-72) and extracellular-signal-regulated-kinase 1/2. Neuroscience. 2010;166:1091–100. doi: 10.1016/j.neuroscience.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 30.Armstead WM, Ganguly K, Kiessling JW, Chen XH, Smith DH, Higazi AA, Cines DB, Bdeir K, Zaitsev S, Muzykantov VR. Red blood cells-coupled tPA prevents impairment of cerebral vasodilatory responses and tissue injury in pediatric cerebral hypoxia/ischemia through inhibition of ERK MAPK activation. J Cereb Blood Flow Metab. 2009;29:1463–74. doi: 10.1038/jcbfm.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–8. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka Y, Imai H, Konno K, Miyagishima T, Kubota C, Puentes S, Aoki T, Hata H, Takata K, Yoshimoto Y, Saito N. Experimental model of lacunar infarction in the gyrencephalic brain of the miniature pig: neurological assessment and histological, immunohistochemical, and physiological evaluation of dynamic corticospinal tract deformation. Stroke. 2008;39:205–12. doi: 10.1161/STROKEAHA.107.489906. [DOI] [PubMed] [Google Scholar]