Abstract
Macrophages can be polarized to exhibit either pro-inflammatory M1 or pro-angiogenic M2 phenotypes, but have high phenotypic plasticity. This pilot study investigated macrophage polarization in the macular retina and choroid of age-related macular degeneration (AMD) and non-AMD subjects, as well as in AMD choroidal neovascular membranes (CNVM). All specimens were evaluated for routine histopathology. Quantitative real-time polymerase chain reaction for representative M1 (CXCL11) and M2 (CCL22) transcripts were performed on macular choroidal trephines (MCT) of 19 AMD and nine non-AMD eye bank eyes, on the microdissected macular retinal cells from the archived slides of five geographic atrophic AMD, five exudative/neovascular AMD, and eight normal autopsied eyes, and on microdissected inflammatory cells from two surgically removed CNVM that did not respond to anti-vascular endothelial growth factor (VEGF) therapy. High M2-chemokine transcript and a low ratio of M1 to M2 chemokine transcript were found in aging non-AMD MCT. Advanced AMD maculae had a higher M1 to M2 chemokine transcript ratio compared to normal autopsied eyes. Macrophages in the two CNVM of patients unresponsive to anti-VEGF therapy were polarized toward either M1 or M2 phenotypes. The number of M2 macrophages was increased compared to M1 macrophages in normal aging eyes. A pathological shift of macrophage polarization may play a potential role in AMD pathogenesis.
Keywords: age-related macular degeneration (AMD), chemokine, choroidal neovascularization, M1 macrophage, M2 macrophage
Age-related macular degeneration (AMD) is the leading cause of irreversible central vision loss in the elderly worldwide.1 Advanced AMD is generally classified into two categories: geographic atrophy (aAMD) or dry AMD; and neovascular/exudative (eAMD) or wet AMD. While genetic and environmental factors, such as aging, diet, inflammation, and oxidative stress, have been linked to AMD pathogenesis, the etiology of the disease is unknown. Recent studies indicate a prominent role for inflammation in AMD development.2 Studies in histological specimens from both humans and animals have demonstrated the presence of macrophages as the main infiltrating inflammatory cells in AMD lesions, particularly in the choroidal neovascular membranes (CNVM).2–6
The precise roles and impacts of macrophages in AMD are unclear and debated. Infiltrating macrophages in the CNVM are capable of expressing pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and pro-inflammatory molecules.7 On the contrary, studies have also shown that macrophages also exert beneficial effects in animal models, such as interleukin (IL)-10 or chemokine Ccl2 deficient mice.8,9 The fact that macrophage populations are heterogeneous and display different phenotypes may explain why macrophages can be both protective and harmful to local tissue in AMD.
Recently, macrophages have been characterized based on their functions, surface markers, and cytokine/chemokine profiles. Classically activated macrophages (M1 macrophages) and alternatively activated macrophages (M2 macrophages) are at opposite ends of this spectrum.10–13 M1 macrophages, driven by Th1 cytokines, are generally pro-inflammatory and secrete M1 chemokines such as CXCL11 (chemotatic for activated T cells). In contrast, M2 macrophages, driven by Th2 cytokines, facilitate tissue repair and neovascularization, and secrete M2 chemokines such as CCL22 (chemoattractant for Th2 cells and enhancing fibrosis). However, due to their plasticity, macrophages with one phenotype can easily convert to another phenotype when placed in a new microenvironment.10–13
In light of these very disparate functional profiles, distinct subclasses of macrophages may exert a specific protective or harmful role in the pathogenesis of AMD.2,4 Here, we study the macrophage phenotypes in the retina and choroid at the macular region from normal eyes, advanced stage AMD eyes, and eAMD-CNVM in a pilot study.
MATERIALS AND METHODS
Human samples
The study was approved by the National Eye Institute (NEI) Institutional Review Board, within which the work was undertaken, and conforms to the provisions of the Declaration of Helsinki. AMD status was graded using the Minnesota Grading System (MGS) from MGS1 (drusen ≤ 63 μm) to MGS4 (aAMD and eAMD).14 Twenty-eight macular choroidal trephines (MCT), 5 mm in diameter, were taken from 19 left eyes with AMD (MGS3-4) and nine left eyes without AMD (MGS1) from the Minnesota Eye Bank. The clinical data and retinas of these eyes were published for proteomics previously.14 The archived slides, including five aAMD (MGS4), five eAMD (MGS4), and eight non-AMD autopsied eyes (MGS1) were from the NEI between 1980 to 2006. These archived slides were autopsied eyes that were fixed in formalin and embedded in paraffin. Two surgical CNVM from two eAMD patients (MGS4) were also included; the specimens were fixed in formalin and embedded in paraffin.
Histology and microdissection
One part of the 28 MCT (about 4/5 MCT) was formalin fixed and paraffin embedded. The other 1/5 portion was frozen and used for quantitative real-time polymerase chain reaction (qRT-PCR). The paraffin-embedded, archived eye sections from 18 subjects (10 AMD and 8 non-AMD) were stained for routine and molecular pathology (microdissection and qRT-PCR). The two CNVM were processed for routine histology, immunohistochemistry, and molecular pathology.
Microdissection was performed on the paraffin-embedded, archived ocular sections of the AMD and control eyes with normal retina, as well as the two CNVM. Under a light microscope, the retinal and choroidal cells of AMD lesions and the normal retinal cells (same number as the cells in the macular lesion) in the mid peripheral retina of 10 AMD eyes, the normal retinal cells in the macular or peripheral retina of eight control eyes, and macrophages and lymphocytes in the two CNVM were identified. A total of 50–100 cells from each location was isolated and carefully collected with a 28-gauge needle. These cells were subjected to molecular analysis as described previously.15 Therefore, there were two samples: one from the macula and one from the periphery for each archived eye. The inflammatory cells (macrophages and lymphocytes) in the CNVM of patient 1 and the inflammatory cells (most of them were CD163+ cells) in the CNVM of patient 2 were also microdissected manually.16
Immunohistochemistry (IHC)
Due to insufficient quantity, quality, and availability of the MCT and archived slides, IHC was only performed on the CNVM and a few archived eyes using the citrate retrieval and avidin-biotin-complex immunoperoxidase techniques.17 The primary antibodies were CD68, a pan macrophage marker (DakoCytomation, Carpinteria, CA, USA), which mainly marks M1 macrophages,18 CD163, a predominant M2 macrophage marker (Novocastra, Newcastle, UK),18–20 and polyclonal rabbit anti-human VEGF (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
RNA isolation and quantitative PCR
Total RNA was extracted from the homogenized frozen MCT (1/5 portion of each MCT) using Trizol according to the manufacturer’s protocol (Micro-to-Midi-Purelink, Invitrogen, Carlsbad, CA, USA). Samples were treated with DNase (Fermentas, Glen Burnie, MD, USA) and then synthesized to cDNA. The macrophages in the two CNVM were manually microdissected under a microscope.
RNA was extracted and reverse-transcribed according to the manufacturer’s protocol (Paradise reagent system, Arcturus, Maintain View, CA, USA). Total RNA was reverse-transcribed using Super Script Reverse Transcriptase II with random primers (Invitrogen). The cDNA from each sample was then used to measure CXCL11 and CCL22 transcripts. The VEGF mRNA was also measured in the two CNVM.
Amplification of cDNA was performed with the MX3000P QPCR system (Agilent, La Jolla, CA, USA) using SYBR Green/Rox PCR master Mix (SABiosciences, Frederick, MD, USA) with qPCR primer assays kits (SABiosciences). SYBR Green fluorescence (PCR product formation) was monitored. The threshold for detection of PCR product was set in the log linear phase of amplification and the threshold cycle (Ct, the number of cycles to reach threshold of detection) was determined for each reaction. The mRNA levels were quantified using masked procedures and compared to the level of β-actin, a housekeeping gene. Due to the limited number and small size of clinic samples, the normalized difference in expression levels between universal human reference RNA (SABiosciences) and tested RNA was calculated as relative expression (ΔΔCT). The calculation was based on the formula: the relative expression level = 10^ΔΔCt/standard curve slope, where ΔΔCt = [Ct (target gene of the tissue)-Ct (β-actin of the tissue)]−[Ct (target gene of the reference)-Ct (β-actin of the reference)]. In the event that an individual mRNA level was more than two standard deviations above the group, it was considered an outlier and excluded.
For the 18 eye sections (archived slides), the cDNA from the microdissected macular and peripheral retinal cells was used to measure CXCL11 and CCL22 mRNAs levels by 32P labeled PCR.15 Briefly, the slides were deparaffinized. The macular cells of AMD lesions, peripheral retina without lesions (AMD cases), normal retina, and retinal pigment epithelium (RPE; control eyes: maculae and periphery) were identified and microdissected manually. RNA isolation from the microdissected samples was performed using the Arcturus Paradise RNA isolation kit (Applied Biosystems, Foster City, CA, USA). Reverse transcription and cDNA synthesis were conducted (Invitrogen). Universal human RNA was used for standardization (BD Biosciences, Palo Alto, CA, USA). Due to inconsistent qualities of the paraffin-embedded archived slides, a great variation of chemokine transcripts was detected among the eyes; thus the macular CXCL11 and CCL22 transcripts were further normalized to the relative expression in the peripheral retina of each eye. The data (expression ratios or relative expressions) were plotted as a bar graph. All primers were purchased from SABiosciences Corporation.
Statistical analysis
Data are presented as mean ± standard error. The unpaired Student’s t-test and one-way ANOVA were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The two methods assumed equal variances. Significance was accepted at P < 0.05.
RESULTS
Clinical data
The nine non-AMD MCT were further divided into two subgroups to better match the ages of the subjects in the AMD and non-AMD groups: a young subgroup (<70 years old, n = 7) and an old subgroup (>70 years old, n = 2). Patient demographics are summarized in Table 1. Of the 19 MCT from AMD patients, three were from eAMD eyes (MGS4), six were from aAMD eyes (MGS4), and 10 were from eyes with MGS3 AMD. None of these patients received anti-VEGF therapy.
Table 1.
Demographics of macular choroidal trephines specimens
| Category | Age (mean ± SE, number) | Sex | ||
|---|---|---|---|---|
| <70-year-old subgroup | >70-year-old subgroup | Female | Male | |
| AMD (19) | 0 | 83.58 ± 1.65 (19) | 10 | 9 |
| Control (9) | 57.57 ± 2.60 (7) | 74.50 ± 0.50 (2) | 4 | 5 |
| Total (28) | 57.57 ± 2.60 (7) | 82.71 ± 1.61 (21) | 14 | 14 |
Data are presented as mean ± SE. AMD, age-related macular degeneration.
For the archived slides of AMD (MGS4) and non-AMD (MGS1) eyes, the average age was 85.86 ± 3.38 for the 10 AMD patients (five aAMD and five eAMD) and 61.75 ± 4.62 for the eight non-AMD subjects. However, only two non-AMD subjects (age, 75.00 ± 2.00) had measurable CXCL11 and CCL22 transcripts. Their ages were not significantly different compared to the average age of the 10 AMD patients (P = 0.15, unpaired Student’s t-test). The average ages of aAMD and eAMD patients were 86.75 ± 4.91 and 84.67 ± 5.55 years, respectively. There was no statistical difference of the average ages between the two AMD groups. None of the AMD patients received anti-VEGF therapy.
One CNVM specimen was obtained from a 93-year-old male AMD patient. The man developed progressive subretinal exudation and hemorrhages after multiple bevacizumab injections within 2 years. He eventually developed sudden loss of vision due to hemorrhagic retinal detachment of the right eye. Pars plana vitrectomy, 180° retinotomy, and excision of the CNVM were performed.
Another CNVM specimen was obtained from a 92-year-old female AMD patient who received six rounds of ranibizumab therapy in the right eye and developed subretinal fibrosis with a sudden decrease in vision from 20/200 to light perception. Clinical examination revealed massive subretinal hemorrhage, retinal detachment, and vitreous hemorrhage. Vitrectomy and excision of the CNVM were performed.
Macrophages and profile of macrophage chemokines in AMD and non-AMD MCT
Small amounts of inner choroidal tissue (collagens, melanocytes and capillaries) and residual Bruch’s membrane were observed in the MCT. Only few RPE cells were adherent to the Bruch’s membrane in eight of the total 28 (28.57%) MCT. No neuroretinal tissue was seen in any of the MCT. More hypocellular or acellular collagens were found in the AMD MCT (14/19, 73.68%) than the non-AMD MCT (1/9, 11.11%).
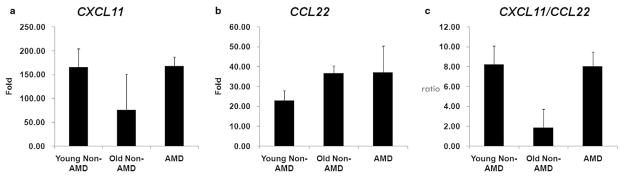
A decreased level of M1 CXCL11, an increased level of M2 CCL22, and a decreased CXCL11/CCL22 ratio (ratio of M1 to M2 chemokine) were observed in the old non-AMD MCT compared to the young non-AMD MCT. There was an increased level of CXCL11, similar amounts of CCL22 and an increased CXCL11/CCL22 ratio in AMD MCT compared to old non-AMD MCT (Fig. 1). No significant differences of chemokine expression were found in the AMD MCT between MGS3 and MGS4.
Figure 1.
Gene expression of macrophage chemokines for (a) CXCL11 (b) CCL22 and (c) ratio of CXCL11/CCL22 in the macular choroidal trephinates. Young non-age-related macular degeneration (AMD) subgroup (n = 7): <70-year-old non-AMD subjects; old non-AMD subgroup (n = 2): >70-year-old non-AMD subjects; AMD subgroup (n = 19): AMD patients. All values are presented as mean ± SE.
Pathology and macrophage chemokines in the retinal cells of the AMD and non-AMD eyes
Histopathology of the 10 AMD eyes revealed classical AMD lesions in the maculae (Fig. 2a,b). Mild macrophage infiltration without neutrophils was observed in AMD lesions of few eyes (Fig. 3). Interestingly, relatively less M2 macrophages (CD163) were found in the eyes with aAMD (Fig. 3b,c) compared to eyes with eAMD (Fig. 3e,f). In the eight non-AMD eyes with normal retinal and choroidal morphology, expression levels of M1 CXCL11 and M2 CCL22 were detected only in two eyes. CXCL11 and CCL22 expression in the retinas of the other six eyes were below detectable levels. Therefore, the AMD eyes showed increased expression of both chemokines. Furthermore, compared to the two non-AMD eyes with detectable chemokine levels, the AMD eyes showed increased CXCL11 and CCL22 expressions (Fig. 2c,d).
Figure 2.
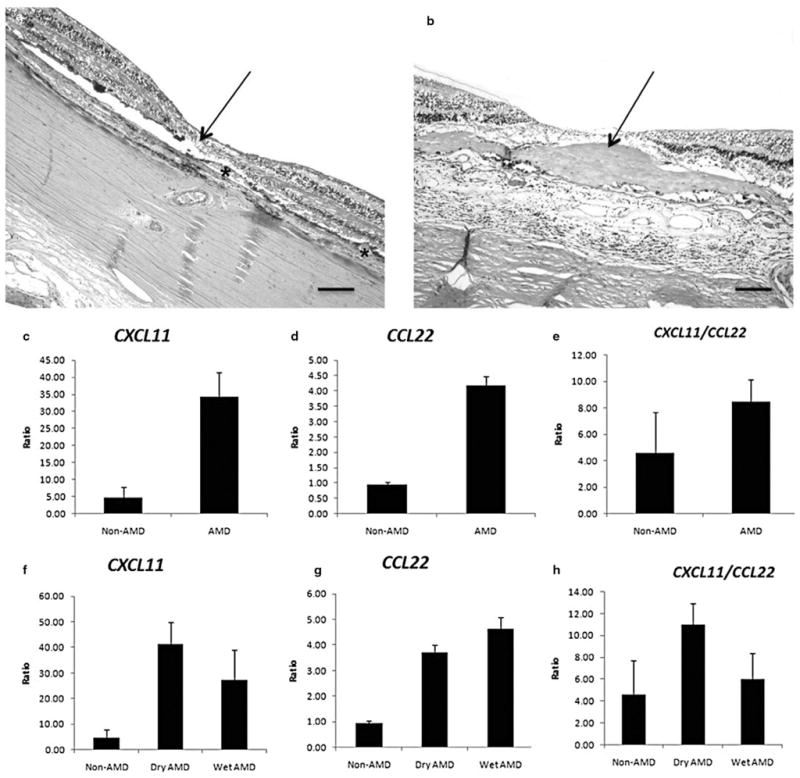
Histology for (a) Geographic atrophy age-related macular degeneration (aAMD) and (b) neovascular/exudative AMD (eAMD), and the relative gene expression ratios between the macular lesions and peripheral retinas of macrophage chemokines for CXCL11 (c and f), CCL22 (d and g) and ratio of CXCL11/CCL22 (e and h) in the archived slides. (a) There is loss of photoreceptor cells (arrow) including the outer and inner segments of the photoreceptors and the outer nuclear layer, abnormal retinal pigment epithelium with hypertrophy and hypotrophy, and many drusen (asterisks) at the fovea and perifovea. (b) Photoreceptor cells and normal RPE are replaced with a thick layer of fibrovascular tissue (arrow) in the maculae. (c–h) Measurable relative expression ratios of chemokines are plotted and compared in bar grafts. Non-AMD group (n = 2): age-matched non-AMD subjects; AMD group (n = 10): AMD patients; Dry AMD (n = 5): aAMD; Wet AMD (n = 5): (eAMD). All values are presented as mean ± SE (a and b, HE, original magnifications: ×100, scale bar, 80 μm).
Figure 3.
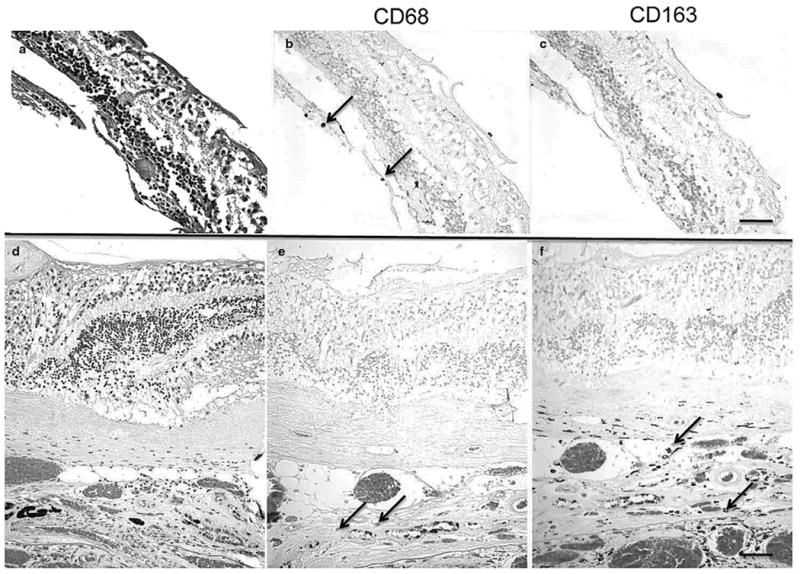
Histopathology of the macular lesions from two age-related macular degeneration patients (AMD; autopsied eyes). (a) A geographic atrophy AMD maculae shows loss of photoreceptors. (b) There is CD68+ macrophage infiltration (arrows). (c) CD163+ cell is not found. (d) A neovascular/exudative AMD maculae shows a thick layer of choroidal neovascularization (CNV). (e) Few CD68+ macrophages (arrows) are indicated in the lesion. (f) More CD163+ macrophages (arrows) are observed in the CNV lesion. (a and d, HE; b, c, e, f, avidin-biotin-complex immunoperoxidase; original magnifications, a–c, ×200, scale bar, 60 μm; d–f, ×100, scale bar, 80 μm).
The mean expression ratios of CXCL11, CCL22, and CXCL11/CCL22 between the maculae and periphery in the 10 advanced AMD eyes were 7.47, 4.35, and 1.85 times higher than the two non-AMD controls with measurable chemokine levels, respectively (Fig. 2c–e). These differences appeared more pronounced in the aAMD eyes than that in the eAMD eyes (Fig. 2f–h). The five aAMD eyes showed CXCL11 of 8.99 and a CXCL11/CCL22 ratio of 2.40 times higher than the non-AMD eyes. The five eAMD eyes showed CCL22 of 4.83 and a CXCL11/CCL22 ratio 1.30 times higher than the non-AMD eyes. The data suggest that while there tends to be a possible polarization towards increased M1 activity in AMD maculae in general, there seems to be a tendency toward greater M1 activity in aAMD and more M2 activity in eAMD.
Patterns of macrophage chemokines in the CNVM with anti-VEGF resistance
The CNVM specimen from patient 1 revealed a fibrovascular membrane infiltrated with many lymphocytes and macrophages, small fresh hemorrhages, and a few degenerate RPE cells (Fig. 4). M1 CXCL11 was very high (29.30 times relative expression) and M2 CCL22 was low (2.84). As expected, VEGF mRNA was highly elevated (330.19). The data suggest M1 polarization.
Figure 4.
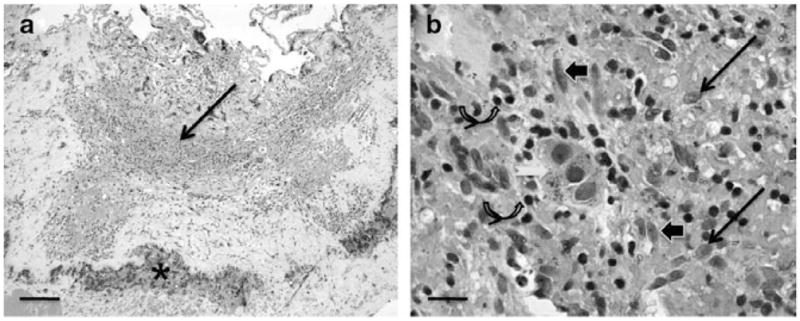
Histopathology of the choroidal neovascular membrane (CNVM) specimens from the first age-related macular degeneration patient who was unresponsive to bevacizumab therapy. (a) The CNVM consists of moderate monocytic infiltrate (arrow) and small hemorrhages (asterisk); (b) Higher magnification showing many macrophages (arrows), lymphocytes (curved arrows), fibroblasts (arrowheads) and degenerated RPE cells (white arrow). (HE; original magnifications: a, ×50, scale bar, 100 μm; b, ×400, scale bar, 50 μm).
The CNVM specimen from patient 2 revealed a fibrotic membrane with few small vessels and old hemorrhage (Fig. 5a). IHC showed expression of VEGF (Fig. 5b) and infiltration of macrophages CD68+/CD163+ cells (Fig. 5c,d). Both M1 CXCL11 and M2 CCL22 were below detectable levels. VEGF mRNA (2.71) was measured in the micro-dissected CD163+ macrophages. The data suggest M2 polarization.
Figure 5.
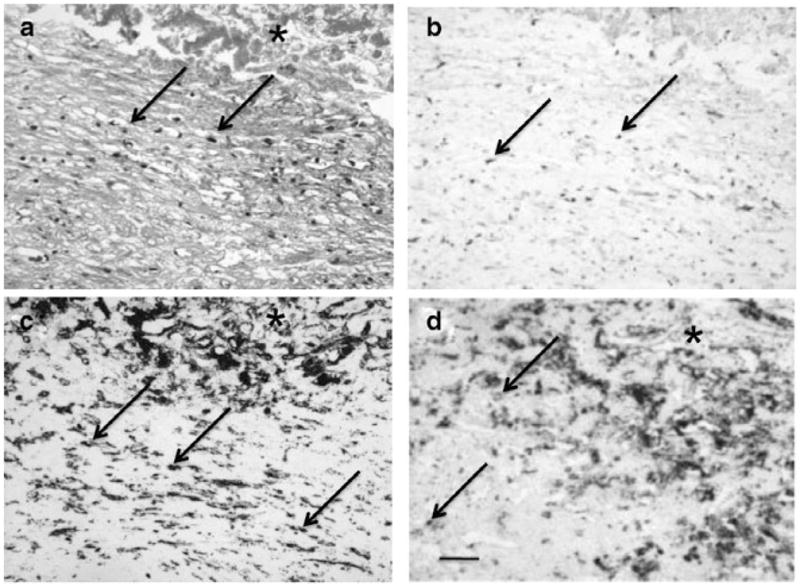
Histopathology of the choroidal neovascular membrane (CNVM) specimens from the second age-related macular degeneration patient who was unresponsive to ranibizumab therapy. (a) The CNVM consists of mild monocytic infiltration (arrows) and hemorrhages (asterisk); (b) Cells in the CNVM express VEGF (arrows); (c and d) Infiltrating cells are CD68+ (c) and CD163+ (d) macrophages. (Original magnifications: ×100, scale bar, 60 μm; a, hematoxylin, b–d, avidin biotin-complex immunoperoxidase).
DISCUSSION
Although this is a pilot study with a small sample size of histological eyes, it is the first attempt at demonstrating macrophage polarization in young and old, non-AMD, as well as AMD eyes. Due to the limited amount of available material, we chose CXCL11 to represent M1 macrophage phenotype and CCL22 to represent M2 macrophage phenotype.10–12
Age-related inflammatory stress occurs in the normal aging process and therefore greater M2 activity may help counter this stress. Our data on MCT support this notion. This observation is compatible with the fact that a higher level of IL-10 has been reported in aging mice eyes.21,22 M1 macrophages are closely related to the Th1 immune response, while M2 macrophages are linked to the Th2 response. Previous findings suggested that, as a consequence of the aging process, a shift from a pro-inflammatory Th1 to an anti-inflammatory Th2 cytokine response occurs.23 These changes can be expected to affect macrophage polarization and activity balance.
Insults such as genetic predisposition and/or photo-oxidation may shift macrophage polarization towards M1, leading to inflammatory damage during age-related disease development. M1 macrophages produce pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α.10,11 Both inflammation and oxidative stress are linked to AMD.2–4,7 In AMD, there are high M1-associated chemokine (CXCL11) levels and a high M1/M2 chemokine (CXCL11/CCL22) ratio in the retina and choroid compared to non-AMD tissues. The lack of an adaptive increase in M2 activity, the relative increase in M1 activity or the combination of both events may contribute to AMD pathogenesis. Moreover, the fact that the M1 activity is higher in aAMD and M2 activity is higher in eAMD may result from the chronic damage of photoreceptor and RPE cells in disease development.
Distinct subclasses of macrophages may exert paradoxical effects on local tissues and represent key mediators in the pathogenesis of AMD. M2 macrophages play a protective role in the aging retina and choroid. A pathological shift away from protective M2 activity toward pro-inflammatory M1 activity may be implicated in the pathogenesis of AMD, specifically in the early stages. However, in later stages, M2 macrophages may actually play a harmful role by promoting fibrosis and angiogenesis. The CNVM with M2 macrophage (both CD68+ and CD163+ cells)18–20 infiltration from patient 2 showed more fibrosis than the CNVM from patient 1. Macrophages are reported to stimulate VEGF production in AMD-associated CNVM and laser-induced CNVM.7,24 VEGF production is closely associated with M2 macrophages.25,26 It is possible that M2 macrophages play a protective role by cleaning waste and inhibiting local inflammation in the aging Bruch’s membrane during the early stages of AMD or before AMD development (MGS2). However, in the later stages (MGS3 and MGS4), the pro-angiogenic and pro-fibrotic roles of M2 macrophages may accelerate neovascularization and fibrosis. It is also possible that macrophage polarization in the two CNVM was secondary to the pro-inflammatory role of bevacizumab and ranibizumab.27
In contrast to patient 2, patient 1 disclosed active inflammation involving primarily M1 macrophage infiltration and production of significant M1-associated chemokines but less fibrosis than patient 2. We hypothesize that failure to respond to anti-VEGF therapy in this patient could be due, at least in part, to overwhelming M1 macrophage infiltration.
A significant increase of macrophage infiltration in CNVM is reported after anti-VEGF therapy.27 Targeting the specific inflammatory molecules has recently been explored.28 When the bio-efficacy of repeated bevacizumab intravitreal injections is decreased, the combined pharmacotherapy with tri-amcinolone, an anti-inflammatory agent, has been reported to negate this effect.29 Intravitreal infliximab has successfully treated two of three patients with neovascular AMD.30 Intra-vitreal rapamycin and daclizumab have also been shown to reduce the need for anti-VEGF therapy.31
Macrophages seem to display a diverse range of phenotypes that can easily change over time.13 Additionally, they are highly plastic depending on the tissue microenvironment.12 Recently macrophage polarization has been reported in the eye with ocular diseases, such as choroidal melanoma and sympathetic ophthalmia.32,33 The finding of whether macrophage polarization is the cause or result of the pathological changes seen in AMD remains elusive and requires further investigation. M1 and M2 macrophages may undergo phenotype switching during normal aging, AMD development, and AMD progression. Our findings may only capture a snapshot in a continuously changing disease process.
The main limitation of this pilot study was the small sample size, which constrained our ability to perform statistical analysis and achieve statistical significance. The sample size was primarily limited by the difficulty in obtaining adequate numbers of human AMD eyes/tissue for histopathological evaluation and/or insufficient material for multiple immunomarkers, cytokines, and chemokine analyses. Therefore, microdissection and RT-PCR, not immunohistochemistry, was chosen as the main tool for the evaluation of macrophage polarization in this preliminary study. Further analysis is warranted to better define the mechanisms of macrophage chemokines and cytokines in AMD. Nevertheless, this is the first attempt to present the potentially critical role of macrophage polarization and diverse functions in AMD and illustrate the potential challenges of using anti-inflammatory therapy for AMD.28
Acknowledgments
The NEI Intramural Research program supported the study.
References
- 1.World Health Organization. Visual impairment and blindness. Geneva, Switzerland: WHO; 2009. [Accessed 9 October 2010]. Available from: http://www.who.int/mediacentre/factsheets/fs282/en/index.html. [Google Scholar]
- 2.Patel M, Chan CC. Immunopathological aspects of age-related macular degeneration. Semin Immunopathol. 2008;30:97–110. doi: 10.1007/s00281-008-0112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dastgheib K, Green WR. Granulomatous reaction to Bruch’s membrane in age-related macular degeneration. Arch Ophthalmol. 1994;112:813–8. doi: 10.1001/archopht.1994.01090180111045. [DOI] [PubMed] [Google Scholar]
- 4.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: The involvement of immunocompetent cells. Graefes Arch Clin Exp Ophthalmol. 1985;223:69–76. doi: 10.1007/BF02150948. [DOI] [PubMed] [Google Scholar]
- 6.Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye. 1990;4:613–21. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- 7.Grossniklaus HE, Ling JX, Wallace TM, et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002;8:119–26. [PubMed] [Google Scholar]
- 8.Apte RS, Richter J, Herndon J, Ferguson TA. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 2006;3:e310. doi: 10.1371/journal.pmed.0030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–97. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: A question of balance. Arterioscler Thromb Vasc Biol. 2009;29:1419–23. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 13.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen TW, Feng X. The Minnesota Grading System of eye bank eyes for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:4484–90. doi: 10.1167/iovs.04-0342. [DOI] [PubMed] [Google Scholar]
- 15.Chan CC, Shen D, Tuo J. Polymerase chain reaction in the diagnosis of uveitis. Int Ophthalmol Clin. 2005;45:41–55. doi: 10.1097/01.iio.0000155936.98330.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen DF, Zhuang Z, LeHoang P, et al. Utility of microdissection and polymerase chain reaction for the detection of immunoglobulin gene rearrangement and translocation in primary intraocular lymphoma. Ophthalmology. 1998;105:1664–9. doi: 10.1016/S0161-6420(98)99036-4. [DOI] [PubMed] [Google Scholar]
- 17.Chan CC, BenEzra D, Hsu SM, Palestine AG, Nussenblatt RB. Granulomas in sympathetic ophthalmia and sarcoidosis. Immunohistochemical study. Arch Ophthalmol. 1985;103:198–202. doi: 10.1001/archopht.1985.01050020050017. [DOI] [PubMed] [Google Scholar]
- 18.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–42. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura K, Komohara Y, Takaishi K, Katabuchi H, Takeya M. Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol Int. 2009;59:300–305. doi: 10.1111/j.1440-1827.2009.02369.x. [DOI] [PubMed] [Google Scholar]
- 20.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 21.Kelly J, Ali Khan A, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Invest. 2007;117:3421–6. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson TA, Apte RS. Angiogenesis in eye disease: Immunity gained or immunity lost? Semin Immunopathol. 2008;30:111–9. doi: 10.1007/s00281-008-0113-8. [DOI] [PubMed] [Google Scholar]
- 23.Burns EA. Effects of aging on immune function. J Nutr Health Aging. 2004;8:9–18. [PubMed] [Google Scholar]
- 24.Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–85. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 25.Noonan DM, De Lerma Barbaro A, Vannini N, Mortara L, Albini A. Inflammation, inflammatory cells and angiogenesis: Decisions and indecisions. Cancer Metastasis Rev. 2008;27:31–40. doi: 10.1007/s10555-007-9108-5. [DOI] [PubMed] [Google Scholar]
- 26.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–61. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 27.Tatar O, Yoeruek E, Szurman P, et al. Effect of bevacizumab on inflammation and proliferation in human choroidal neovascularization. Arch Ophthalmol. 2008;126:782–90. doi: 10.1001/archopht.126.6.782. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Wang VM, Chan CC. The role of anti-inflammatory agents in age-related macular degeneration (AMD) treatment. Eye. 2011;25:127–39. doi: 10.1038/eye.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaal S, Kaplan HJ, Tezel TH. Is there tachyphylaxis to intra-vitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology. 2008;115:2199–205. doi: 10.1016/j.ophtha.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Theodossiadis PG, Liarakos VS, Sfikakis PP, Vergados IA, Theodossiadis GP. Intravitreal administration of the anti-tumor necrosis factor agent infliximab for neovascular age-related macular degeneration. Am J Ophthalmol. 2009;147:825–30. doi: 10.1016/j.ajo.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Nussenblatt RB, Byrnes G, Sen N, et al. A randomized pilot study of systemic immunosuppression in the treatment of age-related macular degeneration with choroidal neovascularization. Retina. 2010;30:1579–87. doi: 10.1097/IAE.0b013e3181e7978e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jager MJ, Ly LV, EI Filali M, Madigan MC. Macrophages in uveal melanoma and in experimental ocular tumor models: Friends or foes? Prog Retin Eye Res. 2011;30:129–46. doi: 10.1016/j.preteyeres.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Furusato E, Shen D, Cao X, Furusato B, Nussenblatt RB, Rushing EJ, Chan CC. Inflammatory cytokine and chemokine expression in sympathetic ophthalmia. Histol Histopathol. 2011 doi: 10.14670/hh-26.1145. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]