Abstract
The University of California at Davis 200 and 206 (UCD-200/206) lines of chickens have proven to be the animal model that best reflects the situation in human systemic sclerosis (SSc). We have demonstrated a disbalance of profibrotic (TGF-β1) and anti-fibrotic (TGF-β2 and 3) TGF-β isoforms as a possible cause for fibrotic alterations in this model. This opens new avenues for diagnosis and therapy for this still intractable condition.
Introduction
Fibrosis, i.e. excessive extracellular matrix (ECM) formation, is a major health problem that receives insufficient attention in basic and clinical research with respect to its aetiology, pathogenesis, diagnosis and therapy.
In principle, fibroses can occur as a consequence of many different pathologic conditions. The most important of these are:
Fibrosis after tissue damage, e.g. post-operative adhesions, burns, alcoholic and post infectious liver cirrhosis, etc.
Fibrosis after inflammatory diseases, e.g. infections, arteriosclerosis, connective tissue diseases, etc.
Fibrosis around foreign body implants, e.g. silicone mammary implants, cardiac pace makers, etc.
“Spontaneous” fibrosis, e.g. keloids, Dupuytren’s contracture, etc.
Tumors, e.g. neurofibromatosis etc.
Systemic sclerosis (SSc, Scleroderma) is a paradigmatic connective tissue disease characterized by two consecutive pathogenetic stages, i.e. a first inflammatory followed by a chronic fibrotic stage, the latter finally leading to death by fibrotic alterations of internal organs, notably the lung.
In contrast to the then current dogma, we have previously shown that the earliest alterations that can be found in SSc are not perivascular mononuclear inflammatory infiltrations but rather consist in apoptosis of microvascular endothelial cells induced by anti-endothelial cell autoantibodies (AECA) entailing the subsequent inflammatory and finally fibrotic complications [1]. This very early endothelial damage is not yet clinically manifest and has therefore received little attention in previous investigations. As a matter of fact, patients with scleroderma are usually only seen by their physicians when overt symptoms occur, most often first apparent as characteristic lesions in the skin. In order to elucidate the exact pathogenetic cause of the disease, the use of appropriate animal models is indispensible. Among these, the University of California at Davis 200 and 206 (UCD200/206) lines of chickens have proven to most closely resemble the human situation in all histopathological, immunological and clinical aspects [2]. In addition, the chicken also provides the unique opportunity to study and easily manipulate the embryo in hatching eggs thus allowing for an extended observation period in a given individual.
The University of California at Davis 200 and 206 lines of chickens – a spontaneously occurring model for scleroderma
UCD 200/206 lines of chickens are selectively bred for the clinical symptoms of a scleroderma-like disease. They are not inbred but homozygous at the major histocompatibility complex (B-locus in chickens), the UCD 200 line carrying B15, the 206 line B17.
Members of both lines develop scleroderma-like clinical symptoms in a timelapse fashion, UCD 206 being slightly more severe affected than UCD 200. Alterations start in the skin within the first week after hatching and then extend to internal organs (notably esophagus, lung, kidney) so that about 90% of the birds are afflicted at the age of 5 weeks.
Since the rest of the skin of chicken is feathered the most impressive lesions can first be observed in the comb characterized by edema followed by Raynaud syndrome-like changes finally leading to complete necrosis, a process called “self-dubbing”. Between 3 to 6 weeks, the esophagus and also other internal organs become involved in the disease process finally leading to severe fibrosis. Due to the fact that the gonads are also affected, breeding of UCD 200/206 chickens is difficult and therefore requires special expertise and careful selection of breeders.
As mentioned above, we have shown that the first stage of the disease consists in microvascular endothelial apoptosis a phenomen that later could also be verified in human patients. AECA-induced apoptosis is not brought about by complement mediated cytotoxicity but by antibody dependent cellular cytotoxicity (ADCC) mediated via the Fas/Fas-ligand rather then the perforin-granzyme pathway. Endothelial cell apoptosis can also be induced by the passive transfer of AECA into embryos via local application onto the chorioallantoic membrane (CAM) or intravenous injection.
The next stage consists in massive perivascular mononuclear cell infiltration, followed by poliferation of fibroblasts and collagenous and non-collagenous extracellular matrix (ECM) deposition. In addition to the pathohistological hallmarks of scleroderma, the UCD200/206 model also presents with serological parameters that are characteristic for the human disease. In addition to AECA, these include antinuclear antibodies (ANA) with a centromeric staining pattern in indirect immunofluorescence as well as anti-phospholipid antibodies.
Among the pro- and antifibrotic cytokines produced by the mononuclear infiltrate, TGF-β has received the greatest attention in our laboratory.
Cellular and molecular characteristics of fibrosis in UCD-200 and 206 chickens
Fibrosis is a characteristic hallmark of SSc and the major cause of functional impairment of the affected organs [3]. In UCD-200/206 chickens, fibrosis is most prominent in skin, esophagus, and lung. It is characterized by accumulation of ECM, mainly collagen types I, III and VI, which are produced by activated fibroblasts [4]. As in human disease, no gross alteration of collagen genes could be demonstrated, as shown by restriction fragment length polymorphism (RFLP) studies [5]. Analyses by RNase protection assays (RPA) of UCD-200 skin, esophagus, and lung revealed two procollagen α2(I) mRNA products, represented by 115bp and 180bp bands, respectively. Compared to healthy controls, the smaller, previously unknown variant was significantly increased in the inflammatory disease stage suggesting that it might be a marker for or even play a role in the initiation of fibrosis [6]. Recently, we have designed appropriate human sense and anti-sense riboprobes that are homologous to the respective chicken proα2(I) probes. Using these probes to analyze human tissues by RPA also resulted in two bands, 207bp and 170bp in size. Whereas the smaller variant was almost absent in normal healthy skin, it was increased in various fibrotic conditions, such as keloids, Dupuytren’s disease, and excessive fibrous capsules that develop around silicone mammary implants. Thus, it seems that the smaller proα2(I) variant is a general marker for early fibrotic processes in human patients too (unpublished data).
The role of TGFβ in avian fibrosis
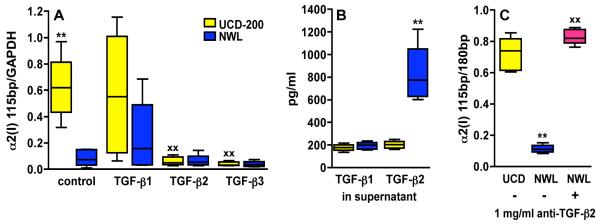
The expression of type I collagen is regulated by various pro- and anti-fibrotic cytokines and growth factors at the transcriptional level. Among these, TGF-β is thought to be a major player in driving fibrosis, and many studies have focussed on its role in the pathogenesis of SSc [7]. There are three TGF-β isoforms, TGF-β1, 2 and 3, which use similar signalling pathways and exert overlapping, albeit not identical biological functions. It is undisputed that TGF-β1 can activate fibroblasts and is a potent stimulator of collagen production, but the specific functions of the other two TGF-β isoforms in the pathogenesis of SSc remains elusive since results from various studies are contradictory. As in human disease, TGF-β1 has a profibrotic activity on chicken fibroblasts reflected by enhanced proliferation and increased procollagen type I expression. Taking advantage of the UCD-200 model we could show that TGF-β2 – in contrast to general belief – can act as an anti-fibrotic cytokine in the pathogenesis of SSc [8]. Chicken embryonic fibroblasts (CEF) from UCD-200 express significantly more of the profibrotic proα2(I) mRNA variant, and show decreased expression of the canonical proα2(I) mRNA transcript compared to fibroblasts from healthy normal White Leghorn (NWL). TGF-β2 and TGF-β3 both reduced the expression of the profibrotic proα2(I) variant in UCD-200-CEF to the same levels seen in healthy controls, whereas TGF-β1 increased its expression. Moreover, TGF-β2 also reduced the 180bp transcript in UCD-200 and NWL-CEF, whereas TGF-β3 reduced the 180bp band only in NWL, but not in UCD-200-CEF. Interestingly, analysis of cell culture supernatants by ELISA revealed that NWL-CEF produced 4.1 times more TGF-β2 than UCD-200-CEF (Figure 1). The constitutive overproduction of the profibrotic proα2(I) mRNA variant and the diminished TGF-β2 synthesis found in untreated UCD-200-CEF suggest that TGF-β2 might be a key cytokine during fibrosis onset.
Figure 1.
Influence of TGF-βs on the expression of a profibrotic α2(I) procollagen variant in chicken embryonic fibroblasts (CEF). (A) α2(I)-mRNA expression in the presence of various TGF-β isoforms, (B) endogenous TGF-β production by CEF, measured in the supernatant, (C) neutralization of endogenous TGF-β2 in NWL-CEF. Boxes represent the 25th to 75th percentiles, lines within the boxes are the median, and lines outside the boxes are 10th and 90th percentiles. ** = p≤0.01 untreated UCD-200-CEF versus untreated NWL-CEF, XX = p≤0.01 treated versus untreated CEF by Mann-Whitney-U test.
Conclusions and therapeutic implications
TGF-β plays essential roles in health and disease regulating cell proliferation and differentiation, immune response, angiogenesis, and tissue repair [9]. Thus, various TGF-β isoforms are considered as promising therapeutic targets. However, a placebo controlled phase I/II trial with anti-TGF-β1 antibody therapy in SSc patients showed no evidence of efficacy, but rather increased morbidity and mortality [10]. This is not to surprising since TGFβ1 not only promotes fibrosis, but also has beneficial effects by inhibiting inflammation. Like TGFβ1, TGFβ2 is anti-inflammatory, but in contrast to TGFβ1, it also can act as an anti-fibrotic cytokine and thus, TGFβ2 seems to be a promising candidate for SSc therapy, especially during the early inflammatory disease stage. The striking immunologic and pathologic similarities found between avian and human SSc make the UCD-200/206 model an ideal tool to test such a novel therapeutic approach.
Key messages.
In avian scleroderma TGF-β1 is profibrotic as expected.
TGF-β2 and 3 are anti-fibrotic.
The TGF-β2 production is significantly diminished in UCD-200 fibroblasts.
Acknowledgement
This work has been continuously supported by the Austrian Research Fund (FWF) most recently projects no. 14466 (to GW) and no. 18726-B05 (to RS).
References
- 1.Sgonc R, Gruschwitz M, Dietrich H, Recheis H, Gershwin ME, Wick G. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J Clin Invest. 1996;98:785–92. doi: 10.1172/JCI118851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wick G, Andersson L, Hala K, et al. Avian models with spontaneous autoimmune diseases. Adv Immunol. 2006;92:71–117. doi: 10.1016/S0065-2776(06)92002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denton CP, Black CM, Abraham DJ. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2:134–44. doi: 10.1038/ncprheum0115. [DOI] [PubMed] [Google Scholar]
- 4.Duncan MR, Wilson TJ, Van De Water J, et al. Cultured fibroblasts in avian scleroderma, an autoimmune fibrotic disease, display an activated phenotype. J Autoimmunity. 1992;5:603–15. doi: 10.1016/0896-8411(92)90157-l. [DOI] [PubMed] [Google Scholar]
- 5.Sgonc R, Dietrich H, Gershwin ME, Colombatti A, Wick G. Genomic analysis of collagen and endogenous virus loci in the UCD-200 and 206 lines of chickens, animal models for scleroderma. J Autoimmunity. 1995;8:763–70. doi: 10.1006/jaut.1995.0057. [DOI] [PubMed] [Google Scholar]
- 6.Ausserlechner MJ, Sgonc R, Dietrich H, Wick G. Altered procollagen mRNA expression during the progression of avian scleroderma. Mol Med. 1997;3:654–62. [PMC free article] [PubMed] [Google Scholar]
- 7.Leask A. Scar wars: is TGFβ the phantom menace in scleroderma? Arthritis Res Ther. 2006;8:213–19. doi: 10.1186/ar1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prelog M, Scheidegger P, Peter S, Gershwin ME, Wick G, Sgonc R. Diminished TGF-β2 production leads to increased expression of a profibrotic procollagen alpha 2 type I mRNA variant in embryonic fibroblasts of UCD-200 chickens, a model for systemic sclerosis. Arthritis Rheum. 2005;52:1804–11. doi: 10.1002/art.21109. [DOI] [PubMed] [Google Scholar]
- 9.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 10.Denton CP, Merkel PA, Furst DE, et al. Cat-192 Study Group; Scleroderma Clinical Trials Consortium Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2007;56:323–33. doi: 10.1002/art.22289. [DOI] [PubMed] [Google Scholar]