Abstract
Autoimmune diseases in human patients only become clinically manifest when the disease process has developed to a stage where functional compensation by the afflicted organ or system is not possible any more. In order to understand the initial etiologic and pathogenic events that are generally not yet accessible in humans, appropriate animal models are required. In this respect, spontaneously developing models - albeit rare – reflect the situation in humans much more closely than experimentally induced models, including knockout and transgenic mice. The present review describes three spontaneous chicken models for human autoimmune diseases, the Obese strain (OS) with a Hashimoto-like autoimmune thyroiditis, the University of California at Davis lines 200 and 206 (UCD-200 and 206) with a scleroderma-like disease and the amelanotic Smyth line with a vitiligo-like syndrome (SLV). Special emphasis is given to the new opportunities to unravel the genetic basis of these diseases in view of the recently completed sequencing of the chicken genome.
1. Introduction
Avian species in general and the chicken in particular have proven to be both, an extremely valuable tool for scientific research as well as a major economic factor (Muir and Aggrey, 2003). With respect to the latter, it is important to note, that the chicken is the largest and most efficient source of animal protein worldwide since it can produce the highest amount of protein in the shortest period of time with the least amount of food (Havenstein et al., 1994). This is the reason why research on various aspects of the lifecycle of chickens in order to improve its economic usefulness is conducted with high speed and efficiency in many laboratories. On the other hand, the chicken as an animal model for the elucidation of many important questions in basic research has lost ground over the past 30 years as compared to other species, notably rodents, especially with the advent of transgenic and knockout murine models. This situation has, unfortunately, led to the extinction of many irretrievable chicken lines since institutional budgets and research grants for maintaining and using these breeds have been severely curtained (Fulton and Delany, 2003). However, the successful completion of the sequencing of the chicken genome in 2004 (International Chicken Genome Sequencing Consortium, 2004) has dramatically changed this situation and will again assign a prime position to this species in many fields of research that take advantage of its unique features and possibilities. Therefore, this review begins with a chapter on chicken genetics with special emphasis on the new horizons that have now been opened for the identification of genetic factors underlying the aetiology and pathogenesis of autoimmune diseases.
This seems to be a good time to review data on three unique lines of chickens that are afflicted with genetically determined, spontaneously occurring organ-specific or systemic autoimmune disease, respectively,
the Obese strain (OS) of chickens that develops a spontaneous autoimmune thyreoiditis perfectly mimicing human Hashimoto disease,
the University of California at Davis 200 and 206 (UCD-200 and 206) lines that serve as a model for human systemic sclerosis (scleroderma)
the amelanotic Smyth line that develops a vitiligo-like syndrome (Smyth line with vitiligo-SLV).
It also seems a right moment to recall some of the unique advantages and characteristics of chickens with special emphasis on their usefulness for immunological research.
Thus, avian Rous sarcoma virus was the first tumor virus identified and the same is true for the discovery of endogenous viruses (ev-loci) (Crittenden, 1981, Svoboda, 1986).
It should be remembered that the formulation of the B- T-lymphocyte concept heavily depended on the discovery of the immunologic role of the bursa of Fabricius and thus bursa-derived lymphocytes (B cells). It was just a fortunate entymologic coincidence that the bone marrow turned out to be the mammalian correlate as the cradle of B cells.
Phylogenetically, the birds stand between fish and mammals (albeit closer to the latter) in the tree of life and provides evidence that the latter branched of the realm of birds about 310 Mio years ago.
A major asset of the chicken is the possibility to easily observe and manipulate the embryo throughout its development and the most important knowledge in embryology stems from studies in this species and its ontogenetic similarities with mammalian development. In this context, it is also appropriate to mention the exciting discoveries on the ontogenetic development of the central nervous system and the immune system using chicken-quail chimeras (Le Douarin et al., 2000)
Apart from the phylogenetic and ontogenetic aspects, chickens are optimal subjects for genetic studies since many offspring can be produced from one pair of parents, reproduction is fast, phenotypic characteristic can easily be assessed and many valuable mutants still exist, in spite of the loss of a considerable number of these (Somes, 1988).
Chickens were the first species where vaccination against an oncogenic virus (Marek’s disease virus - MDV) was developed on an appropriate immunogenetic background and is now successfully applied on a large industrial scale (Bacon and Witter, 1994). Also, the chicken egg has been and still is used as an “incubator” for the development of human vaccines, e.g. against influenza.
The chicken has also proved to be a good source for the production of viral vectors for molecular biological purposes.
The chicken displays a very interesting solution to the generation of antibody diversity where a very limited original immunoglobulin repertoire is altered by gene conversion events with many pseudo-VH-and VL-segments, respectively (Reynaud et al., 1994).
Antibodies produced in hens are transferred via the yolk into the eggs and can easily be harvested in large amounts from this source. More recently, monoclonal antibodies have also been successfully produced from chicken lymphoid cells.
Decisive studies leading to the discovery of immune tolerance was the chicken parabiosis experiment by Hasek (1953), and parallel investigations in the murine system by Medawar’s group (1953), who also provided the right explanation for the observed immunological phenomenon and was awarded by the Nobel Prize for this work.
The graft-versus-host-reaction (GvHR) has been described by Simonsen using either the chicken embryonic spleen chorionallantoic membrane (CAM) assay (Simonsen et al., 1962).
In addition to the above-mentioned spontaneously occurring chicken models afflicted with autoimmune diseases, there are many other examples of spontaneously developing pathological conditions in this species on a genetic basis. Chickens also serve as important models for experimentally induced diseases, e.g. in tumor research (Fulton and Delany, 2003).
In chickens, the genetic basis for susceptibility for and resistance against viral disease has first been demonstrated (Longenecker et al., 1976). Here, it is also important to remember, that the first example of a possible role of infections for the development of atherosclerosis stems from work in chickens (Fabricant et al., 1983).
Chicken erythrocytes are nucleated and DNA can thus be easily prepared from these nuclei after hemolysis. Chicken erythrocytes also express MHC class I antigens (B-G antigens) on their surface that can be identified by simple hemagglutination techniques (Plachy and Hala, 1997). The chicken major histocompatibility (B) complex was identified as a second MHC by Briles (Miller et al., 2004).
The economic impact of chickens has already been mentioned but it is important to emphasize that old lines with potential economic value are endangered, too. This loss of genetic diversity in poultry in livestock breeds and the endeavours to conserve animal genetic resources for global agriculture is a focus of the Food and Agriculture Organisation (FAO) of the United Nations. Thus, between 1984 and 1998 over 230 poultry stocks were eliminated, presenting approximately 40% of the US stocks and over 60% of Canadian stocks. Further major losses since that time have occurred. In a small country like Austria, three endogenous lines are endangered, but even for this small number no support is available from governmental sources. If one considers the possible impact of special chicken lines that are able to survive under various adverse conditions and thus could play a crucial role in alleviating the lack or food protein in the Third World, these losses are even more deplorable.
However, from a scientific standpoint one also has to realise that chickens have certain disadvantages as laboratory animals. Thus, most research institutions are not equipped for keeping chickens. Chickens have a relatively long generation time, i.e. approximately 1 year. Furthermore, the raising and housing of chickens is expensive, they are noisy and special ornithologic, epidemiologic and virologic expertise is required for their sustainment. Chickens are carriers of diseases that may be pathogenic for humans, such has as the avian flu. Finally, sofar there are fewer immunologic reagents available for this species as compared to mice and rats.
However, an interesting and unorthodox view has been put forward in favour of chickens by the late Morten Simonson, namely that no religion has ever raised reservations that may preclude the use of chickens as a source of animal proteins.
2. Chicken genomics and its application to the genetic dissection of autoimmune disorders
2.1. Introduction
This review paper concerns three unique chicken models for autoimmune disorders in humans. An extensive amount of data on the immunology and pathology of these models has been described but their genetics are still poorly understood. A genetic dissection of the genes and mutations causing these disorders is well justified since genetics can reveal the primary cause(s) of the disease and thereby give a deeper understanding of the pathological process leading to an autoimmune disorder. The rapid progress in chicken genomics during recent years facilitates such genetic studies in several ways:
the access to millions of genetic markers makes high-resolution linkage mapping of loci controlling phenotypic traits possible
the access to a high quality draft sequence of the chicken genome means that once a trait locus has been mapped to a chromosomal region it is easy to generate a complete or near complete list of all genes in the interval
the access to a genome sequence facilitates the resequencing of a chromosome region in the search for mutations underlying phenotypic traits
high-throughput expression analysis can now be carried out using high-density cDNA or oligonucleotide arrays.
Thus, genetic dissections of thyroiditis in the OS line, vitiligo in the Smyth line and scleroderma in the UCD-200 line are now both realistic and highly desirable research goals. This section will describe the strategies that may be employed to successfully accomplish the positional identification of the genetic factors required for the development of these autoimmune disorders. A better understanding of the genetics behind these disorders will increase their value as models for human disease.
2.2. The chicken genome
A high-quality draft sequence of the chicken genome was released in 2004 (International Chicken Genome Sequencing Consortium, 2004). A 6.6X sequence coverage was generated by sequencing a single inbred red junglefowl female. The total size of the chicken genome is ~1,050 million base pairs (Mb) and the assembled genome sequence that was order and assigned to a specific chromosome constituted 907 Mb (86%). The major part of the remaining sequences occurs as unassigned sequence contigs.
The genome sequence can be accessed through the major genome browsers (http://genome.ucsc.edu; http://www.ensembl.org; http://www.ncbi.nlm.nih.gov). This is a high-quality draft sequence which means that the major part of the genome is well assembled but there are regions which are still poorly covered. The assembly of the two sex chromosomes (Z and W) is still far from complete. The reason for this is that a female bird (Z/W) was sequenced and thus there was only one copy each of these chromosomes while two copies of each autosome were sequenced. Furthermore, GC-rich regions and regions containing gene duplications caused problems in the assembly and in particular the MHC region on chromosome 16, which harbors clusters of duplicated genes, is poorly covered in the current assembly. The great majority of these problematic regions will be resolved within the next few years when a finished sequence will be completed.
Chickens have 38 pairs of autosomes, 5 macro-, 5 intermediate and 28 micro-chromosomes, which differ widely in size from about 2 Mb for the smallest microchromosome to about 200 Mb for the largest macrochromosome, chromosome 1. The sequence analysis of the chicken genome revealed many striking differences between the macro- and micro-chromosomes. Microchromosomes have in comparison with macrochromosomes a higher G+C content, a higher density of CpG-islands, a higher gene density, shorter introns, a lower frequency of repetitive sequences and a higher recombination rate. It is still unclear why the chicken genome, and many other bird genomes, show this variation in the size of chromosomes.
The size of the chicken genome is only 30-40% of an average mammalian genome that usually contains ~3,000 Mb. A major reason for this size difference is a lower proportion of repetitive sequence in the chicken genome, ~11% vs. 40-50% in mammalian genomes (International Chicken Genome Sequencing Consortium, 2004). The chicken genome also contains fewer pseudogenes and segmental duplications. About 2.5% of the human genome (70 Mb) can be aligned with chicken genome sequences and basically all of these sequences are expected to be conserved because of their functional significance. Only 44% of the conserved sequence represents protein-coding sequences. Many of the conserved non-coding sequences are located far from well-defined genes and may have important regulatory functions.
Another important progress in chicken genomics has been the development of a rich collection Expressed Sequence Tags (ESTs) (Abdrakhmanov et al., 2000; Boardman et al., 2002; Savolainen et al., 2005; Tirunagaru et al., 2000). Two of these studies have involved transcripts from cells of immunological importance, Abdrakhmanov et al. (2000) sequenced clones from bursal cells while Tirunagaru et al. (2000) sequenced splenocytes enriched for activated T cells. These EST projects have been important for the annotation of the genome sequence, in particular for those transcripts that are novel to birds or show a high sequence divergence to their mammalian homologues. In fact, genes associated with the immune response appear to be the group of proteins that has evolved most rapidly during avian and mammalian evolution (International Chicken Genome Sequencing Consortium, 2004). Thanks to the development of these resources and the generation of the genome sequence it is now possible to carry out genome-wide expression analysis using cDNA or oligonucleotide arrays. This approach can be used to further characterize autoimmune disorders in chicken. However, this is not an alternative to a genetic study but rather a complement. The strength of a genetic study is that it can link a specific gene and a specific mutation to a phenotype through segregation analysis whereas a large scale expression analysis is an excellent tool to study the consequences of such mutations.
Another interesting feature of the chicken genome is that chickens have a fairly high recombination rate and the rate varies as a function of the size of the chromosomes (International Chicken Genome Sequencing Consortium, 2004). The recombination rates have been estimated at 2.8 cM/Mb and 6.4 cM/Mb for macrochromosomes and microchromosomes, respectively; one centiMorgan (cM) corresponds to a recombination rate of 1% per gamete. This is significantly higher than the corresponding estimates for humans (~1 cM/Mb) and for mouse (~0.5 cM/Mb). A high recombination rate is initially a disadvantage in a gene mapping project, since more genetic markers are required to detect linkage to the trait locus. However, it is a major advantage in the final stage of a mapping project since it is the identification of recombination events that defines the borders of a chromosomal region harbouring the causative gene and it is crucial that this region is as narrow as possible to facilitate the identification of the causative mutation (see below). Thus, a mouse pedigree comprising 5,000 – 10,000 animals is required in order to achieve the same mapping resolution as can be obtained using ~1,000 chickens.
2.3. Chicken is a highly polymorphic species
As part of the chicken genome project the level of sequence polymorphism was examined by generating partial genome sequences (0.25X coverage) from three domestic chickens, one White Leghorn, one broiler and one Silkie (International Chicken Polymorphism Map Consortium, 2004). These sequence data were aligned with the near complete genome sequence that was generated from a red jungle fowl (RJF), the wild ancestor of the domestic chicken. The analysis revealed as many as 2.8 million single nucleotide polymorphisms (SNPs) in total. The nucleotide diversity was estimated at five sequence differences per 1,000 bp in comparisons between domestic lines and RJF, between domestic lines as well as within some domestic lines; this figure dropped to four sequence differences per 1,000 bp for two of the domestic lines, which have been maintained as closed populations. These estimates are about 5-fold higher than those found in humans even when comparing humans from different ethnic groups and it is on the same level as observed between mouse subspecies (International Chicken Polymorphism Map Consortium, 2004). Thus, the domestic chicken is a highly polymorphic species, and there is a considerable genetic diversity both within and between lines. The results show that chicken domestication has not involved a severe population bottleneck leading to a drastic loss of genetic diversity and the long-term effective population size must have been much larger for the domestic chicken and its wild ancestor than it has been for humans.
This extensive study revealed on average one SNP every ~350 bp throughout the chicken genome. The crucial question is how many of these are true SNPs and how many of them are segregating within a particular population. An initial evaluation indicated that a very high proportion (>90%) represents true SNPs and not sequence artefacts and a surprisingly high proportion (~70%) are common SNPs that are polymorphic in many chicken populations (International Chicken Polymorphism Map Consortium, 2004). Subsequent studies have confirmed this (L. Andersson et al., unpublished). Thus, the established database comprising 2.8 million SNPs is an outstanding resource for high-resolution genetics in the chicken. The data can be accessed through the major genome browsers (see above) or through the chicken variation database (http://chicken.genomics.org.cn/index.jsp).
2.4. Linkage mapping – a powerful approach for unraveling the genetic basis for phenotypic traits
The vertebrate genome contains on the order of 20,000 genes and the functional roles of many genes are still poorly understood. Thus, for any phenotypic trait there are many potential candidate genes and in the worst case scenario the causative gene(s) may not be identified as a candidate gene simply because it has not yet been studied in any detail. This is a major reason why a genetic investigation is a highly relevant approach for understanding the molecular basis of phenotypic traits, like the three autoimmune disorders that are the subjects of this review. Linkage mapping allows us to define a chromosomal region that harbours one or more genes affecting the manifestation of the phenotype. It is essential that such a region is as narrow as possible to reduce the work required to identify the causative gene(s). In fact, linkage mapping can be seen as a method to exclude candidate genes since all genes that do not show co-segregation with the trait locus can be excluded. It is much easier to exclude a candidate gene by a genetic analysis than by functional studies.
A linkage mapping experiment starts with the collection or generation of an informative pedigree material in which the locus/loci controlling the phenotypic traits is segregating. The OS, the UCD-200 and the Smyth lines are kept as closed populations in which alleles predisposing to disease are expected to be fixed or close to fixation. In this case the best strategy is to cross the autoimmune line with a line that does not express the disorder. The choice of line used for the intercross will determine how many susceptibility loci will be segregating in the intercross pedigree. For instance, the Smyth line appears to share some susceptibility factors for the development of vitiligo with the Brown line but not with the Light Brown Leghorn line (see chapter 5.2.).
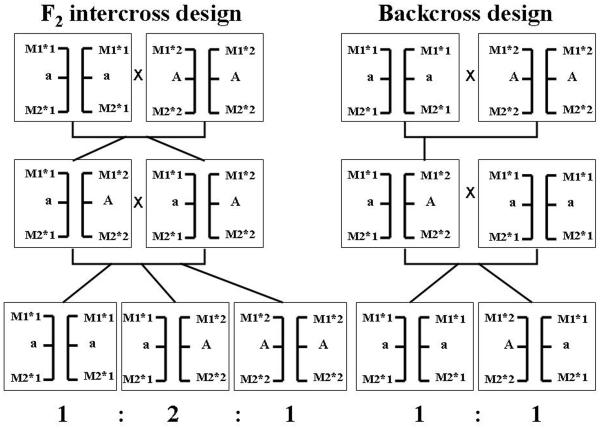
All animals in the first intercross generation (F1) will be heterozygous at the trait locus/loci if the two lines are fixed for alternative alleles (Figure 1). One can then choose to generate an F2 generation by intercrossing F1 animals or a backcross population by backcrossing F1 animals to the susceptible line. An F2 design is generally more powerful because both parents are expected to segregate at the trait loci. However, a backcross design may be preferable if the expression of a disorder is dependent on the interaction between several loci. For instance, let us assume that the expression of an autoimmune disorder is caused by homozygosity for recessive alleles at three unlinked loci and that the lines used in the intercross are fixed for different alleles at all loci. In this case the number of progeny showing disease in an F2 design will be (1/4)3=1/64 whereas (1/2)3=1/8 will express the disease in a backcross design.
Figure 1.
Schematic illustration of the segregation of a recessive allele a predisposing to disease in an F2 intercross design and a backcross design. The co-segregation with the flanking genetic markers M1, with alleles 1 and 2, and M2, with alleles, 1 and 2, is indicated. The genotype at the disease locus can easily be deduced using these flanking markers.
After the collection of DNA samples and phenotypic data from the entire pedigree, a genome scan with genetic markers is carried out. About one informative marker per 20 cM throughout the genome is required to carry out a complete genome scan. Thus, about 200 evenly spaced markers are required in total; the total map distance in chicken is about 4,000 cM. So far highly polymorphic microsatellites have been used for this type of studies (Kerje et al., 2003) but high-throughput analysis of a set of SNPs is today an attractive alternative.
The next step in a linkage mapping experiment is a statistical analysis with the aim to identify which markers co-segregate with the trait locus/loci. Linkage mapping of traits showing a simple monogenic inheritance, one gene with full penetrance, is straightforward. This is because there is a direct relationship between phenotype and genotype for such traits, which makes it possible to directly score recombination events between genetic markers and the trait locus. Thus, the chromosomal position can be determined with high accuracy. For instance, with an F2 design comprising 800 progeny there will be 1600 informative meioses (since each F2 animal receives one gamete from each parent). This makes it possible to map a monogenic trait locus to a fraction of a cM, which is expected to correspond to not more than a few hundred kilobase pairs. The region harbouring a causative mutation is defined as the region between the two closest flanking markers showing at least one recombination event to the trait locus.
Previous data indicate that vitiligo in the Smyth line, thyroiditis in the OS line and scleroderma in the UCD-200 line are all caused by a limited number of genes predisposing to disease. This assumption is based on the observation that lines with a high incidence of disease were established by a limited number of generations of selective breeding and because a fairly high proportion of affected birds have been observed in the limited backcross or intercross experiments that have been carried out. However, the likely presence of multiple susceptibility loci makes the linkage analysis more complicated since there is no more a simple one-to-one relationship between genotype and phenotype. For instance, a bird may be homozygous for a susceptibility allele at one locus but does not express the disorder since it is not homozygous at other loci affecting the disorder. In this case it will be essential to carry out a linkage analysis that does not assume full penetrance at the susceptibility loci or alternatively analyze the data using a Quantitative Trait Locus (QTL) model (Andersson and Georges, 2004). A QTL is defined as a chromosomal region harboring one or more genes affecting a complex trait and is a relevant concept for classical quantitative traits, like weight, or for all-or-none traits, like an autoimmune disorder, determined by multiple genes. It may also be possible to quantify the severity of an autoimmune disorder and use that as the trait in a QTL analysis. A QTL analysis does not require any prior knowledge about the number of loci controlling the trait of interest or the mode of inheritance. It may also be highly relevant to analyze the data using a statistical model that search for epistatic interaction between loci (Carlborg and Haley, 2004), because it is quite likely that an autoimmune disorder is only manifested in those birds that carry a certain genotype combination at two or more loci.
The major challenge in a QTL analysis of complex traits is not the detection of QTLs but the subsequent identification of the underlying genes and mutations (Andersson and Georges, 2004). This is because of the poor precision in the initial mapping experiments even if hundreds of F2 progeny are used. However, several strategies can be used to achieve high-resolution mapping of QTLs, in particular if the number of loci controlling the trait is limited. For instance, let us assume that there are three loci controlling one of the autoimmune disorders described in this review. There should be no problem to identify the approximate chromosomal position of the three loci in an initial mapping experiment. One can then use marker data to select animals that are homozygous at two loci but heterozygous at the third locus (Figure 1) and then use such birds for further breeding experiments. By this approach it may be possible to transform a trait with complex inheritance to a simple monogenic trait that can easily be subjected to high-resolution mapping. An alternative approach is to determine the QTL genotype with high confidence by progeny testing. In this case one select birds that carry recombinant chromosomes for the QTL interval of interest, and these birds are then backcrossed to a line with known QTL status, if dominance occurs one should backcross to birds that are homozygous for the recessive allele. This exercise makes it possible to establish a collection of chromosomes with known QTL status which are then sequenced. For instance, this approach was successfully used for the identification of a single point mutation underlying a major QTL in pigs affecting muscle growth (Van Laere et al., 2003).
2.5. Identification of causative mutations
Once a chromosomal region harbouring a causative gene has been defined the search for the gene itself and the causative mutation is initiated. This phase of a mapping project can be the most laborious one and it is therefore wise to use exploit genetics as far as possible so that the confidence interval for the trait locus becomes tiny. Firstly, one of the web browsers that display the chicken genome sequence is used to download a list of all genes that are present in the actual chromosome region. For most parts of the chicken genome there is a high accuracy regarding gene content and gene order. However, as described above, there are some parts of the genome, like the sex chromosomes, for which the genome assembly is not of high quality yet. Caution is also required since the current annotation of the genome most certainly has missed some genes, the gene sequences may be there but they have not yet been recognized as coding sequences for various reasons.
The first question to ask is of course if there are any obvious candidate genes in the interval. If one of the regions showing linkage for an autoimmune disorder turns out to include the MHC region, it is quite likely that there are one or several mutations in MHC genes that are causing the disorder. Expression analysis may also be used to evaluate the genes present in the defined interval. For instance, it is very unlikely that a gene that is only expressed in the brain is associated with the development of vitiligo, which involves the destruction of melanocytes. DNA sequence analysis is used to search for candidate mutations. This is now greatly facilitated in chicken by the access to a high-quality draft genome sequence, which can be used to design primers for PCR amplifications. Ideally one should resequence the entire chromosome region defined by linkage analysis from individuals with different genotypes at the trait locus. However, if the region is too large for a complete resequencing experiment, the analysis has to be restricted to the top candidate genes but then it is essential to sequence both coding sequences and regulatory regions because the causative may be non-coding and control gene expression. The sequencing of potential regulatory regions for a candidate gene is challenging because such regions may be located hundreds of kilobases from the coding sequence. The outcome of a resequencing experiment is a list of sequence differences between haplotypes associated with different alleles at the trait locus. Ideally this list should be restricted to a single mutation, a result which provides genetic evidence that the causative mutation has been identified. This favorable outcome is possible if one can identify the ancestral haplotype on which the causative mutation occurred. This is not completely unrealistic since it may be known in which line of chicken a disease-causing mutation first occurred and the ancestral haplotype may still be present in that line. In fact, the identification of a non-coding mutation underlying a major QTL in pigs was greatly facilitated by the access to the ancestral haplotypes, which only differed from the mutant haplotype by a single base substitution (Van Laere et al., 2003).
The final stage of a gene mapping experiment is to prove that a candidate mutation is causing the phenotypic effect. The strategy for this depends very much on the nature of the candidate mutation, if it is a coding or non-coding mutation, and the nature of the phenotypic effect, for instance if it can be manifested in a cultured cell or not. The ultimate proof for the causative nature of a candidate mutation may be to replicate the phenotypic effect in a transgenic model. Taking advantages of the new opportunities that have arisen with the successful sequencing of the chicken genome we are now jointly attempting to identify the genes underlying the three avian autoimmune disease models described in the next section of this review.
3. The Obese Strain (OS) of chickens – model for human Hashimoto disease
3.1. Introduction
The OS was originally developed by R.K. Cole at Cornell Veterinary College, Ithaca, New York, in the late 1950s (Cole et al., 1966) when he observed symptoms of hypothyroidism in less than 1% of female birds of the Cornell special C-strain (CS). By selective breeding of such dams with essentially normal appearing CS-roosters, the frequency of spontaneous autoimmune thyroiditis (SAT) increased in male chickens, too (Wick et al., 1981). Following a selective breeding program, the OS was then developed and since 1968, male and female birds are afflicted to about the same percentage up to the present day (Wick et al., 1994).
The phenotypical symptoms of SAT-based hypothyroidism consists in small body size with relatively high body weight (hence the name Obese strain), lipemia, long silky feathers, small combs, low fertility and poor hatchability (Figure 2). These symptoms can be prevented or reversed by supplementation of the diet with thyroxine. As a matter of fact, the OS can only be bred when appropriate thyroid hormone substitution is given (Dietrich et al., 1989).
Figure 2.
5 months old male NWL (left) and OS (right) chickens. The latter shows the typical hypothyroid phenotype, i.e. small body size, silky feathers (especially visible over the legs), small comb. The feathers are ruffled due to cold sensitivity even at normal ambient temperature.
Insert:
Severely infiltrated thyroid gland with prominent germinal centers (dark round structures) and only small thyroid follicles (clear empty structures) remaining. Original magnification x 100
The reason for these clinical symptoms is a severe mononuclear cell (MNC) infiltration of the thyroid gland resulting in the complete destruction of its architecture (Figure 2 insert). After many decades of selective breeding, 100% of OS chicks show severe thyroid infiltration and the first signs of infiltration already appear at one week of age (Dietrich et al., 1997). The natural history of the OS with an extensive documentation of the breeding program has been summarized earlier (Dietrich et al., 1999).
3.2. Histologic and immunologic hallmarks of the OS
Histologically, SAT significantly differs from experimentally induced autoimmune thyroiditis (EAT - using adjuvants) by the presence of numerous germinal centers similar to the situation in human Hashimoto thyroiditis (Wick and Graf, 1972). The severity of SAT is either classified arbitrarily or planimetrically according to a standard scoring schedule where 0 = no infiltration, + = up to 25% of the entire thyroid cross section occupied by infiltrating cells, ++ = 25-50% infiltrated, +++ = 50-75% infiltrated, and ++++ = 75%-total infiltration (Wick et al., 1994). The very first cells infiltrating the thyroid are MHC class II (B-L)+, interleukin-2 receptor (IL-2R)+, CD4+ T cells expressing the T cell receptor α/β (TCR α/β) (Wick et al., 1984, Cihak et al., 1995). Neonatal thymectomy with subsequent depletion of peripheral T cells using specific turkey anti-chicken T cell antibodies prevents the development of SAT (de Carvalho et al., 1981). Injection of monoclonal mouse antibodies against T cell receptor 2 (TCR α/Vβ1) and T cell receptor 3 (TCR α/Vβ2) supports the notion that most of the infiltrating T cells carry the TCR 2 (Cihak et al., 1995). So far, the role of the small number of infiltrating TCR γ/δ (TCR 1)+ cells in the development of SAT is still elusive. Early in the selective breeding program, circulating autoantibodies against thyreoglobulin (TgAAb) were demonstrated in a high percentage of these chickens (Cole et al., 1968). Later, autoantibodies against the second colloid antigen (CA2), microsomal thyroid antigens as well as thyroid hormones were also demonstrated in lower frequency (Khoury et al., 1982). Also, autoantibodies against non-thyroid antigens are found in some OS birds, e.g against proventricular parietal cells, exocrine and endocrine components of the pancreas, antigens of the adrenal cortex and the parathyroid glands (Aichinger et al., 1984). However, no clinically or histopathologically sizeable symptoms are associated with these latter autoantibodies. TgAAb are only produced upon stimulation with autologous Tg, as demonstrated in experiments where OS chicks were first thyreodectomised on the day of hatching and then turned out to be devoid of TgAAb. However, such antibodies did develop after injection of autologous Tg (de Carvalho et al., 1982).
It is also important to note, that the immune system of the OS chicken shows in general hyperreactivity against both, exogenous and autologous antigens as well as against T cell mitogens (Schauenstein et al., 1987). This seems to be partly due to a dominantly encoded hyperproduction of IL-2 and a hyperexpression of IL-2 receptors (Krömer and Wick, 1989). More recently, increased levels of IL-15, another proinflammatory cytokine, has also been shown to be associated with the onset of SAT (Kaiser et al, 2002).
Detailed analyses of the thymic cellular make up revealed a deficit of so-called thymic nurse cells (TNC) in the OS. TNCs are large complexes consisting of thymic epithelial cells (TEC) that contain many T cells in membrane-coated vacudes. This is important, since TNCs have been identified as sites for positive T cell selection that thus may also be disturbed in the OS (Boyd et al., 1984).
3.3. Effector mechanisms and immunoregulation
Several forms of virus infections were excluded as possible causes for the development of SAT. These included Newcastle disease virus, infectious laryngotracheitis virus, reoviruses, infectious bursitis virus and avian encephalomyelitis virus. In addition, serum samples from our OS chicken colony were tested negative for mycoplasma gallisepticum and mycoplasma synovie. Avian leucosis virus (ALV) was also considered and, again our colony proved to be leucosis free. Furthermore, injection of Rous associated virus type I and type II did not change the timing and severity of thyroid infiltration. Also, experiments under germ free conditions did not substantiate the possible role of microbial infection on the course of SAT (Malin et al., 1994; Hala et al., 1996;).
As mentioned above, activated T cells seem to be the first effector cells arriving in the thyroid gland. Adoptive transfer experiments have shown that this can most efficiently be achieved by intravenously injecting T cells harvested from infiltrated donor OS thyroid glands into CS recipients (Kroemer et al., 1985).
However, neonatal and in ovo bursectomy has been shown to significantly delay the development of SAT (Wick et al., 1970a) supporting the concept that TgAAb accelerate disease development. It has been shown by Kofler et al (1983) that complement binding TgAAb are transferred from the mother hen via the egg yolk into the newly hatched chickens were they are deposited in the target organ.
In a series of classical experiments it was shown that neonatal thymectomy of OS chickens entails the development of most severe SAT (Wick et al., 1970b). At that time, this result was unexpected and the author of this part of the present review (G.W.) was forced by his supervisor, Ernest Witebsky, to repeat the experiments about ten times before he was allowed to publish this effect on spontaneously occurring autoimmune thyroiditis, because in experimentally, i.e. with adjuvant, induced autoimmune diseases, such as EAT and experimental autoimmune encephalomyelitis (EAE) (Wick and Steiner, 1972), normal animals became resistant against the induction of disease. We, therefore, hypothesized that neonatal thymectomy of OS chicken resulted in the depletion of what we then called “self recognition-controlling cells” that reside within the thymus for a longer time than effector T cells. Neonatal thymectomy, therefore, seemed to result in the depletion of these cells that were later, when similar experiments were conducted in rodents (Penhale and Ahmed, 1982; Sakaguchi and Sakaguchi, 1989), called T suppressor cells or now T regulatory cells (Tregs) (Sakaguchi and Sakaguchi, 2005). Thymectomy thus apparently allowed previously emigrated T effector cells to exert their autodestructive potential without being surprised by the “controlling cells” that still had not left the thymus in sufficient numbers. The existence of intrathymic suppressor T cells was proven in subsequent experiments that also showed that the kinetics of thymocyte emigration of these cells was indeed severely disturbed in OS chickens (Boyd et al., 1985). Definite proof that this concept was true derived from experiments, where neonatal thymectomy was combined with peripheral T cell depletion resulting in a complete inhibition of SAT-development (de Carvalho et al., 1981). More recently, the idea that T cells initiate SAT has come under renewed scrutiny since immunohistological experiments have shown that macrophages in the OS thyroid gland may not only act as normally functioning antigen presenting cells but also play a decisive early role as effector cells (Hala et al., 1996).
Immune reactivity in general and autoreactivity in particular are known to be regulated by various mechanisms on different levels. On one hand, there are mechanisms intrinsic to the immune system itself, such as Tregs, and the idiotypic network, etc. On the other hand, the potency of the immune reaction is also influenced by regulatory factors such as hormonal effects. In the OS, it has been shown, that the effect of Tregs as, e.g., reflected by hyperproduction of TgAAb and the increased expression of IL-2R, is encoded by dominant genes (Kroemer et al., 1989). In addition to these essential genetic effects, the OS also displays an altered immunoendocrine communication that exacerbates its immunologic hyperreactivity (Wick et al., 1993). Although, basic glucocorticoid (in birds corticosterone) serum levels are equal in OS and normal white Leghorn (NWL) chickens, the former show significantly increased serum concentrations of corticosterone binding globulin (CBG) entailing decreased free metabolically-active corticosterone levels independent of age and sex. Furthermore, in the OS, pathologically altered immunoendocrine feedback regulation via the HPA-axis was first demonstrated in an autoimmune animal model. Similar to the situation in normal rats and mice (Besedovsky and Del Rey, 1996), immunisation with a foreign antigen leads to an increased corticosterone serum concentration that in turn downregulates the immune response. However, in the OS, it was for the first time shown that this post-immunisation glucocorticoid surge is severely blunted and that this represents an additional factor for the overshooting autoimmune reactivity. This phenomenon has not only been shown upon immunisation with foreign antigens but also after injection of so called glucocorticoid increasing factors (GIF) contained, e.g., in conditioned medium of mitogen-activated spleen cells or PBL, or applied in the form of recombinant cytokines such as IL-1, IL-6 or TNFα. Finally, OS thymocytes have been shown to be resistant against the apoptosis-inducing effect of glucocorticoids. Thus, in summary, in addition to the defect of intrinsic T regulatory mechanisms, the OS also suffers from a severely altered dialogue between the immune and the endocrine systems (Wick et al., 1993).
3.4. Endocrinology
Hypothyroid symptoms in the OS are due to a deficiency of triiodothyronine (T3) and thyroxine (tetraiodothyronine, T4) and they can be prevented or reversed by hormonal substitution. OS chickens also display a thyroid-stimulating hormone (TSH)-independent autonomous hyperfunction of thyroid epithelial cells and a defect of iodine organification, i.e. the enzymatically catalysed process that finally results in the iodination of Tg-associated tyrosine and the formation of thyroid hormones, preceding the actual development of SAT (Sundick et al., 1991; Rose et al., 2002). Depletion of dietary iodine leads to a significant delay in the onset of SAT as well as an attenuation of SAT. Conversely, resupplementation of iodine entails a rapid exacerbation of SAT. As will be discussed below, a genetically determined susceptibility of the target organ is an absolute prerequisite for the development of SAT in the OS. So far, the nature of this thyroiditis susceptibility gene has not yet been clarified, but it seems to be associated with the pathologically altered tyrosine iodination process. Recent data by Vasicek et al (2001) point to the possibility that thyrotrophic endogenous viruses play a role in the pathological iodine metabolism that predisposes the OS to SAT.
The altered immunoendocrine communication via the HPA-axis in the OS as compared to chickens of normal strains has already been discussed above. This phenomenon correlates with the presence of an endogenous virus locus (ev-22) that is unique for the OS (Ziemiecki et al., 1988), but seems to have a modulatory role only rather then being the candidate thyroid susceptibility gene: chicks of an F2 generation developed from (OS x NWL) F1 crossings can develop severe thyroiditis without being carriers of ev-22 (Kroemer et al., 1989).
Treatment of OS chicks with glucocorticoids or with specially designed androgen analogues that retain their immunosuppressive potential, but no longer carry their endocrinological side effects, leads to a prevention of the development of SAT or reversal of already manifest disease (Schuurs et al., 1992).
3.5. Immunogenetics with special emphasis of genetically determines target organ susceptibility
3.5.1. Minor modulatory genes
As mentioned above, the original OS colony was established by R.K. Cole in 1957 after beginning selective breeding of CS parental birds for hypothyroid “obese” symptoms in 1955. A colony derived from this OS flock kept at Cornell Veterinary College, Ithaca, New York, was then established in Vienna, Austria, in 1970, and transferred to Innsbruck, Austria, in 1975 where it is still maintained and shows a nearly 100% incidence of SAT independent of sex (Dietrich, 1989).
Immunogenetic analyses of OS by Bacon et al (1974) led to the identification of major histocompatibility complex (MHC, B-locus in chickens), genes as important factors for the development of the disease. Of the three B-haplotypes (B1, B3 and B4, later renamed as B13, B15 and B5, respectively) (Briles et al., 1982), B13 and B15 were associated with severe disease while B5 positive birds were low responders with only mild SAT. At this stage of development, the modulatory effect of the MHC could still be recognized (Wick et al., 1979). In later generations, however, the influence of the B-haplotypes was not evident anymore, indicating that the MHC-haplotype has only a modulatory role and is not a prerequisite for the development of the disease (Hala, 1988).
The OS has been close bred over many generations for the hypothyroid phenotype and the three sublines are only homozygous for the B-locus but on purpose not inbred (Dietrich et al., 1996).
3.5.2. Major genes
Several theories have been put forward to explain the genetic basis of SAT. The first of these was formulated by Cole who – based on classical genetic analyses – concluded that the trait is under the control of more than one gene (Cole, 1966). Subsequently, Rose et al (1976) and then Wick et al (1979) proposed that at least three genetic loci are involved in the natural history of SAT.
This three locus model included: Immune response genes associated with MHC genes, non-MHC immune response genes and gene(s) coding for a primary target organ defect that emerged from previous genetic analyses.
In our experiments, aimed at determining the genetic background of the disease we resorted to crosses between OS and chickens of an inbred healthy normal line (CB-line) (Hala and Plachy, 1997) unrelated to OS and serving as a homogenous genetic background for crossbreeding experiments.
Table 1 summarizes the findings in the F1 generation in a simplified version. From these experiments and further studies of these backcrosses and the F2 generation it was concluded that about three to four genes regulate the full development of SAT. Two to three of these are dominant and responsible for the hyperreactivity of the immune system as already mentioned above. One of them is recessive and encodes susceptibility of the target organ to the autoimmune attack of the immune system (Neu et al., 1985, 1986).
Table 1.
| Disease Phenotype | ||
|---|---|---|
|
| ||
| Chicken Strain | Spontaneous Autoimmune Thyroiditis |
Anti-Thyroglobulin Autoimmunity |
| OS | ++++ | ++++ |
| NWL | − | − |
| (OS x NWL) F1 | − | ++++ |
OS = Obese Strain, NWL = Normal White Leghorn
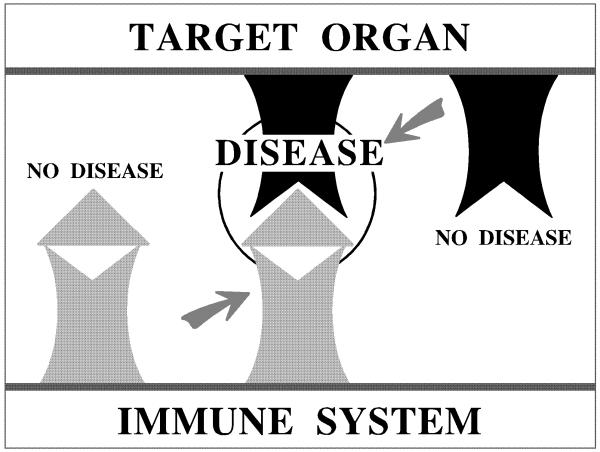
On the basis of these results, a new theory with respect to the development of SAT in the OS in particular but also other autoimmune diseases in general has been formulated (Hala, 1988). This theory postulates the existence of two essential sets of genes that must be present in order that an autoimmune disease develops, viz. genes coding for a hyperreactivity of the immune system on one hand and genes coding for target organ/structure susceptibility to the attack of the immune system, on the other hand. The incidence and severity of a given autoimmune disease based on the presence of these two sets of essential genes can then be fine tuned by modulatory factors that affect either the immune system (such as an altered immunoendocrine feedback regulation via the HPA-axis) or the target organ (such as the iodine content of food) in the OS (Figure 3). The issue of target organ susceptibility as a prerequisite for the development of autoimmune diseases has now pre-occupied our group for many years (Wick et al., 1987) and the two gene family concept has been successfully applied to the study of many other autoimmune diseases in our laboratory, notably scleroderma (Sgonc and Wick, 1999) and atherosclerosis (Wick et al., 2004).
Figure 3.
Schematic representation of “two essential gene family”’ concept for the development of autoimmune disease. Arrows represent modulatory factors affecting either the immune system or the target organ.
3.6. Attempts to identify genes responsible for SAT development
A primary intrinsic expression of MHC class II molecules on the surface of thyroid epithelial cells as originally proposed by Bottazzo et al (1983) for human Graves’ disease and Hashimoto thyroiditis has been excluded in the OS. Aberrant B-L expression was only observed in the neighbourhood of pre-existing mononuclear cell infiltrations, i.e. the presence of interferon-γ (IFN-γ) (Wick et al., 1984; Kuehr et al., 1994). This phenomenon may, therefore, play a major role in the perpetuation rather than the initiation of SAT. We did, however, observe that OS thyroid epithelial cells have a lower threshold for IFN-γ-induced MHC class II antigen expression as compared to those of normal chickens (Wick et al., 1987).
In the thyroid glands of OS but not of CB chickens, infiltrating macrophages and follicular epithelial cells are positive for non-specific esterase. This esterase expression can already be detected on the first day after hatching, i.e. before lymphoid effector cell infiltration (Hala et al., 2000). So far, it is not yet known, if this phenomenon is based on thyroid specific features that reflect the primary target organ susceptibility.
Inflicting mechanical injury on one thyroid gland does not precipitate autoimmune thyroiditis in the injured as well as the contralateral thyroid.
Most probably, the target organ susceptibility has something to do with the tyrosine iodination process as mentioned above (Brown et al., 1998).
In the recent attempt to identify disease-specific transcripts responsible for the initiation of SAT, suppression subtractive hybridisation (SSH) of RNA prepared from OS and CB thyroid lobes, respectively, obtained from three day old chicks (i.e. before the beginning of infiltration), were performed (Vasicek et al., 2001). Final screening and analyses by Northern Blot and sequencing revealed nine clones to be of potential interest (Table 2). Three of these were OS-specific, and four thyroid-gland-specific. We are at present in the process of determining a possible function of these genes in the course of SAT as a different approach. Taking advantage of the now known sequence of the whole chicken genome, parallel microsatelite typing experiments are underway in order to identify the recessive target organ susceptibility gene as well as the dominant genes determining immunologic hyperreactivity in the OS.
Table 2. Sequenced clones from SSH of OS/NWL thyroid cDNA (3 days old).
| Clone | Length (bp) | Specificity of hybridization |
Significant alignment | Accession number |
|---|---|---|---|---|
| 1.2.1. | 385 | No | Chicken EST | AF370360 |
| Human coatomer protein | U24105 | |||
| 1.2.2. | 378 | Thyroid specific | None | AJ414704 |
| 1.1.M3 | 400 | No | None | AJ414705 |
| 1.1.V5 | 505 | Thyroid specific | Human thyroglobulin | X05615 |
| 2.2.6E | 904 | OS specific | ALV ev–6 | AY013305.1 |
| ALV ev–3 | AY013304.1 | |||
| ALV ev–1 | AY013303.1 | |||
| ALV strain NTRE–2 | MI4897.1 | |||
| 3.2.2.A | 514 | OS specific | ALV ev–3 | AY013304.1 |
| ALV ev–1 | AY013303.1 | |||
| 6.2.1.E2 | 854 | Thyroid specific | unknown gene | AJ414706 |
| 6.2.2.C3 | 620 | OS specific (quantitative) |
None | AJ414707 |
| CB8G | 400 | Thyroid specific | Human thyroglobulin | X05615 |
Suppression substractive hybridization of RNA prepared from thyroid glands of OS and normal control (inbred B12 B12 line) chicks at the age of 3 days, i.e. before onset of lymphoid infiltration. Sequences were analysed by BLAST search (National Center for Biotechnology Information). EST = expressed sequence tag, ALV = avian leucosis virus, ev = endogenous virus (adapted from Vasicek et al., 2001)
3.7. Breeding and maintenance of the OS chickens
OS chickens, similar to the other chicken lines described in this review are difficult to breed and maintain. In the special case of the OS, sufficient supplementation of the diet with thyroid hormones is necessary to ascertain sufficient fertility and hatchability. This supplementation should be started only at the age of ten weeks, i.e. a timepoint when assessment of the clinical symptoms of hypothyroidism is possible. At that age, normal looking roosters and dams as well as excessive dwarfs are eliminated from a further breeding program and only birds with an intermediate size displaying a hypothyroid phenotype are retained as future breeders and put on a thyroid hormone supplementation diet. The final selection of breeders is then performed at the age of 20 weeks when the phenotypic symptoms of hypothyroidism are reassessed, B-locus typing is performed and all chickens are tested for the presence and titers of TgAAb and a virus-free state. The breeding program is then performed with artificial insemination. Especially designed cages for housing of single birds by far exceeding the size of commercially available cages are used in our animal unit in order to facilitate the macroscopic observation and the handling for semen collection and artificial insemination (for details see Dietrich, 1989; Wick et al., 1994; Dietrich, et al., 1999).
4. The University of California at Davis (UCD) 200 line of chickens – a model for human systemic sclerosis
4.1. Clinical features and pathogenesis: the enigma of systemic sclerosis (SSc)
Systemic sclerosis (SSc) is a female predominant autoimmune connective tissue disease that is characterized by microvascular alterations, perivascular inflammatory infiltrates, and ultimately fibrosis of the skin and several internal organs, and by the presence of multiple autoantibodies (Jimenez and Derk, 2004). Clinically, SSc can manifest in a wide range of forms ranging from a limited skin involvement with minimal systemic alterations (limited cutaneous SSc) to severe forms (diffuse cutaneous SSc), fulminant in some cases (LeRoy et al., 1988). Nevertheless, a progressive thickening and fibrosis of the skin is universally found in patients with SSc, while internal organs are commonly involved often only subclinically at presentation. In a significant number of cases, however, the cutaneous involvement is confined to the digits and the dorsum of the extremities (acrosclerosis) and slowly progresses to generalized sclerosis; this particular form of SSc was long included in the CREST syndrome (i.e. calcinosis, long-standing Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) (Fritzler and Kinsella, 1980). The Raynaud phenomenon is the second most frequent sign in SSc being referred by the majority of patients (Block and Sequeira, 2001), while musculoskeletal involvement is also common (Pope, 2003). As mentioned above, internal organs are often affected by fibrosis in SSc, including the esophagus (Rose et al., 1998), the lungs (producing severe respiratory failure as a frequent consequence) (Co et al., 2000), the heart and the pericardium (Deswal and Follansbee, 1996), the kidneys (producing the scleroderma renal crisis characterized by the acute onset of hypertension and renal failure) (Steen, 2003), the thyroid (hypothyroidism) (Gordon et al., 1981), and the male reproductive system (causing impotency) (Lally and Jimenez, 1981).
The main pathological features of SSc include an abnormal deposition of collagen in the skin and several internal organs, inflammatory alterations of both the cellular and humoral compartments of acquired immunity, and typical alterations in the microvasculature (Fleischmajer et al., 1977; White, 1996; Herrick, 2000). In advanced stages, progression of the vascular and fibrotic changes is observed alongside with a decrease in inflammation. Similar to other multifactorial diseases, the pathogenesis of SSc appears to be extremely complex. Genetic predisposition is considered as necessary, yet not sufficient, to determine SSc onset, as indicated by the concordance rates among monozygotic twins that are similar for the disease onset and higher for the presence of SSc-associated autoantibodies pattern when compared to concordance rates in dizygotic twins (Feghali-Bostwick et al., 2003). As indicated by pathohistological findings, fibroblasts, endothelial cells, and lymphocytes are the key players in the pathogenesis of the three main observed phenomena, i.e. cutaneous and visceral fibrosis, obliteration of small arteries and arterioles, and immunologic alterations, such as the production of serum autoantibodies, the chronic mononuclear-cell infiltration of affected tissues, and the dysregulation of cytokine and growth factor production (Jimenez and Derk, 2004). Although some progress has been made in the elucidation of the pathogenesis there are still many open questions, and the etiology of SSc remains enigmatic. The search for the ultimate etiology requires animal models.
Several models have been proposed that can be subdivided into two main categories based on whether the disease is induced by exogenous compounds or transmitted genetically as a stable trait. Examples of the former group are represented by the induction of SSc-like disease by the administration of bleomycin, glycosaminoglycans (GAG) derived from patients’ urine, and organic solvents, or the sclerodermatous graft versus host disease (GvHD). On the other hand, genetically transmitted animal models of SSc include the tight-skin (Tsk) mice. The Tsk1/+ mouse was obtained by a dominant mutation in the fibrillin-1 gene on chromosome 2 of the B10.D2 (58N)/SN inbred strain, the Tsk2/+ mouse is a mutant that appeared in the offspring of a 101/H mouse after the administration of the mutagenic agent ethylnitrosourea (for review of the murine models see Jimenez and Christner, 2002). Importantly, all these models display only some of the pathologic features of SSc, e.g. Tsk1/+ mice lack mononuclear cell infiltration and microvascular damage, and Tsk2/+ also do not show any microvascular alterations. Thus neither model is an ideal mimic for human SSc, and they are not useful to understand the etiology of the disease (Sgonc et al., 1999). In contrast, the avian SSc observed in the UCD-200 line of chickens appears as a better model for the human disease, since it manifests similar inflammatory, immunological, vascular, digestive, and articular involvement. In particular, chickens develop the whole spectrum of human SSc, including vascular occlusion, lymphocyte infiltrate, and fibrosis of the skin and internal organs, distal polyarthritis, and serum abnormalities including the appearance of autoantibodies.
4.2. The UCD-200 and 206 chickens
The first chickens showing signs of a genetically determined fibrotic disease were discovered in 1942 by P. Bernier at the Department of Poultry Husbandry, Oregon State University, Corvallis. In 1977, hatching eggs were brought to the University of California at Davis where the line UCD-200 was developed and first described by Gershwin et al (1981). Some years later, the UCD-206 subline was established, which is homozygous for MHC (B locus) B15, thus being histocompatible to the NWL lines UCD 058 and H.B 15 FIN, chicken strains that serve as healthy controls. In 1988, a UCD-200 colony was established in the Experimental Animal Facilities of the Innsbruck Medical University, followed by a colony of UCD-206 chickens in 1993. After the loss of these valuable chicken lines at the University of California at Davis, the colonies at the Innsbruck Medical University are the only still available for research. The natural history of SSc-like disease in this avian model is relatively consistent in all chickens (Van de Water et al., 1995, Sgonc and Wick, 1999). A comparison of the features observed in human and avian SSc is illustrated in Table 3. One to two weeks after hatching UCD-200 and 206 manifest typical comb lesions with a current incidence of 97.5 and 92%, respectively. It starts with swelling and erythema leading to necrosis and the loss of the comb (“self dubbing”) in the majority of cases (Figure 4). By the age of 3-4 weeks 20-40% of UCD-206 chickens develop dermal lesions at the neck followed by necrotic lesion of the toes (Figure 5), and particular involvement in approximately 10% of the birds. UCD-200 animals show dermal lesions of the neck to a lesser degree. Skin inflammation appears early in the natural history of UCD-200 and is later replaced by fibrosis of the dermis and subcutaneous fat and muscle. Involvement of internal organs also occurs in the esophagus and small intestine (wall thickening by collagen deposit), lungs (lymphocyte infiltration and fibrosis is found in 50% of 6-week old chickens), heart (pericardial effusion is detected in 40% of 6-month old animals), kidney (with alterations of renal arterioles in nearly all animals), and testicles. Fibrosis of the reproductive organs, which seems to be more severe in UCD-206 than UCD-200, makes breeding very difficult, and special care has to be taken not to lose the strains.
Table 3. Comparison of clinical, biochemical, immunological, and pathological features of human and avian SSc.
| Human SSc | Av ian SSc | |
|---|---|---|
| Clinical features | ||
| Disease presentation | Subtle, middle-age | Acute, early in life |
| Skin fibrosis | Present | Present |
| Esophageal fibrosis | Present | Present |
| Lung fibrosis | Present | Present |
| Kidney involvement | Present | Present |
| Heart involvement | Present | Present |
| Polyarthritis | Present | Present |
| Eye involvement | Debated | Absent |
|
Autoimmune features
Autoantibodies |
||
| ANA | Present | Present |
| Anti-Scl-70 | Present | Absent |
| Anti-centromere | Present | Present |
| Anti-cytoplasmic | Present | Present |
| Autoreactive T cells | Present | Present |
| Etiology | ||
| Genetic susceptibility | Necessary, not sufficient | Necessary and sufficient |
| Environmental factors | Hypothesized | Not important |
| Pathology | ||
| Fibroblast alterations | Present | Present |
| Endothelial alterations | Present | Present |
| Smooth muscle alterations | Present | Present |
Figure 4.
One week old UCD-200 chickens with early inflammatory SSc showing sequence of erythema, edema, and necrosis of the comb.
Figure 5.
Eight weeks old UCD-206 chickens with chronic fibrotic SSc. Note the loss of feathers, and the extremely thickened skin on the neck (left chicken), the necrotic lesion of the toe resembles the consequence of Raynaud’s phenomenon (right chicken), and the loss of the combs (“self dubbing”).
4.2.1. Genetics
The genetic basis of UCD-200 chickens has been investigated by means of crossed strains and using the comb abnormalities at 4 weeks of age as phenotype determinant (Abplanalp et al., 1990). UCD-200 chickens (in which 100% of male and 60% of female chickens manifested the SSc-like disease at the time of this genetic study) were crossed with partially inbred strains and the obtained chickens (F1) did not show signs of disease. When F1 cocks were backcrossed with UCD-200 hens, the offspring manifested a prevalence of disease varying between 42% and 88%. Importantly, signs of SSc-like disease were consistently observed more frequently in male compared to female chickens in all strains. This is particularly intriguing if one considers that, unlike mammals, male birds are homozygous for sex chromosomes while females are heterozygous, thus possibly explaining the original discrepancy in sex ratios between the human (Whitacre, 2001) and avian SSc. However, due to continuous selective breeding male and female chickens are now affected equally. As in human SSc, the MHC (B locus) haplotype also has some influence on disease development in the avian model. Thus, backcrossing of UCD-200, which are homozygous for MHC-B17, with different MHC haplotypes led to lower disease penetrance, except MHC-B15, which is carried by UCD-206 (Abplanalp et al., 1990).
4.2.2. Immunobiology
Several studies investigated the T cell development and differentiation within the thymus of UCD-200 chickens. The use of monoclonal antibodies recognizing specific areas of the thymus allowed the definition of abnormalities of the thymic ontogenic development in affected chickens at different stages of the disease natural history. The rationale of these studies was based on the critical function of the thymus in the process of T cell negative selection that is key to immunological tolerance. With respect to this, the inappropriate presentation of potentially autoreactive (self) peptides or the disruption of the normal microenvironment of the stromal architecture might ultimately lead to autoreactive T cells and predispose to autoimmunity. UCD-200 chickens have significant and highly specific abnormalities in the thymus subcapsular regions and in the MHC class II expression in the cortex (Boyd et al., 1991; Wilson et al., 1992); these observations led to the hypothesis that T cell maturation might be impaired in affected chickens. Importantly, these alterations were specific to strains manifesting the SSc-like disease and were consistent at all ages, being detectable already prior to the disease onset. The use of monoclonal antibodies further made it possible to determine that the regions most affected in UCD-200 chickens were the subcapsular area and the medulla. Moreover, significantly fewer apoptotic thymocytes are found in UCD-200 chickens compared to healthy controls, strongly suggesting a disturbed negative selection during thymic T cell maturation that might result in an insufficient deletion of autoreactive cells (Sgonc and Wick, 1999).
Most studies on the cellular infiltrates in UCD-200 chickens were performed in the skin, although several lines of evidence were also confirmed in other affected tissues. The prominent cellular infiltrate is composed of lymphocytes while monocytes and macrophages are found in significantly lower proportions. Both types of T cells (i.e. helper, cytotoxic) are detected with CD4+ cells being numerically predominant over CD8+ with 44% by 4 weeks of age. The vast majority of skin-infiltrating cells in the dermis and subcutaneous tissue in affected chickens are TCRα/β+/ CD3+/ CD4+/ MHC class II+ cells with 10% of these also being IL-2R (CD25) positive (Tregs) while the papillary dermis mainly contains TCRγ/δ+/MHC class II-T cells (Van de Water et al., 1989; Gruschwitz et al., 1991). At the same time diseased chickens show significantly reduced percentages and numbers of T cells expressing TCR1, TCR2, CD3, CD4 or IL-2-receptor in the periphery, probably owing to an increased influx into affected tissues (Gruschwitz et al., 1991). In vitro, UCD-200 peripheral blood T cells show a significantly decreased mitogen-induced proliferation rate associated with a decreased capacity to produce IL-2 and to express IL-2 receptors compared with healthy control chickens. In contrast to the deficient in vitro IL-2 production, the sera of UCD-200 chickens contain significantly higher levels or IL-2 bioactivity (Gruschwitz et al., 1991; Wilson et al., 1992). The in vitro vs in vivo discrepancy might be explained by a state of preactivation of peripheral T lymphocytes, either by autoantigens or by non-specific signals resulting in a transient exhaustion of IL-2 secretion that becomes effective in vitro. The increase of MHC class II+ cells in the circulation also points to such an endogenous prestimulation (Gruschwitz et al., 1991). The reduced in vitro T cell response might also derive from a reduced calcium influx following stimulation with mitogens, with or without IL-2 (Wilson et al., 1992). Similar changes in T cell phenotype and function are observed during the course of human SSc, and it is widely accepted that T cells play an important role in the pathogenesis of SSc. There is also some evidence pointing to an antigen driven T cell activation (Sakkas and Platsoucas, 2004). However, the antigen(s) activating T cells is (are) still unknown in human and chicken SSc.
Several autoantibodies characterize human SSc with antinuclear antibodies (ANA) found in over 90% of cases. The highly disease specific anti-topoisomerase-I (anti-Scl-70) antibodies are found in approximately 20% of SSc-sera and are associated with diffuse SSc. Anti-centromere antibodies, which are also rarely detected in other connective tissue diseases, are present in SSc with an overall frequency of 20-30%, and are mainly seen in patients with limited SSc and CREST (Cepeda and Reveille, 2004).
Similarly, circulating autoantibodies are also found in UCD-200 and 206 chickens. Most frequently the ANA display a speckled or nucleolar immunofluorescence pattern on Hep-2 cells. Centromeric staining is found especially with sera from UCD-206 (Sgonc and Wick, 1999). The ANA-subset autoantibody profile shows a chronological increase of antibodies against ssDNA, poly(I) and poly(G), as well as an increase of anti-cardiolipin antibodies. Early in life, the majority of UCD-200 and 206 sera is positive for anti-cytoplasmic antibody staining on HEp-2 cells, and 60% have detectable rheumatoid factor by the age of 6 month. No reactivity has been observed against Scl-70, RNA, SS-A/Ro, SS-B/La or Sm using test kits destined for diagnostic use in humans (Haynes and Gershwin, 1984; Gruschwitz et al., 1993). It is not known if these autoantibodies have any pathophysiological function or are merely epiphenomena. In contrast, anti-endothelial cell antibodies (AECA) that are present in UCD-200 and 206 chickens already before disease onset, and in human SSc mainly in the early inflammatory disease stage, are centrally involved in the induction of endothelial cell injury (Sgonc et al., 1996; Sgonc et al., 2000; Worda et al., 2003).
4.2.3. Vascular alterations
Microvascular damage is found in all involved organs and leads to underperfusion and chronic ischemia, which may play an important role in organ dysfunction. The typical vascular lesions consist of intimal proliferation leading to luminal narrowing of arterioles and capillaries, duplication of the basement membrane, perivascular edema and mononuclear cell infiltration. With disease progression, there is an accumulation of intravascular platelets at progressively damaged endothelial sites, the latter reflected in release of von Willebrand factor (vWF) into the circulation. Lesions progress with increased perivascular collagen deposition leading to fibrosis, obliteration of many capillaries and dilatation of the remaining ones. Further evidences of endothelial cell dysfunction include changes in prostacyclin, thrombomodulin, and angiotensin converting enzyme (ACE). Platelet activation, which can be found in the presence of endothelial damage, is demonstrated by increased levels of thromboxan, β-thromboglobulin, and circulating platelet aggregates (reviewed by Sgonc 1999).
It has long been unclear which of the three salient pathologic features, i.e. vascular abnormalities, perivascular mononuclear cell infiltration, and increased collagen deposition, is the primary pathogenic event in SSc. In looking for the initiating factors in such a complex disease, it is of great value to study animal models sharing as many as possible of the hallmarks of the human disease. The UCD-200 and 206 chicken lines are the only animal model displaying all key symptoms, i.e. endothelial lesions, severe perivascular lymphocytic infiltration of skin and viscera, fibrosis of skin and internal organs, ANA, and AECA.
A comparative study of skin lesion biopsies from UCD-200 and 206 chickens and human SSc patients clearly showed that endothelial cells are the primary target of the autoimmune attack, subsequently undergoing apoptosis (Sgonc et al., 1996). This endothelial cell apoptosis is not a localized phenomenon in the skin, but also the first pathogenic event demonstrable in affected internal organs, thus supporting the hypothesis that endothelial cell apoptosis plays an important role in the initiation of SSc followed by accumulation of mononuclear cells and fibrosis (Nguyen et al., 2000).
Follow-up studies further revealed that endothelial cell apoptosis in SSc is induced by AECA dependent cellular cytotoxicity (ADCC) via the Fas/Fas ligand pathway (Sgonc et al., 2000). NK cells, the effector cells in ADCC, recognize IgG-AECA coated target cells by binding of the antibodies to their Fcγ receptor. Ligation of the Fcγ receptor results in up-regulation of FasL expression, and NK cells can then kill targets that bear Fas (Eischen et al., 1996).
As in human SSc, AECA are the causative principle for the first pathogenic event in avian scleroderma, viz. vascular endothelial cell apoptosis. This was shown by in vivo studies using AECA-positive UCD-200 serum samples for application onto the chorionallantoic membrane (CAM) of various healthy control lines on embryonic day (ED) 10 or for intravenous injection into normal CC-chicken embryos on ED 13. The results revealed that AECA bind to small vessels and that apoptosis of endothelial cells is significantly increased after transfer of AECA-positive sera in comparison to controls (Worda et al., 2003). However, the involved antigen(s) has (have) not yet been identified. The identification of this (these) endothelial cell antigen(s) is a main goal of current research, and should help to elucidate the etiology of this enigmatic disease. It also might lead to the development of new tests for early diagnosis, and to a rational endothelial cell directed therapy.
4.2.4. Fibrosis
Fibroblasts from UCD-200 and 206 fibrotic skin display an activated phenotype producing elevated quantities of collagen, mainly types I, III, and VI, and GAG compared to skin fibroblasts derived from NWL (Duncan et al., 1992). Similar to the human disease, restriction fragment length polymorphism (RFLP) studies did not show any gross alteration of collagen genes (Sgonc et al., 1995).
Cytokines produced by tissue infiltrating immune cells are critical to human SSc onset since they act on growth, migration, and collagen synthesis by smooth muscle cells, endothelial cells, and fibroblasts (Ihn, 2005). Using supernatants from mononuclear cells isolated from lesional UCD-206 skin, a link between infiltrating cells and fibroblast activation has also been shown in the avian model. Supernatants from cultured mononuclear cells isolated from developing fibrotic skin lesions, normal appearing skin, and peripheral blood of UCD-206 chickens were added to normal chicken skin fibroblasts. The results revealed that only mononuclear cells from lesional skin secrete fibroblast-activating cytokines leading to increased collagen and GAG production (Van de Water et al., 1994; Duncan et al., 1995).
Numerous studies have suggested various pro- and antifibrotic cytokines to be involved in the pathogenesis of SSc (Kissin and Korn, 2003), In particular, transforming growth factor beta (TGF-β) emerged as an important mediator in the development of fibrosis, possibly acting locally in a paracrine fashion (Falanga et al., 1987; Kikuchi et al., 1992). The TGF-β superfamily represents a large family of closely related proteins with TGF-β1, 2 and 3 being the most common members. It has been shown in vitro and in vivo that TGF-β is a potent stimulator of collagen production (Cotton et al.; 1998). But although much effort has been made to elucidate the pathogenic role of the TGF-β isoforms in SSc, their specific functions remain elusive since some results are contradictory. A recent study on chicken embryonic fibroblasts (CEF) from UCD-200 and NWL helped to elucidate the contradictory results on TGF-β2 in the pathogenesis of SSc (Prelog et al., 2005). As in human SSc, TGF-β1 has a profibrotic activity on UCD-200 fibroblasts reflected by enhanced proliferation, altered interaction with the surrounding extracellular matrix, increased expression of a profibrotic proα2(I) mRNA variant, and decreased expression of the classic proα2(I) mRNA transcript. These proα2(I) mRNA variants have been detected in an earlier study by RNase protection assays (RPA) where the smaller variant, which is significantly increased in inflammatory lesions of UCD-200 skin and esophagus, is represented by a 115bp band, and the classic proα2(I) mRNA transcript by a 180bp band (Ausserlechner et al., 1997). The smaller proα2(I) mRNA variant seems to play a crucial role in the development of fibrosis, and the 115bp/180bp ratio, which is strongly elevated early in fibrosis, probably is a good marker of fibrosis onset. TGF-β2 and TGF-β3 both reduced the expression of the profibrotic proα2(I) mRNA in UCD-200-CEF to the same levels seen in healthy control NWL-CEF. Interestingly, analysis of cell culture supernatants revealed that NWL-CEF produced 4.1 times more TGF-β2 than UCD-CEF. The constitutive overproduction of profibrotic proα2(I) mRNA variant and diminished TGF-β2 synthesis found in untreated UCD-200-CEF suggest that TGF-β2 – in contrast to general belief – can act as an antifibrotic cytokine and might be a key player during fibrosis onset (Prelog et al., 2005).
4.3. Scientific value of UCD-200 and 206 chicken lines
The strength of the UCD-200 model lies in the spontaneous development of a disease closely resembling human SSc. It is the only model that manifests the whole clinical, histopathological, and serological spectrum of human SSc and thus, can up to date still be regarded as the best animal model for SSc. The studies on UCD-200 and 206 chickens have provided substantial and valuable information on the pathogenesis of the human disease. Thus, only the comparative study of UCD-200 and human SSc made it possible to identify endothelial cell apoptosis as a primary pathogenic event. Although, some progress has been made during the last years, the etiology and pathogenesis of human SSc remain poorly understood and current medical treatments are often unsatisfactory. The striking immunological and pathological similarities found between the avian and human forms of SSc make this model an ideal tool to further investigate the initial pathomechanisms of SSc, and to test novel approaches of evidence based therapies.
5. The Smyth line chicken model for human autoimmune vitiligo
5.1. Introduction to vitiligo
Vitiligo is a common dermatological disorder in humans, affecting at least 1-2 % of the world’s population. It is characterized by destruction of pigment cells (melanocytes) in the skin, generally resulting in patches of depigmentation and, in some individuals, complete depigmentation of the skin. The cosmetic disfiguration resulting from vitiligo leads to psychosocial effects that are particularly severe in the young and in people with darker skin pigmentation. In most cases of vitiligo, the loss of pigmentation is due to an autoimmune, primarily cell-mediated, destruction of melanocytes, and there is a recognized association between vitiligo and a variety of other autoimmune diseases (Nordlund and Lerner, 1982; Ortonne and Bose, 1993; Mason, 1997; Passeron and Ortonne, 2005; Spritz, 2006).
The mutant Smyth line (SL) of chicken (Figure 6), previously known as the delayed amelanosis (DAM) chicken, is the only animal models for autoimmune vitiligo that recapitulates the entire spectrum of clinical and biological manifestations of the human disease. The onset and incidence of SL vitiligo (SLV) are predictable, and the autoimmune lesion is easily accessible (located in the feather). Because the feather regenerates, it provides the opportunity to study the evolving lesion prior to and throughout the development of SLV in the same individuals. As in most autoimmune diseases, several factors are involved in the expression of SLV, including a genetic susceptibility, an immune system component and an environmental component.
Figure 6.
Smyth line animal model for vitiligo. The rooster on the left demonstrates normal pigmentation; the rooster on the right is amelanotic, there is no pigmented new feather growth.
The origin of the SL chicken and the establishment of this animal model for human vitiligo have been reviewed extensively by Smyth (1989). The goal of this review is to briefly summarize key aspects of autoimmune SLV and its relationship to human vitiligo.
5.2. The Smyth line animal model of vitiligo
The mutant Smyth line (SL) chicken was developed by Dr. J. Robert Smyth, Jr., a poultry geneticists at the University of Massachusetts, Amherst, MA. The SL chicken is characterized by a spontaneous, vitiligo-like, post-hatch loss of melanin producing melanocytes in feather and choroidal tissue (Figure 6). Vitiligo occurs in approximately 70% to 95% of hatch-mates, with about 70% of those affected expressing complete depigmentation (amelanosis) in adulthood (Smyth, 1989). The SL of chickens maintained at the University of Arkansas (U of A) originated from the SL101 subline [B101/101 Major Histocompatibility Complex (MHC) haplotype]. This line has been studied most extensively due to its early expression of vitiligo (6-10 weeks of age) (Smyth and McNeil, 1999). Additionally, the U of A maintains two MHC-matched control lines of chickens. One line, the parental Brown line (BL101) has a low incidence of vitiligo (<2%) and is considered genetically susceptible to vitiligo, based on a high incidence of vitiligo (71 %) following i.v. treatment with the DNA methylation inhibitor 5-azacytidine; the other line, the Light Brown Leghorn (LBL101) has no incidence of vitiligo even with 5-azacytidine treatment (Sreekumar et al., 1996). SL101, BL101 and LBL101 lines of chickens (hereafter referred to as SL, BL, and LBL) therefore, offer a unique opportunity to study the factors and mechanisms involved in the susceptibility and expression of autoimmune vitiligo.
There are many similarities between SL and human vitiligo. Both are characterized by a destruction of melanocytes, usually first seen during adolescence and early adulthood. In both SL chickens and humans, pigmentation loss may be either partial or complete. Remelanization of amelanotic tissue occurs, although severe pigment loss and remelanization are more frequent in the chicken (Boissy and Lamoreux, 1988). In addition to vitiligo, SL chickens exhibit uveitis (40 % to 45 %), often resulting in blindness, and have associated autoimmune diseases such as hypothyroidism (4 % to 5 %) and an alopecia areata-like feathering defect (2 % to 3 %) (Smyth and McNeil, 1999). Similarly, in humans it is not uncommon to find thyroidal and other autoimmune diseases associated with vitiligo (Lerner and Nordlund, 1982; Spritz, 2006). Like in human vitiligo, melanocyte loss in SL chickens is accompanied by lymphocyte infiltration into the affected area/target tissue and melanocyte death appears to be mediated by melanocyte-specific cell-mediated immune processes (Wang and Erf, 2003; 2004; Passerone and Ortonne, 2005). Additionally, SL chickens have altered antioxidant capacity, heightened lipid peroxidation, and increased production of reactive oxygen species in feathers and other tissues (Wijesekera, 2004; Erf et al., 2005), phenomenona also reported in vitiligo patients (Agrawal et al., 2004).
The genetic basis of amelanosis, and the other line-associated traits, in SL chickens has long been recognized to be under the complex control of multiple autosomal genes (Smyth et al., 1981). Although multiple MHC types originally existed in the SL and related lines, to eliminate that gene complex as a variable in studies of SL vitiligo, MHC-matched sublines of the SL, BL and LBL were bred to near-homozygosity for different MHC types. Most studies were based on the B101 sublines, and those are the only sublines currently in existence. The exact equivalent of B101 with standard MHC types is not defined. The presence of endogenous viral (ev) genes is related to expression of SLV, which is in agreement with the reported role of ev genes in autoimmune diseases (Nakagawa and Harrison, 1996) as well as the effect of turkey herpesvirus (HVT) vaccination in potentiating the expression of depigmentation in SL chickens (see section III.C.). A greater number of ev genes were expressed in SL and BL birds than in control line LBL birds (Sreekumar et al., 2000). In an F2 resource population (SL X BL), there was linkage disequilibrium between the SLV phenotype and the EV fragments: 16.2-kb SacI and 19-kb HindIII (Sreekumar et al., 2000). To date, however, the exact identity of most of the genes controlling SLV expression remains undefined. Molecular characterization of the SL sublines and their parental controls demonstrated high levels of inbreeding within lines and very high levels of genetic similarity between the SL sublines and their MHC-matched parental control lines (Sreekumar et al., 2001), suggesting that the genes responsible for the SL phenotype reside in a relatively small proportion of the chicken genome. The recent availability of a dense map of single-nucleotide variation in the chicken genome (International Chicken Polymorphism Map Consortium, 2004) combined with the defined SL subline will facilitate mapping the genomic regions and identifying the specific genes responsible for the depigmentation and other abnormalities seen in SL chickens, as described in chapter 2.
5.3. Multifactorial nature of SL vitiligo
5.3.1. Melanocyte defect
Previous studies by J. Robert Smyth, Jr. and co-workers describe the presence of a competent pigment system in SL chicks at hatch. One of the earliest manifestations of SLV, detectable at the light microscope level, was the appearance of histologically abnormal melanocytes in the feather epithelial barb ridge, where melanocytes are located. These melanocytes had thickened, partially retracted dendrites, and an irregular shape. Pigment transfer from melanocyte dendrites to keratinocytes was reduced at this stage. More advanced stages were represented by marked clumping or the absence of melanocytes and further reduction in pigment transfer. The earliest abnormalities within SL melanocytes, prior to visible onset of SLV, were irregularly shaped melanosomes containing pigmented membrane extensions, hyperactive melanization, and selective autophagocytosis of melanosomes. These observations suggested that the synthesis of abnormal melanin granules with pigmented extensions was related to a hyperactive process of melanization, and that this aberrant process, in turn, stimulated the selective autophagocytosis of melanosomes (Boissy et al., 1983; 1985). Similar degenerative processes were also observed in vitro in neural crest-derived melanocytes from embryos of SL chickens (Boissy et al., 1986) and were found to include heightened lipid peroxidation and catalase activity (Lockhart, 2004). The occurrence of these melanocyte malfunctions ex vivo provides strong evidence for an inherent melanocyte defect in SLV. However, as shown through immunosuppression studies, the inherent melanocyte defect alone is not sufficient to cause SLV without a functioning immune system, but appears to be involved in provoking a melanocyte-specific autoimmune response (Lamont and Smyth, 1981; Boissy et al., 1984; Fite et al., 1986; Pardue et al., 1987).
5.3.2. Immune system involvement
Several studies provide evidence supporting a role of the immune system in the pathology of SLV. Phenotypic cell population analysis based on immunohistochemical staining of growing feathers, as well as on flow cytometric analysis of feather pulp cell suspensions, revealed that the majority of feather-infiltrating lymphocytes were T cells (most with αβ1 T cell receptors) and included both T helper cells and cytotoxic T cells, whereby cytotoxic T cells predominated during active SLV (Erf et al., 1995; Shresta et al., 1997). Melanocyte death was shown to occur by apoptosis, apparently induced by cytotoxic T cells (Wang and Erf, 2004). Vitiliginous feathers also exhibited increased numbers of MHC class II-expressing cells (including macrophages, activated T cells and B cells) and heightened levels of MHC class II expression compared to controls, suggesting the presence of inflammatory mediators such as interferon γ (IFN-γ) in affected feathers (Wang et al., 1998). The presence of IFN-γ in feathers with active SLV, but not in normally pigmented or completely amelanotic feathers, was later demonstrated by Northern and Western blots and by quantitative RT-PCR (Plumlee et al., 2006). Vitiligo was not associated with altered numbers and proportions of circulating lymphocytes, although the development of SLV was accompanied by substantial increases in inflammatory blood leukocytes one to two weeks prior to, and at first observation of, SLV (Erf and Smyth, 1996). Similarly, lymphocyte infiltration was not observed in the skin where the undifferentiated melanocyte precursor pool is located (Erf et al., 1995; Bowers, 1988). However, dermal lymphoid aggregates (DLA) contained similar numbers of B and T cells per mm2, but were larger, more abundant, and differed greatly in the proportions among T cell subsets in vitiliginous SL birds compared to controls (Erf et al., 1997). The function of DLA is not established; however, they may serve as local sites for the activation and expansion of melanocyte-specific lymphocytes in SLV.
Overall, the immunopathology of SLV described above is very similar to observations in affected skin of vitiligo patients and supports cell-mediated immune mechanisms in melanocyte destruction. In SL chickens, moreover, direct evidence for a role of cell-mediated immunity in the development of SLV was provided by immunosuppression studies (Fite et al., 1986; Pardue et al., 1987) and by in vivo demonstration of anti-melanocyte cell-mediated immunity in vitiliginous SL, but not in non-vitiliginous SL and control, chickens (Wang and Erf, 2003). In both human vitiligo and SLV, anti-melanocyte autoantibodies have been described (Harning et al., 1991; Park et al., 1996). Although their contribution to the onset and progression of vitiligo is not understood, the observation that bursectomy reduced the incidence of SLV (Lamont and Smyth, 1981) suggests a role of humoral immunity in the chicken model. SL autoantibodies first appear in the peripheral circulation one to two weeks before the onset of SLV, cross-react with mouse and human melanocytes, bind to pigment cells within tissues, and recognize antigens expressed in the cytoplasm and on the surface of melanocytes and melanoblasts (Austin and Boissy, 1995; Searle et al., 1993). Specifically, SL autoantibodies recognize mammalian tyrosinase related protein-1 and, based on molecular studies, its avian homologue (Austin and Boissy, 1995).
5.3.3. Environmental components
In addition to an inherent melanocyte defect and an autoimmune component, Erf et al. (2001a) reported a role of environmental factors, specifically vaccination with live turkey herpesvirus (HVT) at hatch, in the expression of SLV. Without HVT, the incidence of SLV is <20%, but with HVT, the incidence is 70-95% (Erf et al., 2001a). HVT is an alpha herpesvirus commonly used in commercial chicken production as a vaccine to protect chickens from Marek’s disease caused by serotype 1 Marek’s disease viruses (MDV-1). HVT is a non-oncogenic serotype 3 MDV isolated from turkeys that causes only minor inflammatory lesions, but, like other MDV, exhibits strong tropism for feather follicles (Holland et al., 1998). Additional studies on the role of HVT in SLV revealed that killed HVT had no effect on the expression of SLV, nor did other live vaccine viruses that do not exhibit feather follicle tropism, such as Newcastle disease virus and infectious bronchitis virus (Erf, 2002). Moreover, SL chickens appear to have heightened cell-mediated immune activity to live HVT compared to MHC-matched BL chickens (Erf et al., 2001b). It is likely that the local immune response brought to the feather due to HVT presence and, potentially, the direct infection of melanocytes by HVT (Wang et al., 2000), may alter the local and internal melanocyte environment in such a way that an already inherently defective, potentially immunologically active melanocyte, would now become visible to the immune system, thus provoking a melanocyte-specific immune response. The report by Grimes et al. (1996) on the presence of cytomegalovirus DNA in depigmented and normally pigmented skin from some patients with vitiligo and the absence of this herpesvirus DNA in control subjects, suggests that vitiligo may be triggered by a viral infection in selected patients. The establishment of HVT as an environmental trigger of vitiligo in vitiligo-susceptible individuals further underlines the value of SL chickens as a model for human vitiligo and other autoimmune diseases.
5.4. Scientific value of SL chickens
In summary, the SL chicken offers unique opportunities to study the interplay between genetic susceptibility, environmental factors and the immune system, that lead to the development of anti-melanocyte autoimmune activity. The similarities of the clinical manifestations and pathological progression between human and SL vitiligo, together with the easy, non-terminal, repeatable access to the autoimmune lesion, the predictability of the disease, the ability to visually monitor the disease development, and the availability of MHC-matched parental and non-vitiligo-susceptible controls, make the SL animal model an excellent model to study spontaneously developing autoimmune vitiligo.
Conclusions and outlook
Chickens have many advantages as compared to other species as experimental animals for genetic and immunologic research. In the three models described in this review these advantages fortuitously coincide with the spontaneous, genetically determined occurrence of autoimmune diseases with still unknown etiology and pathogenesis. In addition, these models represent both, two organ-specific (OS and SLV) and one systemic (UCD-200 and 206) autoimmune diseases. With the availability of the whole sequence of the chicken genome a resurrection of avian models for these and other human diseases can take place provided that these valuable endangered lines are not lost like so many others in the past. The genetic basis of these crippling autoimmune diseases will soon be defined hopefully opening new diagnostic, preventive and therapeutic horizons for their human counterparts.
Acknowledgements
We would like to devote this review to the late Randall K. Cole, DVM, Cornell Veterinary College, Ithaca, NY, who not only discovered and developed the OS but also continued to work and share his friendship, generosity and expertise with us until his death at the age of 93 during the writing of this review. We would also like to acknowledge the original work of Dr. P. Bernier, Corvalis, OR, who developed the UCD-200 line. The excellent scientific insight and tireless work of Dr. J. Robert Smyth Jr., in recognizing the value of the SLV model, developing the genetic resources for study, conducting the initial research to validate the model, and generously mentoring other scientists using the Smyth Line, are all gratefully acknowledged.
We also thank Ms. Barbara Gschirr for secretarial help and Mrs. Ilona Lengenfelder for artwork during preparation of this manuscript. The work on the OS and UCD-200 and 206 lines has been continuously supported by the Austrian Research Fund (FWF) most recently projects no. 14466 (to GW) and no. 18726-B05 (to RS).
REFERENCES
- Abdrakhmanov I, Lodygin D, Geroth P, Arakawa H, Law A, Plachy J, Korn B, Buerstedde JM. A large database of chicken bursal ESTs as a resource for the analysis of vertebrate gene function. Genome Res. 2000;10:2062–2069. doi: 10.1101/gr.10.12.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abplanalp H, Gershwin ME, Johnston E, Reid J. Genetic control of avian scleroderma. Immunogenetics. 1990;31:291–295. doi: 10.1007/BF02115002. [DOI] [PubMed] [Google Scholar]
- Aichinger G, Kofler H, Diaz-Merida O, Wick G. Non-thyroid autoantibodies in Obese strain (OS) chickens. Clin. Immunol. Immunopathol. 1984;32:57–69. doi: 10.1016/0090-1229(84)90043-6. [DOI] [PubMed] [Google Scholar]
- Andersson L, Georges M. Domestic animal genomics: deciphering the genetics of complex traits. Nature Rev. Genet. 2004;5:202–212. doi: 10.1038/nrg1294. [DOI] [PubMed] [Google Scholar]
- Argawal D, Shajil EM, Marfatia YS, Begum R. Study on antioxidant status of vitiligo patients of different age-groups in Baroda. Pigment Cell Res. 2004;17:289–294. doi: 10.1111/j.1600-0749.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- Ausserlechner MJ, Sgonc R, Dietrich H, Wick G. Altered procollagen mRNA expression during the progression of avian scleroderma. Mol. Med. 1997;3:654–662. [PMC free article] [PubMed] [Google Scholar]
- Austin LM, Boissy RE. Mammalian tyrosinase-related protein-1 is recognized by autoantibodies from vitiliginous Smyth chickens. An avian model for human vitiligo. Am. J. Pathol. 1995;146:1529–1541. [PMC free article] [PubMed] [Google Scholar]
- Bacon LD, Kite JH, Rose NR. Relation between the major histocompatibility (B) locus and autoimmune thyroiditis in Obese chickens. Science. 1974;186:274–275. doi: 10.1126/science.186.4160.274. [DOI] [PubMed] [Google Scholar]
- Bacon LD, Witter RL. Serotype specificity of B-haplotype influence on the relative efficasy of Mareḱs disease vaccines. Avian Dis. 1994;38:65–71. [PubMed] [Google Scholar]
- Besedovsky HO, Del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr. Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Block JA, Sequeira W. Raynaud’s phenomenon. Lancet. 2001;357:2042–2048. doi: 10.1016/S0140-6736(00)05118-7. [DOI] [PubMed] [Google Scholar]
- Boardman PE, Sanz-Ezquerro J, Overton IM, Burt DW, Bosch E, Fong WT, Tickle C, Brown WR, Wilson SA, Hubbard SJ. A comprehensive collection of chicken cDNAs. Curr. Biol. 2002;12:1965–1969. doi: 10.1016/s0960-9822(02)01296-4. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Smyth JR, Jr., Fite KV. Progressive cytologic changes during the development of delayed feather amelanosis and associated choroidal defects in the DAM chicken line. Am. J. Pathol. 1983;111:197–212. [PMC free article] [PubMed] [Google Scholar]
- Boissy RE, Lamont SJ, Smyth JR., Jr. Persistence of abnormal melanocytes in immunosuppressed chickens of the autoimmune “DAM” line. Cell Tissue Res. 1984;235:663–668. doi: 10.1007/BF00226966. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Moellmann G, Smyth JR., Jr. Melanogenesis and autophagocytosis of melanin within feather melanocytes of delayed amelanotic (DAM) chickens. Pigment Cell. 1985;1:731–739. [Google Scholar]
- Boissy RE, Moellmann G, Trainer AT, Smyth JR., Jr. Delayed-amelanotic (DAM or Smyth) chicken: Melanocyte dysfunction in vivo and in vitro. J. Invest. Dermatol. 1986;86:149–156. doi: 10.1111/1523-1747.ep12284190. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Lamoreux ML. Animal models of an acquired pigmentary disorder-vitiligo. Prog. Clin. Biol. Res. 1988;256:207–218. [PubMed] [Google Scholar]
- Bottazzo GF, Pujol-Borrell R, Hanafusa T, Feldman M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983;ii:1115–1118. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Bowers RR. The melanocyte of the chicken: A Review. Prog. Clin. Biol. Res. 1988;256:49–63. [PubMed] [Google Scholar]
- Boyd RL, Oberhuber G, Hala K, Wick G. Obese strain (OS) chickens with spontaneous autoimmune thyroiditis have a deficiency in thymic nurse cells. J. Immunol. 1984;132:718–724. [PubMed] [Google Scholar]
- Boyd RL, Hala K, Wick G. Interactions and quantitative analysis of immunoregulatory cells in the chicken thymus. J. Immunol. 1985;135:3039–3049. [PubMed] [Google Scholar]
- Boyd RL, Wilson TJ, Van De Water J, Haapanen LA, Gershwin ME. Selective abnormalities in the thymic microenvironment associated with avian scleroderma, an inherited fibrotic disease of L200 chickens. J. Autoimmun. 1991;4:369–380. doi: 10.1016/0896-8411(91)90031-7. [DOI] [PubMed] [Google Scholar]
- Briles WE, Bumstead N, Ewert DL, Gilmour DG, Gogusev J, Hala K, Koch C, Longenecker BM, Nordskog AW, Pink JR, Schierman LW, Simonsen M, Toivanen P, Toivanen P, Vainio O, Wick G. Nomenclature for chicken major histocompatibility (B) complex. Immunogenetics. 1982;5:441–447. doi: 10.1007/BF00345903. [DOI] [PubMed] [Google Scholar]
- Brown TR, Zhao G, Palmer KC, Sundick RS. Thyroid injury, autoantigen availability, and initiation of autoimmune thyroiditis. Autoimmunity. 1998;27:1–12. [PubMed] [Google Scholar]
- Cepeda EJ, Reveille JD. Autoantibodies in systemic sclerosis and fibrosing syndromes: clinical indications and relevance. Curr. Opin. Rheumatol. 2004;16:723–732. doi: 10.1097/01.bor.0000144760.37777.fa. [DOI] [PubMed] [Google Scholar]
- Carlborg O, Haley C. Epistasis: too often neglected in complex trait studies? Nat. Rev. Genet. 2004;5:618–625. doi: 10.1038/nrg1407. [DOI] [PubMed] [Google Scholar]
- Cihak J, Hoffman-Fezer G, Koller A, Kaspers B, Merkle H, Hala K, Wick G, Lösch U. Preferential TCR Vβ1 gene usage by autoreactive T cells in spontaneous autoimmune thyroiditis of the Obese strain of chickens. J. Autoimmun. 1995;8:507–520. doi: 10.1016/0896-8411(95)90005-5. [DOI] [PubMed] [Google Scholar]
- Co HT, Block JA, Sequeira W. Scleroderma lung: pathogenesis, evaluation, and current therapy. Am. J. Ther. 2000;7:321–324. [PubMed] [Google Scholar]
- Cole RK. Hereditary hypothyroidism in the domestic fowl. Genet. 1966;53:1021–1033. doi: 10.1093/genetics/53.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RK, Kite JH, Witebsky E. Hereditary autoimmune thyroiditis in the fowl. Science. 1968;160:1357–1358. doi: 10.1126/science.160.3834.1357. [DOI] [PubMed] [Google Scholar]
- Cotton SA, Herrick AL, Jayson MI, Freemont AJ. TGF beta - a role in systemic sclerosis? J. Pathol. 1998;184:4–6. doi: 10.1002/(SICI)1096-9896(199801)184:1<4::AID-PATH968>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Crittenden LB. Exogenous and endogenous leukosis virus genes – a review. Avian Pathol. 1981;10:101–112. doi: 10.1080/03079458108418464. [DOI] [PubMed] [Google Scholar]
- de Carvalho L, Wick G, Roitt IM. Requirement of T cells for the development of spontaneous autoimmune thyroiditis in Obese strain (OS) chickens. J. Immunol. 1981;126:750–753. [PubMed] [Google Scholar]
- de Carvalho LCP, Templeman J, Wick G, Roitt IM. The role of self-antigen in the development of autoimmunity in Obese strain (OS) chickens with spontaneous autoallergic thyroiditis. J. Exp. Med. 1982;155:1255–1266. doi: 10.1084/jem.155.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deswal A, Follansbee WP. Cardiac involvement in scleroderma. Rheum. Dis. Clin. North Am. 1996;22:841–860. doi: 10.1016/s0889-857x(05)70304-5. [DOI] [PubMed] [Google Scholar]
- Dietrich HM. Housing, breeding and selecting chickens of the Obese strain (OS) with spontaneous autoimmune thyroiditis. Lab. Anim. 1989;23:345–352. doi: 10.1258/002367789780745953. [DOI] [PubMed] [Google Scholar]
- Dietrich HM, Oliveira-dos-Santos AJ, Hala K, Wick G. Skin allograft survival in chicken strains with spontaneous autoimmune diseases. Poultry Sci. 1996;75:285–293. doi: 10.3382/ps.0750285. [DOI] [PubMed] [Google Scholar]
- Dietrich HM, Oliveira-dos-Santos AJ, Wick G. Development of spontaneous autoimmune thyroiditis in Obese strain (OS) chickens. Vet. Immunol. Immunopath. 1997;57:141–146. doi: 10.1016/s0165-2427(96)05762-5. [DOI] [PubMed] [Google Scholar]
- Dietrich HM, Cole RK, Wick G. The natural history of the Obese Strain of chickens – An animal model for spontaneous autoimmune thyroiditis. Poultry Sci. 1999;78:1359–1371. doi: 10.1093/ps/78.10.1359. [DOI] [PubMed] [Google Scholar]
- Duncan MR, Wilson TJ, Van De Water J, Berman B, Boyd R, Wick G, Gershwin ME. Cultured fibroblasts in avian scleroderma, an autoimmune fibrotic disease, display an activated phenotype. J. Autoimmun. 1992;5:603–615. doi: 10.1016/0896-8411(92)90157-l. [DOI] [PubMed] [Google Scholar]
- Duncan MR, Berman B, Van de Water J, Boyd RL, Wick G, Gershwin ME. Mononuclear cells isolated from fibrotic skin lesions in avian scleroderma constitutively produce fibroblast-activating cytokines and immunoglobulin M. Int. Arch. Allergy Immunol. 1995;107:519–526. doi: 10.1159/000237094. [DOI] [PubMed] [Google Scholar]
- Eischen CM, Schilling JD, Lynch DH, Krammer PH, Leibson PJ. Fc receptor-induced expression of Fas ligand on activated NK cells facilitates cell-mediated cytotoxicity and subsequent autocrine NK cell apoptosis. J. Immunol. 1996;156:2693–2699. [PubMed] [Google Scholar]
- Erf GF, Trejo-Skalli AV, Smyth JR., Jr. T cells in regenerating feathers of Smyth line chickens with vitiligo. Clin. Immunol. Immunopathol. 1995;76:120–126. doi: 10.1006/clin.1995.1105. [DOI] [PubMed] [Google Scholar]
- Erf GF, Smyth JR., Jr. Alterations in blood leukocyte populations in Smyth line chickens with autoimmune vitiligo. Poult. Sci. 1996;75:351–356. doi: 10.3382/ps.0750351. [DOI] [PubMed] [Google Scholar]
- Erf GF, Trejo-Skalli AV, Poulin M, Smyth JR., Jr. Dermal lymphoid aggregates in autoimmune Smyth line chickens. Vet. Immun. Immunopathol. 1997;58:335–343. doi: 10.1016/s0165-2427(97)00036-6. [DOI] [PubMed] [Google Scholar]
- Erf GF, Bersi TK, Wang X, Sreekumar GP, Smyth JR., Jr. Herpesvirus connection in the expression of autoimmune vitiligo in Smyth line chickens. Pigment Cell Res. 2001a;14:40–46. doi: 10.1034/j.1600-0749.2001.140107.x. [DOI] [PubMed] [Google Scholar]
- Erf GF, Johnson JC, Parcells MS, Wang X, Bersi TK. A role of turkey herpesvirus in autoimmune Smyth line vitiligo. In: Schat KA, editor. Current Progress on Avian Immunology Research. American Association of Avian Pathologists, Inc.; 2001b. pp. 226–231. [Google Scholar]
- Erf GF. Herpesvirus connection in Smyth line vitiligo. Part II. Vitiligo Newsletter. 2002;8(2):8. [Google Scholar]
- Erf GF, Wijesekera HD, Lockhart BR, Golden AL. Antioxidant capacity and oxidative tress in the local environment of feather-melanocytes in vitiliginous Smyth line chickens. Pigment Cell Res. 2005;18(Suppl. 1):69. [Google Scholar]
- Fabricant CG, Fabricant J, Minick CR, Litrenta MM. Herpesvirus-induced atherosclerosis in chickens. Fed. Proc. 1983;42:2476–2479. [PubMed] [Google Scholar]
- Falanga V, Tiegs SL, Alstadt SP, Roberts AB, Sporn MB. Transforming growth factor-beta: selective increase in glycosaminoglycan synthesis by cultures of fibroblasts from patients with progressive systemic sclerosis. J. Invest. Dermatol. 1987;89:100–104. doi: 10.1111/1523-1747.ep12580445. [DOI] [PubMed] [Google Scholar]
- Feghali-Bostwick C, Medsger TA, Jr., Wright TM. Analysis of systemic sclerosis in twins reveals low concordance for disease and high concordance for the presence of antinuclear antibodies. Arthritis Rheum. 2003;48:1956–1963. doi: 10.1002/art.11173. [DOI] [PubMed] [Google Scholar]
- Fite KV, Pardue S, Bengston L, Hayden D, Smyth JR., Jr. Effects of cyclosporine in spontaneous, posterior uveitis. Curr. Eye Res. 1986;5:787–796. doi: 10.3109/02713688609000020. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R, Perlish JS, Reeves JR. Cellular infiltrates in scleroderma skin. Arthritis Rheum. 1977;20:975–984. doi: 10.1002/art.1780200410. [DOI] [PubMed] [Google Scholar]
- Fritzler MJ, Kinsella TD. The CREST syndrome: a distinct serologic entity with anticentromere antibodies. Am. J. Med. 1980;69:520–526. doi: 10.1016/0002-9343(80)90462-3. [DOI] [PubMed] [Google Scholar]
- Fulton JE, Delany ME. Genetics. Poultry genetic resources – operation rescue needed. Science. 2003;300:1667–1668. doi: 10.1126/science.1085407. [DOI] [PubMed] [Google Scholar]
- Gershwin ME, Abplanalp H, Castles JJ, Ikeda RM, van der Water J, Eklund J, Haynes D. Characterization of a spontaneous disease of white leghorn chickens resembling progressive systemic sclerosis (scleroderma) J. Exp. Med. 1981;153:1640–1659. doi: 10.1084/jem.153.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MB, Klein I, Dekker A, Rodnan GP, Medsger TA., Jr. Thyroid disease in progressive systemic sclerosis increased frequency of glandular fibrosis and hypothyroidism. Ann. Intern. Med. 1981;95:431. doi: 10.7326/0003-4819-95-4-431. [DOI] [PubMed] [Google Scholar]
- Grimes PE, Sevall JS, Vojdani A. Cytomegalovirus DNA identified in skin biopsy specimen of patients with vitiligo. J. Am. Acad. Dermatol. 1996;35:21–26. doi: 10.1016/S0190-9622(96)90490-9. [DOI] [PubMed] [Google Scholar]
- Gruschwitz MS, Moormann S, Kroemer G, Sgonc R, Dietrich H, Boeck G, Gershwin ME, Boyd R, Wick G. Phenotypic analysis of skin infiltrates in comparison with peripheral blood lymphocytes, spleen cells and thymocytes in early avian scleroderma. J. Autoimmun. 1991;4:577–593. doi: 10.1016/0896-8411(91)90178-f. [DOI] [PubMed] [Google Scholar]
- Gruschwitz MS, Shoenfeld Y, Krupp M, Gershwin ME, Penner E, Brezinschek H-P, Wick G. Antinuclear antibody profile in UCD line 200 chickens: a model for progressive systemic sclerosis. Int. Arch. Allergy Immunol. 1993;100:307–313. doi: 10.1159/000236430. [DOI] [PubMed] [Google Scholar]
- Hala K. Hypothesis: immunogenetic analysis of spontaneous autoimmune thyroiditis in Obese strain (OS) chickens: a two-gene family model. Immunobiology. 1988;177:354–373. doi: 10.1016/S0171-2985(88)80019-6. [DOI] [PubMed] [Google Scholar]
- Hala K, Malin G, Dietrich H, Loesch U, Boeck G, Wolf H, Kaspers B, Geryk J, Falk M, Boyd RL. Analysis of the initiation period of spontaneous autoimmune thyroiditis (SAT) in Obese Strain (OS) of chickens. J. Autoimmunity. 1996;9:129–138. doi: 10.1006/jaut.1996.0016. [DOI] [PubMed] [Google Scholar]
- Hala K, Plachy J. Inbred lines of chickens. In: Lefkovits I, editor. Immunology Methods Manual. Academic Press Ltd; 1997. pp. 2285–2293. [Google Scholar]
- Hala K, Kubek A, Plachy J, Vasicek D. Expression of nonspecific esterase by thyroid follicular epithelium as a marker for the target organ susceptibility to immune system attack. Immunobiology. 2000;201:598–610. doi: 10.1016/S0171-2985(00)80077-7. [DOI] [PubMed] [Google Scholar]
- Harning R, Cui J, Bystryn JC. Relation between the incidence and the level of pigment cell antibodies and disease activity in vitiligo. J. Invest. Dermatol. 1991;97:1078–1080. doi: 10.1111/1523-1747.ep12492607. [DOI] [PubMed] [Google Scholar]
- Hasek M. Vegetative hybridization of animals by joint blood circulation during embryonal development. CS. Biol. 1953;2:265–277. doi: 10.1097/01.TP.0000101933.59274.B5. Czech. [DOI] [PubMed] [Google Scholar]
- Havenstein GB, Ferket PR, Scheideler SE, Larson BT. Growth, livability, and feed conversion of 1957 vs 1991 broilers when fed “typical” 1957 and 1991 broiler diets. Poultry Sci. 1994;73:1785–1794. doi: 10.3382/ps.0731785. [DOI] [PubMed] [Google Scholar]
- Haynes DC, Gershwin ME. Diversity of autoantibodies in avian scleroderma. An inherited fibrotic disease of White Leghorn chickens. J. Clin. Invest. 1984;73:1557–1568. doi: 10.1172/JCI111362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick AL. Vascular function in systemic sclerosis. Curr. Opin. Rheumatol. 2000;12:527–533. doi: 10.1097/00002281-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Holland MS, Mackenzie CD, Bull RW, Silva RF. Latent turkey herpesvirus infection in lymphoid, nervous, and feather tissues of chickens. Avian Dis. 1998;42:292–299. [PubMed] [Google Scholar]
- Ihn H. Scleroderma, fibroblasts, signalling, and excessive extracellular matrix. Curr. Rheumatol. Rep. 2005;7:156–162. doi: 10.1007/s11926-005-0069-9. [DOI] [PubMed] [Google Scholar]
- International Chicken Genome Sequencing Consortium Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- International Chicken Polymorphism Map Consortium A genetic variation map for chicken with 2.8 million single-nucleotide polymorphisms. Nature. 2004;432:717–722. doi: 10.1038/nature03156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez SA, Christner PJ. Murine animal models of systemic sclerosis. Curr. Opin. Rheumatol. 2002;14:671–680. doi: 10.1097/00002281-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann. Intern. Med. 2004;140:37–50. [PubMed] [Google Scholar]
- Kaiser P, Rothwell L, Vasicek D, Hala K. A role for IL-15 in driving the onset of spontaneous autoimmune thyroiditis. J. Immunol. 2002;168:4216–4220. doi: 10.4049/jimmunol.168.8.4216. [DOI] [PubMed] [Google Scholar]
- Kerje S, Carlborg Ö, Jacobsson L, Schütz K, Hartmann C, Jensen P, Andersson L. The two-fold difference in adult size between the red junglefowl and White Leghorn chickens is largely explained by a limited number of QTLs. Animal Genetics. 2003;34:264–274. doi: 10.1046/j.1365-2052.2003.01000.x. [DOI] [PubMed] [Google Scholar]
- Khoury EL, Bottazzo GF, de Carvalho LC, Wick G, Roitt IM. Predisposition to organ-specific autoimmunity in Obese strain (OS) chickens: Reactivity to thyroid, gastric, adrenal and pancreatic cytoplasmic antigens. Clin. Exp. Immunol. 1982;49:273–282. [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Yamakage A, Smith EA, LeRoy EC, Trojanowska M. Differential modulation of bFGF receptors by TGF-beta in adult skin, scleroderma skin, and newborn foreskin fibroblasts. J. Invest. Dermatol. 1992;99:201–205. doi: 10.1111/1523-1747.ep12650432. [DOI] [PubMed] [Google Scholar]
- Kissin EY, Korn JH. Fibrosis in scleroderma. Rheum. Dis. Clin. North Am. 2003;29:351–369. doi: 10.1016/s0889-857x(03)00018-8. [DOI] [PubMed] [Google Scholar]
- Kofler H, Kofler R, Wolf H, Wick G. Immunofluorescence studies on the codistribution of immune deposits and complement in the thyroid glands of Obese strain (OS) chickens. Immunobiology. 1983;164:390–401. doi: 10.1016/S0171-2985(83)80035-7. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Sundick RS, Schauenstein K, Hala K, Wick G. Analysis of lymphocytes infiltrating the thyroid gland of Obese strain chickens. J. Immunol. 1985;135:2452–2457. [PubMed] [Google Scholar]
- Kroemer G, Wick G. The role of interleukin-2 in autoimmunity. Immunol. Today. 1989;10:246–251. doi: 10.1016/0167-5699(89)90262-4. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Neu N, Kuehr T, Dietrich H, Faessler R, Hala K, Wick G. Immunogenetic analysis of spontaneous autoimmune thyroiditis in Obese strain chickens. Clin. Immunol. Immunopath. 1989;52:202–213. doi: 10.1016/0090-1229(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Kuehr T, Hala K, Dietrich H, Herold H, Wick G. Genetically determined target organ susceptibility in the pathogenesis of spontaneous autoimmune thyroiditis: aberrant expression of MHC-class II antigen and possible role of virus. J. Autoimmunity. 1994;7:13–25. doi: 10.1006/jaut.1994.1002. [DOI] [PubMed] [Google Scholar]
- Lally EV, Jimenez SA. Impotence in progressively systemic sclerosis. Ann. Intern. Med. 1981;95:150–153. doi: 10.7326/0003-4819-95-2-150. [DOI] [PubMed] [Google Scholar]
- Lamont SJ, Smyth JR., Jr. Effect of bursectomy on development of a spontaneous postnatal amelanosis. Clin. Immunol. Immunopathol. 1981;21:407–411. doi: 10.1016/0090-1229(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Dieterlen-Lievre F, Teillet MA, Ziller C. Interspecific chimeras in avian embryos. Methods Mol. Biol. 2000;135:373–386. doi: 10.1385/1-59259-685-1:373. [DOI] [PubMed] [Google Scholar]
- LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr., Rowell N, Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J. Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- Lockhart BR. Antioxidant state and extent of oxidative stress in melanocytes from autoimmune vitiliginous Smyth line and control chickens. University of Arkansas; Fayetteville: 2004. M. S. Thesis. [Google Scholar]
- Longenecker BM, Pazderka F, Gavora JS, Spencer JL, Ruth RF. Lymphoma induced by herpesvirus: resistance associated with a major histocompatibility gene. Immunogenetics. 1976;3:401–407. [Google Scholar]
- Malin G, Loesch U, Dietrich H, Geryk J, Falk M, Wick G, Hala K. Are virus and microbes involved in the induction of autoimmune thyroiditis in chickens? In: Davison TF, Bumstead N, Kaiser P, editors. Advances in avian immunology research. Carfax; Abingdon, UK: 1994. pp. 213–219. [Google Scholar]
- Mason PJ. Vitiligo: the psychosocial effects. Medsburg. Nurs. 1997;6:216–218. [PubMed] [Google Scholar]
- Miller MM, Bacon LD, Hala K, Hunt HD, Ewald SJ, Kaufman J, Zoorob R, Briles WE. Nomenclature for the chicken histocompatibility (B and Y) complex. 56. Immunogenetics. 2004;56:261–279. doi: 10.1007/s00251-004-0682-1. [DOI] [PubMed] [Google Scholar]
- Muir WM, Aggrey SE. Poultry Genetics, Breeding and Biotechnology. CABI Publishing; Wallingford, Oxon, UK: 2003. [Google Scholar]
- Nakagawa K, Harrison LC. The potential role of endogenous retroviruses in autoimmunity. Immunol. Rev. 1996;152:193–236. doi: 10.1111/j.1600-065x.1996.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Neu N, Hala K, Dietrich H, Wick G. Spontaneous autoimmune thyroiditis in obese strain chickens: genetic analysis of target organ abnormalities. Clin. Immunol. Immunopathol. 1985;37:397–405. doi: 10.1016/0090-1229(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Neu N, Hala K, Dietrich H, Wick G. Genetic background of spontaneous autoimmune thyroiditis in the Obese strain of chickens studied in hybrids with an inbred line. Int. Arch. Allergy Appl. Immunol. 1986;80:168–173. doi: 10.1159/000234047. [DOI] [PubMed] [Google Scholar]
- Nguyen VA, Sgonc R, Dietrich H, Wick G. Endothelial injury in internal organs of UCD 200 chickens, an animal model for systemic sclerosis. J. Autoimmun. 2000;14:143–149. doi: 10.1006/jaut.1999.0355. [DOI] [PubMed] [Google Scholar]
- Nordlund JJ, Lerner AB. Vitiligo-It is important. Arch. Dermatol. 1982;118:5–8. doi: 10.1001/archderm.118.1.5. [DOI] [PubMed] [Google Scholar]
- Ortonne JP, Bose SK. Vitiligo: Where do we stand? Pigment Cell Res. 1993;6:61–72. doi: 10.1111/j.1600-0749.1993.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Pardue SL, Fite KV, Bengston L, Lamont SJ, Boyle ML, III, Smyth JR., Jr. Enhanced integumental and ocular amelanosis following termination of cyclosporine administration. J. Invest. Dermatol. 1987;88:758–761. doi: 10.1111/1523-1747.ep12470453. [DOI] [PubMed] [Google Scholar]
- Park YK, Kim NS, Hann SK, Im S. Identification of autoantibody to melanocytes and characterization of vitiligo antigen in vitiligo patients. J. Dermatol. Sci. 1996;11:111–129. doi: 10.1016/0923-1811(95)00427-0. [DOI] [PubMed] [Google Scholar]
- Passeron T, Ortonne J-P. Physiopathology and genetics of vitiligo. J. Autoimmun. 2005;25:63–68. doi: 10.1016/j.jaut.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Penhale WJ, Ahmed SA. Animal model of human disease. Lymphocytic thyroiditis. Autoimmune thyroiditis in rats induced by thymectomy and irradiation. Am. J. Pathol. 1982;106:300–302. [PMC free article] [PubMed] [Google Scholar]
- Plachy J, Hala K. Comparative aspects of the chicken immunogenetics (review) Fol. Biologica (Praha) 1997;43:133–151. [PubMed] [Google Scholar]
- Plumlee BLX, Wang X, Erf GF. Interferon-gamma expression in feathers from vitiliginous Smyth line chickens. FASEB J. 2006 in press. [Google Scholar]
- Pope JE. Musculoskeletal involvement in scleroderma. Rheum. Dis. Clin. North Am. 2003;29:391–408. doi: 10.1016/s0889-857x(03)00017-6. [DOI] [PubMed] [Google Scholar]
- Prelog M, Scheidegger P, Peter S, Gershwin ME, Wick G, Sgonc R. Diminished TGF-β2 production leads to increased expression of a profibrotic procollagen alpha 2 type I mRNA variant in embryonic fibroblasts of UCD-200 chickens, a model for systemic sclerosis. Arthritis Rheum. 2005;52:1804–1811. doi: 10.1002/art.21109. [DOI] [PubMed] [Google Scholar]
- Reynaud CA, Bertocci B, Dahan A, Weill JC. Formation of the chicken B-cell repertoire: ontogenesis, regulation of Ig gene rearrangement, and diversification by gene conversion. Adv. Immunol. 1994;57:353–378. doi: 10.1016/s0065-2776(08)60676-8. [DOI] [PubMed] [Google Scholar]
- Rose NR, Bacon LD, Sundick RS. Genetic determinants of thyroiditis in the OS chickens. Transpl. Rev. 1976;31:264–285. doi: 10.1111/j.1600-065x.1976.tb01457.x. [DOI] [PubMed] [Google Scholar]
- Rose NR, Bonito R, Burek CL. Iodine: an environmental trigger of thyroiditis. Autoimmun. Rev. 2002;1:97–103. doi: 10.1016/s1568-9972(01)00016-7. [DOI] [PubMed] [Google Scholar]
- Rose S, Young MA, Reynolds JC. Gastrointestinal manifestations of scleroderma. Gastroenterol. Clin. North Am. 1998;27:563–594. doi: 10.1016/s0889-8553(05)70021-2. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N. Organ-specific autoimmune disease induced in mice by elimination of T cell subsets. V. Neonatal administration of cyclosporin A causes autoimmune disease. J. Immunol. 1989;142:471–480. [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N. Regulatory T cells in immunologic self-tolerance and autoimmune disease. Int. Rev. Immunol. 2005;24:211–226. doi: 10.1080/08830180590934976. [DOI] [PubMed] [Google Scholar]
- Sakkas LI, Platsoucas CD. Is systemic sclerosis an antigen-driven T cell disease? Arthritis Rheum. 2004;50:1721–1733. doi: 10.1002/art.20315. [DOI] [PubMed] [Google Scholar]
- Savolainen P, Fitzsimmons C, Arvestad L, Andersson L, Lundeberg J. ESTs from brain and testis of White Leghorn and red junglefowl: annotation, bioinformatic classification of unknown transcripts and analysis of expression levels. Cytogenet. Genome Res. 2005;111:79–87. doi: 10.1159/000085674. [DOI] [PubMed] [Google Scholar]
- Schauenstein K, Kroemer G, Boeck G, Ross K, Hala K, Wick G. T cell hyperreactivity in Obese strain (OS) chickens. Different mechanism operative in spleen and peripheral blood lymphocyte activation. Immunobiol. 1987;175:226–235. doi: 10.1016/S0171-2985(87)80031-1. [DOI] [PubMed] [Google Scholar]
- Schuurs AHWM, Dietrich H, Gruber J, Wick G. Effects of sex steroid analogs on spontaneous autoimmune thyroiditis in Obese strain chickens. Int. Arch. Allergy Immunol. 1992;97:337–344. doi: 10.1159/000236142. [DOI] [PubMed] [Google Scholar]
- Searle EA, Austin LM, Boissy YL, Zhoa H, Nordlund JJ, Boissy RE. Smyth chicken melanocyte autoantibodies: cross-species recognition, in vivo binding, and plasma membrane reactivity of the antiserum. Pigm. Cell Res. 1993;6:145–157. doi: 10.1111/j.1600-0749.1993.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Sgonc R, Dietrich H, Gershwin ME, Colombatti A, Wick G. Genomic analysis of collagen and endogenous virus loci in the UCD-200 and 206 lines of chickens, animal models for scleroderma. J. Autoimmun. 1995;8:763–770. doi: 10.1006/jaut.1995.0057. [DOI] [PubMed] [Google Scholar]
- Sgonc R, Gruschwitz MS, Dietrich H, Recheis H, Gershwin ME, Wick G. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J. Clin. Invest. 1996;98:785. doi: 10.1172/JCI118851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgonc R. The vascular perspective of systemic sclerosis: Of chickens, mice and men. Int. Arch. Allergy Immunol. 1999;120:169–176. doi: 10.1159/000024264. [DOI] [PubMed] [Google Scholar]
- Sgonc R, Wick G. What can we learn from an avian model for scleroderma? In: Shoenfeld Y, editor. The decade of autoimmunity. Elsevier; Amsterdam: 1999. pp. 209–217. [Google Scholar]
- Sgonc R, Dietrich H, Sieberer C, Wick G, Christner PJ, Jimenez SA. Lack of endothelial cell apoptosis in the dermis of tight skin 1 and tight skin 2 mice. Arthritis Rheum. 1999;42:581–584. doi: 10.1002/1529-0131(199904)42:3<581::AID-ANR28>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sgonc R, Gruschwitz MS, Boeck G, Sepp N, Gruber J, Wick G. Endothelial cell apoptosis in systemic sclerosis is induced by antibody-dependent cell-mediated cytotoxicity via CD95. Arthritis Rheum. 2000;43:2550–2562. doi: 10.1002/1529-0131(200011)43:11<2550::AID-ANR24>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Shresta S, Smyth JR, Jr., Erf GF. Profiles of pulp infiltrating lymphocytes at various times throughout feather regeneration in Smyth line chickens with vitiligo. Autoimmunity. 1997;25:193–201. doi: 10.3109/08916939708994728. [DOI] [PubMed] [Google Scholar]
- Simonsen M. Graft-versus-host reactions. Their natural history and applicability as tools of research. Progr. Allergy. 1962;6:349–467. [PubMed] [Google Scholar]
- Smyth JR, Jr., Boissy RE, Fite KV. The DAM chicken: a model for spontaneous postnatal cutaneous and ocular amelanosis. J. Hered. 1981;72:150–156. [PubMed] [Google Scholar]
- Smyth JR., Jr. The Smyth chicken: a model for autoimmune amelanosis. Poult. Biol. 1989;2:1–19. [Google Scholar]
- Smyth JR, Jr., McNeil M. Alopecia areata and universalis in the Smyth chicken model for spontaneous autoimmune vitiligo. J. Invest. Dermatol. Symp. Proc. 1999;4:211–215. doi: 10.1038/sj.jidsp.5640213. [DOI] [PubMed] [Google Scholar]
- Somes RG. International Registry of Poultry Genetics Stocks. Bull. 476, Storrs Agricultural Experiment Station, Iniv. of Connecticut, Storrs. 1988:98. [Google Scholar]
- Spritz RA. The genetics of generalized vitiligo and associated autoimmune diseases. J. Dermatol. Sci. 2006 doi: 10.1016/j.jdermsci.2005.10.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Sreekumar GP, Erf GF, Smyth JR., Jr. 5-Azacytidine treatment induces autoimmune vitiligo in the parental control strains of the Smyth line chicken model for autoimmune vitiligo. Clin. Immun. Immunopathol. 1996;81:136–144. doi: 10.1006/clin.1996.0169. [DOI] [PubMed] [Google Scholar]
- Sreekumar GP, Smyth JR, Jr., Ambady S, Ponce de Leon FA. Analysis of the effect of endogenous viral genes in the Smyth line chicken model for autoimmune vitiligo. Am. J. Pathol. 2000;156:1099–1107. doi: 10.1016/S0002-9440(10)64978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar GP, Smyth JR, Jr., Ponce de Leon FA. Molecular characterization of the Smyth chicken sublines and their parental controls by RFLP and DNA fingerprint analysis. Poult. Sci. 2001;80:1–5. doi: 10.1093/ps/80.1.1. [DOI] [PubMed] [Google Scholar]
- Steen VD. Scleroderma renal crisis. Rheum. Dis. Clin. North Am. 2003;29:315–333. doi: 10.1016/s0889-857x(03)00016-4. [DOI] [PubMed] [Google Scholar]
- Sundick RS, Herdegen D, Brown TR, Dhar A, Bagchi N. Thyroidial iodine metabolism in Obese strain chickens prior to immune-mediated damage. J. Endocrinol. 1991;128:239–244. doi: 10.1677/joe.0.1280239. [DOI] [PubMed] [Google Scholar]
- Svoboda J. Rous sarcoma virus. Intervirology. 1986;26:1–60. doi: 10.1159/000149682. [DOI] [PubMed] [Google Scholar]
- Tirunagaru VG, Sofer L, Cui J, Burnside J. An expressed sequence tag database of T-cell enriched activated chicken splenocytes: sequence analysis of 5251 clones. Genomics. 2000;66:144–151. doi: 10.1006/geno.2000.6189. [DOI] [PubMed] [Google Scholar]
- Van de Water J, Haapanen L, Boyd R, Abplanalp H, Gershwin ME. Identification of T cells in early dermal lymphocytic infiltrates in avian scleroderma. Arthritis Rheum. 1989;32:1031–1040. doi: 10.1002/anr.1780320813. [DOI] [PubMed] [Google Scholar]
- Van de Water J, Boyd R, Wick G, Gershwin ME. The immunologic and genetic basis of avian scleroderma, an inherited fibrotic disease of line 200 chickens. Int. Rev. Immunol. 1994;11:273–282. doi: 10.3109/08830189409061732. [DOI] [PubMed] [Google Scholar]
- Van de Water J, Jimenez SA, Gershwin ME. Animal models of scleroderma: contrasts and comparisons. Int. Rev. Immunol. 1995;12:201–216. doi: 10.3109/08830189509056713. [DOI] [PubMed] [Google Scholar]
- Van Laere AS, Nguyen M, Braunschweig M, Nezer C, Collette C, Moreau L, Archibald AL, Haley CS, Buys N, Andersson G, Georges M, Andersson L. Positional identification of a regulatory mutation in IGF2 causing a major QTL effect on muscle growth in the pig. Nature. 2003;425:832–836. doi: 10.1038/nature02064. [DOI] [PubMed] [Google Scholar]
- Vasicek D, Vasickova K, Kaiser P, Drozenova R, Citek J, Hala K. Analysis of genetic regulation of chicken spontaneous autoimmune thyroiditis, an animal model of human Hashimotós thyroiditis. Immunogenetics. 2001;53:776–785. doi: 10.1007/s00251-001-0388-6. [DOI] [PubMed] [Google Scholar]
- Wang X, Smyth JR, Jr., Bersi TK, Erf GF. MHC class II expression by T cells present in growing feathers from Smyth line chickens with vitiligo. Poult. Sci. 1998;77(Suppl. 1):26. [Google Scholar]
- Wang X, Parcells MS, Erf GF. Marek’s disease virus infection of cultured chicken melanocytes. Pigm. Cell Res. 2000;13:217. [Google Scholar]
- Wang X, Erf GF. Melanocyte-specific cell mediated immune response in vitiliginous Smyth line chickens. J. Autoimmun. 2003;21:149–160. doi: 10.1016/s0896-8411(03)00087-8. [DOI] [PubMed] [Google Scholar]
- Wang X, Erf GF. Apoptosis in feathers of Smyth line chickens with autoimmune vitiligo. J. Autoimmun. 2004;22:21–30. doi: 10.1016/j.jaut.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nat. Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- White B. Immunopathogenesis of systemic sclerosis. Rheum. Dis. Clin. North Am. 1996;22:695–708. doi: 10.1016/s0889-857x(05)70296-9. [DOI] [PubMed] [Google Scholar]
- Wick G, Kite JH, Cole RK, Witebsky E. Spontaneous thyroiditis in the Obese strain of chickens. III. The effect of bursectomy on the development of the disease. J. Immunol. 1970a;104:45–54. [PubMed] [Google Scholar]
- Wick G, Kite JH, Witebsky E. Spontaneous thyroiditis in the Obese strain of chickens. IV. The effect of thymectomy and thymo-bursectomy on the development of the disease. J. Immunol. 1970b;104:54–62. [PubMed] [Google Scholar]
- Wick G, Graf J. Electron microscopic studies in chickens of the Obese strain with spontaneous hereditary autoimmune thyroiditis. Lab. Invest. 1972;27:400–411. [PubMed] [Google Scholar]
- Wick G, Steiner R. Bursectomy and thymectomy of Obese strain (OS) chickens with spontaneous autoimmune thyroiditis and simultaneous experimental and allergic encephalomyelitis. J. Immunol. 1972;109:1031–1035. [PubMed] [Google Scholar]
- Wick G, Gundolf R, Hala K. Genetic factors in spontaneous autoimmune thyroiditis in OS chickens. J. Immunogenet. 1979;6:177–183. doi: 10.1111/j.1744-313x.1979.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Wick G, Boyd R, Hala K, de Carvalho L, Kofler R, Mueller PU, Cole RK. The Obese Strain (OS) of chickens with spontaneous autoimmune thyroiditis: Review of recent data. Curr. Topics Microbiol. Immunol. 1981;91:109–128. doi: 10.1007/978-3-642-68058-8_5. [DOI] [PubMed] [Google Scholar]
- Wick G, Hala K, Wolf H, Boyd RL, Schauenstein K. Distribution and functional analysis of B-L/Ia positive cells in the chicken: expression of B-L/Ia antigens on thyroid epithelial cells in spontaneous autoimmune thyroiditis. Mol. Immunol. 1984;21:1259–1265. doi: 10.1016/0161-5890(84)90019-1. [DOI] [PubMed] [Google Scholar]
- Wick G, Kroemer G, Neu N, Faessler R, Ziemiecki A, Mueller RG, Ginzel M, Beladi I, Kuehr T, Hala K. The multi-factorial pathogenesis of autoimmune disease. Immunol. Lett. 1987;16:249–257. doi: 10.1016/0165-2478(87)90154-4. [DOI] [PubMed] [Google Scholar]
- Wick G, Brezinschek H-P, Hala K, Dietrich H, Wolf H, Kroemer G. The Obese strain (OS) of chickens. An animal model with spontaneous autoimmune thyroiditis. Adv. Immunol. 1989;47:433–500. doi: 10.1016/s0065-2776(08)60666-5. [DOI] [PubMed] [Google Scholar]
- Wick G, Hu Y, Schwarz S, Kroemer G. Immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in autoimmune diseases. Endocrine Rev. 1993;14:539–563. doi: 10.1210/edrv-14-5-539. [DOI] [PubMed] [Google Scholar]
- Wick G, Cole RL, Dietrich H, Maczek C, Müller P-U, Hala K. The Obese strain of chickens with spontaneous autoimmune thyroiditis as a model for Hashimoto disease. In: Cohen IR, Miller A, editors. Autoimmune Disease Models – A Guidebook. Academic Press; London: 1994. pp. 107–122. [Google Scholar]
- Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu. Rev. Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- Wijesekera DH. Antioxidant state and extent of oxidative stress in autoimmune vitiliginous Smyth line and control chickens. University of Arkansas; Fayetteville: 2004. M. S. Thesis. [Google Scholar]
- Wilson TJ, Van de Water J, Mohr FC, Boyd RL, Ansari A, Wick G, Gershwin ME. Avian scleroderma: evidence for qualitative and quantitative T cell defects. J. Autoimmun. 1992;5:261–276. doi: 10.1016/0896-8411(92)90142-d. [DOI] [PubMed] [Google Scholar]
- Worda M, Sgonc R, Dietrich H, Niederegger H, Sundick RS, Gershwin ME, Wick G. In vivo analysis of the apoptosis-inducing effect of anti-endothelial cell antibodies in systemic sclerosis by the chorionallantoic membrane assay. Arthritis Rheum. 2003;48:2605–2614. doi: 10.1002/art.11179. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A, Kroemer G, Mueller RG, Hala K, Wick G. Ev22, a new endogenous avian leukosis virus locus found in chickens with spontaneous autoimmune thyroiditis. Arch. Virol. 1988;100:267–271. doi: 10.1007/BF01487689. [DOI] [PubMed] [Google Scholar]