Abstract
Postural instability occurs in HIV infection, but quantitative balance tests in conjunction with neuroimaging are lacking. We examined whether infratentorial brain tissue volume would be deficient in nondemented HIV-infected individuals and whether selective tissue deficits would be related to postural stability and psychomotor speed performance. The 123 participants included 28 men and 12 women with HIV infection without dementia or alcohol use disorders, and 40 men and 43 women without medical or psychiatric conditions. Participants completed quantitative balance testing, Digit Symbol test, and a test of finger movement speed and dexterity. An infratentorial brain region, supratentorial ventricular system, and corpus callosum were quantified with MRI-derived atlas-based parcellation, and together with archival DTI-derived fiber tracking of pontocerebellar and internal and external capsule fiber systems, brain measures were correlated with test performance. The tissue ratio of the infratentorium was ~3% smaller in the HIV than control group. The HIV group exhibited performance deficits in balancing on one foot, walking toe-to-heel, Digit Symbol substitution task, and time to complete all Digit Symbol grid boxes. Total infratentorial tissue ratio was a significant predictor of balance and Digit Symbol scores. Balance scores did not correlate significantly with ventricular volumes, callosal size, or internal or external capsule fiber integrity but did so with indices of pontocerebellar tract integrity. HIV-infected individuals specifically recruited to be without complications from alcohol use disorders had pontocerebellar tissue volume deficits with functional ramifications. Postural stability and psychomotor speed were impaired and attributable, at least in part, to compromised infratentorial brain systems.
Keywords: MRI, DTI, Cerebellum, Pons, Vermis, Gait, Postural stability, HIV infection, Motor speed, Dexterity, Falling, Ataxia
Introduction
Human immunodeficiency virus (HIV) infection causes mild to severe impairment of cognitive and motor abilities in about one-third of people infected (for reviews Cysique and Brew 2009; Ellis et al. 2009; Woods et al. 2009). These HIV-Associated Neurocognitive Disorders (HAND) have reduced in severity with the introduction of medications for successful viral suppression, but the prevalence of milder forms of asymptomatic neurocognitive impairment (ANI) remains substantial and is estimated to be between 5–20% (for reviews, Ellis et al. 2009; Goodkin et al. 2001; Woods et al. 2009) even in patients with longstanding viral suppression (Simioni et al. 2010). The functional consequences for activities of everyday living can be insidious and disruptive (Gorman et al. 2009), underscoring the relevance of their detection with quantitative assessment and identification of their neural underpinnings through in vivo neuroimaging examination.
Prominent in the constellation of compromised function are movement disorders (e.g., Mattos et al. 2002; Nath et al. 1987) involving signs of extrapyramidal dysfunction manifest as slowed psychomotor speed, impaired manual dexterity (e.g., Fama et al. 2007; Gorman et al. 2009; Heaton et al. 1995; Maki et al. 2009; Sacktor et al. 2003; Sassoon et al. 2007; Woods et al. 2009), and disturbance of gait and balance (e.g., Fama et al. 2007; Robertson et al. 2006). Motor slowing has been attributed, for example, to disturbance of frontostriatal systems, affected early in the course of HIV infection (Chang et al. 2000; Paul et al. 2007; Sclar et al. 2000; von Giesen et al. 2001), or to diffuse white matter abnormalities, noted as hyperintense signal on MRI (Archibald et al. 2004; Ernst et al. 1999; Heindel et al. 1994). Commonly, however, the attribution of functional impairment to a specific brain abnormality is assumed but not directly tested within the same study. Examples of exceptions are two DTI fiber tracking studies: the first reported a relation between compromise of callosal fibers of the splenium but not genu as selectively correlating with tests of finger speed and dexterity in HIV-infected individuals (Pfefferbaum et al. 2007); the second derived global fractional anisotropy tractography maps in HIV-infected individuals and found that lower anisotropy, an index of compromised fiber microstructure, correlated with poorer scores on a number of measures of cognitive and motor functions, including finger tapping rates and speed of switching attentional focus (Tate et al. 2010).
Disturbance of gait and balance is recognized as a feature of HIV-related motor compromise and was initially noted in case series (Castellanos et al. 1994; Graus et al. 1990; Yebra et al. 1988). Studies of static posturography using a force platform revealed longer sway paths in ~25% of asymptomatic HIV patients compared with uninfected controls, although the authors discounted the clinical relevance of this finding (Arendt et al. 1994). In contrast with this conclusion, another study examined postural reflexes in response to force platform perturbation and found that neurologically intact HIV-infected patients displayed normal postural reflexes when responding to predictable but not to unpredictable perturbations to stability; these authors considered this abnormality as a precursor of more serious postural stability problems with advancing involvement of frontostriatal dysfunction (Beckley et al. 1998; also see Dellepiane et al. 2005). This interpretation was consistent with the observation that the incidence of postural instability measured as sway during quiet standing increased in advanced stages of infection (Trenkwalder et al. 1992).
To meet the challenge of simple quantitative assessment without extensive physiological recording systems of gait, at least in terms of velocity, the Timed Gait test was developed, requiring patients to walk a prescribed distance quickly (Price and Sidtis 1993). A large-scale study (N= 1,549) using the Time Gait test found a systematic relation between slowed gait and increasing HIV dementia stage (Robertson et al. 2006). Using another simple yet quantitative test of balance, we observed a deficit of one standard deviation in nondemented HIV-infected men as they attempted to balance on one foot with their eyes open (Fama et al. 2007). The fact that these HIV-infected men were not demented underscores the utility of quantitative assessment in identifying specific deficits in asymptomatic patients. A multi-site, longitudinal exploration of HIV dementia revealed progressive worsening of motor signs of gait instability, slurred speech, and clumsiness along with cerebellar atrophy noted on MRI or CT, and ultimately neuropathological evidence of granular cell layer degeneration of the cerebellum and axonal swelling of the brain stem (Tagliati et al. 1998). Nonetheless, quantitative, controlled investigation of postural stability with concurrently collected MRI assessment of the condition of the cerebellum or pons is lacking in groups of nondemented HIV-infected individuals. Also lacking are studies that have explicitly selected HIV infected groups to be without history of alcohol use disorder, which is a highly prevalent concomitant of HIV infection (Galvan et al. 2002; Samet et al. 2007) and a common source of ataxia associated with cerebellar and other brainstem pathology (for review, Sullivan et al. 2010a). A recent structural MRI study found small but reliable volume deficits of 3–6% in the vermis of an HIV-infected group compared with a healthy control group (Klunder et al. 2008). Although the extent of the posterior vermis shrinkage correlated with severity of depressive symptoms, relations with motor or balance testing were not presented.
Here, we used structural MRI and quantitative tests of postural stability and finger movement and psychomotor speed and dexterity to examine whether an infratentorial brain volume, comprising the pons, cerebellar hemispheres, and vermis, would be marked by regional tissue shrinkage in HIV-infected men and women compared with healthy controls, and whether tissue deficits would be related to functional impairment in postural stability or psychomotor speed. As a further test of the selectivity of a pontocerebellar substrate of postural instability and psychomotor slowing, we sought correlations between these motor measures and previously reported regional brain volumes of the ventricular system and corpus callosum (Pfefferbaum et al. 2006) and white matter fiber integrity status of the internal and external capsules, pontocerebellar tracts, and cerebellar hemisphere fibers (Pfefferbaum et al. 2010). Because alcohol use disorders are highly prevalent in HIV-infected individuals (Galvan et al. 2002; Samet et al. 2007) and because alcohol abuse exerts a deleterious effect on gait, balance, and cerebellar integrity (Harper and Kril 1990; Sullivan et al. 2000; Sullivan et al. 2010b; Victor et al. 1989), the HIV-infected group was selected to be free of alcohol use disorder.
Methods
Subjects
Participants included 28 men and 12 women (mean age 41 years) with HIV infection; controls included 40 men and 43 women (mean age 44 years) without medical or psychiatric conditions. Motor data from smaller samples of these groups appeared in other publications (Fama et al. 2007; Sullivan et al. 2010b), and MRI data of the control group were used in another study (Sullivan et al. 2010b).
Means±standard deviations (S.D.) of demographic variables for the control and HIV-infected men and women are presented in Table 1 as are the statistical results of the group comparisons on these variables. On average, the groups had about two or more years of post-high school education, although the HIV-infected men and women had significantly fewer years of education than the controls. The groups did not differ significantly, however, in socioeconomic status (Hollingshead 1975), handedness (Crovitz and Zener 1962), or body mass index (Table 1). The HIV-infected group endorsed more depressive symptoms on the Beck Depression Inventory II (Beck et al. 1996), had lower Global Assessment of Functioning taken from the SCID interview, and had a higher incidence of smoking than controls. On average, the estimated HIV infection onset occurred in their 30 s for men and women; no participant scored below 80 on the Karnofsky scale (Karnofsky 1949), and all but 3 scored 90 or 100. Of the 28 HIV-infected men, 8 had had an AIDS-defining event (CD4+ count < 200 or AIDS-related infection), and 2 of the 12 women had such a history (sex-by-diagnosis χ2=.159, n.s.). Medication status was characterized as HAART (N=25) [e.g., two nucleoside reverse transcriptase inhibitors (NRTIs) and a protease inhibitor, or two NRTIs and a nonnucleoside reverse transcriptase inhibitor (NNRTI)], non-HAART (N=6), or none (N=9).
Table 1.
Subject characteristics of the four study groups: Mean(±SD) or frequency count
| Control men | HIV-infected men | Control women | HIV-infected women | ANOVA p-value Follow-up t-tests | |
|---|---|---|---|---|---|
| Sample size | 40 | 28 | 43 | 12 | |
| Age | 41.9 (10.43) | 40.3 (10.14) | 46.0 (8.90) | 45.7 (8.13) | n.s. |
| Education (years) | 15.9 (2.27) | 14.1 (3.05) | 15.3 (2.00) | 13.8 (2.67) | p=.0061 CM=CW>HM=HW |
| Socioeconomic status (SES)a | 26.6 (14.97) | 33.2 (13.73) | 28.0 (10.97) | 35.5 (13.25) | n.s. |
| Handednessb | 22.8 (9.27) | 26.2 (12.52) | 23.4 (14.79) | 23.1 (7.74) | n.s. |
| Body mass index | 26.9 (4.83) | 25.4 (3.34) | 24.7 (4.49) | 26 (3.16) | n.s. |
| Current smokers | 4 of 40 | 12 of 28 | 0 of 43 | 2 of 10 | p=.0001 CW<CM=HW<HM |
| Beck depression inventory | 2.08 2.33 |
10.5 (8.33) | 2.9 3.08 |
12.8 (9.26) | p=.0001 CM=CW<HM=HW |
| Global assessment of function | 79.3 (10.25) | 72.3 (11.15) | 84.3 (6.42) | 72.2 (12.63) | p=.0009 CM=CW>HM=HW |
| Lifetime alcohol consumption (kg) | 58.9 (76.84) | 60.5 (54.68) | 26.3 (31.99) | 50.2 (62.99) | p=.0364 |
| HIV-infection onset | – | 32 (7.78) | – | 36.7 (3.45) | n.s. |
| Karnofsky rating | – | 97.1 (6.00) | – | 92.5 (1.79) | p=.0325 |
| CD4+ count | – | 537.4 (258.97) | – | 583.4 (103.55) | n.s. |
| Viral load | – | 13597.6 (4654.88) | – | 4609.7 (3226.36) | n.s. |
Lower scores indicate higher SES
Right handedness=14–32; left handedness=50–70
p≤.01 required for statistical significance
As determined from the SCID interview and a time-back calendar history of alcohol drinking, none of the subjects had a history of alcohol dependence or abuse, none of these subjects drank even near an alcoholic level (>80 g alcohol daily for men and >60 g daily for women for at least 30 days). Consumption of 80 g/day of alcohol in men, the equivalent of 6 standard drinks, has been widely identified as a cut-off for greatly increased risk of developing liver disease (Savolainen et al. 1993) and various forms of cancer. The lower level for women (for body weight and fat:water content) of 60 g or 4.5 drinks a day recognizes the greater sensitivity of women than men to alcohol. More recent cut-offs provided by NIAAA for at-risk or harmful drinking are more than 4 drinks on any day or 14 per week for men and more than 3 drinks on any day or 7 per week for women <http://rethinkingdrinking.niaaa.nih.gov>. None of the patients or controls reported drinking at that level over a sustained period.
To determine whether HIV-infected participants met criteria for HIV-associated neurocognitive disorder (HAND) (Antinori et al. 2007; Woods et al. 2009) or for AIDS Dementia Complex (ADC) Stage 1 or beyond (Brew 1999), we used the computer-based MicroCog neuropsychological test battery (Powell et al. 1993) to assess attention (digits forward and backward), working memory (alphabet tracking, math computation), and episodic memory (immediate and delayed recall of stories); in addition, language was assessed with letter fluency (FAS) (Benton and Hamsher 1976), and psychomotor speed was assessed with Digit Symbol Substitution test (Wechsler 1981) and alternated finger tapping (Sullivan et al. 2001). MicroCog scores were available for 37 and FAS and Digit Symbol scores were available for 38 participants; scores for each measure were adjusted for age and education based on our laboratory controls. The HIV -infected group performed within 1 SD on all measures. Consideration of individual performance indicated that no HIV-infected patient achieved scores below 2 SD of the control mean on 2 functional domains, although one HIV-infected man had a fluency score of −2.075 and an HIV-infected woman had an alternated finger tapping score of −2.05. No HIV-infected participant was unable to perform activities of daily living. Of those with these cognitive and motor scores, none met criteria for HAND as determined with these measures, and no participant was at ADC Stage 1 or beyond.
MRI acquisition protocol
MR imaging was conducted on a General Electric (Waukesha, MI) 1.5 T whole body clinical system. Images used for quantification were acquired with a volumetric SPoiled Gradient Recalled (SPGR) sequence (94, 2 mm thick slices; TR/TE=25/5 ms, flip angle=30°, matrix=256×192) for morphometry and a late-echo fast spin-echo (FSE) sequence (94, 2 mm thick slices; TR/TE=11050/98 ms, matrix=256×192) for automated fluid-tissue delineation. Before any participant was included in the study, their images were read by a clinical neuroradiologist to identify space occupying lesions or other dysmorphology indicative of neuropathology that could interfere with morphometric analysis. In addition, all images were reviewed to identify studies of quality too poor for quantification. Any participants showing dysmorphology or poor scan quality were excluded from this analysis.
MRI quantification of infratentorial tissue volume
A structural mask of cerebellum and pons was defined on the SRI24 atlas (Rohlfing et al. 2010) [http://nitrc.org/projects/sri24/] by manual outlining. This mask was propagated to the FSE images of all subjects via registrations from the SRI24 atlas to the subject SPGR images, concatenated with the transformation from the subject SPGR to subject FSE image space. The resulting subject-space masks were then used to extract cerebellum and pons from each subject. The extracted cerebellum and pons FSE data for 66 normal controls were then used to construct a study-specific high-resolution template of pons and cerebellum for further analysis. To this end, the 66 pons and cerebellum images were co-registered using the same template-free group-wise registration algorithm that we previously applied to generate the SRI24 whole-brain atlas (Rohlfing et al. 2010). This software is made available as part of the Computational Morphometry Toolkit (CMTK) [http://nitrc.org/projects/cmtk/].
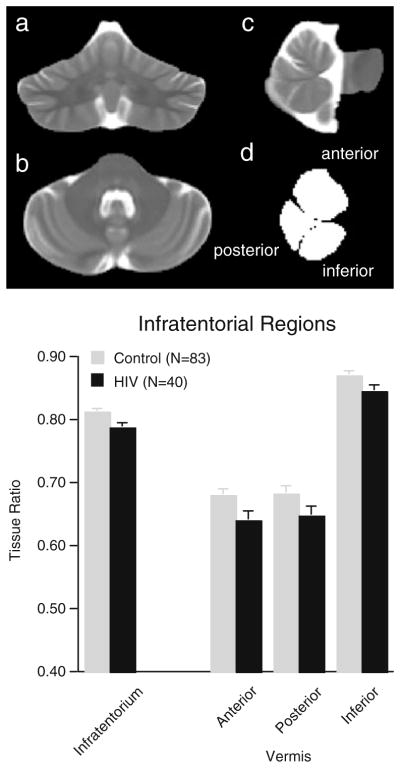
The resulting template, which had a resolution of 0.9375 mm isotropic pixel size, was used for manual delineation of the borders of the infratentorial volume, which included the pons, cerebellar hemispheres, vermis, and CSF-filled fissures, cisterns, and fourth ventricle. Further manual delineation divided the vermis into three regions (anterior, posterior, and inferior sectors) on 9 midsagittal slices (Fig. 1).
Fig. 1.
Coronal a, axial b, and sagittal c slices through the infratentorial volume from the FSE group template; d sagittal view of the region-of-interest masks for the superior, posterior, and inferior sectors of the cerebellar vermis. Bar graphs: Mean±SEM of the tissue volume ratios of the four regions of interest for the four subject groups
Each subject’s extracted pons and cerebellum FSE image was finally registered via nonrigid image registration (Rohlfing and Maurer 2003) to the study-specific template, and the registration transformation was used to project the infratentorial and vermis masks from the template back into each subject’s native space for fluid-tissue segmentation. Computation and application of the registration transformations were done using inhouse software, which is also available as part of the CMTK software package.
To reduce differences attributable to individual variability in intracranial volumes, especially between men and women, regional tissue volumes were expressed as ratios (or percentages) of the amount of tissue to tissue plus CSF in each region of interest, i.e., [tissue/(tissue+CSF)] of the region.
Volumetry of the ventricular system and corpus callosum
These acquisition and analysis procedures appear in Pfefferbaum et al. (Pfefferbaum et al. 2006). In short, these measurements were made using automatic atlas-based parcellation of the aforementioned SPGR images acquired at 1.5 T. To create the parcellation template, the ventricular system and corpus callosum were manually delineated on the SRI24 atlas (Rohlfing et al. 2010). The ventricular system was divided into frontal, body, and occipital regions, and the corpus callosum was divided geometrically to correspond to six, anatomically based sectors (after Pandya and Seltzer 1986). The SRI24 atlas was automatically registered to the SPGR data of each subject using the same nonrigid image registration software also used for registering pons and cerebellum between subjects and atlas. The transformations between subject SPGR data and SRI24 atlas were then applied to the ventricular system and corpus callosum template in SRI24 space to obtain the corresponding segmentations of these structures in each subject. The volumes of each region in each subject were then expressed as standardized Z-scores, corrected for normal variation in intracranial volume and age based on the control group of 121 men and women used in our earlier report (Pfefferbaum et al. 2006).
DTI acquisition protocol
The DTI acquisition and fiber tracking analysis procedures appeared previously (Pfefferbaum et al. 2009; Sullivan et al. 2010c). In short, 1.5 T DTI data were collected contemporaneously with the structural data. Diffusion-weighted imaging was performed with the same slice location as the dual-echo FSE described above, using a single shot spin-echo echo-planar imaging technique (47 contiguous, 4 mm thick slices, TR/TE=10,000/103 ms, matrix=128×128, in-plane resolution=1.875 mm2, b-value= 860 s/mm2). Diffusion was measured along six non-collinear directions (6 NEX) with alternating signs to minimize the need to account for cross-terms between imaging and diffusion gradients (Bodammer et al. 2004). For each slice, six images with no diffusion weighting (b=0 s/mm2) were also acquired.
DTI models water diffusion within each voxel as a zero-mean multivariate Gaussian distribution, which is parameterized by its symmetric 3×3 covariance matrix, the diffusion tensor. The diffusion-weighted data measured with respect to each of the diffusion gradient directions exhibits a signal attenuation relative to b=0 data acquired without diffusion gradients. The logarithm of this signal attenuation is a linear combination of the six unique elements of the diffusion tensor, their weights determined by the direction of the diffusion gradients. Computing a least squares solution for this over-determined linear equation system independently at each voxel yielded the elements of the diffusion tensor for that voxel.
DTI fiber tracking
We applied fiber tracking to white matter systems involving motor systems, including the internal and external capsules, pontocerebellar tracts, and cerebellar hemisphere fibers. Fiber tracking was performed with the software by Gerig et al. (2005) based on the method of Mori and colleagues (Mori and van Zijl 2002; Xue et al. 1999; Xu et al. 2002). This approach requires the identification of a group of “source” voxels from which streamlines (i.e., fiber tracts) are initiated and propagated throughout the brain following the orientation of the principal eigenvector of the diffusion tensor at each point along the fiber. To describe a particular fiber bundle selectively, a group of “target” voxels is also identified, and only the streamlines that pass through the target are retained. Thus, each identified fiber bundle is required to originate in a source voxel and pass through at least one target voxel. Fiber tract targets were identified on a laboratory standard group average FA image; sources were planes perpendicular to the orientation of the fiber tracts.
The tensor data as well as targets and sources were passed to the fiber tracking routine in native space, thus avoiding the need for tensor field resampling and tensor reorientation. Fiber tracking parameters included white matter extraction threshold (minimum FA) of .17, minimum fiber length of 37.5 mm, maximum fiber length of 187.5 mm, fiber tracking threshold of .125 (terminates a fiber if the vector field in the local neighborhood is too noisy), and angle of maximum deviation of .80 (~37°Δ maximum angle between successive fiber segments).
DTI measures of white matter fiber integrity of striatal and cerebellar motor systems
Examples of the internal and external capsules, pontocerebellar tracts, and cerebellar hemisphere fiber tracts are presented in Fig. 2. DTI metrics quantifying the condition of the tracts were fractional anisotropy (FA), longitudinal or axial diffusivity (λL), and transverse or radial diffusivity (λT). These measures are computed from the three eigenvalues, λ1≥λ2≥λ3, of the diffusion tensor. FA quantifies the degree to which diffusion exhibits a predominant orientation: the more linear and organized the fibers in a region, the higher the FA. Where diffusion has a preferential orientation, the largest eigenvalue of the diffusion tensor is referred to as longitudinal, or axial, diffusivity (λL=λ1), which has been shown to be an indicator of axonal integrity. The mean of the two smaller eigenvalues of the diffusion tensor is referred to as transverse, or radial, diffusivity (λT = [λ2 + λ3]/2), which is considered a marker of myelin integrity (Song et al. 2002; Sun et al. 2006). Higher FA and lower diffusivity in white matter are in the direction of higher microstructural integrity. These measures were expressed as standardized Z-scores, corrected for normal variation in age, based on the 120 controls used in our earlier report (Pfefferbaum et al. 2007).
Fig. 2.
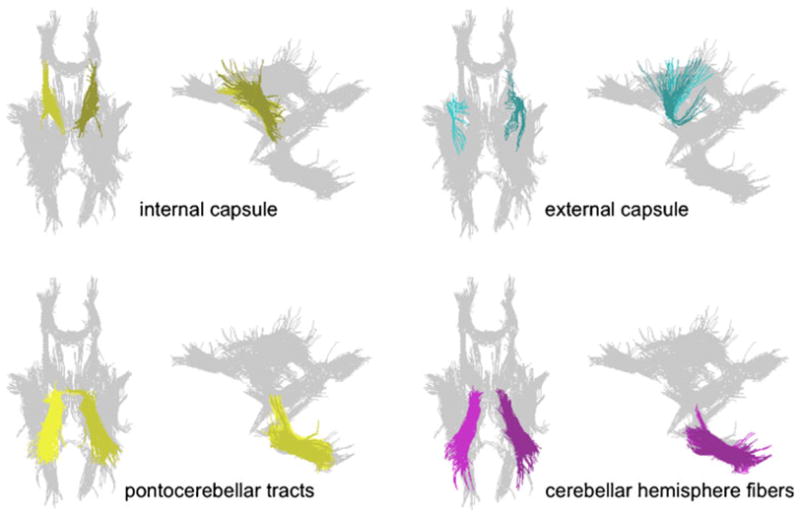
Examples of fiber tracts for the internal capsule, external capsule, pontocerebellar tracts, and cerebellar hemisphere fibers
Motor tests
Test of gait and balance
Most participants completed the Walk-a-Line Battery of gait and balance (Fregly et al. 1972), which consisted of three tasks, each performed first with eyes open and then eyes closed, and always with arms folded across the chest. Each condition was tested twice, unless a perfect score was achieved on the initial trial, in which case the subject received full credit on that condition. The conditions were as follows: Stand Heel-to-Toe for 60 s. (maximum score=120 s.), a measure sensitive to proprioceptive sensitivity of the lower extremities; Walk Heel-to-Toe for 10 steps (maximum score=20 steps); and Stand on One Foot, first the right foot and then the left, each for 30-sec. trials (maximum score=60 s. for each foot).
Digit symbol task
This subtest of the Wechsler Adult Intelligence Scale (Wechsler 1981) assesses cognitive-motor speed and required speeded scribing of symbols matched to single-digit numbers. Performance was scored in two ways: number of correct symbols written in boxes in 90 s. and time (in seconds) to complete filling in all 93 boxes (Sassoon et al. 2007).
Fine finger movement test
Subjects turned a knurled rod with their forefinger and thumb, unimanually and then bimanually (Cooper et al. 1991; Corkin et al. 1986). Three 30-second trials for each condition were administered. For correlational analyses, we reduced the data to two summary scores: the mean of the left and right unimanual conditions and the mean of the left and right bimanual conditions.
Statistical analysis
Regional brain volume ratios and balance testing scores were subjected to a series of two-way (diagnosis-by-sex) analyses of variance (ANOVAs). Follow-up t-tests identified specific group differences in brain metrics or performance. Correlations between regional brain volumes or DTI metrics and balance scores or disease factors were tested with Pearson correlations. Multiple-regression analysis was used to test for specificity of brain structure-function relationships. Given the multiple comparisons, we considered p≤.01 as significant.
Results
Group differences in regional infratentorial volumes
A diagnosis-by-sex ANOVA for the tissue ratio of the total infratentorium yielded a significant group effect (F(1,119)=6.655, p=.0111) but neither a sex effect (F(1,119)=.434, p=.51) nor group-by-sex interaction (F(1,119)=.589, p=.44). On average, this tissue ratio was ~3% smaller in the HIV than control group. A 2 diagnosis-by-3 region analysis of the vermis yielded group (F(1,121)=4.693, p=.0322) and region (F(2,242)= 677.197, p=.0001) effects but no group-by-region interaction (F(2,242)=.495, p=.61), indicating that the volume deficits, which ranged from 3% to 6% in the HIV group, were not disproportionately different across the three vermian sectors from the control group’s profile of vermian sector ratios.
Group differences in balance, dexterity, and speeded motor performance
Group differences in performance were examined with diagnosis-by-sex ANOVAs. Descriptive and group statistics are presented in Table 2.
Table 2.
Group mean±SE and diagnosis-by-sex ANOVA results for cognitive and motor tests
| Control
|
HIV-infected
|
ANOVA Diagnosis effecta |
|||||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | ||||
| Balance test measures | |||||||
| Stand Heel-to-Toe eyes open | mean= | 118.2 | 109.3 | 109.6 | 97.7 | F= | 4.493 |
| SE= | 1.15 | 3.63 | 5.72 | 9.92 | p= | 0.0363 | |
| Eyes closed | mean= | 69.9 | 63.3 | 65.0 | 48.7 | F= | 1.129 |
| SE= | 7.11 | 7.3 | 8.32 | 12.26 | p= | 0.29 | |
| Walk Heel-to-Toe eyes open | mean= | 18.7 | 17.5 | 16.9 | 14.5 | F= | 5.488 |
| SE= | 0.58 | 0.74 | 1.14 | 1.91 | p= | 0.0209 | |
| Eyes closed | mean= | 5.8 | 5.3 | 3.9 | 3.1 | F= | 7.672 |
| SE= | 0.66 | 0.67 | 0.50 | 0.56 | p= | 0.0066* | |
| Stand on Left Foot eyes open | mean= | 55.9 | 54.7 | 49.6 | 41.8 | F= | 8.426 |
| SE= | 1.96 | 2.13 | 3.86 | 6.51 | p= | 0.0045* | |
| Eyes closed | mean= | 28.9 | 22.6 | 12.6 | 17.9 | F= | 8.133 |
| SE= | 3.41 | 3.15 | 1.46 | 4.93 | p= | 0.0052* | |
| Stand on right Foot eyes open | mean= | 58.3 | 55.5 | 54.3 | 51.5 | F= | 2.739 |
| SE= | 1.17 | 1.87 | 2.59 | 4.70 | p= | 0.10 | |
| Eyes closed | mean= | 30.5 | 24.7 | 17.8 | 24.5 | F= | 2.519 |
| SE= | 3.19 | 3.25 | 3.20 | 6.61 | p= | 0.12 | |
| Fine finger movements | |||||||
| Unimanual | mean= | 92.6 | 77.9 | 84.5 | 70.1 | F= | 11.682 |
| SE= | 2.01 | 1.55 | 2.35 | 2.97 | p= | 0.0009* | |
| Bimanual | mean= | 90.6 | 75.1 | 82.0 | 67.5 | F= | 11.261 |
| SE= | 2.04 | 1.53 | 2.66 | 2.74 | p= | 0.0011* | |
| Digit symbol task | |||||||
| Output in 90 s. | mean= | 52.9 | 60.1 | 50.8 | 48.6 | F= | 8.228 |
| SE= | 2.07 | 1.62 | 2.15 | 3.41 | p= | 0.0049* | |
| Time to complete grid in sec. | mean= | 159.4 | 136.7 | 163.5 | 165.4 | F= | 5.955 |
| SE= | 6.25 | 3.57 | 6.63 | 11.16 | p= | 0.0162 | |
Only the diagnosis and diagnosis-by-sex effects were of interest. None of the interactions were significant and are therefore not reported.
p-values meeting conservative significance level of ≤.01
Tests of postural stability
In no case was the diagnosis-by-sex interaction significant; therefore, only group differences were pursued. Significant group differences were forthcoming on 5 of the 10 balance measures and were most robust for the conditions requiring toe-to-heel walking on a line and standing on the left foot (in this predominantly right-handed subject group). The HIV group was not impaired on the Romberg test (standing heel-to-toe) assessing proprioception. HIV-infected participants were asked for self-reports of peripheral neuropathy, and 39 of the 40 participants provided responses: 7 of 27 men and 2 of 12 women reported such symptoms. In no test condition, however, did self-reported peripheral neuropathy account for HIV-related deficits, nor did it account for deficits in the tests of hand and finger dexterity and speed, described next.
Fine finger movement test
Group and sex differences were significant on all four measures, but group-by-sex interactions were not significant. For these measures, the controls were faster than the HIV-infected men and women, and the men were faster than the women, regardless of diagnosis, in turning the dowel either unimanually or bimanually.
Digit symbol test
The HIV group scribed significantly fewer symbols in 90 s. and took longer to complete all Digit Symbol grid boxes than controls. Modest diagnosis-by-sex interactions were due to the particularly fast scribing by the control women relative to the remaining three groups.
Relation between infratentorial volumes and motor performance in the HIV-infected group
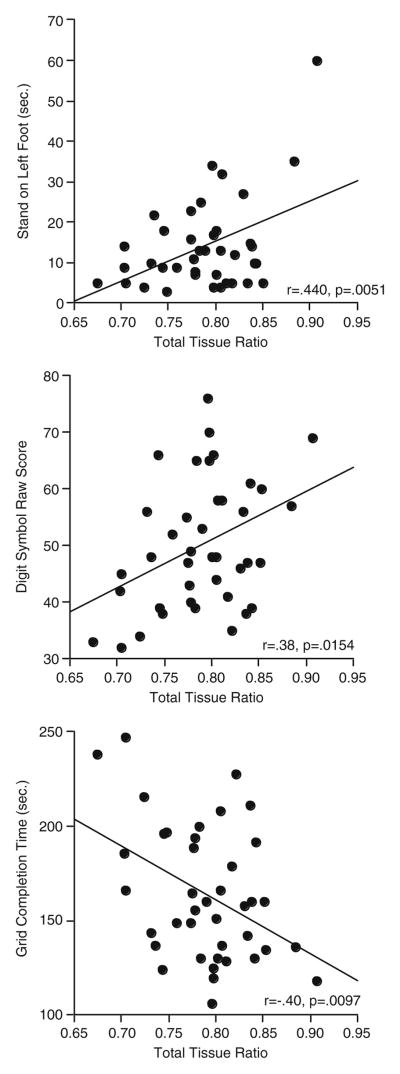
Because of the insubstantial diagnosis-by-sex effects on the primary performance variables, test scores of the men and women were combined in the brain structure-function analyses. Despite lack of correlation with any of the 3 regional vermian volumes, the total infratentorial tissue ratio was a significant predictor of balancing on the left foot with eyes closed (r=.44, p=.0051) and Digit Symbol grid completion time (r=−.40, p=.0097) and showed a trend with Digit Symbol scribing (90 s.) (r=.38, p=.0154) (Fig. 3).
Fig. 3.
Correlations between test scores and the total infratentorial tissue volume ratio in the HIV-infected participants who completed MRI and testing protocols
Tests of selectivity
To test for selectivity of the pontocerebellar volume-motor correlations, the motor test scores were correlated with volume measures of the lateral ventricles and corpus callosum. None of the correlations reached statistical significance (which required p≤.01), even if uncorrected for multiple comparisons. Similar correlations with fiber tracking metrics failed to identify any significant relations between motor scores and internal or external capsule FA or diffusivity metrics. By contrast, shorter time balancing on one foot correlated significantly with higher longitudinal diffusivity in the pontocerebellar tracts (r=−.48, p=.0019) and showed a trend to be related to cerebellar hemisphere tracts (r=−.39, p=.015).
Effect of demographic and HIV-related variables on infratentorial volumes and balance scores
Multiple-regression analyses eliminated depressive symptom (BDI) scores, CD4+ cell count, viral load, age of HIV onset, duration of HIV infection, smoking status, BMI, and history of an AIDS-defining event as significant independent contributors to the identified bivariate brain structure-function relations between the total pontocerebellar volume and the three balance and psychomotor measures. Further analysis focusing on one-foot balancing revealed that neither age nor amount of alcohol consumed over a lifetime made a significant independent contribution to the relation between pontocerebellar volume and balance. No correlation between MRI volume ratios and fine finger movement scores even approached significance.
Pontocerebellar volume ratios were related to type of antiviral medication the HIV-infected subjects were taking, but neuropsychological test performance was not. The subgroup of HIV-infected patients taking NNRTI (N=14) tended to have smaller pontocerebellar ratios in three of the four regions than those not on NNRTI (N=26). These differences were identified with t-tests, but none met the criterion for significance (total pontocerebellar tissue ratio t(38)=2.582, p=.0138; anterior vermis t(38)=2.24, p=.031; posterior vermis t(38)=2.142, p=.0387; inferior vermis t(38)=1.758, p=.0868).
Discussion
HIV infected individuals specifically recruited to be without complications from alcohol use disorders or clinically evident dementia had pontocerebellar tissue volume deficits with functional ramifications. The most robust findings in both HIV-infected men and women were volume deficits in the combined pons, cerebellar hemispheres, and vermis tissue, expressed as the ratio of their own infratentorial tissue+CSF volume. Functional deficits in motor performance were present in static postural stability and tandem walking, especially when tested with eyes closed, and psychomotor speed and finger dexterity. Quantitative assessment of both brain structure and function enabled a test of their relationship and the novel observation that poorer balance and slower psychomotor performance correlated with smaller pontocerebellar tissue volume ratios in the HIV-infected group. Selectivity of these brain structure-function relations was supported by significant correlations between motor measures and fiber tract integrity of the pontocerebellar system and absence of relations between motor performance and internal or external capsule fiber condition. Further, these relations could not be attributed to the general condition of the brain, as indexed by ventricular volume, which did not correlate with either upper limb speeded or lower limb balancing performance.
Psychomotor slowing has been documented in HIV infection since the initial neuropsychological studies of the disease (Butters et al. 1990), but a brain structural substrate for this impairment is typically assumed to be of frontostriatal origin. Postural stability, less often quantitatively tested than cognitive or upper limb performance, was moderately impaired. The performance correlations with smaller infratentorial brain tissue volume suggest a pontocerebellar system substrate for these movement deficits. Other motor systems, notably those involving the basal ganglia, known to be affected by HIV infection (for review, Nath 2010), may contribute as well, although our function-fiber tracking correlations provide no support for this speculation.
Factors associated with signs of extrapyramidal dysfunction in HIV infection include age (Valcour et al. 2008), history of AIDS (Ramachandran et al. 1997), and certain antiretroviral medications (Sacktor et al. 2003). Didanosine, stavudine (Cherry et al. 2006; but see Schifitto et al. 2002), and some protease inhibitors (Crabb 2004) are also associated with sensory peripheral neuropathy in HIV infection (Lopez et al. 2004). Although metabolic syndrome is another associate of sensory neuropathy (Ances et al. 2009), we found no evidence for associations between BMI (as a surrogate of metabolic syndrome) and any brain or behavioral measure. In the present study, patients who were currently taking antiretroviral medications did not have significantly different motor test scores or pontocerebellar volume ratios from those not taking medications. Type of medication, however, was a significant differentiator with respect to tissue volume ratios but not motor performance. In this case, NNRTI medication was associated with smaller pontocerebellar tissue volumes.
Gait and balance problems can be related to, or exacerbated by, HIV-related neuropathy of the peripheral nervous system (cf., Goodkin et al. 2001; Richardson and Hurvitz 1995), but concurrent lower limb neuropathy does not necessarily account for postural instability (Bauer et al. 2005). Indeed, pathology in the central nervous system, the cerebellum and pons in particular, is known to be involved in HIV infection (Abe et al. 1996; Anders et al. 1993; Kinzel et al. 2004; Kuchelmeister et al. 1993; Ruiz et al. 1997; Tagliati et al. 1998). Early studies that identified cerebellar pathology did so in severe and end-stage cases of HIV infection and reported evidence for Purkinje cell damage (Everall et al. 1995) or in cases with focal cerebellar lesions associated with opportunistic infections (e.g., Scatliff et al. 1997; Tagliati et al. 1998). Yet, another study found significant degeneration of the cerebellar granular cell layer and axonal swelling in HIV-infected cases free of opportunistic infection (Tagliati et al. 1998). Following early reports of HIV-positive cases with cerebellar granule cell destruction from polyomavirus JC (Du Pasquier et al. 2003; Otis and Moral 2005), study of a large series of cases revealed an unexpectedly high incidence of JC virus-infected cerebellar granule cell neurons, suggesting an under-appreciated site of neuropathology in HIV infection (Wuthrich et al. 2009). Other mechanisms of infratentorial volume shrinkage that could influence postural stability include axonal injury, which can occur without myelin damage, associated with opportunistic infection (for review, Medana and Esiri 2003) and even central pontine myelinolysis, which, while rare, has been reported in HIV infection (e.g., Miller et al. 1998).
In vivo studies, too, provide evidence for pontocerebellar involvement in HIV infection. One of the earliest and largest studies used computed tomography (CT) and later MRI to examine 47 patients with AIDS or AIDS-related complex (Flowers et al. 1990). In addition to HIV-related infections, relevant findings included visually-detectable focal or diffuse white matter lesions and cerebral, cerebellar, and brainstem atrophy. The pontocerebellar volume deficits of 3–6% in the HIV-infected group relative to the controls we observed herein are consistent with those recently reported by Klunder and colleagues (Klunder et al. 2008). A phosphorous MR spectroscopic imaging study reported alkaline pH in a larger cerebellar volume of asymptomatic HIV-infected patients relative to controls, interpreted as a precursor of neuronal cell death salient in the cerebellum (Patton et al. 2001). Based on MRI and proton MR spectroscopy, frontal and cerebellar sites, notably at the pontocerebellar junction, showed the greatest evidence for demyelination in AIDS patients with a clinical diagnosis of progressive multifocal leukoencephalopathy (Chang et al. 1997). Our previous DTI study of white matter fiber systems (Pfefferbaum et al. 2009) failed to identify statistically significant microstructural differences in the HIV-infected relative to the control group in tracts involving the pontocerebellar or cerebellar hemisphere fiber systems but did find evidence for diffusivity abnormalities in the internal and external capsules, suggestive of axonal involvement. Despite the fact that integrity of the pontocerebellar fiber systems did not differ from controls, unlike that of the internal or external capsules, the pontocerebellar fibers predicted postural stability and psychomotor speed in the HIV-infected group. We speculate that these relationships may be indicative of preclinical disruption of the pontocerebellar fiber systems or of relatively intact connectivity to compromised infratentorial motor sites.
In conclusion, HIV-infected individuals specifically recruited to be without complications from clinically-detectable dementia or alcohol use disorders have pontocerebellar tissue volume deficits with functional ramifications. Postural stability, finger dexterity and speed, and psychomotor quickness were impaired and attributable, at least in part, to compromised infratentorial brain systems. The condition of the microstructure of pontocerebellar fiber systems also contributed to the motor deficits. Taken together, these brain structure-function relations provide evidence for the selectivity of these infratentorial brain systems in associated functional impairment. The notable postural instability identified should raise awareness of the relevance of advising HIV-infected patients to take safety precautions while engaging in activities of daily living requiring balance.
Acknowledgments
Support for this work was provided by the United States National Institutes of Health grants AA017347, AA017168, EB008381. The registration tools are available as source code in the Computational Morphometry Toolkit: http://nitrc.org/projects/cmtk/. The SRI24 atlas is available from http://nitrc.org/projects/sri24/.
Footnotes
Financial Disclosures None of the authors have any conflicts to disclose.
Contributor Information
Edith V. Sullivan, Email: edie@stanford.edu, Department of Psychiatry & Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Road, Stanford, CA 94305-5723, USA
Margaret J. Rosenbloom, Department of Psychiatry & Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Road, Stanford, CA 94305-5723, USA. Neuroscience Program, SRI International, Menlo Park, CA, USA
Torsten Rohlfing, Neuroscience Program, SRI International, Menlo Park, CA, USA.
Carol A. Kemper, Department of Infectious Diseases, Stanford University School of Medicine, Stanford, CA, USA. Santa Clara Valley Medical Center, San Jose, CA, USA
Stanley Deresinski, Department of Infectious Diseases, Stanford University School of Medicine, Stanford, CA, USA. Santa Clara Valley Medical Center, San Jose, CA, USA.
Adolf Pfefferbaum, Department of Psychiatry & Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Road, Stanford, CA 94305-5723, USA. Neuroscience Program, SRI International, Menlo Park, CA, USA.
References
- Abe H, Mehraein P, Weis S. Degeneration of the cerebellar dentate nucleus and the inferior olivary nuclei in hiv-1-infected brains: a morphometric analysis. Acta Neuropathologica. 1996;92:150–155. doi: 10.1007/s004010050502. [DOI] [PubMed] [Google Scholar]
- Ances BM, Vaida F, Rosario D, Marquie-Beck J, Ellis RJ, Simpson DM, et al. Role of metabolic syndrome components in hiv-associated sensory neuropathy. AIDS. 2009;23:2317–2322. doi: 10.1097/QAD.0b013e328332204e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders KH, Becker PS, Holden JK, Sharer LR, Cornford ME, Hansen LA, et al. Multifocal necrotizing leukoencephalopathy with pontine predilection in immunosuppressed patients: a clinicopathologic review of 16 cases. Human Pathology. 1993;24:897–904. doi: 10.1016/0046-8177(93)90140-c. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for hiv-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, et al. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Archivesof Neurology. 2004;61:369–376. doi: 10.1001/archneur.61.3.369. [DOI] [PubMed] [Google Scholar]
- Arendt G, Maecker HP, Purrmann J, Homberg V. Control of posture in patients with neurologically asymptomatic hiv infection and patients with beginning hiv-1-related encephalopathy. Archives of Neurology. 1994;51:1232–1235. doi: 10.1001/archneur.1994.00540240076019. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Ceballos NA, Shanley JD, Wolfson LI. Sensorimotor dysfunction in hiv/aids: effects of antiretroviral treatment and comorbid psychiatric disorders. AIDS. 2005;19:495–502. doi: 10.1097/01.aids.0000162338.66180.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-ii. Psychological Corporation, Psychological Corporation; 1996. [Google Scholar]
- Beckley DJ, Bloem BR, Martin EM, Panzer VP, Remler MP. Postural reflexes in patients with hiv-1 infection. Electroencephalography and Clinical Neurophysiology. 1998;109:402–408. doi: 10.1016/s0924-980x(98)00040-x. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher KD. Multilingual aphasia examination (manual, revised 1978) University of Iowa, University of Iowa; 1976. [Google Scholar]
- Bodammer N, Kaufmann J, Kanowski M, Tempelmann C. Eddy current correction in diffusion-weighted imaging using pairs of images acquired with opposite diffusion gradient polarity. Magnetic Resonance in Medicine. 2004;51:188–193. doi: 10.1002/mrm.10690. [DOI] [PubMed] [Google Scholar]
- Brew BJ. Aids dementia complex. Neurologic Clinics. 1999;17:861–881. doi: 10.1016/s0733-8619(05)70170-5. [DOI] [PubMed] [Google Scholar]
- Butters N, Grant I, Haxby J, Judd LL, Martin A, McClelland J, et al. Assessment of aids-related cognitive changes: recommendations of the nimh workshop on neuropsychological assessment approaches. Journal of Clinical and Experimental Neuropsychology. 1990;12:963–978. doi: 10.1080/01688639008401035. [DOI] [PubMed] [Google Scholar]
- Castellanos F, Mallada J, Ricart C, Zabala JA. Ataxic neuropathy associated with human immunodeficiency virus seroconversion. Archives of Neurology. 1994;51:236. doi: 10.1001/archneur.1994.00540150022010. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Tornatore C, Aronow H, Melchor R, Walot I, et al. Metabolite abnormalities in progressive multifocal leukoencephalopathy by proton magnetic resonance spectroscopy. Neurology. 1997;48:836–845. doi: 10.1212/wnl.48.4.836. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Leonido-Yee M, Speck O. Perfusion mri detects rcbf abnormalities in early stages of hiv-cognitive motor complex. Neurology. 2000;54:389–396. doi: 10.1212/wnl.54.2.389. [DOI] [PubMed] [Google Scholar]
- Cherry CL, Skolasky RL, Lal L, Creighton J, Hauer P, Raman SP, et al. Antiretroviral use and other risks for hiv-associated neuropathies in an international cohort. Neurology. 2006;66:867–873. doi: 10.1212/01.wnl.0000203336.12114.09. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Jordan N, Harvey N, Sullivan EV. Cognitive impairment in early, untreated parkinson’s disease and its relationship to motor disability. Brain. 1991;114:2095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Corkin S, Growdon JH, Sullivan EV, Nissen MJ, Huff FJ. Assessing treatment effects from a neuropsychological perspective. In: Poon L, editor. Handbook of clinical memory assessment in older adults. Washington DC: American Psychological Association; 1986. pp. 156–167. [Google Scholar]
- Crabb C. Protease inhibitors and risk of developing hiv-related sensory neuropathy. AIDS. 2004;18:N10. doi: 10.1097/00002030-200409240-00001. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Zener KA. Group test for assessing hand and eye dominance. The American Journal of Psychology. 1962;75:271–276. [PubMed] [Google Scholar]
- Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in hiv/aids: a review. Neuropsychology Review. 2009 doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- Dellepiane M, Medicina MC, Mora R, Salami A. Static and dynamic posturography in patients with asymptomatic hiv-1 infection and aids. Acta Otorhinolaryngologica Italica. 2005;25:353–358. [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier RA, Corey S, Margolin DH, Williams K, Pfister LA, De Girolami U, et al. Productive infection of cerebellar granule cell neurons by jc virus in an hiv+individual. Neurology. 2003;61:775–782. doi: 10.1212/01.wnl.0000081306.86961.33. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Calero P, Stockin MD. Hiv infection and the central nervous system: a primer. Neuropsychology Review. 2009 doi: 10.1007/s11065-009-9094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, Witt M, Walot I, Aronow H, Leonido-Yee M, et al. Progressive multifocal leukoencephalopathy and human immunodeficiency virus-associated white matter lesions in aids: magnetization transfer mr imaging. Radiology. 1999;210:539–543. doi: 10.1148/radiology.210.2.r99fe19539. [DOI] [PubMed] [Google Scholar]
- Everall IP, Hudson L, al-Sarraj S, Honavar M, Lantos P, Kerwin R. Decreased expression of ampa receptor messenger rna and protein in aids: a model for hiv-associated neurotoxicity. Natural Medicines. 1995;1:1174–1178. doi: 10.1038/nm1195-1174. [DOI] [PubMed] [Google Scholar]
- Fama R, Eisen JC, Rosenbloom MJ, Sassoon SA, Kemper CA, Deresinski S, et al. Upper and lower limb motor impairments in alcoholism, hiv-infection, and their comorbidity. Alcoholism, Clinical and Experimental Research. 2007;31:1038–1044. doi: 10.1111/j.1530-0277.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Flowers CH, Mafee MF, Crowell R, Raofi B, Arnold P, Dobben G, et al. Encephalopathy in aids patients: evaluation with mr imaging. American Journal of Neuroradiology. 1990;11:1235–1245. [PMC free article] [PubMed] [Google Scholar]
- Fregly AR, Graybiel A, Smith MS. Walk on floor eyes closed (wofec): a new addition to an ataxia test battery. Aerospace Medicine. 1972;43:395–399. [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, et al. The prevalence of alcohol consumption and heavy drinking among people with hiv in the united states: results from the hiv cost and services utilization study. Journal of Studies on Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- Gerig G, Corouge I, Vachet C, Krishnan KR, MacFall JR. Quantitative analysis of diffusion properties of white matter fiber tracts: a validation study. 13th Proceedings of the International Society for Magnetic Resonance in Medicine; Miami, FL. 2005. p. Abstract no. 1337. [Google Scholar]
- Goodkin K, Wilkie FL, Concha M, Hinkin CH, Symes S, Baldewicz TT, et al. Aging and neuro-aids conditions and the changing spectrum of hiv-1-associated morbidity and mortality. Journal of Clinical Epidemiology. 2001;54(Suppl 1):S35–S43. doi: 10.1016/s0895-4356(01)00445-0. [DOI] [PubMed] [Google Scholar]
- Gorman AA, Foley JM, Ettenhofer ML, Hinkin CH, van Gorp WG. Functional consequences of hiv-associated neuropsychological impairment. Neuropsychology Review. 2009;19:186–203. doi: 10.1007/s11065-009-9095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus F, Ribalta T, Abos J, Alom J, Cruz-Sanchez F, Mallolas J, et al. Subacute cerebellar syndrome as the first manifestation of aids dementia complex. Acta Neurologica Scandinavica. 1990;81:118–120. doi: 10.1111/j.1600-0404.1990.tb00945.x. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ. Neuropathology of alcoholism. Alcohol and Alcoholism. 1990;25:207–216. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, et al. The hnrc 500–neuropsychology of hiv infection at different disease stages. Hiv neurobehavioral research center. Journal of the International Neuropsychological Society. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Jernigan TL, Archibald SL, Achim CL, Masliah E, Wiley CA. The relationship of quantitative brain magnetic resonance imaging measures to neuropathologic indexes of human immunodeficiency virus infection. Archives of Neurology. 1994;51:1129–1135. doi: 10.1001/archneur.1994.00540230067015. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four-factor index of social status. Yale University, Department of Sociology, Yale University, Department of Sociology; 1975. [Google Scholar]
- Karnofsky DA. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of chemotherapeutic agents. New York: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- Kinzel N, Strike D, Clark HB, Cavert W. Cerebellopontine degeneration as an immune restoration disease in hiv infection. AIDS and Behavior. 2004;18:2348–2350. doi: 10.1097/00002030-200411190-00025. [DOI] [PubMed] [Google Scholar]
- Klunder AD, Chiang MC, Dutton RA, Lee SE, Toga AW, Lopez OL, et al. Mapping cerebellar degeneration in hiv/aids. NeuroReport. 2008;19:1655–1659. doi: 10.1097/WNR.0b013e328311d374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchelmeister K, Bergmann M, Gullotta F. Cellular changes in the cerebellar granular layer in aids-associated pml. Neuropathology and Applied Neurobiology. 1993;19:398–401. doi: 10.1111/j.1365-2990.1993.tb00460.x. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Dew MA, Caldararo R. Risk modifiers for peripheral sensory neuropathy in hiv infection/aids. European Journal of Neurology. 2004;11:97–102. doi: 10.1046/j.1351-5101.2003.00713.x. [DOI] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, et al. Impairments in memory and hippocampal function in hiv-positive vs hiv-negative women: a preliminary study. Neurology. 2009;72:1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos JP, Rosso AL, Correa RB, Novis SAP. Movement disorders in 28 hiv-infected patients. Arquivos de Neuropsiquiatria. 2002;60:525–530. [PubMed] [Google Scholar]
- Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human cns diseases. Brain. 2003;126:515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- Miller RF, Harrison MJ, Hall-Craggs MA, Scaravilli F. Central pontine myelinolysis in aids. Acta Neuropathologica (Berlin) 1998;96:537–540. doi: 10.1007/s004010050931. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC. Fiber tracking: principles and strategies-a technical review. NMR in Biomedicine. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Annals of the New York Academy of Sciences. 2010;1187:122–128. doi: 10.1111/j.1749-6632.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- Nath A, Jankovic J, Pettigrew LC. Movement disorders and aids. Neurology. 1987;37:37–41. doi: 10.1212/wnl.37.1.37. [DOI] [PubMed] [Google Scholar]
- Otis CN, Moral LA. Images in pathology: granule cell loss in aids-associated progressive multifocal leukoencephalopathy. International Journal of Surgical Pathology. 2005;13:360. doi: 10.1177/106689690501300409. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. The topography of commissural fibers. In: Lepore F, Ptito M, Jasper HH, editors. Two hemispheres-one brain: Functions of the corpus callosum. New York: Alan R. Liss, Inc; 1986. pp. 47–74. [Google Scholar]
- Patton HK, Chu WJ, Hetherington HP, den Hollander J, Stewart KE, Raper JL, et al. Alkaline ph changes in the cerebellum of asymptomatic hiv-infected individuals. NMR in Biomedicine. 2001;14:12–18. doi: 10.1002/nbm.677. [DOI] [PubMed] [Google Scholar]
- Paul RH, Yiannoutsos CT, Miller EN, Chang L, Marra CM, Schifitto G, et al. Proton mrs and neuropsychological correlates in aids dementia complex: evidence of subcortical specificity. The Journal of Neuropsychiatry and Clinical Neurosciences. 2007;19:283–292. doi: 10.1176/jnp.2007.19.3.283. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, et al. Contribution of alcoholism to brain dysmorphology in hiv infection: effects on the ventricles and corpus callosum. Neuroimage. 2006;33:239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fiber tracking in hiv infection and alcoholism comorbidity: synergistic white matter damage. Brain. 2007;130:48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV. Frontostriatal fiber bundle compromise in hiv infection without dementia. AIDS. 2009;23:1977–1985. doi: 10.1097/QAD.0b013e32832e77fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Fama R, Sassoon SA, Sullivan EV. Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholic men and women: selective relations to dissociable functions. Alcoholism, Clinical and Experimental Research. 2010;34:1201–1211. doi: 10.1111/j.1530-0277.2010.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D, Kaplan E, Whitla D, Weintraubm S, Caitlin R, Funkenstein H. Microcog assessment of cognitive functioning manual. San Antonio: Psych Corp; 1993. [Google Scholar]
- Price RL, Sidtis JJ. Evaluation of the aids dementia complex in clinical trials. JAIDS. 1993;3:551–560. [PubMed] [Google Scholar]
- Ramachandran G, Glickman L, Levenson J, Rao C. Incidence of extrapyramidal syndromes in aids patients and a comparison group of medically ill inpatients. The Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9:579–583. doi: 10.1176/jnp.9.4.579. [DOI] [PubMed] [Google Scholar]
- Richardson JK, Hurvitz EA. Peripheral neuropathy: a true risk factor for falls. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1995;50:M211–M215. doi: 10.1093/gerona/50a.4.m211. [DOI] [PubMed] [Google Scholar]
- Robertson KR, Parsons TD, Sidtis JJ, Hanlon Inman T, Robertson WT, Hall CD, et al. Timed gait test: normative data for the assessment of the aids dementia complex. Journal of Clinical and Experimental Neuropsychology. 2006;28:1053–1064. doi: 10.1080/13803390500205684. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Maurer CR. Nonrigid image registration in shared-memory multiprocessor environments with application to brains, breasts, and bees. IEEE Transactions on Information Technology in Biomedicine. 2003;7:16–25. doi: 10.1109/titb.2003.808506. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A. The sri24 multi-channel atlas of normal adult human brain structure. Human Brain Mapping. 2010;31:798–819. doi: 10.1002/hbm.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Post MJ, Bundschu CC. Dentate nuclei involvement in aids patients with cns cryptococcosis: imaging findings with pathologic correlation. Journal of Computer Assisted Tomography. 1997;21:175–182. doi: 10.1097/00004728-199703000-00003. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Tarwater PM, McArthur JC, Selnes OA, Becker J, et al. Response to systemic hiv viral load suppression correlates with psychomotor speed performance. Neurology. 2003;61:567–569. doi: 10.1212/01.wnl.0000076477.71313.6e. [DOI] [PubMed] [Google Scholar]
- Samet JH, Walley AY, Bridden C. Illicit drugs, alcohol, and addiction in human immunodeficiency virus. Panminerva Medica. 2007;49:67–77. [PubMed] [Google Scholar]
- Sassoon SA, Fama R, Rosenbloom MJ, O’Reilly A, Pfefferbaum A, Sullivan EV. Component cognitive and motor processes of the digit symbol test: differential deficits in alcoholism, hiv infection and their comorbidity. Alcoholism, Clinical and Experimental Research. 2007;31:1315–1324. doi: 10.1111/j.1530-0277.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- Savolainen VT, Liesto K, Mannikko A, Penttila A, Karhunen PJ. Alcohol consumption and alcoholic liver disease: evidence of a threshold level of effects of ethanol. Alcoholism, Clinical and Experimental Research. 1993;17:1112–1117. doi: 10.1111/j.1530-0277.1993.tb05673.x. [DOI] [PubMed] [Google Scholar]
- Scatliff JH, Kwock L, Chancellor K, Bouldin TW, Kapoor CC, Castillo M. Postmortem mr imaging of the brains of patients with aids. Neuroimaging Clinics of North America. 1997;7:297–320. [PubMed] [Google Scholar]
- Schifitto G, McDermott MP, McArthur JC, Marder K, Sacktor N, Epstein L, et al. Incidence of and risk factors for hiv-associated distal sensory polyneuropathy. Neurology. 2002;58:1764–1768. doi: 10.1212/wnl.58.12.1764. [DOI] [PubMed] [Google Scholar]
- Sclar G, Kennedy CA, Hill JM, McCormack MK. Cerebellar degeneration associated with hiv infection [letter] Neurology. 2000;54:1012–1013. doi: 10.1212/wnl.54.4.1012. [DOI] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, et al. Cognitive dysfunction in hiv patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through mri as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and korsakoff’s syndrome: relation to ataxia. Neuropsychology. 2000;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, et al. Equivalent disruption of regional white matter microstructure in aging healthy men and women. Neuro-Report. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harris RA, Pfefferbaum A. Alcohol’s effects on brain and behavior. Alcohol Research & Health. 2010a;33:127–143. [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Pontocerebellar volume deficits and ataxia in alcoholic men and women: no evidence for “Telescoping”. Psychopharmacology. 2010b;208:279–290. doi: 10.1007/s00213-009-1729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiology of Aging. 2010c;31:464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magnetic Resonance in Medicine. 2006;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- Tagliati M, Simpson D, Morgello S, Clifford D, Schwartz RL, Berger JR. Cerebellar degeneration associated with human immunodeficiency virus infection. Neurology. 1998;50:244–251. doi: 10.1212/wnl.50.1.244. [DOI] [PubMed] [Google Scholar]
- Tate DF, Conley J, Paul RH, Coop K, Zhang S, Zhou W, et al. Quantitative diffusion tensor imaging tractrography metrics are associated with cognitive performance among hiv-infected patients. Brain Imaging and Behavior. 2010 doi: 10.1007/s11682-009-9086-z. Epub online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenkwalder C, Straube A, Paulus W, Krafczyk S, Schielke E, Einhaupl KM. Postural imbalance: an early sign in hiv-1 infected patients. European Archives of Psychiatry and Clinical Neuroscience. 1992;241:267–272. doi: 10.1007/BF02195975. [DOI] [PubMed] [Google Scholar]
- Valcour V, Watters MR, Williams AE, Sacktor N, McMurtray A, Shikuma C. Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. Journal of Neurovirology. 2008;14:362–367. doi: 10.1080/13550280802216494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The wernicke-korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition. 2. F.A. Davis Co, F. A. Davis Co; 1989. [Google Scholar]
- von Giesen HJ, Wittsack HJ, Wenserski F, Koller H, Hefter H, Arendt G. Basal ganglia metabolite abnormalities in minor motor disorders associated with human immunodeficiency virus type 1. Archives of Neurology. 2001;58:1281–1286. doi: 10.1001/archneur.58.8.1281. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale - revised. The Psychological Corporation, The Psychological Corporation; 1981. [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of hiv-associated neurocognitive disorders. Neuropsychology Review. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich C, Cheng YM, Joseph JT, Kesari S, Beckwith C, Stopa E, et al. Frequent infection of cerebellar granule cell neurons by polyomavirus jc in progressive multifocal leukoencephalopathy. Journal of Neuropathology and Experimental Neurology. 2009;68:15–25. doi: 10.1097/NEN.0b013e3181912570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Mori S, Solaiyappan M, van Zijl PC, Davatzikos C. A framework for callosal fiber distribution analysis. NeuroImage. 2002;17:1131–1143. doi: 10.1006/nimg.2002.1285. [DOI] [PubMed] [Google Scholar]
- Xue R, van Zijl PC, Crain BJ, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magnetic Resonance in Medicine. 1999;42:1123–1127. doi: 10.1002/(sici)1522-2594(199912)42:6<1123::aid-mrm17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Yebra M, Garcia-Merino A, Albarran F, Varela JM, Echevarria JM. Cerebellar disease without dementia and infection with the human immunodeficiency virus (hiv) Annals of Internal Medicine. 1988;108:310–311. doi: 10.7326/0003-4819-108-2-310. [DOI] [PubMed] [Google Scholar]