Abstract
Background
Sonchus asper (SA) is traditionally used for the treatment of various ailments associated with liver, lungs and kidneys. This study was aimed to investigate the therapeutic potential of nonpolar (hexane, SAHE; ethyl acetate, SAEE and chloroform, SACE) and polar (methanol, SAME) crude extracts of the whole plant.
Methods
To achieve these goals, several parameters including free-radical (DPPH•, ABTS•+, H2O2 and •OH) scavenging, iron chelating activity, scavenging of superoxide radicals, total flavonoids and total phenolic content (TPC) were examined.
Results
The SA extracts presented a remarkable capacity to scavenge all the tested reactive species with IC50 values being found at the μg ⁄ ml level. The SAME was shown to have the highest TPCs while lowest IC50 values for the DPPH•, ABTS•+ radical scavenging capacities and iron chelating scavenging efficiency, moreover, SAME had best activities in scavenging of superoxide radicals and hydrogen peroxide as well as potently scavenged the hydroxyl radicals.
Conclusion
These results suggest the potential of S. asper as a medicine against free-radical-associated oxidative damage.
Keywords: Sonchus asper, Antioxidant activities, Solvent extraction, Phenolics
Background
Sonchus asper (Compositae) is used in the treatment of wound healing and possesses anti-burning properties [1]. It has diuretic, refrigerant, sedative and antiseptic properties, used in the treatment of cough, bronchitis and asthma [2], tonsils [3], kidney inflammation [4], erectile dysfunction in male [5], fever, constipation, diabetes, scabies and heart diseases [6]. Chemical studies of the SA revealed the presence of ascorbic acid, carotenoids and fatty acids [7].
Phenolic compounds which are secondary metabolites in plants are one of the most widely occurring groups of phytochemicals that exhibit antiallergenic, antimicrobial, antiartherogenic, antithrombotic, antiinflammatory, vasodilatory and cardioprotective effects [8,9]. Due to the presence of the conjugated ring structures and hydroxyl groups; many phenolic compounds have the potential to function as antioxidants by scavenging or stabilizing free radicals involved in oxidative processes through hydrogenation or complexing with oxidizing species that are much stronger than those of vitamins C and E [10]. Tannic acid, quercetin and catechin were reported earlier in SA [11], and could therefore; contribute to antioxidant activities [7]. With increasing recognition of herbal medicine as an alternative form of health care, screening of medicinal plants for biologically active compounds has become an important source of antibiotic prototypes and cancer-related drugs [7]. Hence, for selecting crude plant extracts with potential useful properties, in vitro screening methods have been used for further in-depth chemical elucidation and pharmacological investigations [12]. Based on the traditional claims surrounding SA and the lack of scientific studies of its potential pharmacological properties, the objective of this study was to evaluate the antioxidant activity through direct free radical scavenging methods and also elucidate total phenolic content (TPC) and polyphenolic flavonoids constituents.
Results
Total phenolics, total flavonoids and % yield contents (TPC)
Content of phenolics compounds, flavonoids and % yield contents in SA are exhibited in (Table 1). The % yield extractions are in descending order of methanol > chloroform > ethyl acetate > n-hexane showing that methanol possesses a significant high amount of % yield contents. Table 1 also summarized that methanolic extract have the highest total phenolic (P < 0.01) (332 ± 1.53 mg GAE/g dry extract) and (11.4 ± 0.45 mg rutin/g dry extract) in comparison with other fractions of SA extract.
Table 1.
Total phenolic content in different extracts of SA
| Sample | Total flavonoids compounds as rutin equivalent (mg/g dry extract) | Total phenolic compounds as mg gallic acid equivalent (GAE mg/g extract) | % yield extraction |
|---|---|---|---|
| SAME | 11.4 ± 0.45c | 332 ± 1.53b | 6.4 ± 0.12b |
| SACE | 8.66 ± 1.9b | 325 ± 2.3b | 3.3 ± 0.2a |
| SAEE | 7.57 ± 0.09b | 192 ± 3.0a | 2.45 ± 0.32a |
| SAHE | 5.16 ± 0.9a | 325 ± 2.3b | 3.3 ± 0.2a |
Each value in the table is represented as Mean ± SD (n = 3) Means not sharing the same letter are significantly different (LSD) at P < 0.01 probability level in each column
DPPH scavenging activity
Different fractions of SA for free radicals of 1, 1-diphenyl 1-2-picryl-hydrazyl (DPPH) showed remarkable scavenging activities (Figure 1) and Table 2. SAME showed the highest scavenging activity (lowest IC50; 2.5 ± 0.05) followed by SACE, SAEE and SAHE. DPPH scavenging activity was significantly correlated with phenolics (P < 0.01, r2 = 0. 9762) with the TPC and flavonoids (P < 0.01, r2 = 0. 8843) in different extracts (Table 3).
Figure 1.
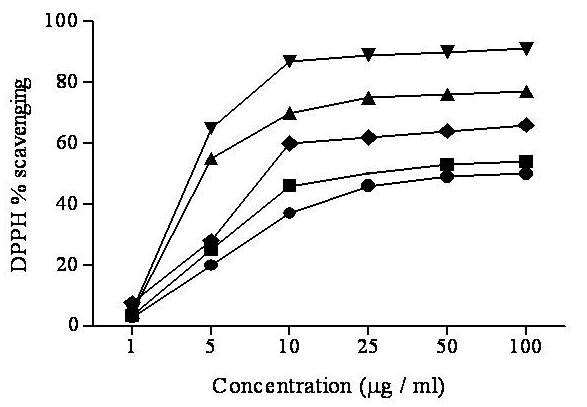
DPPH radical scavenging activity of different extracts from the methanol extract of Sonchus asper by different solvents at different concentrations. Each value represents a Mean ± SD (n = 3) SAHE; SAEE; SACE; SAME and ascorbic acid.
Table 2.
IC50 of different extracts of SA for various antioxidant systems
| Treatments | Iron chelating activity assay | Super oxide radical scavenging assay | DPPH radical scavenging assay activity | ABTS+ radical scavenging assay | Hydroxyl scavenging assay | Hydrogen peroxide scavenging assay |
|---|---|---|---|---|---|---|
| SAME | 64 ± 2.12a | 57.34 ± 3.21a | 2.5 ± 0.05a | 53.4 ± 4.2a | 66.8 ± 1.7a | 67.34 ± 3.21a |
| SACE | 87.8 ± 2.56c | 70.2 ± 4.56b | 3.8 ± 0.2b | 74.2 ± 2.6b | 86.3 ± 1.56b | 80.2 ± 4.56b |
| SAEE | 100.4 ± 2.21d | 84.34 ± 1.05d | 4.1 ± 0.32b | 83.4 ± 1. 5c | 79.1 ± 3.9b | 70.3 ± 1.5a |
| SAHE | 110.6 ± 1.67e | 92.21 ± 2.45d | 12.2 ± 1.43c | 90.21 ± 2.8c | 115 ± 3.2c | 92.21 ± 2.45c |
| ASA | 73.7 ± 3.4b | 76.3 ± 2.15c | 3.61 ± 23b | 76.3 ± 2.15b | 62.2 ± 2.65a | 66.3 ± 2.15a |
Each value in the table is represented as Mean ± SD (n = 3) Means not sharing the same letter are significantly different (LSD) at P < 0.01 probability level in each column
Table 3.
Correlations between the IC50 values of antioxidant activities, phenolics and flavonoids content of S.asper
| Assays (IC50 μg/ml) | Correlations R2 | |
|---|---|---|
| Phenolics | Flavonoids | |
| DPPH | 0.9762b | 0.8843b |
| Iron chelating assay | 0.8101b | 0.7657a |
| Super oxide radical scavenging | 0.6987a | 0.6765b |
| Hydrogen peroxide scavenging assay | 0.1223 | 0.1056 |
| Hydroxyl radical scavenging activity | 0.2003 | 0.2060 |
| ABTS+ radical scavenging assay | 0.8821b | 0.7797a |
S.asper methanolic extract and its soluble fractions were used in the correlation. a, b indicate significance at P < 0.05 and P < 0.01 respectively
Superoxide radical scavenging activity
Superoxide (O2•-) radical is known to be very harmful to cellular components as a precursor of the more reactive oxygen species, contributing to the tissue damage and various diseases. SAME (57.34 ± 3.21 μg/ml) possessed the most potent superoxide activity followed by SACE (70.2 ± 4.56 μg/ml) with decreasing order of solvent polarity (Figure 2) and Table 2. A remarkable nonsignificant correlation (P > 0.01, r2 = 0.6987) was recorded between TPC of SA and superoxide radical (O2•-) scavenging activity and with polyphenolic flavonoids (P > 0.01, r2 = 0.6765) as shown in Table 3.
Figure 2.
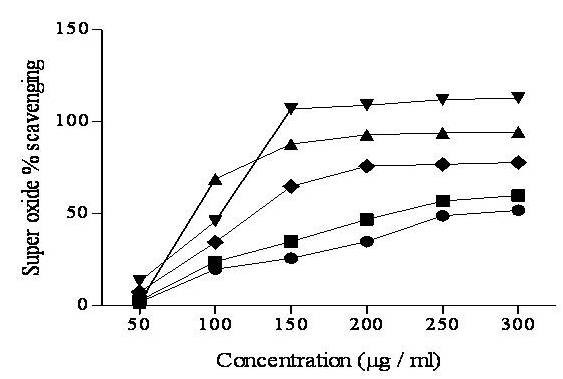
Super oxide radical scavenging activity of different extracts from the methanol extract of Sonchus asper by different solvents at different concentrations. Each value represents a Mean ± SD (n = 3) SAHE; SAEE; SACE; SAME and ascorbic acid.
ABTS radical assay
Differences for the ABTS•+ (2, 2 azobis-(3-ethylbenzothiozoline-6-sulphonic acid) radical cation scavenging capacities of each sample and reference compounds, including ascorbic acid was recorded in this study Table 2. Among various fractions of S. asper; SAME possessed the highest ABTS radical scavenging activity (53.4 ± 4.21 μg/ml), while SAHE showed the lowest ABTS radical scavenging activity (90.21 ± 2.8 μg/ml). Significant and positive correlation of TPCs and flavonoids for ABTS antioxidant capacity was obtained for various fractions (P < 0.01, r2 = 0.8821) and (P > 0.01, r2 = 0.7797) as shown in Table 3.
Hydrogen peroxide scavenging activity
Hydrogen peroxide non reactive, but sometimes it can be toxic to living cells, because in living cell it is converted into free radical called hydroxyl radicals (•OH), react with biomolecules, cause tissue damage and cell death. Table 2 shows the IC50 of (•OH) scavenging effect by various fractions along with ascorbic acid. SAME (67.34 ± 3.21 μg/ml) markedly scavenged the free radical and was most potent than reference chemicals; ascorbic acid (76.3 ± 2.15 μg/ml respectively. Nonsignificant level of association (P > 0.05, r2 = 0.1223) and (P > 0.05, r2 = 0.1056) was recorded between the TPC, flavonoids of different extracts of SA and H2O2 scavenging activity (Table 3).
Hydroxyl radical scavenging activity
The •OH radical is an extremely reactive in biological systems and has been implicated as highly damaging species in free radical pathology, capable of damaging biomolecules of the living cells. These radical combines with nucleotides in DNA and cause strand breakage leading to carcinogenesis, mutagenesis and cytotoxicity. Hydroxyl radical (•OH) scavenging capacity of an extract is directly related to its antioxidant activity. SAME was the most effective (66.8 ± 1.7) for hydroxyl radical scavenging activity followed by SAEE (79.1 ± 3.9 μg/ml), SACE (86.3 ± 1.56 μg/ml) and SAHE (115 ± 3.2 μg/ml) (Table 2). The scavenging activity obtained for ascorbic acid is 57.2 ± 2.65 μg/ml. Extremely low level of association (P > 0.05, r2 = 0.2003) and (P > 0.05, r2 = 0.2060) was determined between the •OH radical scavenging activity, TPCs and flavonoids of different extracts shown in Table 3.
Iron chelating assay
Reduction of the iron ion is an indicator of electron-donating activity, which is an important mechanism of phenolic antioxidant action. Yellow color of test solution changes to various shades of green and blue depending upon the reducing power of each extract. The presence of reductant (antioxidants) in the herbal extracts causes the reduction of Fe3+/Ferric cyanide complex to ferrous form. Therefore, the Fe2+ complex can be monitored by measuring the formation of Perl's Prussian blue. The IC50 values of iron reduction ability of the SAME, SACE, SAEE and SAHE are (64 ± 2.12 μg/ml), (87.8 ± 2.56 μg/ml), (100.4 ± 2.21 μg/ml), (110.6 ± 1.67 μg/ml while that of ascorbic acid have (73.7 ± 3.4 μg/ml) respectively (Table 2). TPCs and total flavonoids content of SA were significantly correlated (P > 0.01, r2 = 0.8101) and (P > 0.01, r2 = 0.8101) with the reducing power (Table 3) respectively.
Discussion
Sonchus asper is used ethnopharmacologically for the treatment of various complaints. The therapeutic benefit of medicinal plants is usually contributed to their antioxidant properties. The biochemical investigation reported that SA constitute of antioxidant compounds such as carotenoids, catechin, rutin, quercetin and other phenolics [7,11]. Moreover, SA activities against oxidative stress, antibacterial and antitumor were yet to be explored. Different free-radical generating systems were used in this study to assess the free-radical scavenging and reducing properties of the crude polar and non-polar extracts of SA along with evaluation of the total phenolic content. Quantitative estimation proved that the SAME possesses the highest concentration of phenolic compounds in SAME fraction of the extract. Similar results were described by other studies in the literature for other extracts of plants [13]. The SA provided us with plentiful of different sorts of polyphenolic compounds as an incredible source of antioxidant, exhibited by the remarkable IC50 values in different extracts. The observed differential scavenging activities of the extracts against various systems may be referred to the different mechanisms of the radical antioxidant reactions in the different assays. Hagerman et al. [14] have reported that the high molecular weight phenolics (tannins) have more abilities to quench free radicals (ABTS•+) and their effectiveness depends on the molecular weight, the number of aromatic rings and nature of hydroxyl group's substitution than the specific functional groups. Free radical (ABTS•+) scavenging activity of SA extracts might be due to the presence of high molecular weight phenolics such as catechin, and rutin derivatives. The SA extracts exhibit remarkable H2O2 and •OH radical scavenging capacity rendering, their utilization in different ailments associated with oxidative stress [15,16]. Recent investigations have shown that many flavonoids and related polyphenols contribute significantly to the antioxidant activity of medicinal plants. Our results revealed that there is a strong and significant correlation between TPC and DPPH• free radical scavenging activity and H2O2 scavenging activity for the SA extracts, while the other assays have nonsignificant correlation with the TPC. This could be due to the difference in the stoichiometry of reactions between the antioxidant compounds in the extracts and the various radicals, which may be inferred as a reason for the difference in their scavenging potential. The diversity in radical scavenging shown in these assays may also be due to factors like stereo selectivity of the radicals or the differential solubility that may be justified in case of crude extracts, which contain a variety of antioxidants.
Materials and methods
Plant material
Sonchus asper at maturity was collected from District Bannu (Pakistan), identified and a specimen was submitted vide voucher # R-147 at Herbarium of Pakistan, Quaid-I-Azam University Islamabad, Pakistan for future reference. Aerial parts of plant (leaves, stem, flowers and seeds) were shades dried at room temperature for two weeks, chopped and ground mechanically of mesh size 1 mm.
Preparation of plant extracts
For extract preparation, 1.5 kg of dried sample was extracted twice with 5 litre of 95% methanol at 25°C for 48 h. The extracts were filtrated through Whatman No. 1 filter paper and combined followed by concentration (SAME) using a rotary evaporator (Panchun Scientific Co., Kaohsiung, Taiwan) under reduced pressure at 40°C. A portion of the filtrates (SAME) obtained was suspended in water (50 ml) and n-hexane (100 ml) was added, shake well, and the layers were allowed to separate for 6 h in a separating funnel. N-hexane layer was separated and evaporated to obtain the n-hexane fraction (SAHE). Similar, protocol was repeated with ethyl acetate (SAEE) and the remaining solvents, i.e., chloroform (SACE). Each of the fractions obtained were dried using a rotary evaporator. The dry extract obtained with each solvent was weighed. The percentage yield was expressed in terms of air dried weight of plant material.
Total phenolic contents
The total phenolic content was determined using the method [17] with certain modifications. Calibration curve was prepared by mixing methanolic solution of gallic acid (1 ml; 0.025-0.400 mg/ml) with 5 ml Folin-Ciocalteu reagent (diluted ten fold). The mixture was incubated for 5 min before the addition of sodium carbonate (4 ml, 0.115 mg/ml). Absorbance was measured at 765 nm. 1 ml of plant extracts (SAME, SAHE, SAEE, SACE) dissolved in respective solvent (0.5-5.0 mg/ml) was also mixed with the reagents above, and after 2 h the absorbance was measured to determine total plant phenolic contents. All determinations were carried out in triplicate. The total content of phenolic compounds in the extract was expressed as gallic acid equivalents (GAE) mg/g of the dry extract.
Determination of the total flavonoids
Total flavonoids content was determined by using a method described [13]. Briefly, 0.25 ml of each fraction (1-5 mg/ml; dissolved in respective solvent) and rutin standard solution (15-250 μg/ml) was mixed with 1.25 ml of distilled water in a test tube, followed by addition of 75 μl of a 5% (w/v) sodium nitrite solution. After 6 min, 150 μl of 10% (w/v) aluminum chloride solution was added, and the mixture was allowed to stand for a further 5 min before 0.5 ml of 1 M NaOH was added. The mixture was made up to 2.5 ml with distilled water and mixed well. The absorbance was measured immediately at 510 nm. The results of samples were expressed as mg of rutin equivalents of total extractable compounds. All fractions were run in triplicate.
In vitro antioxidant activity
Diphenylpicrylhydrazyl radical scavenging activity
The free-radical scavenging activity of the various fractions, gallic acid and ascorbic acid was measured with the stable radical diphenylpicrylhydrazyl (DPPH) in terms of hydrogen-donating or radical-scavenging activity. 3 ml of DPPH (in methanol) solution having 0.980 (± 0.02) O.D. was added to 100 μl of different fractions (dissolved in respective solvent), at different concentrations (1-250 μg/ml). After 30 min, the absorbance was measured at 517 nm according to a described procedure [18] with some modifications. Lower absorbance of the reaction mixture indicates higher free radical scavenging activity. The antioxidant activity of the extract was expressed as IC50, which was defined as the concentration (μg/ml) of extract that inhibits the formation of DPPH radicals by 50%. Ascorbic acid was used as a positive control. Each study corresponded to three experiments, performed in duplicate. The scavenging activity was estimated based on the percentage of DPPH radicals scavenged by the following formula:
where A0 is absorption of control, AS is absorption of tested extract solution.
ABTS radical scavenging activity
ABTS assay was performed according to the protocol [19]. The stock solution was prepared by mixing equal volumes of 7 mM ABTS solution and 2.45 mM potassium persulfate solution followed by incubation for 12 h at room temperature in the dark to yield a dark-colored solution containing ABTS•+ radicals. Working solution was prepared freshly before each assay by diluting the stock solution by mixing of stock solution to 50% methanol for an initial absorbance of about 0.700 (± 0.02) at 745 nm, with temperature control set at 30°C. Free radical scavenging activity was assessed by mixing 300 μl of different fractions (25-250 μg/ml in respective solvents) with 3.0 ml of ABTS working standard. The decrease in absorbance was measured exactly 1 min after mixing the solution, the final absorbance was noted up to 6 min. Data for each assay was recorded in triplicate. Ascorbic acid was used as positive controls. The scavenging activity was estimated based on the percentage of ABTS radicals scavenged by the following formula:
where A0 is absorption of control, AS is absorption of tested extract solution.
Determination of superoxide radical scavenging activity
Superoxide scavenging was determined by the nitroblue tetrazolium reduction method [20]. The reaction mixture consisted of 1 ml of nitroblue tetrazolium (NBT) solution (l M NBT in 100 mM phosphate buffer, pH 7.4), 1 ml NADH solution (l M NADH in 100 mM phosphate buffer, pH 7.4) and 0.1 ml of different fractions and ascorbic acid (50 mM phosphate buffer, pH 7.4) was mixed. The reaction was started by adding 100 μl of (PMS) solution (60 μM PMS in 100 mM phosphate buffer, pH 7.4) in the mixture. The tubes were uniformly illuminated with an incandescent visible light for 15 minutes and the optical density was measured at 530 nm before and after the illumination. The percentage inhibition of superoxide generation was evaluated by comparing the absorbance values of the control and experimental tubes. The abilities to scavenge the superoxide radical were calculated by using the following formula:
where Ao is the absorbance without sample, and Ae is absorbance with sample.
Hydrogen peroxide scavenging activity
The scavenging capacity for hydrogen peroxide was measured according to the method [21]. A solution of hydrogen peroxide (2 mM) was prepared in 50 mM phosphate buffer (pH 7.4). Hydrogen peroxide concentration was determined spectrophotometrically at 230 nm absorption using the molar extinction coefficient for H2O2 of 81 mol-1cm-1. 0.1 ml of various fractions (25-250 μg/ml in respective solvents), ascorbic acid was transferred into the test tubes and their volumes were made up to 0.4 ml with 50 mM phosphate buffer (pH 7.4) or solvent (methanol). After addition of 0.6 ml hydrogen peroxide solution, tubes were vortexed and absorbance of the hydrogen peroxide at 230 nm was determined after 10 min, against a blank. 50 mM phosphate buffer without hydrogen peroxide was used as blank. Hydrogen peroxide scavenging ability (in triplicate) was calculated by the formula:
where Ao is the absorbance without sample, and Ae is absorbance with sample.
Hydroxyl radical scavenging activity
The effect of extracts on hydroxyl radicals was assayed by using the deoxyribose method [22]. 2-Deoxyribose is degraded on exposure to hydroxyl radicals generated by Fenton's reaction. Each fraction and ascorbic acid (ASA) was prepared in methanol. The reaction mixture contained 450 μl of 0.2 M sodium phosphate buffer (pH 7.0), 150 μl of 10 mM 2-deoxyribose, 150 μl of 10 mM FeSO4-EDTA, 150 μl of 10 mM H2O2, 525 μl of H2O, and 75 μl of sample solution (0.050-0.250 mg/ml in respective solvents). The reaction was started by the addition of H2O2. After incubation at 37°C for 4 h, the reaction was stopped by adding 750 μl of 2.8% trichloroacetic acid and 750 μl of 1% TBA in 50 mM NaOH, the solution was boiled for 10 min, and then cooled in water. The absorbance of the solution was measured at 520 nm. Ascorbic acid (0.05-0.250 mg/ml) was used as a positive control. The ability to scavenge the hydroxyl radical was calculated using the following equation:
2.5.6. Chelating activity on Fe2+
The extracts were assessed for their ability to compete with ferrozine for iron (II) ions in free solution. The chelating ability of ferrous ions by various fractions was estimated by the method [23]. Extracts (0.05-250 mg/ml), 2.5 ml were added to a solution of 2 mM FeCl2.4H2O (0.05 ml). The reaction was initiated by the addition of 5 mM ferrozine (0.2 ml); the mixture was shaken vigorously and left standing at room temperature for 10 min. Absorbance of the solution was then measured at 562 nm against the blank performed in the same way using FeCl2 and water. EDTA (0.625-5 μg/ml) served as the positive control, and a sample without extract or EDTA served as the negative control. All tests were run in triplicate and averaged. The percentage of inhibition of ferrozine-Fe2+complex formation was calculated using the formula:
Statistical analysis
Data were expressed as mean and standard error (SE). Statistical analysis of parameteric data for IC50 was carried out using graph prism pad software. Experimental results were further analyzed for Pearson correlation coefficient between TPCs, flavonoids and different antioxidant assays and tested for significance by Student's t-test (P < 0.05). SPSS ver. 14.0 (Chicago, IL, USA) and Microsoft Excel 2007 (Roselle, IL, USA) were used for the statistical and graphical evaluations.
Conclusion
Presence of phenolics in SAME might be responsible for DPPH, ABTS radical scavenging capacities and reducing power. Moreover, scavenging activities of SAEE for superoxide radicals and hydrogen peroxide are remarkable. This indicated that SA contained potential antioxidant bioactive compounds, which if properly and extensively studied, could provide many chemically interesting and biologically active drug candidates, including some with potential antiproliferative properties.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RAK made a significant contribution to acquisition of data, analysis, drafting of the manuscript.
MRK has made a substantial contribution to conception and design, interpretation of data, drafting and revising the manuscript for intellectual content.
SS participated in the design and collection of data and analysis. All authors read and approved the final manuscript.
MA participated in the design and collection of data and experimental work and analysis. All authors read and approved the final manuscript.
Contributor Information
Rahmat Ali Khan, Email: rahmatalikhan2011@yahoo.com.
Muhammad Rashid Khan, Email: mrkhanqau@yahoo.com.
Sumaira Sahreen, Email: Sumairasahreen@gmail.com.
Mushtaq Ahmed, Email: mushtaq213@yahoo.com.
References
- Rehman EU. Indigenous knowledge on medicinal plants, village Barali Kass and its allied areas, District Kotli Azad Jammu and Kashmir, Pakistan. Ethnobotanical Leaflets. 2006;10:254–264. [Google Scholar]
- Khan RA, Khan MR, Sahreen S. Protective effect of Sonchus asper extracts against experimentally-induced lung injuries in rats: A novel study. Exp Toxicol Pathol. 2011. in press . [DOI] [PubMed]
- Jeruto P, Lukhoba C, Ouma G, Otieno D, Mutai C. An ethnobotanical study of mericinal plants used by the Nandi people in Kenya. J Ethnopharm. 2008;116:370–376. doi: 10.1016/j.jep.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Khan RA, Khan MR, Sahreen S, Bukhari J. Prevention of CCl4-induced nephrotoxicity with Sonchus asper in rat. Food Chem Toxicol. 2010;23:1304–1321. doi: 10.1016/j.fct.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Kareru PG, Kenji GM, Gachanja AN, Keriko JM, Mungai G. Traditional medicine among the Embu and Mbeere peoples of Kenya. Afric J Trad Compl Alter Med. 2007;4:75–86. doi: 10.4314/ajtcam.v4i1.31193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MR, Haroon J, Khan RA, Bokhari J, Rashid U. Prevention of KBrO3-induced cardiotoxicity by Sonchus asper in rat. J Med Plants Res. 2011;12:2514–2520. [Google Scholar]
- Afolayan AJ, Jimoh FO. Nutritional quality of some wild leafy vegetables in South Africa. Intl J Food Sci Nutr. 2008;60:424–431. doi: 10.1080/09637480701777928. [DOI] [PubMed] [Google Scholar]
- Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Alpinar K, Ozyurek M, Kolak U, Guclu K, Aras C, Altun M, Celik SE, Berker KI, Bektasoglu B, Apak R. Antioxidant Capacities of Some Food Plants Wildly Grown in Ayvalik of Turkey. Food Sci Tech Res. 2009;15:59–64. [Google Scholar]
- Amic D, Davidovic-Amic D, Beslo D, Trinajstic N. Structure-radical scavenging activity relationship of flavonoids. Croat Chem Acta. 2003;76:55–61. [Google Scholar]
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agro industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. [Google Scholar]
- El-Zalabani SM, Mahmoud II, Ahmed FI, Shehab NG. Protein carbohydrate, mineral and vitamin contents of Sonchus oleraceus L. growing in Egypt. J Pharm Sci. 1999;23:46–54. [Google Scholar]
- Sakanaka S, Tachibana Y, Okada Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha) Food Chem. 2005;89:569–575. [Google Scholar]
- Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agri Food chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao M, Wanga J, Yangb B, Jiang Y. Antioxidant activity of methanolic extract of emblica fruit (Phyllanthus emblica L.) from six regions in China. J Food Comp Anal. 2008;21:219–228. [Google Scholar]
- Ozsoy N, Can A, Yanardag R, Akev N. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem. 2008;110:571–583. [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1996;16:144–153. [Google Scholar]
- Gyamfi MA, Yonamine M, Aniya Y. Free radical scavenging activity of medicinal herb of Ghana: Thonningia sanguinea on experimentally induced liver injuries. General Pharmacol. 1999;32:661–667. doi: 10.1016/s0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yong M, Rice-Evas C. Antioxidant activity applying an improved ABTS radical cation decoloursation assay. Free Rad Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechin isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Nagai T, Myoda T, Nagashima T. Antioxidative activities of water extract and ethanol extract from field horsetail (tsukushi) Equisetum arvense L. Food Chem. 2005;91:389–394. [Google Scholar]
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]


