Abstract
The zebrafish is an ideal model organism for investigating the molecular mechanisms underlying cardiogenesis, due to the powerful combination of optical access to the embryonic heart and plentiful opportunities for genetic analysis. A continually increasing number of studies are uncovering mutations, morpholinos, and small molecules that cause striking cardiac defects and disrupt blood circulation in the zebrafish embryo. Such defects can result from a wide variety of origins including defects in the specification or differentiation of cardiac progenitor cells; errors in the morphogenesis of the heart tube, the cardiac chambers, or the atrioventricular canal or problems with establishing proper cardiac function. An extensive arsenal of techniques is available to distinguish between these possibilities and thereby decipher the roots of cardiac defects. In this chapter, we provide a guide to the experimental strategies that are particularly effective for the characterization of cardiac henotypes in the zebrafish embryo.
I. Introduction
Cardiac birth defects are found in as many as 1 in 100 infants (Hoffman and Kaplan, 2002). The prevalence of these defects provides a strong motivation for studies of the molecular mechanisms responsible for normal cardiac form and function. Although it is suspected that many congenital heart diseases have a genetic basis (Pierpont et al., 2007), only a modest number of genes have been identified as disease loci (Bruneau, 2008; Ransom and Srivastava, 2007). Therefore, an increasingly growing community of investigators is eager to employ effective methods for determining the precise roles of essential genes during cardiogenesis.
In this regard, the zebrafish is an ideal model organism for studying the genetic regulation of heart development (Schoenebeck and Yelon, 2007). The zebrafish embryo is externally fertilized and transparent, allowing easy visualization of the heart and its contractility. The simple architecture of the zebrafish heart also aids analysis: it is composed of two major chambers, a ventricle and an atrium, each of which contains an inner layer of endocardium and an outer layer of myocardium. The particular characteristics of each cardiac tissue can be readily visualized with cellular resolution, facilitating distinctions between normal and aberrant phenotypes. In addition, since the zebrafish embryo does not require a functional cardiovascular system for its survival (Pelster and Burggren, 1996), cardiac defects can be analyzed in detail throughout embryogenesis.
To date, hundreds of studies have taken advantage of these benefits of the zebrafish for identifying crucial cardiac genes. Classical genetic screens have unearthed a prodigious number of mutations causing a variety of defects in the embryonic zebrafish heart (Alexander et al., 1998; Beis et al., 2005; Chen et al., 1996; Stainier et al., 1996; Warren et al., 2000). Notably, genetic screens have been particularly effective at identifying two large categories of mutant phenotypes: mutations that disrupt the initial formation of the midline heart tube (Alexander et al., 1998; Chen et al., 1996; Stainier et al., 1996) and mutations that disrupt cardiac function (Chen et al., 1996; Stainier et al., 1996; Warren et al., 2000). Additionally, knockdown of gene function via injection of gene-specific morpholinos has been instrumental in revealing the functions of numerous cardiac genes (Bill et al., 2009; Eisen and Smith, 2008). Small molecule screens have also contributed to the identification of essential cardiac pathways (Kaufman et al., 2009; Peal et al., 2010): for instance, several compounds have been shown to disrupt cardiac valve morphogenesis (Scherz et al., 2008), and others have been shown to influence cardiac action potential (Milan et al., 2003, 2009).
Whether caused by mutations, morpholinos, or small molecules, cardiac defects typically manifest as pericardial edema and faulty blood circulation that are evident by 48 h postfertilization (hpf), if not earlier. This common phenotype can result from a wide variety of origins including defects in the specification or the differentiation of cardiac progenitors (Fig. 1A,B); errors in the morphogenesis of the heart tube, the cardiac chambers, or the atrioventricular canal (AVC) (Fig. 1C–E); or problems with establishing proper cardiac function. Recent studies have pioneered a variety of experimental strategies for distinguishing between these possibilities and thereby deciphering the roots of cardiac defects. In this chapter, we provide a guide for investigators who aspire to use state-of-the-art techniques to characterize cardiac phenotypes in zebrafish embryos.
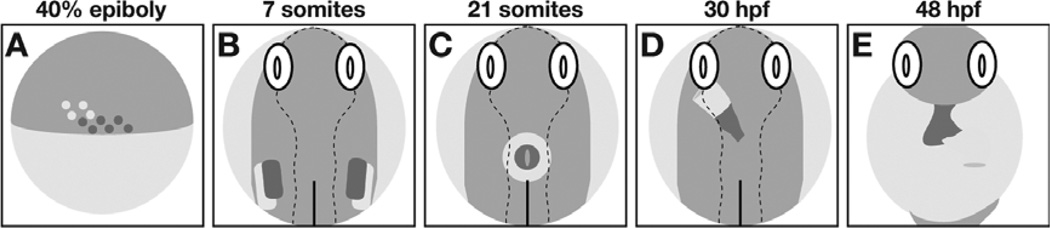
Fig. 1.
Phases of heart formation in the zebrafish embryo. (A) Schematic lateral view, dorsal to the right, of the late blastula. Ventricular (red) and atrial (yellow) myocardial progenitor cells are spatially organized within bilateral marginal zones at this stage. (B–D) Schematic dorsal views, anterior up, depicting the locations of ventricular (red) and atrial (yellow) cardiomyocytes during cardiac morphogenesis. (B) Myocardial progenitors migrate to form bilateral heart fields within the ALPM. (C) The process of cardiac fusion recruits cardiomyocytes to the embryonic midline, where they form a cardiac cone. (D) Continued migration of cardiomyocytes elongates the cardiac cone to create the heart tube. (E) Schematic frontal view, anterior up, depicting the morphologically distinct ventricle (red) and atrium (yellow) that are separated by a constriction at the atrioventricular canal following chamber emergence. Adapted from Schoenebeck and Yelon (2007).
II. Defects in Heart Size
Heart size defects can readily be assessed in the zebrafish embryo: an enlarged heart, a shrunken heart, or an apparent absence of cardiac tissue can easily be detected even on a dissecting microscope (Chen et al., 1996; Stainier et al., 1996). The perceptible size of the organ is a product of the number of cardiomyocytes and the morphology and arrangement of those individual cells. This section focuses on the possible causes of production of an inappropriate number of cardiac cells.
To determine whether heart size defects are a function of cell number defects, it is useful to employ a reporter transgene, Tg(cmlc2:DsRed2-nuc) (Mably et al., 2003), that expresses DsRed in the nuclei of all differentiated cardiomyocytes. Nuclear localization of the fluorescent signal facilitates counting of cardiomyocytes in individual embryos; combining this technique with the use of an antibody recognizing an atrium-specific myosin heavy chain (S46; anti-atrial myosin heavy chain (Amhc) (Berdougo et al., 2003)) allows quantitative analysis of the number of cells in each chamber (Schoenebeck et al., 2007). However, nuclear DsRed expression is most robust and resolvable in embryos that are 48 hpf or older, since the DsRed protein requires approximately 24 h to properly fold and localize after initial cardiac myosin light chain 2 (cmlc2) promoter activation (Baird et al., 2000; Bevis and Glick, 2002; Lepilina et al., 2006). To count cells at earlier stages, an alternate approach visualizes cardiomyocyte nuclei with an antibody recognizing the transcription factor Mef2, which is detectable in cardiomyocytes as early as 24 hpf (Targoff et al., 2008).
Increased or decreased numbers of cardiomyocytes could be the result of defects in the specification of cardiac progenitor cells. Fate maps of the late blastula have demonstrated that cardiac progenitors arise from bilateral multipotential zones within the embryonic margin (Fig. 1A) (Keegan et al., 2004). During gastrulation, the cardiac progenitors migrate to reside in bilateral fields in the anterior lateral plate mesoderm (ALPM) (Fig. 1B) (Keegan et al., 2004; Schoenebeck et al., 2007). Gastrula fate maps have shown that the dimensions of these heart fields correspond well with the expression pattern of hand2 in the ALPM (Schoenebeck et al., 2007). Differentiation of progenitors into cardiomyocytes begins around the 14 somite stage, as indicated by the expression of myocardial markers such as cmlc2 (Yelon et al., 1999). Ventricular and atrial progenitors are spatially organized both within the lateral margin and the ALPM (Fig. 1A,B) (Keegan et al., 2004; Schoenebeck et al., 2007). Once they differentiate, ventricular and atrial cardiomyocytes can be distinguished by molecular markers, such as ventricular myosin heavy chain (vmhc) and amhc (Fig. 1C) (Berdougo et al., 2003; Yelon et al., 1999). Thus, a failure to produce the right numbers of differentiated ventricular or atrial cardiomyocytes could reflect an alteration in the number of cardiac progenitors selected from within a multipotential zone, a change in the size of the heart fields within the ALPM, or a modification of the effective production of differentiated cells by the heart fields.
Fate mapping techniques can be employed to distinguish between these possibilities. Construction of a fate map in mutant, morphant, or drug-treated embryos can reveal alterations in the size of the cardiac progenitor pool, the organization of the progenitors, and their productivity in terms of the number of cardiomyocytes generated (Keegan et al., 2004, 2005; Schoenebeck et al., 2007; Thomas et al., 2008; Waxman et al., 2008). In these experiments, embryos are injected at the one-cell stage with a caged fluorescein dextran lineage tracer that can subsequently be photoactivated in selected cells using a laser (Keegan et al., 2004; Schoenebeck et al., 2007). The locations of the labeled cells are noted, and their contributions to the myocardium are assessed at later stages. In addition to determining whether the progeny of selected cells become ventricular and/or atrial cardiomyocytes, it is possible to count the number of cardiomyocytes derived from the labeled cells, particularly when using anti-fluorescein immunohistochemistry to detect the lineage tracer. This sensitive technique can resolve differences from the wild-type fate map and thereby indicate the origin of a defect in heart size: cardiac progenitors might be missing from their usual locations or might be found in atypical locations, ventricular and atrial progenitors might be disorganized, and individual progenitors might give rise to fewer or more cardiomyocytes than usual.
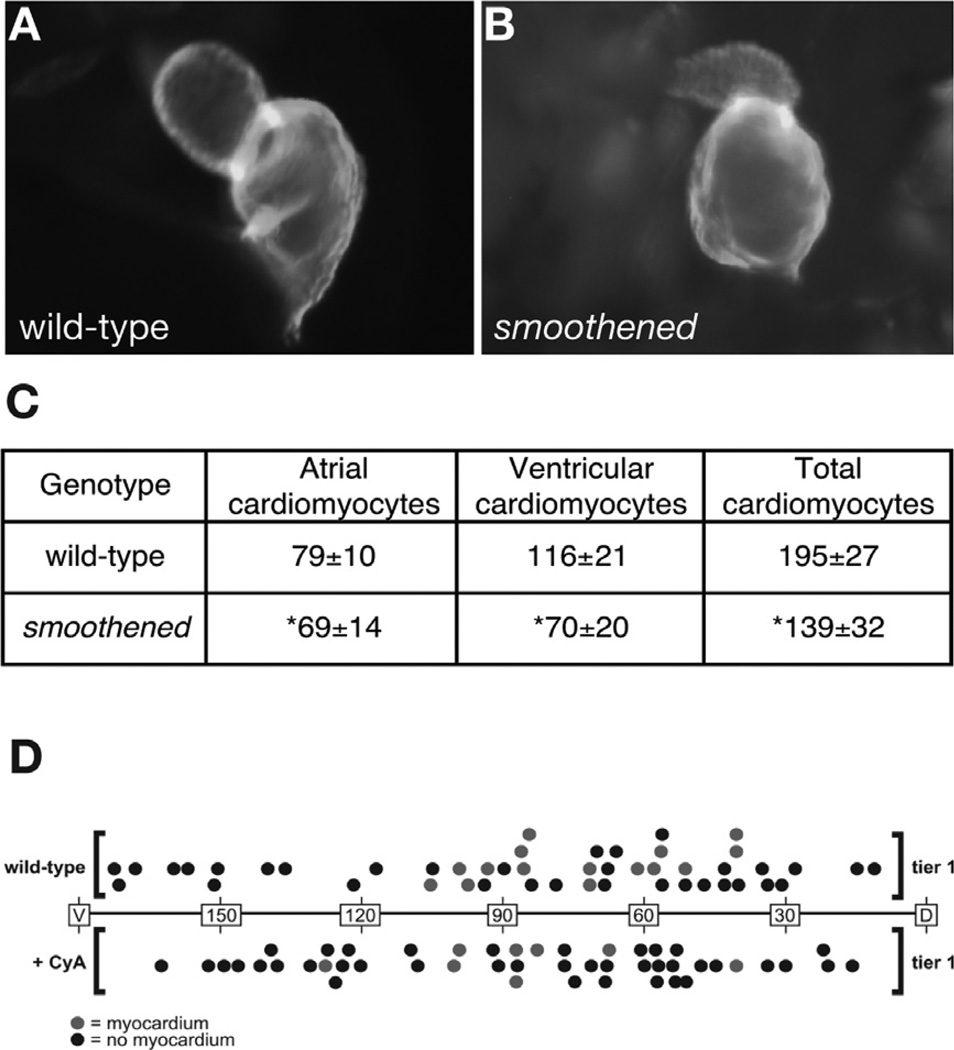
Several studies have utilized cell counting and fate mapping techniques to characterize heart size defects and their origins in zebrafish embryos (Schoenebeck et al., 2007; Thomas et al., 2008; Waxman et al., 2008). In one example, this combination of experimental strategies was used to show that hedgehog (Hh) signaling promotes the specification of cardiac progenitor cells (Thomas et al., 2008). In zebrafish smoothened mutants, Hh signaling is disrupted by the loss of function of the Smoothened receptor (Chen et al., 2001; Varga et al., 2001). These mutant embryos exhibit misshapen hearts that appear smaller than their wild-type counterparts (Fig. 2A,B) (Thomas et al., 2008). Cell-counting experiments demonstrated that the shrunken appearance of the smoothened mutant heart is a consequence of a reduced number of cardiomyocytes in both the ventricle and atrium (Fig. 2C) (Thomas et al., 2008). These results suggested that Hh signaling could modulate chamber size either through regulating specification of cardiac progenitors or proliferation of cardiomyocytes. Fate mapping in embryos treated with cyclopamine, a Smoothened antagonist, detected cardiac progenitor cells in the lateral margin only half as often as in wild-type control embryos (Fig. 2D), indicating inadequate specification of the cardiac progenitor population (Thomas et al., 2008). In contrast, the productivity of successfully specified progenitor cells remained normal in cyclopamine-treated (CyA) embryos (4.6-labeled cardiomyocytes per experiment in wild-type embryos and 4.5-labeled cardiomyocytes per experiment in cyclopamine-treated embryos), indicating that Hh signaling is not essential for cardiomyocyte proliferation at the stages examined (Thomas et al., 2008). These data, together with additional results, led to the conclusion that Hh signaling plays an important role in directing multipotential cells into the myocardial lineage.
Fig. 2.
Hedgehog signaling promotes the specification of myocardial progenitor cells. (A, B) Lateral views of wild-type (A) and smoothened mutant (B) hearts at 48 hpf. Immunofluorescence with MF20 and S46 antibodies allows visualization of the ventricle (red) and atrium (yellow), both of which are misshapen in smoothened mutants. (C) Quantification of cardiomyocyte number at 52 hpf in wild-type and smoothened mutant hearts. Values represent the mean cell number (± standard deviation) for each category, and asterisks indicate statistically significant differences from wild type. (D) Fate maps of myocardial progenitors at 40% epiboly in wild-type and cyclopamine-treated embryos. Longitude coordinates of the first tier of the embryonic margin are depicted with dorsal as the origin (0°) and ventral as 180°. Each dot represents an individual labeling experiment. Red dots represent embryos in which labeled blastomeres contributed to ventricular myocardium, and black dots represent embryos in which the labeled cells did not become cardiomyocytes. Adapted from Thomas et al. (2008).
In addition to resulting from altered progenitor specification or cardiomyocyte production, defects in heart size could be a consequence of inappropriate timing of myocardial differentiation. A recent study has shown that the zebrafish myocardium forms during two distinct phases ofmyocardial differentiation (de Pater et al., 2009). The first phase creates the primitive heart tube, beginning with the differentiation of the ventricle and progressing to the atrium. During the second phase, a separate population of late-differentiating cardiomyocytes forms the outflow tract at the arterial pole of the ventricle (Fig. 3). Therefore, defects in the number of cardiomyocytes could reflect ineffective execution of the timing of myocardial differentiation; for example, loss of Fgf signaling has been shown to inhibit the second phase of differentiation and thereby lead to an inadequate number of ventricular cardiomyocytes (de Pater et al., 2009).
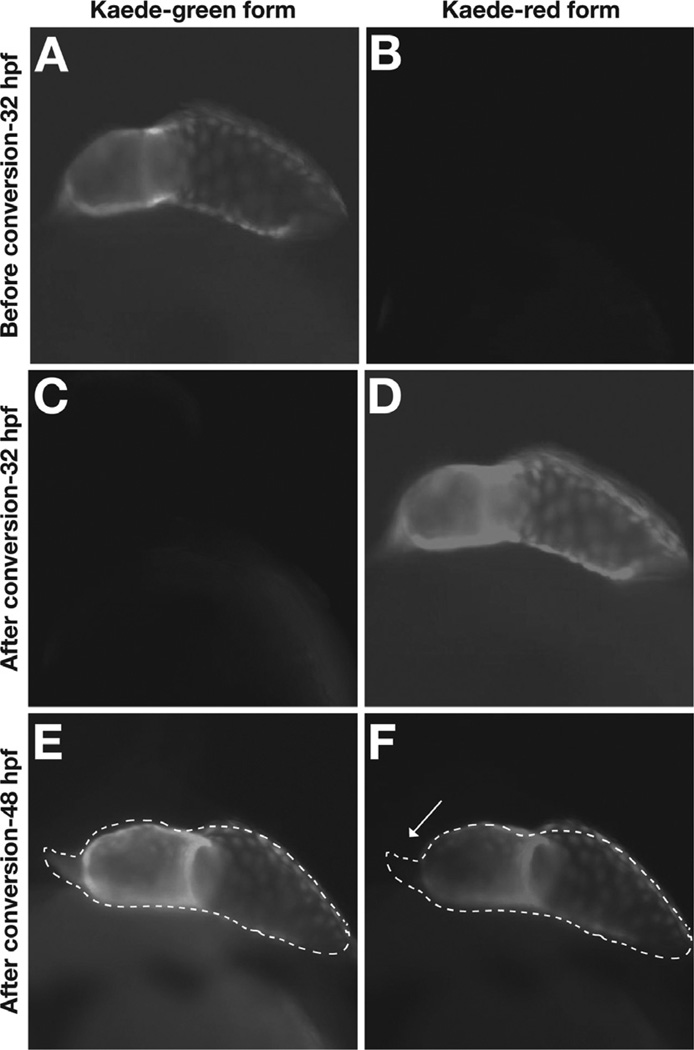
Fig. 3.
Photoconversion of Kaede provides an assay for the timing of cardiomyocyte differentiation. Lateral views of Tg(cmlc2:kaede) embryos, ventricle to the left. (A, B) Prior to photoconversion, the heart exhibits green, but not red, Kaede fluorescence. (C, D) After photoconversion, green Kaede fluorescence has been converted into red Kaede fluorescence. (E, F) Sixteen hours later, red Kaede fluorescence persists in the cells that were expressing Tg(cmlc2:kaede) at 32 hpf. These cells also contain newly generated green Kaede fluorescence. In contrast, newly differentiated cells in the outflow tract at the arterial pole (arrow) exhibit only green, and not red, Kaede fluorescence. Adapted from de Pater et al. (2009).
Two types of transgenic assays have been developed to monitor the timing of myocardial differentiation in zebrafish embryos, and both can be effective for detecting defects in timing. One strategy uses a pair of independent reporter transgenes (de Pater et al., 2009): a transgene that expresses GFP in differentiated cardiomyocytes under the control of the cmlc2 promoter, Tg(cmlc2:egfp) (Huang et al., 2003), and a transgene that expresses DsRed under the control of the same promoter, either Tg(cmlc2:DsRed2-nuc) (Mably et al., 2003) or Tg(cmlc2:dsRed) (Kikuchi et al., 2010). This assay takes advantage of the difference in GFP and DsRed protein-folding kinetics to distinguish early-differentiating and late-differentiating populations of cells (de Pater et al., 2009; Lepilina et al., 2006). Since DsRed requires more time to mature and fluoresce than does GFP, early-differentiating cardiomyocytes express both GFP and DsRed at timepoints when the late-differentiating cells express only GFP (de Pater et al., 2009). Thus, confocal imaging of DAPI-stained double transgenic embryos facilitates counting of both early-differentiated and late-differentiated cardiomyocyte nuclei; for example, analysis of double transgenic embryos at 48 hpf has been used to examine whether heart size defects are due to an altered number of late-differentiating cells at the arterial pole of the ventricle (de Pater et al., 2009).
A second, complementary strategy uses a reporter transgene, Tg(cmlc2:kaede) (de Pater et al., 2009), that expresses the green-to-red photoconvertible fluorescent protein Kaede under the control of the cmlc2 promoter. Prior to photoconversion, differentiated cardiomyocytes exhibit green fluorescence but not red fluorescence (Fig. 3A,B). Upon exposure to UV light, the Kaede protein is cleaved and converts from its green form to its red form (Fig. 3C,D). At later stages, any cells exhibiting green fluorescence, but not red fluorescence, are interpreted as having initiated differentiation after the time of photoconversion. For example, photoconversion of embryos at 32 hpf, followed by the examination of fluorescence at 48 hpf, reveals green fluorescent cardiomyocytes at the arterial pole of the ventricle (Fig. 3E,F). Thus, this assay can be used to identify embryos with defects in the late differentiation of arterial pole cardiomyocytes.
III. Defects in Heart Shape
In order for the heart to be an effective pump, the cardiac chambers and the atrioventricular valve (AVV) need to acquire specific morphologies that are crucial for their function. Because of the dynamic nature of cardiac morphogenesis, defects in heart shape can have a variety of origins, ranging from complete failure of cardiomyocyte migration at early stages to subtle displacement of atrioventricular cushions at later stages. In this section, we discuss a series of experimental strategies for determining the possible causes of a misshapen heart in a zebrafish embryo.
A number of zebrafish mutations have been shown to cause dramatic defects in cardiomyocyte migration during early steps of heart morphogenesis (Kikuchi et al., 2000, 2001; Kupperman et al., 2000; Reiter et al., 1999). Instead of exhibiting a single heart at the midline, these embryos have a pair of separate hearts in bilateral positions, a condition known as cardia bifida. During wild-type development, differentiated cardiomyocytes move from their lateral positions in the ALPM toward the embryonic midline, where they merge to assemble the heart tube through a process called cardiac fusion (Fig. 1C) (Glickman and Yelon, 2002). In embryos with cardia bifida, cardiac fusion fails because cardiomyocytes fail to migrate to the midline; this phenotype is typically evident in the aberrant expression patterns of myocardial markers, such as cmlc2, between 18 and 22 hpf. Analyses of cardia bifida mutants have highlighted two different requirements for medial cardiomyocyte movement. One group of cardia bifida mutations (e.g., casanova, bonnie and clyde, faust, miles apart) disrupt the formation of the anterior endoderm that is adjacent to the migrating myocardium (Alexander et al., 1999; Kikuchi et al., 2000, 2001; Kupperman et al., 2000; Reiter et al., 1999), and a second group of mutations (e.g., natter, hands off) disrupt the organization of the extracellular matrix (ECM) that surrounds the cardiomyocytes (Garavito-Aguilar et al., 2010; Trinh and Stainier, 2004). Together, these phenotypes indicate the importance of myocardium–endoderm and myocardium–ECM interactions during cardiac fusion. Therefore, when investigating new cardia bifida phenotypes, it is valuable to examine the specification and morphogenesis of the anterior endoderm, using appropriate markers (e.g., Tg(-0.7her5:EGFP), axial, sox17 (Kawahara et al., 2009; Kupperman et al., 2000; Osborne et al., 2008; Tallafuss and Bally-Cuif, 2003)), as well as the deposition and composition of the ECM, using immunofluorescent detection of relevant components (e.g., fibronectin and laminin (Arrington and Yost, 2009; Garavito-Aguilar et al., 2010; Trinh and Stainier, 2004)).
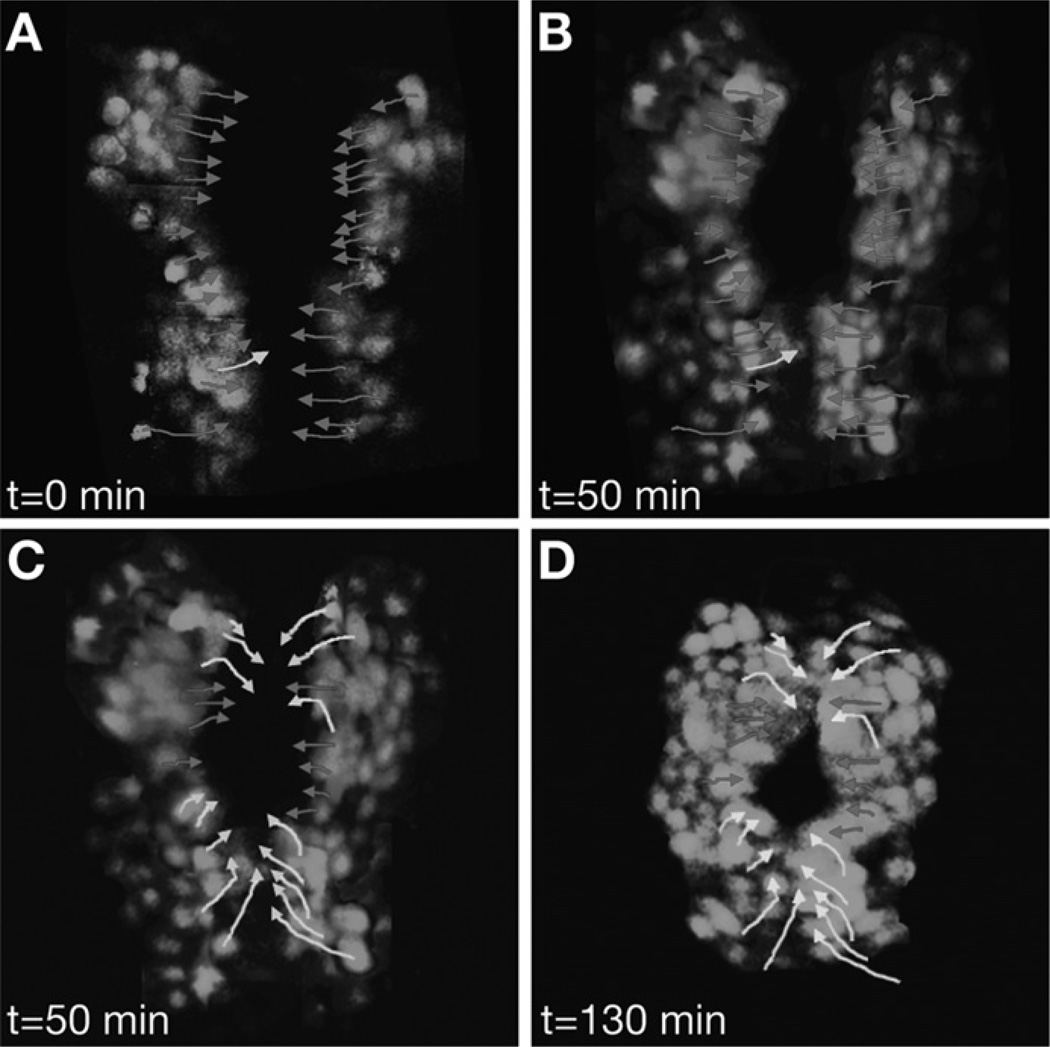
Subtle errors in cardiomyocyte movement do not necessarily result in a cardia bifida phenotype but can still interfere with the shape of the heart tube. Time-lapse confocal microscopy has been instrumental in elucidating the wild-type patterns of directed cardiomyocyte movements that underlie heart tube dimensions (Baker et al., 2008; de Campos-Baptista et al., 2008; Holtzman et al., 2007; Smith et al., 2008). Wild-type cardiac fusion begins with medially directed movements of the cardiomyocytes that recruit the bilateral populations toward each other (Fig. 4A,B) (Holtzman et al., 2007). Then, anterior and posterior subsets of cardiomyocytes change to an angular direction of movement in order to construct a ring-like configuration of myocardium at the midline, referred to as the cardiac cone (Figs. 1C and 4C,D) (Holtzman et al., 2007). Finally, the cardiac cone tilts to the left, due to the differential leftward migration of cardiomyocytes: cells in the posterior region of the cone exhibit a greater overall leftward displacement compared with cells in the anterior region of the cone (Fig. 5) (Smith et al., 2008). Continued leftward and anterior migration of cardiomyocytes completes the elongation of the heart tube (Fig. 1D). Thus, heart tube morphology depends on the coordination of carefully choreographed patterns of cell movement, and disruption of these patterns can result in defects in heart shape.
Fig. 4.
Tracking the paths of individual cardiomyocytes during cardiac fusion. Selected images from a time-lapse of cardiac fusion in a wild-type embryo expressing Tg(cmlc2:egfp) between the 16 and 20 somite stages; dorsal views, anterior to the top. Arrows indicate paths traveled by individual cells from their starting positions (A, C) to their ending positions (B, D). Red arrows indicate medial movement, and yellow arrows indicate angular movement. (A, B) Cardiac fusion initiates with medial movement toward the midline. (C, D) Cardiomyocytes in anterior and posterior regions and then transition into angular patterns of movement that create the cardiac cone. Adapted from Holtzman et al. (2007).
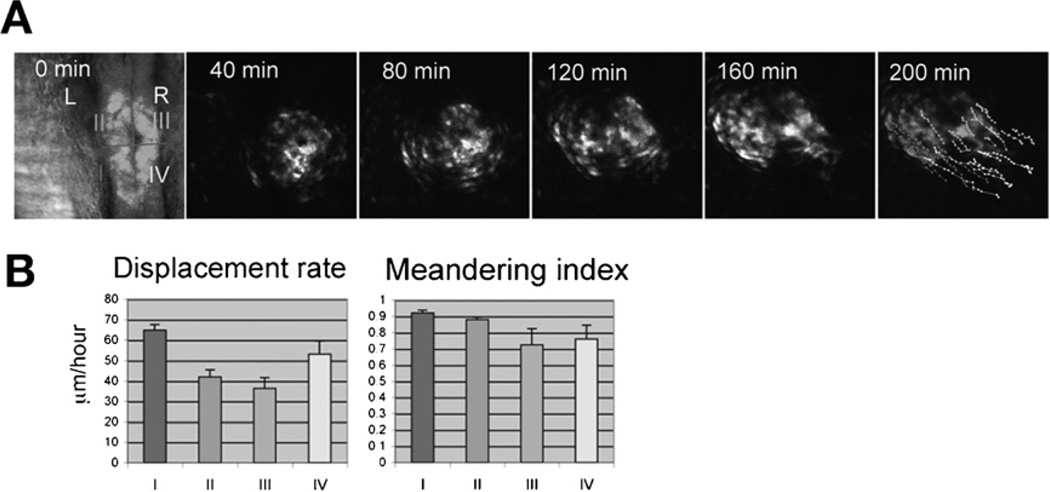
Fig. 5.
Patterns of cardiomyocyte movement during heart tube elongation. (A) Selected images from a time-lapse of heart tube elongation in a wild-type embryo expressing Tg(cmlc2:egfp) beginning at the 23-somite stage; dorsal views, anterior to the top. The cardiac cone was divided into four regions (I–IV), and the movements of individual cardiomyocytes from each region were tracked. (B) Bar graphs indicate the mean displacement rate and meandering index for tracked cardiomyocytes; error bars indicate standard errors. Comparisons of the displacement rate (displacement/time) and meandering index (displacement/track length) for each quadrant indicate that posterior cardiomyocytes exhibit higher rates of displacement than anterior cardiomyocytes, whereas the meandering index is comparable for each subset of cardiomyocytes. Adapted from Smith et al. (2008).
To distinguish whether a dysmorphic heart originates with problems during cardiac fusion or tube elongation, it is sufficient to use myocardial markers, such as cmlc2, vmhc, and amhc, at a series of timepoints between 16 and 30 hpf to identify the first signs of abnormal cardiac morphology. Additional information about the cellular mechanisms underlying morphological defects can come from examination of markers that exhibit apicobasal polarity in cardiomyocytes, such as aPKC, β-catenin, and ZO-1 (Rohr et al., 2006; Trinh and Stainier, 2004); apicobasal polarity is disrupted by mutations in heart and soul (has; prcki) and nagie oko (nok; mpp5) (Horne-Badovinac et al., 2001; Peterson et al., 2001; Rohr et al., 2006), both of which block heart tube elongation. Finally, to determine the specific type of cell movement defects disrupting cardiac cone or heart tube formation, it is necessary to conduct appropriate time-lapse analyses. Several studies have utilized the transgene Tg(cmlc2:egfp) (Huang et al., 2003) to track individual cardiomyocyte movements through time-lapse confocal microscopy (Baker et al., 2008; de Campos-Baptista et al., 2008; Holtzman et al., 2007; Rohr et al., 2008; Smith et al., 2008). Such analyses can ascertain multiple parameters related to cell movement including direction of cell displacement (medial, angular, leftward, anterior, etc.), rate of cell displacement, and degree of meandering (Figs. 4 and 5). For example, time-lapse analysis of cardiomyocyte movements during cardiac fusion in cloche mutant embryos revealed normal medial movement toward the midline but failure to execute angular movements, resulting in a dysmorphic cardiac cone (Holtzman et al., 2007). Since cloche mutants lack endocardium, these data suggested that myocardium–endocardium interactions play an important role in directing cardiomyocyte behavior. In other examples, reduction of BMP signaling and reduction of Nodal signaling were shown to alter the direction, speed, and coherence of cardiomyocyte displacement during tube elongation, demonstrating the importance of these signaling pathways for driving leftward cell movement (de Campos-Baptista et al., 2008; Smith et al., 2008).
Although many types of heart shape defects originate during heart tube assembly, dysmorphic phenotypes can also appear during the stages of cardiac chamber emergence. Between 24 and 48 hpf, the heart tube transforms into morphologically evident cardiac chambers that exhibit distinct curvatures: a bulging concave curvature designated as the outer curvature (OC) and a recessed convex curvature called the inner curvature (IC) (Fig. 1D,E) (Auman et al., 2007). Regional changes in cardiomyocyte size and shape create the differences between the chamber curvatures. In the linear heart tube (LHT), ventricular cardiomyocytes appear relatively small and round. During chamber emergence, cells in the ventricular OC become enlarged and elongated, creating a bulging morphology, whereas cells in the ventricular IC retain a rounded morphology and exhibit a smaller increase in size (Fig. 6) (Auman et al., 2007). Errors in the execution of these cell shape changes can result in abnormal ventricular shape: a compact and narrow ventricle if cells fail to expand normally or a dilated and round ventricle if expansion is excessive (Auman et al., 2007).
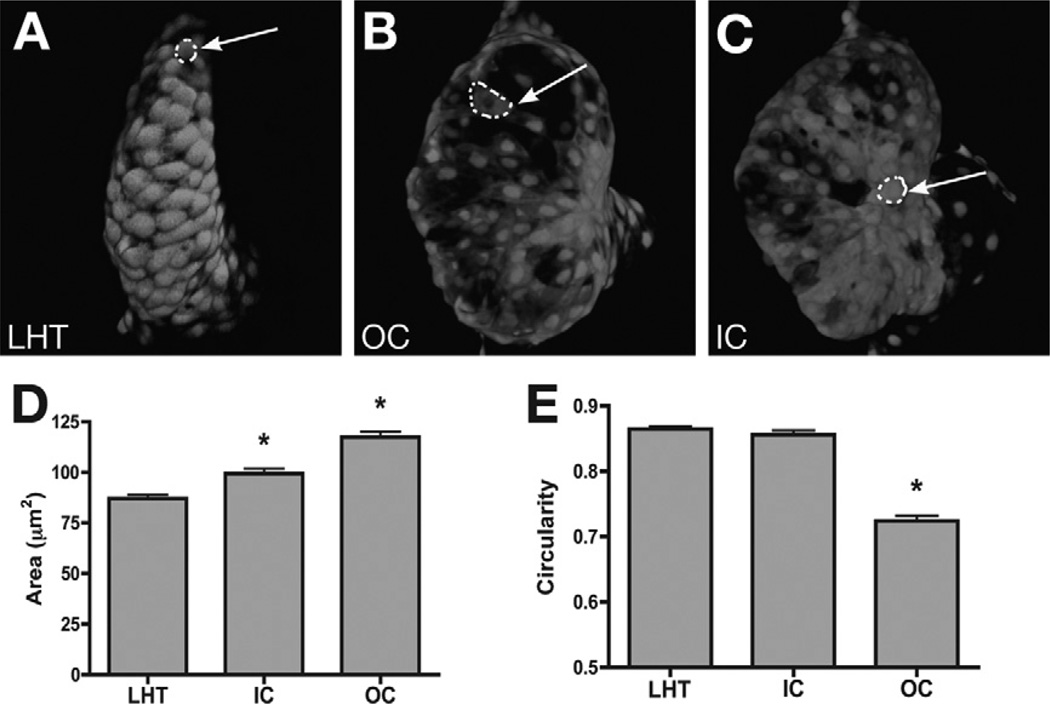
Fig. 6.
Regionally confined cell shape changes underlie the emergence of chamber curvatures. (A–C) Confocal projections of live hearts expressing Tg(cmlc2:egfp) with mosaic expression of Tg(cmlc2:dsRed). Arrows point to representative cells expressing both dsRed and egfp. Ventricular cells in the LHTat 28 hpf (A) and in the IC at 52 hpf (C) are relatively cuboidal, whereas cells in the OC at 52 hpf (B) are flattened and elongated. (D, E) Bar graphs indicate the mean surface area and circularity index of cells in the LHT, IC, and OC. Error bars indicate standard error, and asterisks indicate statistically significant differences from LHT values. OC cells exhibit an increase in surface area with an accompanying decrease in circularity. Adapted from Auman et al. (2007).
To determine if a dysmorphic chamber phenotype is caused by defects in cardiomyocyte morphology, it is necessary to visualize and assess cardiomyocyte size and shape using a marker that outlines cell boundaries (e.g., phalloidin or an anti-Dm-grasp antibody) together with a myocardial reporter transgene like Tg(cmlc2:egfp) (Auman et al., 2007; Deacon et al., 2010). Size is typically expressed in terms of cardiomyocyte surface area, and shape is typically evaluated using a circularity index that quantifies divergence from a perfectly circular morphology (Fig. 6D, E). For example, morphometric analysis has shown that knockdown of the microRNA mir-143 results in ventricular OC cells that are small and circular, having failed to undergo the expansion and elongation seen in wild-type embryos; these results indicate an important role of this microRNA in modulating the expression of determinants of cardiomyocyte morphology (Deacon et al., 2010). When evaluating the origins of chamber emergence defects, it is also important to keep in mind that defects in cardiac function, such as reduced blood flow and/or reduced contractility, can have a significant impact on cardiomyocyte morphology (Auman et al., 2007). In this regard, it is valuable to consider whether an observed dysmorphic chamber could be a secondary consequence of a primary defect in heart function.
In addition to being caused by defects in heart tube assembly and chamber emergence, dysmorphic hearts can result from specific errors in the development of the AVC, a constriction of the heart tube found at the boundary between the atrium and the ventricle (Fig. 1E). In wild-type embryos, specification of the AVC is made evident by the restricted expression of markers such as bmp4 and versican in the myocardium and notch1b and has2 in the endocardium (Hurlstone et al., 2003; Walsh and Stainier, 2001). As AVC differentiation proceeds, the adhesion molecule Dm-grasp is detectable in the AVC endocardial cells, but not in the remainder of the endocardium (Beis et al., 2005). This is accompanied by distinct cell morphology changes in both the endocardium and myocardium: endocardial cells undergo transition from a squamous shape to a cuboidal shape, while myocardial cells undergo apical constriction and extend their basolateral surfaces (Beis et al., 2005; Chi et al., 2008a). The AVC endocardial cushions will subsequently be remodeled into the leaflets of the AVV (Scherz et al., 2008).
Errors in AVC formation frequently lead to a failure of constriction at the atrioventricular boundary that is morphologically apparent by 48 hpf. Additionally, AVC defects often result in retrograde blood flow, visible as blood vacillating in between the chambers (Beis et al., 2005; Stainier et al., 1996). To determine whether these phenotypes indicate an error in AVC specification or differentiation, it is appropriate to use the AVC molecular markers described above, as well as fluorescent reporter transgenes (such as Tg(cmlc2:ras-gfp) and Tg(flk1:ras-cherry)) to mark myocardial and endocardial cell borders and thereby visualize AVC cell shapes (Chi et al., 2008a). For example, one group of mutations (e.g., jekyll, slipjig) have been shown to disrupt the initial specification of the AVC; these mutants fail to exhibit restricted expression of AVC molecular markers and fail to undergo AVC-specific myocardial and endocardial cell shape changes (Chi et al., 2008a; Walsh and Stainier, 2001). Conversely, adenomatous polyposis coli (apc) mutants fail to restrict AVC differentiation to the proper location: the cardiac chambers in apc mutant embryos exhibit expanded expression of AVC markers and excessive endocardial cushions that extend beyond their normal boundaries (Hurlstone et al., 2003).
Subtle errors in valve function can cause retrograde blood flow even when initial AVC specification and differentiation occur normally. The use of specialized fluorescent microscopy techniques such as selective plane illumination microscopy (SPIM), which can capture optical sections in thick samples rapidly and with minimal photobleaching (Huisken and Stainier, 2009; Huisken et al., 2004), has been instrumental in visualizing valve function defects in live embryos. SPIM analysis of wild-type embryos expressing Tg(flk1:egfp) (Jin et al., 2005) in endocardial cells and Tg(gata1:dsRed) (Traver et al., 2003) in erythrocytes has revealed key features of AVV maturation and function (Fig. 7) (Scherz et al., 2008). At 55 hpf, the AVV undergoes three phases of motion: myocardial contractions bring the sides of the AVC together (Fig. 7A), then the juxtaposed sides of the endocardium engage in a rolling motion (Fig. 7B), and, finally, the AVC relaxes (Fig. 7C). At this stage, the relatively immature AVV is unable to prevent retrograde blood flow. Over time, the increasing thickness of the AVV begins to occlude the AVC lumen, preventing retrograde blood flow upon relaxation (Fig. 7D–F). Thus, examination via SPIM can reveal regulators of specific aspects of AVV morphology and behavior. For example, treatment of embryos with Cox2 inhibitors has been shown to displace the AVV toward the ventricle and thereby interfere with AVV rolling and lumen occlusion, indicating the importance of the Cox2 signaling pathway for the prevention of retrograde blood flow (Scherz et al., 2008).
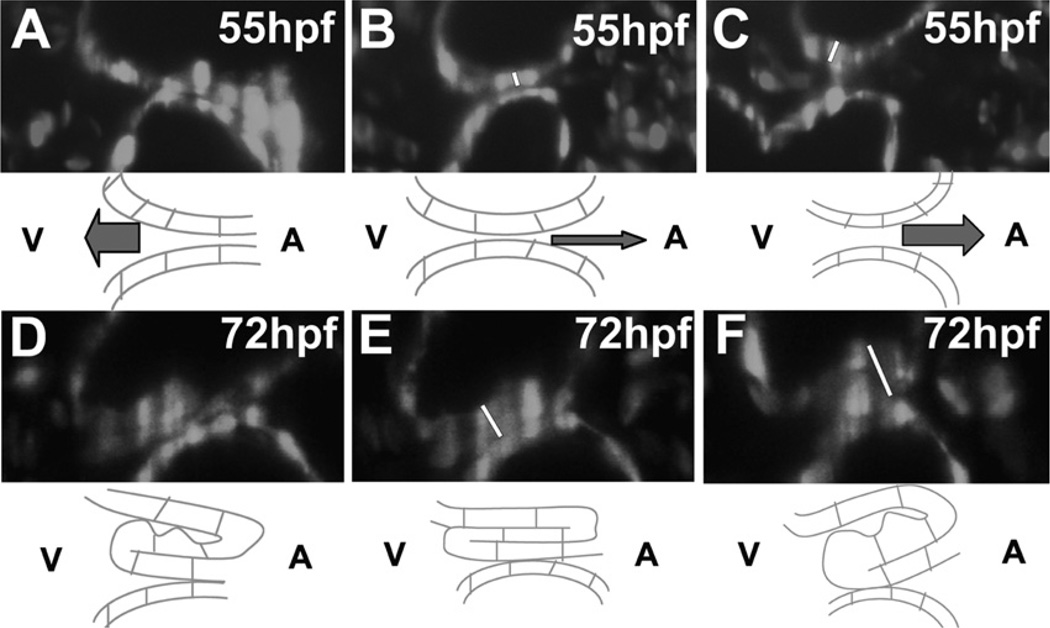
Fig. 7.
Time-lapse imaging of atrioventricular valve function during development. Selected images from SPIM analysis of embryos expressing Tg(flk1:egfp) and Tg(gata1:dsRed) at 55 hpf (A–C) and 72 hpf (D–F), with accompanying schematics. (A–C) At 55 hpf, the AVC initially closes as a consequence of myocardial contraction (A), then closes further through a rolling motion (B), and finally relaxes in order to reopen (C). (D–F) Similar motions take place at 72 hpf, but the increased thickness of the AVC endocardium occludes the lumen and prevents retrograde blood flow during the steps of rolling and relaxation. Adapted from Scherz et al. (2008).
IV. Defects in Cardiac Function
The heart must regulate circulation efficiently with appropriately robust contractions triggered by propagated electrical impulses. Defects in cardiac function can range from absence of heartbeat to subtle arrhythmia, and these problems can be caused by abnormalities in the contractile apparatus or the conduction system. In this section, we discuss several experimental techniques that are suitable for determining the causes of defective heart function.
It is convenient to monitor heart function in the zebrafish embryo, since the contracting heart and flowing blood are readily visible on a dissecting microscope. The heart tube begins to contract by 24 hpf, and robust, serial contractions of the atrium and ventricle are apparent by 48 hpf. Correspondingly, sarcomere assembly begins within the primitive heart tube, and mature sarcomeres are organized by the time of chamber emergence (Huang et al., 2009). The cardiac conduction system matures over the same timeframe. Within the heart tube, electrical activity travels unidirectionally and smoothly from the venous pole to the arterial pole (Chi et al., 2008b;Milan et al., 2006). Once the cardiac chambers form, a cardiac conduction delay separates the atrial and ventricular rhythms; this delay is a consequence of the formation of specialized conduction tissue at the AVC (Fig. 8) (Chi et al., 2008b; Milan et al., 2006).
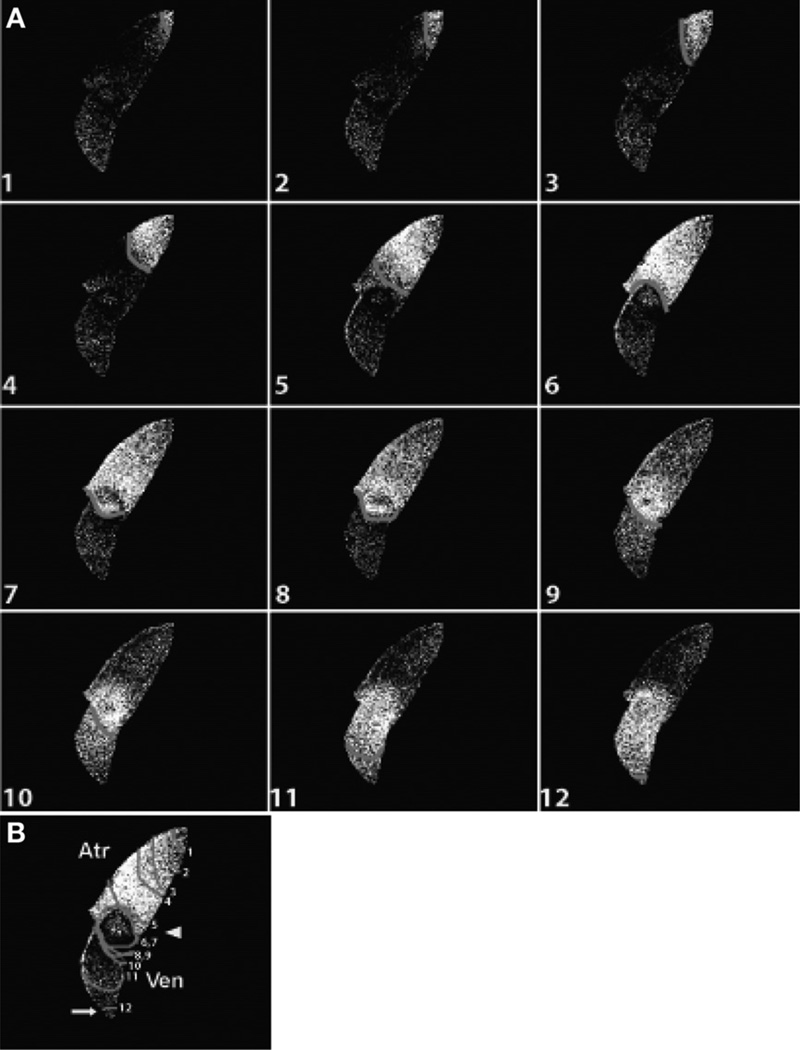
Fig. 8.
Optical mapping of cardiac conduction. (A) Sequence of images depicts calcium activation initiating in the sinus venosus (top) and concluding in the ventricle (bottom) of a live heart expressing Tg (cmlc2:gCaMP) at 48 hpf. (B) Optical map of the pattern of calcium excitation shown in (A); isochronal lines represent every 60 ms. Images 5–10 (A) and isochronal lines 5–10 (B) indicate conduction delay at the AVC. Adapted from Chi et al. (2008).
Qualitative assessment of heart function can rapidly discern the presence of severe defects such as failure of chamber contraction or lack of blood flow through the dorsal aorta. Quantitative methods can provide a more refined understanding of less dramatic phenotypes. Assessment of heart rate is straightforward and can elucidate even subtle alterations in the speed and rhythm of contraction. Additionally, degree of contractility can be quantified using high-speed video microscopy to determine ventricular fractional shortening, a measurement of systolic contractile function normalized to the diameter of the heart (Fink et al., 2009; Rottbauer et al., 2005, 2006). Finally, dynamics of blood flow can be measured by tracking movement of erythrocytes or fluorescent molecules introduced into circulation (Hove et al., 2003; Schwerte and Pelster, 2000); alterations in the pace or pattern of blood flow can provide a complementary indication of subtle aberrations in cardiac function.
To determine whether functional defects originate with contractile apparatus abnormalities, it is effective to use transmission electron microscopy to inspect sarcomere structure directly. Examination of sarcomere formation between 24 and 48 hpf can distinguish between errors in myofibril assembly and maintenance. For example, in silent heart mutants, which lack the cardiac troponin T gene tnnt2, myofibrils fail to assemble, as the thick filaments are disorganized in the absence of normal thin filaments (Sehnert et al., 2002). In contrast, in pickwick mutants, which lack titin, nascent myofibrils form normally but higher-order sarcomeric structures are absent, suggesting a key role of Titin in sarcomere organization and maintenance (Xu et al., 2002).
For heart function phenotypes that are not associated with defects in the contractile apparatus, it is logical to investigate whether cardiac conduction is aberrant. Electrical currents can be assayed in vivo by electrocardiography, and similar patch clamp techniques can be used to stimulate the heart to test its excitability (Rottbauer et al., 2001). For optical mapping of cardiac conduction, calcium flux can be monitored using fluorescent dyes (Ebert et al., 2005; Langenbacher et al., 2005; Milan et al., 2006) or with a fluorescent calcium indicator transgene (Tg(cmlc2:gCaMP)) (Chi et al., 2008b), and transmembrane action potential can be evaluated using voltage-sensitive dyes (Panáková et al., 2010). This combination of techniques can detect a wide variety of conduction abnormalities, ranging from cell-intrinsic defects in calcium handling to failure to develop specific types of conduction tissue. For example, the arrhythmia observed in tremblor (ncx1h) mutants is caused by defects in calcium extrusion that result in elevated intracellular calcium in cardiomyocytes (Ebert et al., 2005; Langenbacher et al., 2005). In a different case, slipjig (foxn4) mutants fail to specify the AVC and therefore do not develop specialized AVC conduction tissue; as a consequence, they exhibit an absence of atrioventricular conduction delay (Chi et al., 2008a).
V. Summary
A wide variety of techniques are available to investigators seeking to determine the origins of defects in heart size, shape, and function in the zebrafish embryo. Whereas some of the applicable techniques require specific reporter transgenes or specialized microscopes, most are readily accessible and should facilitate characterization of cardiac phenotypes in a broad range of laboratories. Experimental strategies will continue to evolve as more molecular markers, new transgenes, and advances in microscopy enhance the resolution of our inspection of cardiac development. These future prospects, together with the always increasing number of relevant mutations, morpholinos, and small molecules, promise a strong continuation of the utility of the zebrafish for illuminating the molecular mechanisms responsible for cardiogenesis.
References
- Alexander J, Stainier DY, Yelon D. Screening mosaic F1 females for mutations affecting zebrafish heart induction and patterning. Dev. Genet. 1998;22:288–299. doi: 10.1002/(SICI)1520-6408(1998)22:3<288::AID-DVG10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Alexander J, Rothenberg M, Henry GL, Stainier DY. casanova plays an early and essential role in endoderm formation in zebrafish. Dev. Biol. 1999;215:343–357. doi: 10.1006/dbio.1999.9441. [DOI] [PubMed] [Google Scholar]
- Arrington CB, Yost HJ. Extra-embryonic syndecan 2 regulates organ primordia migration and fibrillogenesis throughout the zebrafish embryo. Development. 2009;136:3143–3152. doi: 10.1242/dev.031492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auman HJ, Coleman H, Riley HE, Olale F, Tsai HJ, Yelon D. Functional modulation of cardiac form through regionally confined cell shape changes. PLoS Biol. 2007;5:e53. doi: 10.1371/journal.pbio.0050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird GS, Zacharias DA, Tsien RY. Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11984–11989. doi: 10.1073/pnas.97.22.11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K, Holtzman NG, Burdine RD. Direct and indirect roles for nodal signaling in two axis conversions during asymmetric morphogenesis of the zebrafish heart. Proc. Natl. Acad. Sci. U.S.A. 2008;105:13924–13929. doi: 10.1073/pnas.0802159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beis D, Bartman T, Jin SW, Scott IC, D’Amico LA, Ober EA, Verkade H, Frantsve J, Field HA, Wehman A, Baier H, Tallafuss A, Bally-Cuif L, Chen JN, Stainier DY, Jungblut B. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development. 2005;132:4193–4204. doi: 10.1242/dev.01970. [DOI] [PubMed] [Google Scholar]
- Berdougo E, Coleman H, Lee DH, Stainier DY, Yelon D. Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development. 2003;130:6121–6129. doi: 10.1242/dev.00838. [DOI] [PubMed] [Google Scholar]
- Bevis BJ, Glick BS. Rapidly maturing variants of the discosoma red fluorescent protein (DsRed) Nat. Biotechnol. 2002;20:83–87. doi: 10.1038/nbt0102-83. [DOI] [PubMed] [Google Scholar]
- Bill BR, Petzold AM, Clark KJ, Schimmenti LA, Ekker SC. A primer for morpholino use in zebrafish. Zebrafish. 2009;6:69–77. doi: 10.1089/zeb.2008.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451:943–948. doi: 10.1038/nature06801. [DOI] [PubMed] [Google Scholar]
- Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Nusslein-Volhard C. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293–302. doi: 10.1242/dev.123.1.293. [DOI] [PubMed] [Google Scholar]
- Chen W, Burgess S, Hopkins N. Analysis of the zebrafish smoothened mutant reveals conserved and divergent functions of hedgehog activity. Development. 2001;128:2385–2396. doi: 10.1242/dev.128.12.2385. [DOI] [PubMed] [Google Scholar]
- Chi NC, Shaw RM, De Val S, Kang G, Jan LY, Black BL, Stainier DY. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 2008a;22:734–739. doi: 10.1101/gad.1629408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NC, Shaw RM, Jungblut B, Huisken J, Ferrer T, Arnaout R, Scott I, Beis D, Xiao T, Baier H, Jan LY, Tristani-Firouzi M, Stainier DY. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol. 2008b;6:e109. doi: 10.1371/journal.pbio.0060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Campos-Baptista MI, Holtzman NG, Yelon D, Schier AF. Nodal signaling promotes the speed and directional movement of cardiomyocytes in zebrafish. Dev. Dyn. 2008;237:3624–3633. doi: 10.1002/dvdy.21777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pater E, Clijsters L, Marques SR, Lin YF, Garavito-Aguilar ZV, Yelon D, Bakkers J. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development. 2009;136:1633–1641. doi: 10.1242/dev.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon DC, Nevis KR, Cashman TJ, Zhou Y, Zhao L, Washko D, Guner-Ataman B, Burns CG, Burns CE. The miR-143-adducin3 pathway is essential for cardiac chamber morphogenesis. Development. 2010;137:1887–1896. doi: 10.1242/dev.050526. [DOI] [PubMed] [Google Scholar]
- Ebert AM, Hume GL, Warren KS, Cook NP, Burns CG, Mohideen MA, Siegal G, Yelon D, Fishman MC, Garrity DM. Calcium extrusion is critical for cardiac morphogenesis and rhythm in embryonic zebrafish hearts. Proc. Natl. Acad. Sci. U.S.A. 2005;102:17705–17710. doi: 10.1073/pnas.0502683102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen JS, Smith JC. Controlling morpholino experiments: don’t stop making antisense. Development. 2008;135:1735–1743. doi: 10.1242/dev.001115. [DOI] [PubMed] [Google Scholar]
- Fink M, Callol-Massot C, Chu A, Ruiz-Lozano P, Izpisua Belmonte JC, Giles W, Bodmer R, Ocorr K. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques. 2009;46:101–113. doi: 10.2144/000113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavito-Aguilar ZV, Riley HE, Yelon D. Hand2 ensures an appropriate environment for cardiac fusion by limiting Fibronectin function. Development. 2010 doi: 10.1242/dev.052225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman NS, Yelon D. Cardiac development in zebrafish: coordination of form and function. Semin. Cell Dev. Biol. 2002;13:507–513. doi: 10.1016/s1084952102001040. [DOI] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Holtzman NG, Schoenebeck JJ, Tsai HJ, Yelon D. Endocardium is necessary for cardiomyocyte movement during heart tube assembly. Development. 2007;134:2379–2386. doi: 10.1242/dev.02857. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, Lin D, Waldron S, Schwarz M, Mbamalu G, Pawson T, Jan Y, Stainier DY, Abdelilah-Seyfried S. Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr. Biol. 2001;11:1492–1502. doi: 10.1016/s0960-9822(01)00458-4. [DOI] [PubMed] [Google Scholar]
- Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- Huang CJ, Tu CT, Hsiao CD, Hsieh FJ, Tsai HJ. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev. Dyn. 2003;228:30–40. doi: 10.1002/dvdy.10356. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang R, Xu X. Myofibrillogenesis in the developing zebrafish heart: A functional study of tnnt2. Dev. Biol. 2009;331:237–249. doi: 10.1016/j.ydbio.2009.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 2004;305:1007–1009. doi: 10.1126/science.1100035. [DOI] [PubMed] [Google Scholar]
- Huisken J, Stainier DY. Selective plane illumination microscopy techniques in developmental biology. Development. 2009;136:1963–1975. doi: 10.1242/dev.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH, Clevers H. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–637. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- Kaufman CK, White RM, Zon L. Chemical genetic screening in the zebrafish embryo. Nat. Protoc. 2009;4:1422–1432. doi: 10.1038/nprot.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- Keegan BR, Meyer D, Yelon D. Organization of cardiac chamber progenitors in the zebrafish blastula. Development. 2004;131:3081–3091. doi: 10.1242/dev.01185. [DOI] [PubMed] [Google Scholar]
- Keegan BR, Feldman JL, Begemann G, Ingham PW, Yelon D. Retinoic acid signaling restricts the cardiac progenitor pool. Science. 2005;307:247–249. doi: 10.1126/science.1101573. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Trinh LA, Reiter JF, Alexander J, Yelon D, Stainier DY. The zebrafish bonnie and clyde gene encodes a Mix family homeodomain protein that regulates the generation of endodermal precursors. Genes Dev. 2000;14:1279–1289. [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Agathon A, Alexander J, Thisse C, Waldron S, Yelon D, Thisse B, Stainier DY. casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001;15:1493–1505. doi: 10.1101/gad.892301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupperman E, An S, Osborne N, Waldron S, Stainier DY. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature. 2000;406:192–195. doi: 10.1038/35018092. [DOI] [PubMed] [Google Scholar]
- Langenbacher AD, Dong Y, Shu X, Choi J, Nicoll DA, Goldhaber JI, Philipson KD, Chen JN. Mutation in sodium-calcium exchanger 1 (NCX1) causes cardiac fibrillation in zebrafish. Proc. Natl. Acad. Sci. U.S.A. 2005;102:17699–17704. doi: 10.1073/pnas.0502679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Mably JD, Mohideen MA, Burns CG, Chen JN, Fishman MC. heart of glass regulates the concentric growth of the heart in zebrafish. Curr. Biol. 2003;13:2138–2147. doi: 10.1016/j.cub.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003;107:1355–1358. doi: 10.1161/01.cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- Milan DJ, Giokas AC, Serluca FC, Peterson RT, MacRae CA. Notch1b and neuregulin are required for specification of central cardiac conduction tissue. Development. 2006;133:1125–1132. doi: 10.1242/dev.02279. [DOI] [PubMed] [Google Scholar]
- Milan DJ, Kim AM, Winterfield JR, Jones IL, Pfeufer A, Sanna S, Arking DE, Amsterdam AH, Sabeh KM, Mably JD, Rosenbaum DS, Peterson RT, Chakravarti A, Kaab S, Roden DM, MacRae CA. Drug-sensitized zebrafish screen identifies multiple genes, including GINS3, as regulators of myocardial repolarization. Circulation. 2009;120:553–559. doi: 10.1161/CIRCULATIONAHA.108.821082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne N, Brand-Arzamendi K, Ober EA, Jin SW, Verkade H, Holtzman NG, Yelon D, Stainier DY. The spinster homolog, two of hearts, is required for sphingosine 1-phosphate signaling in zebrafish. Curr. Biol. 2008;18:1882–1888. doi: 10.1016/j.cub.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panákovaá D, Werdich AA, MacRae CA. Wnt11 patterns a myocardial electrical gradient through regulation of the L-type Ca2+ channel. Nature. 2010;466:874–878. doi: 10.1038/nature09249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peal DS, Peterson RT, Milan D. Small molecule screening in zebrafish. J. Cardiovasc. Transl. Res. 2010;3:454–460. doi: 10.1007/s12265-010-9212-8. [DOI] [PubMed] [Google Scholar]
- Pelster B, Burggren WW. Disruption of hemoglobin oxygen transport does not impact oxygen-dependent physiological processes in developing embryos of zebra fish (Danio rerio) Circ. Res. 1996;79:358–362. doi: 10.1161/01.res.79.2.358. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Mably JD, Chen JN, Fishman MC. Convergence of distinct pathways to heart patterning revealed by the small molecule concentramide and the mutation heart-and-soul. Curr. Biol. 2001;11:1481–1491. doi: 10.1016/s0960-9822(01)00482-1. [DOI] [PubMed] [Google Scholar]
- Pierpont ME, Basson CT, Benson DW, Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- Ransom J, Srivastava D. The genetics of cardiac birth defects. Semin. Cell. Dev. Biol. 2007;18:132–139. doi: 10.1016/j.semcdb.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Reiter JF, Alexander J, Rodaway A, Yelon D, Patient R, Holder N, Stainier DY. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999;13:2983–2995. doi: 10.1101/gad.13.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr S, Bit-Avragim N, Abdelilah-Seyfried S. Heart and soul/PRKCi and nagie oko/Mpp5 regulate myocardial coherence and remodeling during cardiac morphogenesis. Development. 2006;133:107–115. doi: 10.1242/dev.02182. [DOI] [PubMed] [Google Scholar]
- Rohr S, Otten C, Abdelilah-Seyfried S. Asymmetric involution of the myocardial field drives heart tube formation in zebrafish. Circ. Res. 2008;102:e12–e19. doi: 10.1161/CIRCRESAHA.107.165241. [DOI] [PubMed] [Google Scholar]
- Rottbauer W, Baker K, Wo ZG, Mohideen MA, Cantiello HF, Fishman MC. Growth and function of the embryonic heart depend upon the cardiac-specific L-type calcium channel alpha1 subunit. Dev. Cell. 2001;1:265–275. doi: 10.1016/s1534-5807(01)00023-5. [DOI] [PubMed] [Google Scholar]
- Rottbauer W, Just S, Wessels G, Trano N, Most P, Katus HA, Fishman MC. VEGF-PLCgamma1 pathway controls cardiac contractility in the embryonic heart. Genes Dev. 2005;19:1624–1634. doi: 10.1101/gad.1319405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottbauer W, Wessels G, Dahme T, Just S, Trano N, Hassel D, Burns CG, Katus HA, Fishman MC. Cardiac myosin light chain-2: a novel essential component of thick-myofilament assembly and contractility of the heart. Circ. Res. 2006;99:323–331. doi: 10.1161/01.RES.0000234807.16034.fe. [DOI] [PubMed] [Google Scholar]
- Scherz PJ, Huisken J, Sahai-Hernandez P, Stainier DY. High-speed imaging of developing heart valves reveals interplay of morphogenesis and function. Development. 2008;135:1179–1187. doi: 10.1242/dev.010694. [DOI] [PubMed] [Google Scholar]
- Schoenebeck JJ, Keegan BR, Yelon D. Vessel and blood specification override cardiac potential in anterior mesoderm. Dev. Cell. 2007;13:254–267. doi: 10.1016/j.devcel.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenebeck JJ, andYelon D. Illuminating cardiac development: Advances in imaging add new dimensions to the utility of zebrafish genetics. Semin. Cell. Dev. Biol. 2007;18:27–35. doi: 10.1016/j.semcdb.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerte T, Pelster B. Digital motion analysis as a tool for analysing the shape and performance of the circulatory system in transparent animals. J. Exp. Biol. 2000;203:1659–1669. doi: 10.1242/jeb.203.11.1659. [DOI] [PubMed] [Google Scholar]
- Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 2002;31:106–110. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- Smith KA, Chocron S, von der Hardt S, de Pater E, Soufan A, Bussmann J, Schulte-Merker S, Hammerschmidt M, Bakkers J. Rotation and asymmetric development of the zebrafish heart requires directed migration of cardiac progenitor cells. Dev. Cell. 2008;14:287–297. doi: 10.1016/j.devcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Fouquet B, Chen JN, Warren KS, Weinstein BM, Meiler SE, Mohideen MA, Neuhauss SC, Solnica-Krezel L, Schier AF, Zwartkruis F, Stemple DL, Malicki J, Driever W, Fishman MC. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development. 1996;123:285–292. doi: 10.1242/dev.123.1.285. [DOI] [PubMed] [Google Scholar]
- Tallafuss A, Bally-Cuif L. Tracing of her5 progeny in zebrafish transgenics reveals the dynamics of midbrain-hindbrain neurogenesis and maintenance. Development. 2003;130:4307–4323. doi: 10.1242/dev.00662. [DOI] [PubMed] [Google Scholar]
- Targoff KL, Schell T, Yelon D. Nkx genes regulate heart tube extension and exert differential effects on ventricular and atrial cell number. Dev. Biol. 2008;322:314–321. doi: 10.1016/j.ydbio.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NA, Koudijs M, van Eeden FJ, Joyner AL, Yelon D. Hedgehog signaling plays a cell-autonomous role in maximizing cardiac developmental potential. Development. 2008;135:3789–3799. doi: 10.1242/dev.024083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, Zon LI. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev. Cell. 2004;6:371–382. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- Varga ZM, Amores A, Lewis KE, Yan YL, Postlethwait JH, Eisen JS, Westerfield M. Zebrafish smoothened functions in ventral neural tube specification and axon tract formation. Development. 2001;128:3497–3509. doi: 10.1242/dev.128.18.3497. [DOI] [PubMed] [Google Scholar]
- Walsh EC, Stainier DY. UDP-glucose dehydrogenase required for cardiac valve formation in zebrafish. Science. 2001;293:1670–1673. doi: 10.1126/science.293.5535.1670. [DOI] [PubMed] [Google Scholar]
- Warren KS, Wu JC, Pinet F, Fishman MC. The genetic basis of cardiac function: dissection by zebrafish (Danio rerio) screens. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000;355:939–944. doi: 10.1098/rstb.2000.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman JS, Keegan BR, Roberts RW, Poss KD, Yelon D. Hoxb5b acts downstream of retinoic acid signaling in the forelimb field to restrict heart field potential in zebrafish. Dev. Cell. 2008;15:923–934. doi: 10.1016/j.devcel.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Meiler SE, Zhong TP, Mohideen M, Crossley DA, Burggren WW, Fishman MC. Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of titin. Nat. Genet. 2002;30:205–209. doi: 10.1038/ng816. [DOI] [PubMed] [Google Scholar]
- Yelon D, Horne SA, Stainier DY. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev. Biol. 1999;214:23–37. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]