Abstract
DNA methylation may mediate persistent changes in gene function following chronic stress. To examine this hypothesis, we evaluated African American subjects matched by age and sex, and stratified into four groups by post-traumatic stress disorder (PTSD) diagnosis and history of child abuse. Total Life Stress (TLS) was also assessed in all subjects. We evaluated DNA extracted from peripheral blood using the HumanMethylation27 BeadChip and analyzed both global and site-specific methylation. Methylation levels were examined for association with PTSD, child abuse history, and TLS using a linear mixed model adjusted for age, sex, and chip effects. Global methylation was increased in subjects with PTSD. CpG sites in five genes (TPR, CLEC9A, APC5, ANXA2, and TLR8) were differentially methylated in subjects with PTSD. Additionally, a CpG site in NPFFR2 was associated with TLS after adjustment for multiple testing. Notably, many of these genes have been previously associated with inflammation. Given these results and reports of immune dysregulation associated with trauma history, we compared plasma cytokine levels in these subjects and found IL4, IL2, and TNFα levels associated with PTSD, child abuse, and TLS. Together, these results suggest that psychosocial stress may alter global and gene-specific DNA methylation patterns potentially associated with peripheral immune dysregulation. Our results suggest the need for further research on the role of DNA methylation in stress-related illnesses.
Keywords: PTSD, epigenetic, total life stress, TPR, APC5, TLR8, NPFFR2
INTRODUCTION
Post-traumatic stress disorder (PTSD) is a debilitating, stress-related psychiatric disorder, with US prevalence rates of 7–8% [Kessler et al., 1994; Kessler, 2000]. Cumulative exposure to traumatic or adverse events across the lifespan is an identified risk factor for developing PTSD, and studies suggest a dose–response relationship between the level of lifetime trauma exposure and development of PTSD [Breslau et al., 1999; Brewin et al., 2000; Ozer et al., 2003; Binder et al., 2004]. This relationship is especially relevant if the trauma was experienced during childhood since child abuse increases the risk for developing PTSD in adulthood [Duncan et al., 1996; Kessler et al., 1997].
Exposure to child abuse has been associated with increased physiological reactivity to stress [Weiss et al., 1999; Heim et al., 2001], which results in dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis that persists into adulthood. These observations are consistent with both human and animal studies that highlight the mechanisms by which early maternal care experience can permanently alter HPA axis activity through DNA methylation of the glucocorticoid receptor promoter [Francis et al., 1999; Weaver et al., 2004]. Additionally, early maltreatment of rat pups increases methylation and decreases expression of BDNF [Roth et al., 2009]. Similar to these preclinical studies, the glucocorticoid receptor promoter in DNA extracted from umbilical cord blood from children born to depressed mothers was reported to be differentially methylated in children, depending on their cortisol levels and cortisol reactivity to stress measured at 3 months of age [Oberlander et al., 2008].
In adults, Uddin et al. [2010] reported associations between PTSD and methylation patterns from DNA extracted from peripheral blood, noting that differentially methylated CpG sites were over-represented in genes related to immune function and inflammation. They then observed in the same subjects that PTSD associates with a greater immune response to cytomegalovirus infection. Because the HPA axis interacts with the immune system to maintain stress-related allostasis, dysregulation of the HPA axis may result in excessive inflammation [Gill et al., 2009]. Subjects with PTSD have been reported to have increased levels of circulating inflammatory cytokines including IL-6 [Maes et al., 1999], TNFα [von Kanel et al., 2007], IL1B [Spivak et al., 1997], and lower anti-inflammatory cytokines such as IL4 [Kawamura et al., 2001; von Kanel et al., 2007] than individuals without PTSD. Further, case–control studies of PTSD have been shown to have distinct expression patterns in genes involved in immune activation in peripheral blood cells [Segman et al., 2005; Zieker et al., 2007].
This convergent data suggest that epigenetic alterations may contribute to the inflammatory and immune dysregulation observed in subjects with PTSD. The current study examines genome-wide methylation in order to confirm and extend previous studies. We hypothesize that DNA methylation will differ in subjects with PTSD. We further hypothesize that childhood trauma or high levels of cumulative stress over the lifetime will also result in independent changes in DNA methylation. Finally, we hypothesize that plasma cytokine levels will associate with PTSD as well as history of childhood trauma and lifetime stress in these subjects.
MATERIALS AND METHODS
Subject Recruitment
We evaluated African American subjects recruited as part of a larger study investigating the influence of genetic and environmental factors on response to stressful life events in a predominantly African-American, urban population of low socioeconomic status (SES) [Binder et al., 2008; Bradley et al., 2008; Gillespie et al., 2009]. Briefly, research participants were approached in the waiting rooms of the primary care clinic or obstetrical-gynecological clinic of a large urban, public hospital while either waiting for their medical appointments or while waiting with others who were scheduled for medical appointments. Subjects willing to participate provided written informed consent and participated in a verbal interview.
In this study, we assessed methylation in 110 subjects selected from the cohort described above such that they could be stratified into four categories: PTSD diagnosis (Clinician-Administered Post-traumatic Stress Disorder Scale; CAPS) [Blake et al., 1995] with (N = 25) and without (N = 25) a history of childhood trauma (Childhood Trauma Questionnaire; CTQ) and controls with (N = 26) and without (N = 34) childhood trauma. All subjects were matched for age and sex. All procedures in this study were approved by the Institutional Review Boards of Emory University School of Medicine and Grady Memorial Hospital.
Subject Assessments
Demographic information including subject age, sex, and race was provided on a self-administered form. The CAPS [Blake et al., 1995] is an interviewer-administered diagnostic instrument measuring PTSD and has been demonstrated to have excellent psychometric properties [Weathers et al., 2001] The CAPS provides a diagnostic measure of PTSD and assesses lifetime and current PTSD. Subjects were scored as having PTSD if they met DSM-IV PTSD criteria for current PTSD from the CAPS interview.
The CTQ [Bernstein et al., 1994; Bernstein 1998; Bernstein et al., 2003] is a 28-item, self-report inventory assessing three types of child abuse: sexual, physical, and emotional. Cutoff scores for each category have shown excellent sensitivity and specificity in correctly classifying cases of abuse in psychiatric patients. The CTQ yields a total score and subscale scores for each of the three types of child abuse. Bernstein and Fink [Bernstein et al., 1994; Bernstein 1998] established scores for mild, moderate, and severe for each type of abuse. In this study, the data from the CTQ were used to classify participants with physical, sexual, and/or emotional abuse into two categories: (1) Those with CTQ scale scores in the none to mild range, and (2) those with scores in the moderate to severe range.
The Stressful Events Questionnaire (SEQ) is a 39-item instrument assessing recent and lifetime Total Life Stress (TLS) via exposure to stressful life events. The SEQ was developed for the purposes of the current study and covers stressful events ranging from divorce, unemployment, crime, financial, and interpersonal stressors to knowing someone who was murdered. The SEQ collects data on whether participants have experienced these events in the last year or ever (coded as no = 0 to yes = 1). These events are summed to yield a total score reflecting the number of types of stressful life events experienced over the last year or prior to the last year (lifetime), revealing a continuous score of 0 (none) to 39 (most severe). In our larger study of over 3,000 participants, lifetime stressful events are normally distributed (mean = 14.96 ± 6.64). Supplementary Table 1 lists the 39 items making up the SEQ, and the clinical and demographic characteristics of subjects whose DNA samples passed QC (as described below) are detailed in Supplementary Table 2.
DNA Methylation
DNA was extracted from whole blood at the Emory University Biomarker Service Core with a KingFisher Flex Magnetic Particle Processor (ThermoFisher Scientifics) and the Mag-Bind SQ Blood DNA kit (Omega Biotek, Norcross, GA) or with a Qiagen M48 BioRobot Workstation using the MagAttract DNA Blood M48 kit (Qiagen, Valencia, CA). DNA concentration was determined by PicoGreen quantitation using the Quant-iT dsDNA Assay Kit (Invitrogen, Carlsbad, CA) on a SpectraMax Gemini XPS plate reader (Molecular Devices, Sunnyvale, CA). Samples were resolved on a 1% agarose gel to verify that the DNA was of high molecular weight (at least 2 kb). One microgram DNA was bisulfite-treated for cytosine to thymine conversion using the EZ DNA Methylation-Gold kit (Zymo Research, Orange, CA). The DNA was then whole-genome amplified, fragmented, and hybridized to the HumanMethylation27 BeadChip (Illumina, San Diego, CA). The XStain was performed on a Tecan Evo 150 liquid handling robot. The BeadChips were scanned using a BeadStation 500GX, and the methylation level (beta value) calculated for each queried CpG locus using the Methylation Module of BeadStudio software.
Samples with probe detection call rates <90% were excluded from further analysis as were those with an average intensity value of either <50% of the experiment-wide sample mean or <2,000 arbitrary units (AU). Hierarchical clustering was then performed to identify extreme outliers and global trends in methylation. One sample of pooled female DNA was included on each BeadChip as a technical control throughout the experiment and assessed for reproducibility using a Pearson R2 coefficient. For each individual sample and CpG site, the signals from methylated (M) and unmethylated (U) bead types are used to calculate a β value: β=M/U + M + 100, which can be treated as an approximation of the proportion of CpG dinucleotides methylated at a particular site. Prior to computing β values, we performed quantile normalization on the signal data to adjust for any technical variability between samples.
Statistical analysis
We worked with the log ratio of β-values log (β/1− β), which is the same as the log signal ratio log(M/U) for quantile-normalized values. To identify CpG sites for which methylation varied significantly with each outcome (PTSD, child abuse, or TLS), we fit a separate linear mixed effects model for each CpG site. For each CpG site, we regressed log (β=1− β) on the variable of interest (PTSD diagnosis, child abuse, or TLS), as well as sex and age. We included chip-specific random effects to allow for potential batch effects due to chip in measurement of the proportion of DNA methylated [Leek et al., 2010]. We fit the above model for all 27,578 CpG sites. To define an initial set of CpG sites for which methylation varied significantly with each outcome, we applied a false discovery rate (FDR) cutoff of 0.05 using the method described by Storey [2002]. To examine global methylation, we also fit the above model with a measure of average methylation across all 27,578 CpG sites as the dependent variable log (β̄/1−β̄), where β̄ represents the average β value over all 27,578 sites for an individual.
Cytokine Measures
Fasting blood was drawn into EDTA tubes between 8 and 9 AM by trained phlebotomists and then placed immediately on ice. Plasma was separated from blood cells via centrifugation, aliquoted into 1 ml aliquots and frozen at −80°C until thawed for cytokine analysis. Both pro-inflammatory (IL6, IFNa, IL1b, IL2, and TNF-α) and anti-inflammatory (IL4 and IL10) cytokines were measured in plasma using a multiplex ELISA (R&D Systems) according to the manufactures’ instructions. Each sample was measured in duplicate and the results were averaged and log transformed prior to analysis.
Because of sample availability, 51 subjects had samples available for both DNA methylation and plasma cytokines. Because this may limit power to detect associations, plasma cytokine levels were also evaluated in an additional 126 subjects that did not have methylation data. The potential demographic and clinical confounding factors (age, sex, years of education, employment, disability, past history of substance abuse, current substance abuse, and past suicide attempt(s)) were not different between the groups in which cytokines were measured or the 110 subjects in methylation dataset. We then examined the association between these potential confounders and each outcome (PTSD, child abuse, or TLS) by applying a general linear model. To avoid adjusting for collinear variables, the associated factors were then entered into a forward and reverse stepwise regression, and only those factors that remained associated with the outcome at P < .05 were considered as covariates in the final analysis model. Because of independent associations (P < 0.05), we adjusted the analysis of PTSD for history of suicide attempts and the analysis of TLS for history of substance abuse.
RESULTS
Global Changes in DNA Methylation
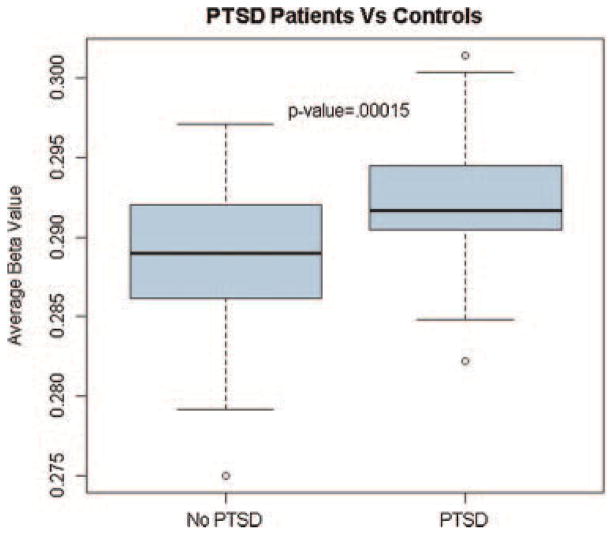
We noted an increase in global methylation in subjects with PTSD compared to controls (T = 3.95; P = 1.5 × 10−4; Fig. 1). There was no change in global methylation levels in subjects with a history of childhood trauma (T = −0.17; P = 0.87) or with increased total life stress (T = −0.29; P = 0.77).
FIG. 1.
Global methylation changes are associated with PTSD diagnosis. Box plots of the average methylation level (β-values averaged across all 27,578 CpG sites) for patients with PTSD (N = 50) and control subjects without PTSD (N = 60).
Gene-Specific Changes in DNA Methylation
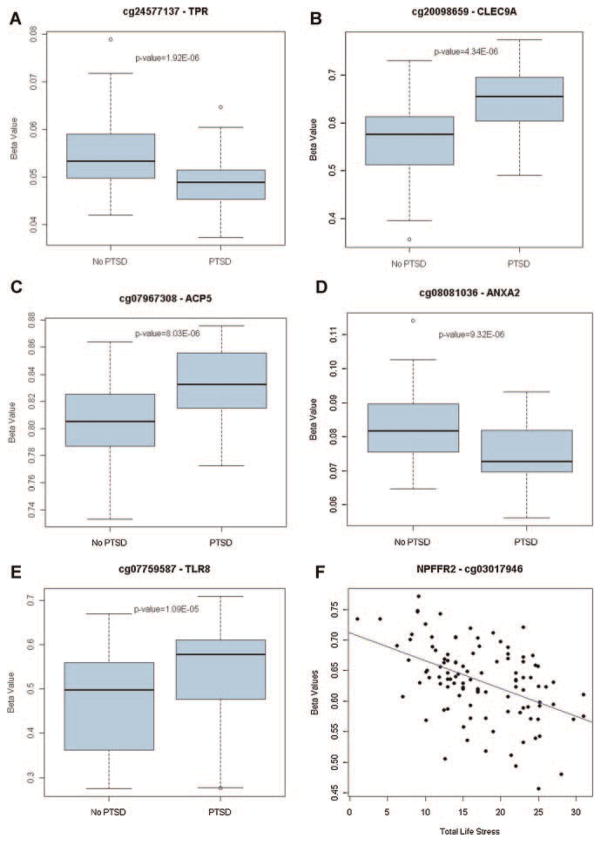
We examined the association between each CpG site and PTSD diagnosis (Supplementary Table 3), child abuse (Supplementary Table 4), and TLS (Supplementary Table 5). CpG sites in five genes met experiment-wide criteria (FDR < 0.05) for association with PTSD diagnosis. CpG sites in translocated promoter region (TPR; T = −5.08; P = 1.9 × 10−6) and annexin A2 (ANXA2; T = −4.69; P = 9.3 × 10−6) demonstrated decreased methylation while CpG sites in C-type lectin domain family 9, member A (CLEC9A; T = 4.89; P = 4.3 × 10−6), acid phosphatase 5, tartrate resistant (ACP5; T = 4.73; P = 8.0 × 10−6), and toll-like receptor 8 (TLR8; T = 4.65; P = 1.1 × 10−5) showed increased methylation in PTSD subjects compared to controls (Supplementary Table 1; Fig. 2). Additionally, one CpG site met criteria for experiment-wide significant association with TLS (Supplementary Table 3; Fig. 2). This site, cg03017946, is located near neuropeptide FF receptor 2 (NPFFR2), and its DNA methylation was inversely associated with TLS scores (T = −5.33, P = 6.6 × 10−7). No CpG site reached experiment-wide criteria for significance for association with child abuse.
FIG. 2.
CpG sites in TPR, CLEC9A, ACP5, ANXA2, and TLR8 are differentially methylated in subjects with PTSD, and NPFFR2 methylation is associated with Total Life Stress. The methylation level (β-value, box plot of average methylation value) for each subject is plotted on the vertical axis while PTSD diagnosis is plotted on the horizontal axis. Box plots of methylation values in PTSD cases (N = 50) and controls (N = 60) are shown for TPR (A), CLEC9A (B), ACP5 (C), ANXA2 (D), and TLR8 (E). F: Total life stress (TLS) scores for all subjects (N = 110) are plotted on the horizontal axis and NPFFR2 methylation β values are presented as a scatter plot on the vertical axis. All six CpG sites met experiment-wide criteria for significance (FDR < 0.05).
Replication of Differentially Methylated Genes from the Literature
To complement our hypothesis-neutral approach, we evaluated the association between CpG sites in genes that had been previously associated with PTSD, early life stress, or child abuse in the literature [McGowan et al., 2009; Roth et al., 2009; Beach et al., 2010; Uddin et al., 2010] and PTSD diagnosis, child abuse, and TLS in our population. In examining previously reported CpG sites in genes related to immune function and inflammation, we focused on the CpG sites identified by a gene ontology analysis of PTSD subjects [Uddin et al., 2010]. The results of the literature replication are presented in Table I. Of the 60 genes examined, 19 were differentially methylated with respect to PTSD diagnosis in these subjects. Interestingly, while none were associated with both PTSD and child abuse, CpG sites in both BDNF and CXCL1 were associated with both PTSD diagnosis and TLS. We did not observe differential methylation of CpG sites in NR3C1 or SLC6A4 with any outcome.
TABLE I.
Evaluation of CpG Sites in Differentially Methylated Genes From the Literature
| Probe ID | Gene | Reference | PTSD diagnosis
|
Child abuse
|
TLS
|
|||
|---|---|---|---|---|---|---|---|---|
| T-statistic | P-value | T-statistic | P-value | T-statistic | P-value | |||
| cg13384396 | ADCY5 | Uddin et al. [2010] | 2.71 | 0.0080 | NS | NS | NS | NS |
| cg16967583 | AGXT | Uddin et al. [2010] | 2.11 | 0.038 | NS | NS | NS | NS |
| cg19224278 | ALDH1A3 | Uddin et al. [2010] | 2.03 | 0.045 | NS | NS | NS | NS |
| cg24921089 | AMPD3 | Uddin et al. [2010] | 2.73 | 0.0075 | NS | NS | NS | NS |
| cg11098259 | AQP9 | Uddin et al. [2010] | NS | NS | NS | NS | NS | NS |
| cg27351358 | BDNF | Roth et al. [2009] | 3.33 | 0.0012 | NS | NS | −2.00 | 0.049 |
| cg16257091 | BDNF | Roth et al. [2009] | 2.01 | 0.047 | NS | NS | NS | NS |
| cg17118262 | CCL1 | Uddin et al. [2010] | NS | NS | −2.38 | 0.019 | NS | NS |
| cg16719404 | CD2 | Uddin et al. [2010] | −3.18 | 0.002 | NS | NS | NS | NS |
| cg00298951 | CMKLR1 | Uddin et al. [2010] | NS | NS | NS | NS | NS | NS |
| cg02029926 | CXCL1 | Uddin et al. [2010] | 2.13 | 0.036 | NS | NS | 2.80 | 0.0062 |
| cg04451770 | ENTPD1 | Uddin et al. [2010] | 3.00 | 0.0034 | NS | NS | NS | NS |
| cg13471990 | ENTPD1 | Uddin et al. [2010] | NS | NS | −3.13 | 0.0022 | NS | NS |
| cg06306751 | F8 | Uddin et al. [2010] | NS | NS | −2.03 | 0.045 | NS | NS |
| cg09001777 | FUT3 | Uddin et al. [2010] | 3.39 | 0.0010 | NS | NS | NS | NS |
| cg07970007 | GBP1 | Uddin et al. [2010] | −1.98 | 0.050 | NS | NS | NS | NS |
| cg13406950 | GBP1 | Uddin et al. [2010] | NS | NS | −2.59 | 0.011 | NS | NS |
| cg15125472 | HSPB6 | Uddin et al. [2010] | 3.51 | 6.93 × 10−4 | NS | NS | NS | NS |
| cg24673765 | HSPB6 | Uddin et al. [2010] | 2.03 | 0.045 | NS | NS | NS | NS |
| cg07463059 | IFI16 | Uddin et al. [2010] | NS | NS | NS | NS | 2.23 | 0.028 |
| cg08090640 | IFI35 | Uddin et al. [2010] | NS | NS | NS | NS | 3.15 | 0.0021 |
| cg23915111 | KIF14 | Uddin et al. [2010] | NS | NS | NS | NS | 3.23 | 0.0017 |
| cg14913610 | KLRG1 | Uddin et al. [2010] | 2.10 | 0.038 | NS | NS | NS | NS |
| cg21092324 | MMRN1 | Uddin et al. [2010] | NS | NS | NS | NS | NS | NS |
| cg25482967 | MRPS10 | Uddin et al. [2010] | NS | NS | 2.66 | 0.0092 | NS | NS |
| cg20318748 | NANP | Uddin et al. [2010] | NS | NS | 2.30 | 0.023 | NS | NS |
| cg07042144 | NLRP12 | Uddin et al. [2010] | NS | NS | NS | NS | NS | NS |
| cg20842040 | PRPS2 | Uddin et al. [2010] | 3.16 | 0.0022 | NS | NS | NS | NS |
| cg07874284 | PRPS2 | Uddin et al. [2010] | 2.24 | 0.028 | NS | NS | NS | NS |
| cg19769182 | PRRT2 | Uddin et al. [2010] | 2.44 | 0.017 | NS | NS | NS | NS |
| cg02740947 | RAD51L3 | Uddin et al. [2010] | NS | NS | NS | NS | −2.19 | 0.031 |
| cg07837085 | SLAMF7 | Uddin et al. [2010] | 2.16 | 0.034 | NS | NS | NS | NS |
| cg21554249 | SUOX | Uddin et al. [2010] | 2.01 | 0.047 | NS | NS | NS | NS |
| cg03430998 | TLR1 | Uddin et al. [2010] | NS | NS | NS | NS | 2.11 | 0.037 |
NS indicates genes that were not significant with respect to the outcome at P < 0.05. Additionally, CpG sites in ATP1A1, BBS7, CD1D, CSNK1D, CYP2J2, DGAT2L6, GALR2, HOXD10, IL8, ISLR2, LST1, LTA4H, MRI1, MTPN, NALP2, NR3C1, NUMB, PTGIS, PTPN22, PYDC1, RIF1, SCN5A, SLC6A4, SOSTDC1, SRXN1, STAP1, STC1, TREM1, TFPI, TLR3, TNC, and TRAP1 were not associated at P < 0.05 with any outcome examined.
Plasma Cytokine Measures
Because a number of genes related to inflammation were differentially methylated with numerous stress-related outcomes, and because of numerous reports of immune system dysregulation in subjects with trauma history, we measured pro-inflammatory (IL6, IFNa, IL1b, IL2, and TNFα) and anti-inflammatory (IL4 and IL10) cytokine levels in a subset of these subjects for which plasma was available (N = 51) using a multiplex ELISA. The association between these immune response markers and PTSD, child abuse, and TLS were evaluated (Table II). Interestingly, IL4 was decreased in subjects with PTSD. Plasma IL2 and TNFα levels were increased in subjects with a history of child abuse. Consistently, subjects with higher TLS scores also have higher TNFα levels.
TABLE II.
Association of Plasma Cytokine Levels With PTSD, Child Abuse, and TLS.
| Methylation sample N = 51
|
Expanded sample N = 177
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTSD
|
Child abuse
|
TLS
|
PTSD
|
Child abuse
|
TLS
|
|||||||
| T-statistic | P-value | T-statistic | P-value | T-statistic | P-value | T-statistic | P-value | T-statistic | P-value | T-statistic | P-value | |
| IL4 | −2.73 | 0.0088 | NS | NS | NS | NS | −2.29 | 0.023 | NS | NS | NS | NS |
| IL2 | NS | NS | 2.53 | 0.015 | NS | NS | NS | NS | NS | NS | NS | NS |
| TNFα | NS | NS | 2.78 | 0.0076 | 3.32 | 0.0017 | NS | NS | 2.42 | 0.017 | 3.05 | 0.0026 |
We then investigated the association of these three cytokines (IL4, IL2, and TNFα) in a larger sample of subjects that included the original 51 subjects and an additional 126 independent subjects (total N = 177; Table II). Decreased plasma IL4 levels remained associated with PTSD in this larger group of subjects when adjusted for previous history of suicide attempts. Also in these subjects, increased plasma TNFα levels are associated with a history of child abuse and with higher TLS scores when adjusted for past history of substance abuse. However, the association between history of child abuse and IL2 levels did not replicate in the larger sample.
DISCUSSION
When examining individual CpG sites, thousands were nominally associated with PTSD diagnosis and symptoms, child abuse, and TLS (Supplementary Tables 3–5). Many of these associations were consistent with previous reports from the literature. For example, Roth et al. [2009] reported that early maltreatment of rat pups produced increased methylation of BDNF that resulted in persistent decreases in gene expression in the adult prefrontal cortex. In this study we observed a nominal association between increased methylation at a CpG site (cg27351358) in BDNF and current PTSD. A recent study [Uddin et al., 2010] identified a number of immune-related genes that were differentially methylated in subjects with PTSD; we also observed evidence of association between CpG sites in 19 of these genes with PTSD, 6 with child abuse, and an additional 5 with TLS. However, CpG sites in 34 genes were not associated with any outcome examined. The failure of more of these CpG sites to replicate may be due to a number of differences in how this study was conducted. For example, we controlled for factors that have been previously shown to alter DNA methylation including age, race, and early life stress [McGowan et al., 2009; Rakyan et al., 2010; Teschendorff et al., 2010; Adkins et al., 2011], and accounted for potential batch effects due to chip [Leek et al., 2010]. Another key difference is that we examined CpG sites in which the % methylation varied from 21–79%. As such, we evaluated a greater number of CpG sites but also set a more stringent threshold for statistical significance.
We further identified five additional genes that may be differentially methylated in PTSD. For example, TPR, which has lower levels of methylation in subjects with PTSD, interacts with the stress-induced transcription factor heat shock transcription factor 1 (HSF1) to upregulate HSP70 [Skaggs et al., 2007], which facilitates folding of the glucocorticoid receptor (NR3C1) [Dittmar et al., 1997]. Additionally, genes related to immune function were also identified. Specifically, TLR8 is expressed in the white matter of periventricular, subcortical, and cerebellar regions of the brain and has been implicated in neurogenesis and neurite outgrowth in the developing brain [Mishra et al., 2006; Mallard et al., 2009]. It remains expressed in adults and participates in inflammatory processes along with suppression of neurite outgrowth and induction of neuronal apoptosis [Ma et al., 2007]. Also, ACP5, also known as TRAP, is expressed in the brain [Sun et al., 2008] and is stimulated by monocyte chemoattractant protein 1 (MCP1), which is produced during neuroinflammation and associated with neuronal death [Kim et al., 2006]. MCP1 increases the excitability of nociceptive neurons and is increased in rodent models of neuropathic pain [White et al., 2009].
A CpG site in NPFFR2 met criteria for experiment-wide significance. NPFF is a neurotransmitter that modulates the opioid system, which has been heavily implicated in PTSD [Holbrook et al., 2010]. Additionally, NPFFR2 is activated in response to inflammatory pain [Yang and Iadarola 2003; Yang et al., 2008; Lameh et al., 2010]. PTSD and other stress-related disorders are associated with increased reports of pain and pain-related disability, as well as increased healthcare utilization and lower quality of life [Bryant et al., 1999; Shipherd et al., 2007] in veteran populations [Asmundson et al., 2004]. We have reported a similar relationship between PTSD and chronic pain in civilian populations [Schwartz et al., 2006]. Also in civilians, PTSD has been shown to be a risk factor for headache chronicity [Peterlin et al., 2008]. Thus, PTSD appears to associate with abnormal regulation of pain responses. We hypothesize that stress-related methylation of NPFFR2 contributes to dysregulation of pain, and to the abnormal activation of inflammatory pathways in chronic stress conditions [Bauer et al., 2010].
While we observed an overall increase in global methylation, CpG sites in TPR and ANXA2 demonstrated decreased methylation. Since DNA methylation is often inversely correlated with gene expression, these data suggest that while transcription is generally reduced overall in peripheral blood, these two genes as well as NPFFR2 may be activated in subjects with PTSD or significant TLS and warrant further investigation.
To determine whether changes in DNA methylation could also be associated with immune dysregulation, we evaluated both pro-inflammatory and anti-inflammatory cytokines in plasma. In two groups of subjects, we observed an association between PTSD and lower IL4 levels, consistent with previous reports [Kawamura et al., 2001; von Kanel et al., 2007]. We also observed increased plasma TNFα levels associated with both child abuse and higher TLS scores. TNFα crosses the blood–brain barrier to stimulate the HPA axis, resulting in increased production of cortisol and symptoms including malaise, fatigue, and changes in sleep and appetite [Raison and Miller 2003; Silverman et al., 2005; Dunn et al., 2006; Sternberg 2006].
Our study only examined DNA extracted from peripheral blood. While this is appropriate for assessing differential methylation of immune-related genes, it is not clear to what degree methylation changes in the blood correlate with the brain. Stress-related outcomes such as PTSD, child abuse, or TLS should also be assessed in brain tissue through animal or post-mortem studies. Interestingly, NPFFR2, which is expressed in hypothalamus, amygdala, forebrain, and brainstem [Goncharuk and Jhamandas, 2008], has also been identified in primary diffuse large B-cell lymphoma [Sung et al., 2011] suggesting that the peripheral blood may be a reasonable proxy for some genes expressed in the brain. Finally, in order to present a complete picture of the factors that may affect DNA methylation in this population, three phenotypes were examined, potentially increasing the possibility of type 1 error.
In conclusion, this study provides evidence suggesting that psychosocial stress may alter global and gene-specific DNA methylation patterns associated with peripheral immune dysregulation and is consistent with previous reports. Further studies are necessary to evaluate the potential role of DNA methylation in chronic activation of the immune system in response to stress-related illness.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the participants of the study. This work was supported, in part, by the Emory Biomarker Service Center.
Grant sponsor: National Institutes of Mental Health MH071537; Grant sponsor: National Institute of Mental Health (to CFG) MH082256 and (to AKS) MH085806; Grant sponsor: Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources M01RR00039; Grant sponsor: NARSAD (CFG); Grant sponsor: The Burroughs Wellcome Fund (KJR).
Footnotes
Additional supporting information may be found in the online version of this article.
References
- Adkins RM, Krushkal J, Tylavsky FA, Thomas F. Racial differences in gene-specific DNA methylation levels are present at birth. Birth Defects Res A Clin Mol Teratol. 2011 doi: 10.1002/bdra.20770. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJ, Wright KD, Stein MB. Pain and PTSD symptoms in female veterans. Eur J Pain. 2004;8(4):345–350. doi: 10.1016/j.ejpain.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Bauer ME, Wieck A, Lopes RP, Teixeira AL, Grassi-Oliveira R. Interplay between neuroimmunoendocrine systems during post-traumatic stress disorder: A minireview. Neuroimmunomodulation. 2010;17(3):192–195. doi: 10.1159/000258721. [DOI] [PubMed] [Google Scholar]
- Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA. Methylation at SLC6A4 is linked to family history of child abuse: An examination of the Iowa Adoptee sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(2):710–713. doi: 10.1002/ajmg.b.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatr. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood trauma questionnaire manual. San Antoinio, TX: Psychological Corporation; 1998. [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Bruckl T, Muller N, Pfister H, Lieb R, Mueller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36(12):1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatr. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Chilcoat HD, Kessler RC, Davis GC. Previous exposure to trauma and PTSD effects of subsequent trauma: Results from the Detroit Area Survey of Trauma. Am J Psychiatr. 1999;156(6):902–907. doi: 10.1176/ajp.156.6.902. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:317–336. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Marosszeky JE, Crooks J, Baguley IJ, Gurka JA. Interaction of posttraumatic stress disorder and chronic pain following traumatic brain injury. J Head Trauma Rehabil. 1999;14(6):588–594. doi: 10.1097/00001199-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Dittmar KD, Demady DR, Stancato LF, Krishna P, Pratt WB. Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. The role of p23 is to stabilize receptor.hsp90 heterocomplexes formed by hsp90.p60. hsp70. J Biol Chem. 1997;272(34):21213–21220. doi: 10.1074/jbc.272.34.21213. [DOI] [PubMed] [Google Scholar]
- Duncan RD, Saunders BE, Kilpatrick DG, Hanson RF, Resnick HS. Childhood physical assault as a risk factor for PTSD, depression, and substance abuse: Findings from a national survey. Am J Orthopsychiatr. 1996;66(3):437–448. doi: 10.1037/h0080194. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6(11):836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45(4):262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatr. 2009;31(6):505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharuk V, Jhamandas JH. Neuropeptide FF2 receptor distribution in the human brain. An immunohistochemical study. Peptides. 2008;29(9):1544–1553. doi: 10.1016/j.peptides.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatr. 2001;158(4):575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med. 2010;362(2):110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- Kawamura N, Kim Y, Asukai N. Suppression of cellular immunity in men with a past history of posttraumatic stress disorder. Am J Psychiatr. 2001;158(3):484–486. doi: 10.1176/appi.ajp.158.3.484. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: The burden to the individual and to society. J Clin Psychiatr. 2000;61:4–12. [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med. 1997;27(5):1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshelman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-IIIR psychiatric disorders in the United States: Results from the national comorbidity survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kim MS, Day CJ, Selinger CI, Magno CL, Stephens SR, Morrison NA. MCP-1-induced human osteoclast-like cells are tartrate-resistant acid phosphatase, NFATc1, and calcitonin receptor-positive but require receptor activator of NFkappaB ligand for bone resorption. J Biol Chem. 2006;281(2):1274–1285. doi: 10.1074/jbc.M510156200. [DOI] [PubMed] [Google Scholar]
- Lameh J, Bertozzi F, Kelly N, Jacobi PM, Nguyen D, Bajpai A, Gaubert G, Olsson R, Gardell LR. Neuropeptide FF receptors have opposing modulatory effects on nociception. J Pharmacol Exp Ther. 2010;334(1):244–254. doi: 10.1124/jpet.109.164384. [DOI] [PubMed] [Google Scholar]
- Leek JT, Scharpf RB, Bravo HC, Simcha D, Langmead B, Johnson WE, Geman D, Baggerly K, Irizarry RA. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat Rev Genet. 2010;11(10):733–739. doi: 10.1038/nrg2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Haynes RL, Sidman RL, Vartanian T. TLR8: An innate immune receptor in brain, neurons and axons. Cell Cycle. 2007;6(23):2859–2868. doi: 10.4161/cc.6.23.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R, Bosmans E. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry. 1999;45(7):833–839. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- Mallard C, Wang X, Hagberg H. The role of Toll-like receptors in perinatal brain injury. Clin Perinatol. 2009;36(4):763–772. v–vi. doi: 10.1016/j.clp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BB, Mishra PK, Teale JM. Expression and distribution of Toll-like receptors in the brain during murine neurocysticercosis. J Neuroimmunol. 2006;181(1–2):46–56. doi: 10.1016/j.jneuroim.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychol Bull. 2003;129(1):52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- Peterlin BL, Tietjen G, Meng S, Lidicker J, Bigal M. Post-traumatic stress disorder in episodic and chronic migraine. Headache. 2008;48(4):517–522. doi: 10.1111/j.1526-4610.2008.00917.x. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160(9):1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Rakyan VK, Down TA, Maslau S, Andrew T, Yang TP, Beyan H, Whittaker P, McCann OT, Finer S, Valdes AM, Leslie RD, Deloukas P, Spector TD. Human aging-associated DNA hypermethylation occurs preferentially at bivalent chromatin domains. Genome Res. 2010;20(4):434–439. doi: 10.1101/gr.103101.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65(9):760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AC, Bradley R, Penza KM, Sexton M, Jay D, Haggard PJ, Garlow SJ, Ressler KJ. Pain medication use among patients with post-traumatic stress disorder. Psychosomatics. 2006;47(2):136–142. doi: 10.1176/appi.psy.47.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segman RH, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N, Shalev AY. Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol Psychiatry. 2005;10(5):500–513. 425. doi: 10.1038/sj.mp.4001636. [DOI] [PubMed] [Google Scholar]
- Shipherd JC, Keyes M, Jovanovic T, Ready DJ, Baltzell D, Worley V, Gordon-Brown V, Hayslett C, Duncan E. Veterans seeking treatment for posttraumatic stress disorder: What about comorbid chronic pain? J Rehabil Res Dev. 2007;44(2):153–166. doi: 10.1682/jrrd.2006.06.0065. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Pearce BD, Biron CA, Miller AH. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 2005;18(1):41–78. doi: 10.1089/vim.2005.18.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs HS, Xing H, Wilkerson DC, Murphy LA, Hong Y, Mayhew CN, Sarge KD. HSF1-TPR interaction facilitates export of stress-induced HSP70 mRNA. J Biol Chem. 2007;282(47):33902–33907. doi: 10.1074/jbc.M704054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A, Valevski A, Weizman A. Elevated levels of serum interleukin-1 beta in combat-related posttraumatic stress disorder. Biol Psychiatry. 1997;42(5):345–348. doi: 10.1016/S0006-3223(96)00375-7. [DOI] [PubMed] [Google Scholar]
- Sternberg EM. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6(4):318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey J. A direct approach to false discovery rates. J Roy Stat Soc: Sr B. 2002;64:479–498. [Google Scholar]
- Sun P, Sleat DE, Lecocq M, Hayman AR, Jadot M, Lobel P. Acid phosphatase 5 is responsible for removing the mannose 6-phosphate recognition marker from lysosomal proteins. Proc Natl Acad Sci USA. 2008;105(43):16590–16595. doi: 10.1073/pnas.0807472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung CO, Kim SC, Karnan S, Karube K, Shin HJ, Nam DH, Suh YL, Kim SH, Kim JY, Kim SJ, Kim WS, Seto M, Ko YH. Genomic profiling combined with gene expression profiling in primary central nervous system lymphoma. Blood. 2011;117(4):1291–1300. doi: 10.1182/blood-2010-07-297861. [DOI] [PubMed] [Google Scholar]
- Teschendorff AE, Menon U, Gentry-Maharaj A, Ramus SJ, Weisenberger DJ, Shen H, Campan M, Noushmehr H, Bell CG, Maxwell AP, Savage DA, Mueller-Holzner E, Marth C, Kocjan G, Gayther SA, Jones A, Beck S, Wagner W, Laird PW, Jacobs IJ, Widschwendter M. Age-dependent DNA methylation of genes that are suppressed in stem cells is a hallmark of cancer. Genome Res. 2010;20(4):440–446. doi: 10.1101/gr.103606.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, Goldmann E, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci USA. 2010;107(20):9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41(9):744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: A review of the first ten years of research. Depress Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weiss B, Catron T, Harris V, Phung TM. The effectiveness of traditional child psychotherapy. J Consult Clin Psychol. 1999;67(1):82–94. doi: 10.1037//0022-006x.67.1.82. [DOI] [PubMed] [Google Scholar]
- White FA, Feldman P, Miller RJ. Chemokine signaling and the management of neuropathic pain. Mol Interv. 2009;9(4):188–195. doi: 10.1124/mi.9.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HY, Iadarola MJ. Activation of spinal neuropeptide FF and the neuropeptide FF receptor 2 during inflammatory hyperalgesia in rats. Neuroscience. 2003;118(1):179–187. doi: 10.1016/s0306-4522(02)00931-4. [DOI] [PubMed] [Google Scholar]
- Yang HY, Tao T, Iadarola MJ. Modulatory role of neuropeptide FF system in nociception and opiate analgesia. Neuropeptides. 2008;42(1):1–18. doi: 10.1016/j.npep.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Zieker J, Zieker D, Jatzko A, Dietzsch J, Nieselt K, Schmitt A, Bertsch T, Fassbender K, Spanagel R, Northoff H, Gebicke-Haerter PJ. Differential gene expression in peripheral blood of patients suffering from post-traumatic stress disorder. Mol Psychiatr. 2007;12(2):116–118. doi: 10.1038/sj.mp.4001905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.