Abstract
Dendritic cells (DCs) regulate both innate and adaptive immune responses. Here we exploit the unique avascularity of the cornea to examine a role for local or very early infiltrating DCs in regulating the migration of blood-derived innate immune cells towards herpes simplex virus type 1 (HSV-1) lesions. A single systemic diphtheria toxin (DT) treatment 2 days before HSV-1 corneal infection transiently depleted CD11c+DCs from both the cornea and lymphoid organs of CD11c-DTR bone marrow chimeric mice for up to 24 hours after infection. Transient DC depletion significantly delayedHSV-1 clearance from the corneathrough 6 days post infection(dpi). No further compromise of viral clearance was observed when DCs were continuously depleted throughout the first week of infection. DC depletion did not influenceextravasation of NK cells, inflammatory monocytes, orneutrophils into the peripheral cornea,but did significantly reduce migration of NK cells and inflammatory monocytes, but not neutrophils towards the HSV-1 lesion in the central cornea. Depletion of NK cells resulted in similar loss of viral control to transient DC ablation. Our findings demonstrate resident corneal DC and/or those that infiltrate the cornea during the first 24 hours after HSV-1 infection contribute to the migration of NK cells and inflammatory monocytes into the central cornea, and are consistent with a role for NK cells and possibly inflammatory monocytes, but not PMN in the clearing HSV-1 from the infected cornea.
Introduction
The body’s innate immune system is rapidly mobilized to combat infection by a variety of pathogens. Dendritic cells (DCs) are present in skin and mucosal surfaces, where they serve as sentinels that alert the body of the invasion of pathogens.They are widely recognized for their ability to bridge innate and adaptive immunity by sampling antigens during infection, transporting them to the lymphoid organs, and mobilizing the adaptive immune response (1,2). In recent years the phenotypic and functional diversity of the DC population has become increasingly apparent. Distinct DC subpopulations occupy different strata of mucosa and skin, and vary in their ability to migrate and cross-present antigens(3-5). DCs residing in lymphoid organs are also functionally and phenotypically diverse. Moreover, recent reports implicate DCs in orchestration of innate immunity(6,7). For example, depletion of DCs during HSV-1 infection of the footpad resulted in a diminished NK cell responsewithin the spleen during the first 24 hours after infection(7,8).
Previous studies have suggested NK cells and neutrophils are primarily responsible for clearing HSV-1 from infected corneas (9-12). However, neutrophils were implicated in HSV-1 clearance in studies involving in vivo depletion of neutrophils using the RB6-8C5 (anti-Gr-1) antibody, which was subsequently shown to deplete a variety of cell types in addition to neutrophils(13,14). In fact two recent studies demonstrated impaired HSV-1 clearance in mice that were depleted of Gr-1+ cells, but not those specifically depleted of neutrophils with antibody to Ly6G, suggesting that neutrophils probably do not play a major role in HSV-1 clearance (14,15).
NK cells provide a rapid response to viral infections through their ability to recognize generic changes that occur in virally infected cells such as reduced expression of MHC (16,17). NK cells can inhibit virus replication through generally lethal release of lytic granules or non-lethal release of antiviral cytokines including IFN-γ(17). Because NK cells are potentially lethal and not activated by specific pathogen-derived antigens, their activity is closely regulated by the immune system. It is now apparent that NK cells require activation, or licensing, to gain full effector functionality(18). DC production of IL-12 and IL-15 has been implicated in NK cell activation(19,20).In fact, IL-15 transpresentation by DC appears to be required for both the the activation and homeostatic proliferation of NK cells (6,21).
The interactions between DCs and NK cells have been largely studied in lymphoid organs. A separate role for DCs in regulating NK cell function locally within a viral lesion is more difficult to assess. DCs rapidly accumulate at the edges of HSV-1 and HSV-2 lesions in skin, genital mucosa, and cornea (5,22-24). Due to the avascularity of the cornea, infiltrating cells must extravasate from blood vessels in the limbal region abutting the peripheral cornea, and then migrate into the central cornea where HSV-1 lesions are established in the mouse model of HSV-1 keratitis(25,26). This model affords a unique opportunity to separately observe tissue extravasation andin situ migration of innate immune responders to sites of viral infection within the central cornea. HSV-1 infection of the corneas of CD11c-DTR mice that express a high affinity diphtheria toxin receptor from the CD11c promoter afforded the opportunity to observe the effect of DC ablation during the early stages of the innate response that clears the virus. We demonstrate that DCs are required during the first 24 hours after HSV-1 corneal infection to orchestrate an innate immune response that clears virus from the cornea from 2-6 dpi. Depleting DCs during the first 24 hours after infection inhibits the migration of NK cells and inflammatory monocytes to the site of the lesion in the central cornea. This study reveals a previously unappreciated local role of dendritic cells in directing the innate immune response to viral infection.
Materials and Methods
Reagents
The following fluorochrome labeled antibodies were used: against PerCP-conjugated anti-CD45 (30-F11), FITC-conjugated anti-CD69 (H1.2F3), PE-conjugated anti-CD3 (clone 17A-2); anti-CD49b (DX-5), and APC-conjugated anti-granzyme B (GB11);anti-CD49b (DX-5) were purchased from BD Pharmingen (San Diego, CA). V450 conjugated anti-CD11b (M1/70), APC-conjugated anti-CD11c (N418), APC-eFlour780-conjugated anti-GR-1 (RB6-8CA), and PE-Cy7-conjugated F4/80 (BM-8) (were purchased from eBiosciences (San Diego, CA). The appropriate isotype control antibodies were purchased from their respective vendors.
Mice
Six- to 8-week-old female wild type (WT) BALB/cJ and CD11c-DTR (C.FVB-Tg(Itgax-DTR/EGFP)57Lan/J) mice were purchased from Jackson laboratories (Bar Harbor, ME). Bone marrow chimeras were created by transferring bone marrow from CD11c-DTR mice into lethally irradiated wild type BALB/cJ mice to avoid lethality associated with multiple DT treatments of CD11c-DTR mice(27). Briefly, BALB/cJ hosts underwent two treatments of 500 rads in an animal γ-irradiator and 2.5×105 bone marrow cells from a CD11c-DTR donor were transferred intravenously (iv). The resulting mice (referred to herein as CD11c-DTR chimeras) were housed under immunocompromised mouse conditions, and treated regularly with 2mg/mL neomycin from Sigma Laboratories (St. Louis, MO) in their drinking water. The CD11c-DTR chimeraswere fully reconstituted and ready for experimental use after 6 weeks. All experimental animal procedures were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research
In vivoDiphtheria toxin and anti-ASGM-1 treatment
Diphtheria toxin (DT) purchased from Sigma Laboratories (St. Louis, MO) was prepared in a sterile solution of PBS at a concentration of 1mg/mL. Transient DC depletion was effected inCD11c-DTR chimeras by a single intraperitoneal injection (i.p.) of175ng DT; continuous DC depletion involved additional ip treatments of 100 ng DT/mouse every three days. NK cell depletion was accomplished by a single ip treatment with 40 ul of rabbit anti-ASGM-1 antibody (Wako Pure Chemicals, Osaka, Japan) at −1 dpi.
Ocular HSV-1 infection
HSV-1 strain RE or HSV-1 KOS RFP-K26was grown in Vero cells, and intact virions were isolated on Optiprep gradients according to manufacturer’s instructions (Accurate Chemical and Scientific Corp, Westbury, NY). For ocular HSV-1 infection, mice were anesthetized by i.p. injection of 2.0 mg ketamine hydrochloride and 0.04 mg of xylazine (Butler Schein, Pittsburgh, PA) in 0.2 mL of Hanks balanced salt solution (Mediatech Inc., Manassas, VA). The abraded central corneas of anesthetized mice were infected by topical application of 3μL of RPMI (Lonza, Walkersville, MD) containing 1 × 105 plaque-forming units (PFU) of virus. All animal experiments were conducted in accordance with guidelines established by the University of Pittsburgh Institutional Animal Care and Use Committee.
Flow Cytometry for Phenotypic Analysis
At indicated times, draining lymph nodes(DLN), spleens, and corneas were harvestedfrom euthanized mice. DLN and spleenswere minced and incubated with 500uL DMEM (Lonza) containing 10% FCS (Atlanta Biologicals, Atlanta, GA) and 420U/mL collagenase type I (Sigma) for 30 minutes at 37°C.
Dissected corneas were treated with PBS 2mM EDTA for 15 minutes to separate the epithelium from the cornealstroma. Cells were then released from the corneal stromaby incubation for 1 hour in 100ul of collagenase type 1 (Sigma) at a concentration of 840U/mL followed by trituration with a micropipette. In some experiments leukocytic infiltration of the conjunctiva, peripheral cornea, and central cornea were examined individually. The cornea and conjunctiva were excised, the conjunctiva dissected from the cornea based on anatomical differences, and the central cornea dissected from the peripheral cornea with a 2 mm trephine. Data were collected on a FACSAria cytometer and analyzed by FACSDiva software (BD Biosciences).
Whole mount fluorescent microscopy
Whole corneas were excised, flattened by making radial incisions, washed in PBS 4% FBS, fixed in 1% paraformaldehyde (PFA) for 2 hours, washed once more in PBS 4% FBS, and mounted. Images were acquired on an Olympus Fluoview 1000X confocal microscope with a 1.4NA 60X oil objective or a 0.85NA 20X oil objective. Images were acquired by sequential scanning to avoid fluorescence crossover, and Z stacks were acquired at Nyquist sampling frequency through the tissue. All image reconstructions were made using Metamorph.
Detection of infectious virus from corneas
Mouse corneas were swabbed with sterile Weck-Cel surgical spears (Medtronic Solan, Jacksonville, FL) at 2, 4, 6, and 8 days post infection (dpi), spears were placed in 0.5 ml RPMI, and frozen at −80° C until assayed. Samples were added to confluent Vero cells, incubated for 1 hour at 37° C, and overlayed with 0.5% methylcellulose. The cultures were incubated for 72 hours, fixed with formalin, stained with crystal violet and viral cytopathic effect was detected with the aid of a dissecting microscope.
Gene expression profiles in infected corneas using GeneChip
Total RNA was isolated from whole corneas using RNeasy and Qiashredder kits (Qiagen, Valencia, CA) and the cDNA was generated using Affymetrix 3′ IVT express kit (Afymetrix Inc., Santa Clara, CA). The resulting cDNA was transcribed in vitro in the presence of biotin-labeled ribonucleotides and fragmented. Hybridization controls were added, and was hybridized overnight to a MG 430 2.0 GeneChip (Afymetrix Inc.). The chips were then washed, developed and scanned in an Agilent ChipScanner (AffymetrixInc). Raw data was processed and analyzed using AffymetrixGeneChip Operating Software (GCOS) v 1.4 with default statistical settings. GCOS 1.4 was used to assess the presence or absence of the target sequence of each panel, its expression level, and then to make all relevant pairwise statistical comparisons among samples. Processed data was sorted and inspected using Microsoft Excel.The unscaled mean values were 464.6 ± 36.7 (mean ± S.D. n=6), and were scaled to a target value of 150 using the GCOS default method (2% trimmed mean). For the highly expressed housekeeping gene GAPDH the coefficient of variation was 6.9% ± 1.3% (mean ± SD for 3 redundant panels per chip), while the housekeeping gene Pcx (pyruvate carboxylase) which has an expression level >20 fold less than GAPDH showed a coefficient of variation of 26.7% ± 5.0% (mean ± SD for 3 redundant panels per chip which showed substantial presence of transcript). Using Pcx as an exemplar, a requirement for two-fold change selects values > 3.7 SD distant from the control mean, i.e. at > 99th percentile.This microarray data set has been deposited in the NCBI’s Gene Expression Omnibus and is accessible through GEO accession number GSE33991 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE33991).
Gene expression profiles in infected corneas using quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was performed using supplies from Applied Biosystems (ABI: Foster City, CA). Between 0.47 and 1.12 μg of total RNA was reverse transcribed using a random primer protocol (High Capacity cDNA kit, Applied Biosystems). The resultant cDNA was diluted to 2 ng / μL with nuclease-free water. Diluted samples were mixed with an equal volume of reaction medium of TaqMan Universal MasterMix (Roche, Indianapolis, IN). Primer probe sets used were Pcx (Mm00442834_m1 or Mm00500992_m1) as the normalizing housekeeping gene and Ifng (Mm00801778_m1) to measure the response of the tissue to viral infection. Both these kits target amplicons which span intron-exon boundaries and are essentially free of interference from genomic DNA. Measurements were also made of the viral gene gH using a custom primer probe kit (26). Since the gH gene is intronless, in virus-infected cultured trigeminal cells approximately 25% of the Ct signal is due to viral DNA; however the uncorrected signal is a good general indicator of infection (26)
Statistical Analysis
All statistical analyses for non-microarray experiments were computed with GraphPad Prism software using unpaired t-tests. P values <0.05 were considered statistically significant.
Results
DTtreatment ablates DC from both lymphoid tissue and the cornea
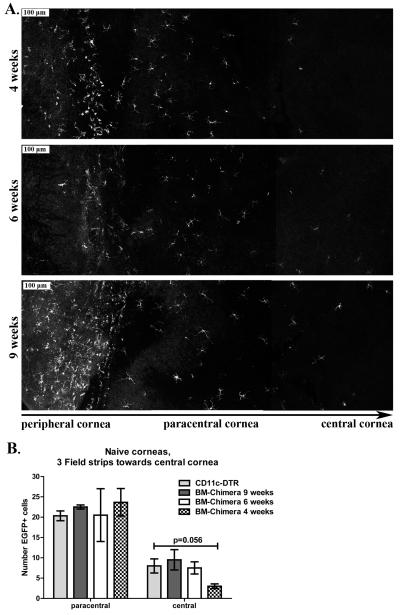
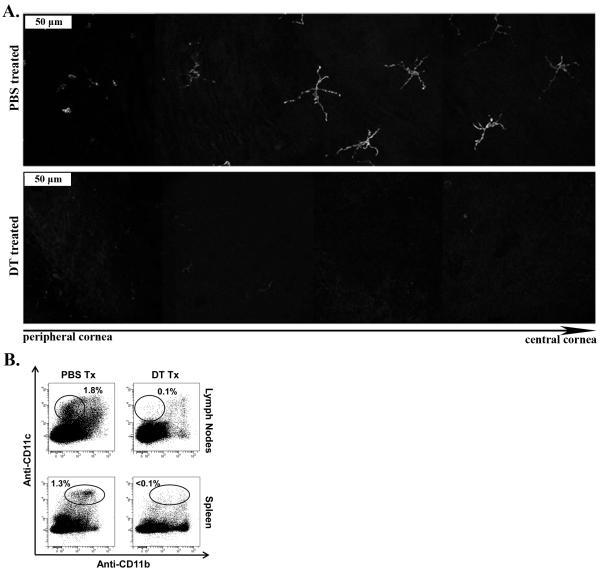
CD11c-DTR chimeras (CD11c-DTR bone marrow into wild type mice) were generated as described in methods to avoid the lethality of DT treatment in CD11c-DTR mice (27). Both the cornea (Fig 1A, B) and lymphoid organs (data not shown) were fully reconstituted with DCs that expressed EGFP+from the CD11c-promoter (from here on designated pCD11c) by 6 weeks, which is similar to the kinetics of reconstitution observed previously(28). DT treatment (175 ng, ip) effectively depleted CD11c+ DC from the corneas and lymph nodes ofreconstituted CD11c-DTR chimeras for 3 days (Fig 2A, 2B). These chimeric CD11c-DTR chimeras were used in all experiments described herein.
Figure 1. CD11c-DTR bone marrow chimeras require 6 weeks for full reconstitution of the cornea.
WT BALB/C mice were lethally irradiated and reconstituted with 5×105 bone marrow derived cells from CD11c-DTR donors. Whole corneas were excised 4, 6, and 9 weeks after bone marrow transfer, mounted, and analyzed via confocal microscopy observing pCD11c-EGFP+ cells. A) Serial images were taken starting at the corneal periphery, moving to the central cornea. Representative serial image sets of corneas of CD11c-DTR bone marrow chimera at 4, 6, and 9 weeks post-reconstitutionvisualizing pCD11c-EGFP+ cell recovery. B) Quantified imaging data of reconstitution; bars present mean ± SEM total number of EGFP-CD11c+ DCs per each of the two fields closest to the central corneal. (n=3-4 corneas/group, 2 independent experiments).
Figure 2. (Diphtheria toxin) DT treatment ablates DCs from the lymphoid organs and the cornea.
CD11c-DTR chimeraswere treated ip with 175ng of DT, or mock treated with PBS. A) At 2 days post injection, whole corneas were harvested, mounted and analyzed via confocal microscopy for pCD11c-EGFP. Serial images of whole mounted corneas were taken starting at the cornea periphery, moving to the central cornea. Data arerepresentative serial image sets of DT or mock treated corneas visualizing pCD11c-EGFP+ ablation. B) At 2 days post injection, spleens and draining lymph nodes were harvested, dispersed, stained with anti-CD11c and CD11b antibodies, and analyzed via flow cytometry. Representative flow plots depicting DCs direct the NK cell response to HSV-1 infection CD11c+ cells within the cornea-draining lymph nodes and spleen following DT and PBS treatment.(n=4-6 mice/group, 2 independent experiments).
Dendritic cells are required for optimal early clearance of HSV-1 from the cornea
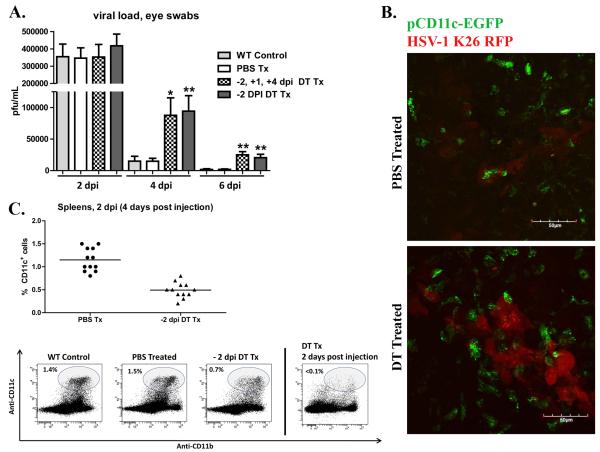
Viral titers drop precipitously from 2-4 dpiin BALB/c mice with HSV-1 corneal infections, suggesting deployment of the innate immune response during this period. Mice that were depleted of DC through 6 dpi by DT treatment at −2, +1, and +3 dpi exhibited markedly impaired viral clearance, harboring a significantly elevated viral load at 4 and 6 dpi (Fig. 3A). A nearly identical increase in viral titers was observed when DC were transiently depleted through 1 dpi by a single DT treatment at −2 dpi (Fig. 3A). Following this treatment, DCs were found within the viral lesions in the central cornea by 2 dpi as well as the spleens at 3 dpi (Fig. 3B, C). Thus, DCs that are recruited to the viral lesion within the first 24 hours after HSV-1 infection appear to play an important role in mobilizing the innate response that clears HSV-1 from the cornea. The virus replicates in the corneal epithelium which in normal corneas is endowed with a population of DCs (4). The rapid involvement of DCs in viral clearance would suggest the participation of these corneal residentDCs.
Figure 3. Dendritic cells are required for optimal early clearance of HSV-1 from the cornea.
Wild type (WT) BALB/c mice andCD11c-DTR chimeras were infected with HSV-1-RE. CD11c-DTR chimers were treated with PBS or DT 2 days before infection (−2 dpi); or treated with DT at −2 dpi, +1 dpi, and + 4 dpi. A) At indicated time eyes were swabbed with a sterile surgical spear and assayed for live virus by standard plaque assay. The data are presented as the mean ± SEM viral plaque forming units (pfu) per cornea.B) CD11c-DTR chimeras were treated with PBS or DT 2 days before corneal infection with HSV-1 K26-RFP. At 2 dpi, whole corneas were excised, mounted, and analyzed via confocal microscopy. Representative images depicting virally infected cells (red), and pCD11c-EGFP+ DCs (green) within the viral lesion in the central cornea .C) Spleens were harvested at 4 dpi from WT mice or CD11c-DTR chimeras that received ip PBS or DT treatment 2 days before HSV-1 corneal infection; cells were dispersed, stained with antibodies to CD11c and analyzed via flow cytometry. DCs had partially recovered by 4 days after treatment (2 dpi) but remained significantly reduced relative to PBS treated controls (p<0.01). (** p<0.01, * p<0.05), n=4-12 mice per group, 2 independent experiments).
DC depletion does not alter extravasation of leukocytes into the infected cornea
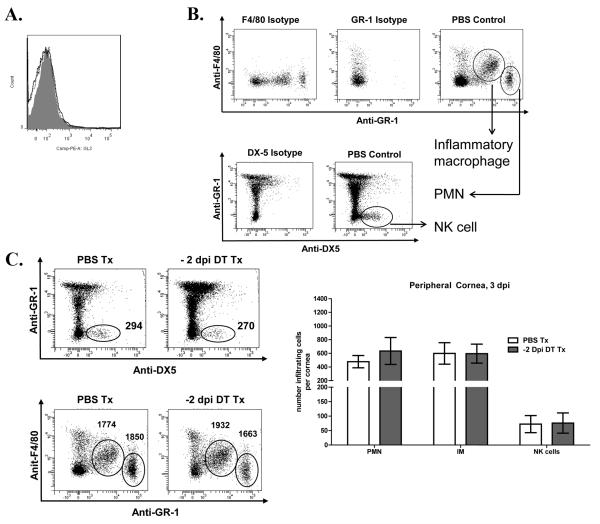
We proposed that DC depletion influenced viral clearance by controlling leukocytic infiltration of the cornea. Normal corneas are devoid of neutrophils, NK cells, Gr-1INT monocytes, and γδ T cells (not shown), but these cells reportedly infiltrate the cornea after infection. At 3 dpi we did not detect infiltrating γδ T cells (4A), but did detect a robust infiltration of Gr-1high F4/80− neutrophils (PMN), Gr-1int F4/80+ inflammatory monocytes (IM), and Gr1− DX5+ NK cells (Fig. 4B). To determine if extravasation of these cells into the peripheral cornea is altered by DC depletion, CD11c-DTR chimeras received a single DT or PBS treatment 2 days before infection, infected corneas were excised at 3 dpi, and the central 2 mm of each cornea was separated from the remaining peripheral cornea using a trephine. Individual peripheral corneas were dispersed with collagenase, and analyzed by flow cytometry. As illustrated in Figure 4C, DT treatment 2 days before infection did not significantly influence the migration of neutrophils, inflammatory monocytes, or NK cells into the peripheral cornea.
Figure 4. DC depletion does not alter extravasation of leukocytes into the peripheral cornea.
Wild type (WT) and CD11c-DTR mice were infected with HSV-1 RE. CD11c-DTR chimeras were treated with PBS or DT 2 days before infection. At 3 dpicorneas were excised, and the central 2 mm dissected away from the remaining peripheral cornea with a trephine. Pools of 3 DCs direct the NK cell response to HSV-1 infection peripheral corneas were dispersed with collagenase, stained with antibodies to CD45,GL-3, CD49b, F4/80, and GR-1, and infiltrating leukocytes identified by flow cytometry (A)Representative histogram overlays depicting GL-3+ γδ T cells within the peripheral cornea. Histograms: Isotype control is grey shaded, CD11c-DTR PBS Tx is black solid, and −2 dpi DT Tx is black dotted. (B)Representative flow plots gated on CD45+ cells (numbers are for three corneas). (C)bars represent the mean ± SEM absolute number of neutrophils (PMN), inflammatory monocytes (IM), and NK cells per individual peripheral cornea (n=4-6 pooled corneas, 3 independent experiments).
The activation phenotype of NK cells in the peripheral cornea is not DC dependent
Recent findings suggest an important role for DC in the migration of activated NK cellsinto sites of infection(6). Although DC depletion did not influence NK cell numbers in the peripheral cornea, impaired viral clearance could reflect DC regulation of their activation status. CD11c-DTR chimeras were treated with PBS or DT 2 days prior to HSV-1 corneal infection. At 3 dpi the corneas were excised and the activation phenotype of the DX5+ NK cells in the corneal periphery was determined based on expression of CD69 and granzyme B (GrB). As illustrated in Figure 5A & B, approximately 65% of the NK cells in the infected corneas were activated as indicated by CD69 and GrB expression, and their activation phenotype was not altered by DC depletion. However, as noted above a single DT treatment at −2 dpi only maintains DC depletion through 1 dpi. It was possible, therefore that NK cells acquired the activation phenotype during DC reconstitution of the cornea (1-3 dpi). To address this, CD11c-DTR mice received DT treatments at −2 and +1 dpi to maintain DC depletion through 3 dpi. However, even continuous DC depletion through 3 dpi did not influence the activation status of NK cells in the peripheral cornea (Fig. 5B).
Figure 5. NK cells in the infected cornea acquire an activation phenotype in the absence of DC.
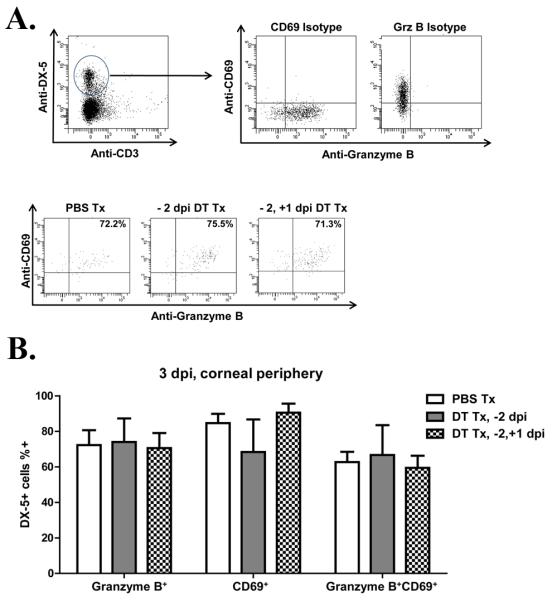
CD11c-DTR chimeras were treated with PBS or DT 2 days before corneal infection with HSV-1-RE (−2 dpi). At 3 dpi the central 2 mm of cornea was removed with a trephine and pools of 3 peripheral corneas were dispersed with collagenase, stained for the NK cell marker DX5, the activation markers CD69 and granzyme B (GrB), and analyzed by flow cytometry. A) flow plots depicting isotype controls for CD69 and Granzyme B expression on NK cells, and representative flow plots depicting peripheral NK cell CD69 and Granzyme B expression in PBS Tx; −2 dpi DT Tx; −2 and +1 DT Tx CD11c-DTR mice. B) Bars represent mean frequency ± SEM of CD69+, Granzyme B+, or CD69+GranzymeB+ NK cells within the corneal periphery. (n=6-8 pooled corneas, 2 independent experiments)
Fewer NK cells and inflammatory monocytes migrate into the central cornea following DC depletion
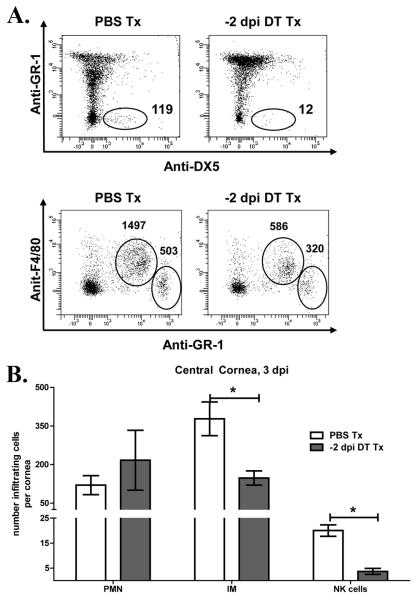
In our corneal infection model, HSV-1 lesions are largely restricted to the central area of the cornea (Fig 6A, B). Because the cornea is devoid of blood vessels, leukocytes must extravasate from blood vessels in the peripheral cornea and then migrate to the site of the lesion within the central cornea. We proposed, therefore, that the failure of DC-depleted corneas to control virus replication might reflect an inability of the extravasated leukocytes to traffic to the central cornea. To test this, we treated mice with PBS or DT 2 days before infection, and then quantified NK cells, inflammatory monocytes, and neutrophils in the central corneas at 3 dpi. DC depletion significantly reduced migration of NK cells and inflammatory monocytes into the central cornea, while neutrophil migration was not affected (Fig. 7).
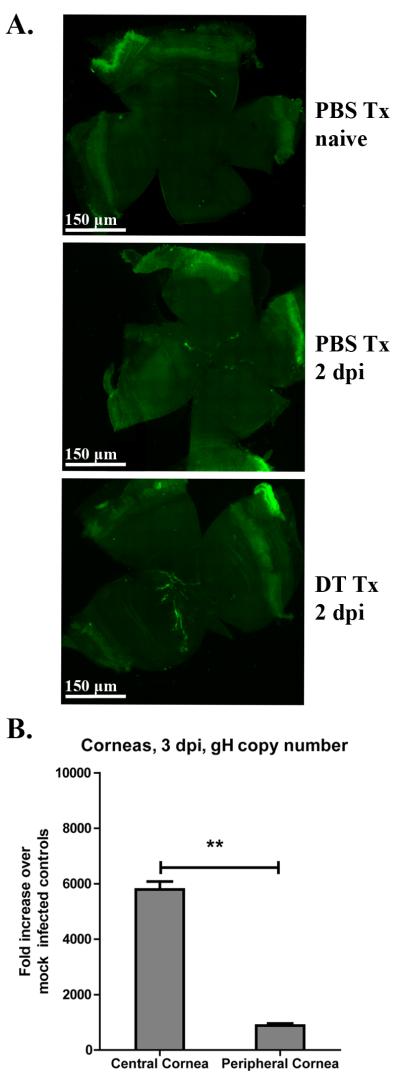
Figure 6. Viral replication is confined to the central cornea.
CD11c-DTR mice were infected with HSV-1-K26 or HSV-1-RE. Mice were either treated with PBS or DT at −2 dpi. For mice infected with HSV-1 K26-RFP, at 2 dpi whole corneas were excised, mounted, and the entire cornea was imaged by confocal microscopy. A) Representative whole corneal images depicting virally infected cells (green) within naive, 2 dpi PBS Tx, or 2 dpi DT Tx corneas. B) In HSV-1 RE infected mice, whole corneas were excised at 3 dpiand the central and peripheral cornea were separated using a 2mm trephine. DNA was extracted from pools of 3 corneas and the amount of HSV-1 genome harbored within the central and peripheral corneas was determined by quantitative real time PCR for the gH gene. Bars represent mean fold increase ± SEM of gH copies over mock infected. (** p<0.01, n=4 mice per group, 2 independent experiments (panel A), (4 pooled corneas, 2 independent experiments, Panel B)
Figure 7. Fewer NK cells and inflammatory monocytes migrate into the central cornea following DC depletion.
Wild type (WT) and CD11c-DTR mice were infected with HSV-1 RE. CD11c-DTR chimeras were treated with PBS or DT 2 days before infection (−2 dpi). At 3 dpi corneas were excised, and the central 2 mm dissected away from the remaining peripheral cornea with a trephine. Pools of 3 central corneas were dispersed with collagenase and infiltrating leukocytes were identified by flow cytometry as described in Fig. 5. (A) Representative flow plots gated on CD45+ cells (numbers are for three corneas). (B)bars represent the mean ± SEM absolute number of neutrophils (PMN), inflammatory monocytes (IM), and NK cells per individual central cornea (*p<0.05, n=4-6 pooled corneas, 3 independent experiments)
Depletion of NK cells results in increased viral burden within the cornea
The above findings were consistent with the notion that DC indirectly contributed to HSV-1 clearance by regulating NK cell migration to the site of the lesion in the central cornea. Therefore, we predicted that NK cell depletion would have a similar impact on HSV-1 clearance from the cornea as was seen with DC depletion. To test this prediction, WT mice were depleted of NK cells by treatment with anti-ASGM-1 antibody or mock depleted with PBS 1 day before corneal infection,and viral clearance was monitored through 8 dpi. Depletion of NK cells delayed clearance of virus from the cornea in a pattern similar to that seen following DC depletion (Fig. 8).
Figure 8. Depletion of NK cells results in increased viral burden within the cornea.
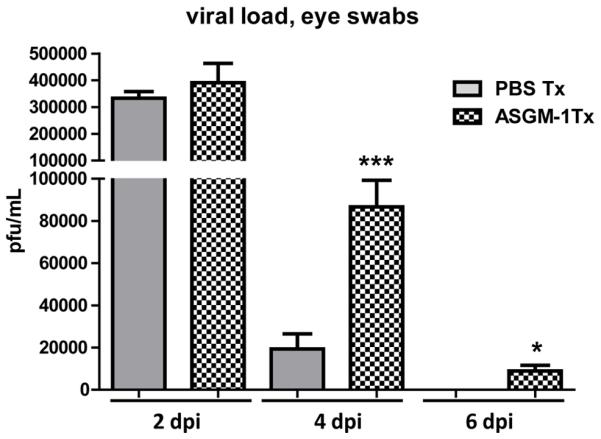
WT BALB/C mice received ip injections of PBS or ASGM-1 antibody 1 day before corneal infection with HSV-1 RE. At indicated dpi, eyes were swabbed with a sterile surgical spear and assayed for live virus by standard plaque assay. Data are presented as the mean ± SEM viral plaque forming units (pfu) per cornea of PBS and anti-ASGM-1 treated mice. (* p<0.05, *** p<0.005,n=8-10 mice per group, 2 independent experiments)
DC depletion alters gene expression in the infected cornea
Gene array analyses following DC depletion revealed changes in gene expression that were consistent with the observed changes in NK cell, monocyte, and neutrophil migration to the central cornea. DC depletion from infected corneas resulted in significant (>2-fold) reductions in expression of several genes whose products are involved in attracting and activating NK cells and monocytes (supplemental table 1). These include the chemokines CCL5 (activates NK cells), CCL7 (attracts and activates macrophages), CCL8 (attracts and activates monocytes and NK cells), CXCL9 (attracts NK cells) CXCL10 (attracts macrophages and NK cells), and CXCL11 (attracts NK cells). In contrast CXCL2, the chemokine primarily responsible for neutrophil migration into the HSV-1 infected cornea,was up-regulated in infected corneas, but was not reduced in infected corneas of DC-depleted mice(29). We did not observe a consistent reduction of IFN-γ mRNA in DC depleted corneas (data not shown). However, we did observe a consistent reduction in mRNA for several IFN-γ inducible genes (supplementary Table 1).
Discussion
It is now clear that DCs do not represent a homogeneous cell population, but rather are comprised of phenotypically and functionally diverse cells. For instance, distinct populations of Langerin positive DCs reside in the epidermal and dermal layers of the skin, with the DEC-205+ CD11b+ epidermal Langerhans cells (LC) implicated in inhibition of contact hypersensitivity reactions, and the CD103+CD11bdim dermal DCs implicated in the stimulation of contact hypersensitivity responses (30,31). The cornea of the eye represents a mucosal surface that enjoys a degree of immune privilege that was previously attributed in part to a lack of professional antigen presenting cells. However, recent studies have demonstrated that the normal mouse cornea contains a small, but clearly detectable population of CD11c+CD11b− DCs and F4/80+CD11b+ macrophages (4,32). The small number of these cornea resident DCs and macrophages has made it difficult to directly define their function. However, we reasoned that by transiently depleting the resident corneal DC population at the time of HSV-1 corneal infection, we could begin to interrogate the functions ofresident and/or very early infiltratingDCs within the HSV-1 infected cornea.
Following corneal infection of BALB/c mice, HSV-1 transiently replicates in corneal epithelial cells, with the majority of viral clearance occurring between 2 and 4 dpi. In our model, the virus is applied topically to the abraded central cornea, and the resulting epithelial lesions are largely restricted to the central corneal epithelium. We show that a single DT treatment 2 days before HSV-1 corneal infection depletes the resident DC population from the cornea, that replenishment of the DC population begins 3 days after treatment (1 day after infection), and that DCs are prominently present in the HSV-1 lesions by 2 dpi. This transient depletion of the cornea resident DC population did not influence HSV-1 titers in the cornea as measured at 2 dpi, but did markedly delay viral clearance at 4 and 6 dpi. An identical delay in viral clearance from the cornea was observed when DCs were persistently depleted by DT treatment at −2, +1, and +3 dpi, suggesting a specific role in viral clearance for the cornea resident DC population or those infiltrating the cornea during the first 24 hours after infection. Although plasmacytoid DCs (pDC) are a major source of type I interferon in HSV-1 infected mice (33,34), pDCs are not present in the normal cornea, and are not depleted by DT treatment of CD11c-DTR mice(35). Therefore, we proposed that the delay in HSV-1 clearance following DC depletion did not reflect direct viral clearance by DCs, but rather DC regulation of another cell type that is the proximal mediator of viral clearance from the cornea.
In HSV-1 corneal infection, γδ T cells, NK cells, and neutrophils have all been assigned important roles in control of HSV-1 replication (11,36,37).We observed virtually no γδ T cells in the infected corneas during viral clearance, and neutrophil infiltration was not altered by DC depletion. Therefore, we hypothesized that DC depletion influenced viral clearance by influencing NK cellfunction in the cornea. This hypothesis was supported by the observation that a very similar loss of HSV-1 control occurred when NK cells were depleted with ASGM-1 antibody before infection. These observations establish NK cells as an important proximal regulator of viral control within the cornea, and are consistent with the notion that DC control HSV-1 replication in the cornea indirectly by regulating NK cells.
Although CD11c can be expressed on activated CD8+ T cells, we do not see CD11c or EGFP expression in CD8+ T cells in the draining lymph nodes of HSV-1 infected CD11c -DTR mice (not shown). Moreover we see very few, if any CD8+ T cells in the infected corneas of BALB/cmice, and never observe them in the cornea as early as 4 dpi when viral titers are augmented by DT treatment of CD11c DTR mice. These findings support the view that DT treatment augments viral titers indirectly by depleting the DCs that regulate NK cell infiltration of the cornea.
We considered that DCs could influence NK cell function by controlling their extravasation, directed migration, or activation within the HSV-1 infected cornea. A recent study demonstrated that DCs are required for NK cell activation and their subsequent migration into inflamed tissue (6). However, we observed normal numbers of NK cells in the peripheral corneas of DC-depleted mice, demonstrating that DCs are not needed for the initial extravasation of NK cells into HSV-1 infected corneas. Thus in this infection model DC priming of NK cells in lymphoid organs appears unnecessary for their subsequent migration into HSV-1 infected corneas.
The potential lethality of NK cells dictates that their effector functions be closely regulated. Indeed NK cell effector mechanisms are regulated by a balance of positive and negative signals that are delivered through surface receptors(38).There is also strong evidence supporting direct activation of NK cells through toll-like receptors (TLR). For instance, viral sensing TLR-2 and TLR-7 can directly activate NK cells, and TLR-2 mediated NK cell activation is critical for control of vaccinia virus in vivo (39,40). Moreover, DCs have been assigned a critical role in the survival and function of NK cells(8,19,21,41). Cross-presentation ofIL-15 appears to be the primary mechanism of DC-mediated activation of NK cell function (13). Thus the relative contribution of direct versus DC-mediated activation to NK cell function at sites of infection remains unclear. We noted that NK cells that infiltrated the peripheral cornea at 3 dpi expressed an activated phenotype (approximately 70% CD69+ Granzyme B+) regardless of DC depletion. These phenotypic findings suggest that any NK cell functional deficiencies resulting from inadequate DC priming in the lymphoid organs can be at least partially compensated for by stimulation within the infected tissue. Moreover, since the majority of NK cells in the corneas of DC depleted mice express an activated phenotype, the delayed HSV-1 clearance in these corneas is probably not due to inadequate activation of NK cells.
In vascularized tissue it can be difficult to distinguish extravasation of leukocytes from the blood and their subsequent directed migration to the site of the infection. However, following HSV-1 infection of the avascular cornea leukocytes must extravasate from blood vessels in the limbal region adjacent to the peripheral cornea and then migrate through approximately 3 mm of avascular tissue to reach the lesion in the central cornea. Thus, one can readily differentiate an effect on extravasation of leukocytes into the peripheral cornea and their directed migration into the central cornea (26,42). Although both DC and NK cells are attracted to the site of HSV-1 lesions, it is not clear if they are attracted simultaneously by the same or concurrently produced chemokines, or if the two cell types are sequentially attracted. We show that when DC are depleted from the cornea NK cell migration from the peripheral to the central cornea is markedly diminished, and control of virus replication is delayed. Our observations establish an important role for DCs in regulating the directed migration of NK cells to sites of viral infection. Since HSV-1 replication in this model occurs primarily in the central region of the avascular cornea, a lack of directed NK cell migration might have a more dramatic effect on viral clearance in the cornea than would be observed in a more vascularized tissue. For instance a recent report established that DC depletion influenced NK cell function in lymphoid organs, but did not influence HSV-1 clearance from infected footpads of mice (8).
The migration of inflammatory monocytes (F4/80+ Gr-1int) from the peripheral to the central region of infected corneas was also impaired by DC depletion. Inflammatory monocytes produce antiviral compounds including tumor necrosis factor and nitric oxide that contribute to the control of HSV-1 replication in the trigeminal ganglion (43). Therefore, the reduction in infiltration of inflammatory monocytes into the central cornea might also have contributed to the delayed clearance of HSV-1 from the cornea.
The impaired HSV-1 clearance following DC depletion was not associated with reduced neutrophil infiltration into the peripheral or central cornea. Although a previous study suggested an important role for neutrophils in controlling HSV-1 replication in the cornea, that study employed the Gr-1 antibody for neutrophil depletion, which is now known to deplete a variety of cell populations in addition to neutrophils (13). While not ruling out a contribution of neutrophils to viral clearance from the cornea, our findings are consistent with recent reports demonstrating a lack of neutrophil involvement in HSV-1 clearance from the lungs and skin (14,15).
The reduced migration of NK cells and inflammatory monocytes into the central cornea that was observed following DC depletion was associated with a significant reduction in several chemokines that are known to direct the migration of these cell types. As noted in supplemental table 1, the chemokines CCL5, CCL7, CCL8, CXCL9, CXCL10, and CXCL11 were up-regulated in infected corneas, and reduced by > 2 fold by DC depletion. It is not clear if these chemokines are produced directly by the DC, or if DC induce their production by other cells, such as monocytes. A number of these chemokines are induced by IFN-γ, and expression of several other IFN-γ inducible genes was reduced in DC depleted corneas. We saw reduced IFN-γ mRNA in some experiments, but this was not a consistent finding in DC-depleted corneas (data not shown). However, the mRNA was obtained from whole corneas so it remains possible that local IFN-γ production at the site of the lesion was reduced by DC depletion. This would be consistent with the reduced NK cell migration to the lesion, and with the observation that NK cell depletion dramatically reduced IFN-γ mRNA in the infected corneas (supplemental Figure 1).
DC depletion did not alter neutrophil migration into the central cornea. This is consistent with the finding that expression ofmacrophage inflammatory protein 2(MIP-2/CXCL2)that is primarily responsible for directing neutrophil migration into the HSV-1 infected cornea(29) was not affected by DC depletion. A previous study showed that early MIP-2 production in HSV-1 infected corneas is regulated by IL-17 that was detected in corneas within 24 hours of infection(44). The source of IL-17 in infected corneas was not established, but our studies suggest that its production is DC independent.
Taken together, our data suggest that the cornea resident or an early responding blood-derived DC population reacts rapidly to HSV-1 infection by directly or indirectly inducing production of several chemokines that are needed to attract NK cells and inflammatory monocytes to the location of the viral lesion within the central cornea. Following a single DT treatment 2 days before HSV-1 corneal infection DCs begin to recover by 1 dpi, and are found in and around the HSV-1 lesion in the central cornea by 2 dpi. However, viral clearance remains impaired at 4 dpi and to a lesser extent at 6 dpi. The impaired viral clearance is associated with a nearly complete exclusion of NK cells and a significant reduction of inflammatory monocytes from the central cornea at 3 dpi. The absence of NK cells in the central cornea either as a result of DC depletion or direct NK cell depletion resulted in similar impairment of viral clearance. This strongly suggests a role for NK cells incontrolling HSV-1 replication in the cornea. We believe the unique requirements of the avascular cornea for NK cell trafficking allowed us to uncover an important function of DCs in controlling NK cell migrationfollowing their extravasation from the blood that would be more difficult to detect in a vascularized tissue.
Supplementary Material
Acknowledgements
We thank Jessica Spehar for technical assistance,Nancy Zurowski for flow cytometry acquisition, and Kira Lathrop for assistance with confocal microscopy.
Grant Support: This work was supported by National Eye Institute grants R01-EY010359 (RLH), P30-EY08099 (RLH), and 5T32-EY017271-02 (GMF); an unrestricted research grant (RLH) from Research to Prevent Blindness, Inc.; a grant from the Eye and Ear Foundation of Pittsburgh (RLH)
Footnotes
The authors have no conflicting financial interests.
Reference List
- 1.Kaisho T, Akira S. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 2001;22:78–83. doi: 10.1016/s1471-4906(00)01811-1. [DOI] [PubMed] [Google Scholar]
- 2.Kaisho T, Akira S. Toll-like receptors and their signaling mechanism in innate immunity. Acta Odontol. Scand. 2001;59:124–130. doi: 10.1080/000163501750266701. [DOI] [PubMed] [Google Scholar]
- 3.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 4.Knickelbein JE, Watkins SC, McMenamin PG, Hendricks RL. Stratification of Antigen-presenting Cells within the Normal Cornea. Ophthalmol. Eye Dis. 2009;1:45–54. doi: 10.4137/oed.s2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eidsmo L, Allan R, Caminschi I, van Rooijen N, Heath WR, Carbone FR. Differential migration of epidermal and dermal dendritic cells during skin infection. J. Immunol. 2009;182:3165–3172. doi: 10.4049/jimmunol.0802950. [DOI] [PubMed] [Google Scholar]
- 6.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kassim SH, Rajasagi NK, Zhao XY, Chervenak R, Jennings SR. In vivo ablation of CD11c-positive dendritic cells increases susceptibility to herpes simplex virus type 1 infection and diminishes NK and T-cell responses. J. Virol. 2006;80:3985–3993. doi: 10.1128/JVI.80.8.3985-3993.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kassim SH, Rajasagi NK, Ritz BW, Pruett SB, Gardner EM, Chervenak R, Jennings SR. Dendritic cells are required for optimal activation of natural killer functions following primary infection with herpes simplex virus type 1. J. Virol. 2009;83:3175–3186. doi: 10.1128/JVI.01907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J. Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiligenhaus A, Bauer D, Zheng M, Mrzyk S, Steuhl KP. CD4+ T-cell type 1 and type 2 cytokines in the HSV-1 infected cornea. Graefes Arch. Clin. Exp. Ophthalmol. 1999;237:399–406. doi: 10.1007/s004170050251. [DOI] [PubMed] [Google Scholar]
- 11.Ghiasi H, Cai S, Perng GC, Nesburn AB, Wechsler SL. The role of natural killer cells in protection of mice against death and corneal scarring following ocular HSV-1 infection. Antiviral Res. 2000;45:33–45. doi: 10.1016/s0166-3542(99)00075-3. [DOI] [PubMed] [Google Scholar]
- 12.Carr DJ, Harle P, Gebhardt BM. The immune response to ocular herpes simplex virus type 1 infection. Exp. Biol. Med. (Maywood.) 2001;226:353–366. doi: 10.1177/153537020122600501. [DOI] [PubMed] [Google Scholar]
- 13.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 14.Wojtasiak M, Pickett DL, Tate MD, Londrigan SL, Bedoui S, Brooks AG, Reading PC. Depletion of Gr-1+, but not Ly6G+, immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. J. Gen. Virol. 2010;91:2158–2166. doi: 10.1099/vir.0.021915-0. [DOI] [PubMed] [Google Scholar]
- 15.Wojtasiak M, Pickett DL, Tate MD, Bedoui S, Job ER, Whitney PG, Brooks AG, Reading PC. Gr-1+ cells, but not neutrophils, limit virus replication and lesion development following flank infection of mice with herpes simplex virus type-1. Virology. 2010;407:143–151. doi: 10.1016/j.virol.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Quinnan GV, J E. Manischewitz. The role of natural killer cells and antibody-dependent cell-mediated cytotoxicity during murine cytomegalovirus infection. J. Exp. Med. 1979;150:1549–1554. doi: 10.1084/jem.150.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh RM. Regulation of virus infections by natural killer cells. Nat. Immun. Cell Growth. Regul. 1986;5:169–199. [PubMed] [Google Scholar]
- 18.Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Munz C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J. Immunol. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 19.Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, Bougras G, Muller WA, Moretta L, Munz C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi H, Dubois S, Sato N, Sabzevari H, Sakai Y, Waldmann TA, Tagaya Y. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105:721–727. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 21.Hochweller K, Striegler J, Hammerling GJ, Garbi N. A novel CD11c.DTR transgenic mouse for depletion of dendritic cells reveals their requirement for homeostatic proliferation of natural killer cells. Eur. J. Immunol. 2008;38:2776–2783. doi: 10.1002/eji.200838659. [DOI] [PubMed] [Google Scholar]
- 22.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JK, Laycock KA, Nash MM, Pepose JS. Corneal Langerhans cell dynamics after herpes simplex virus reactivation. Invest Ophthalmol. Vis. Sci. 1993;34:2282–2290. [PubMed] [Google Scholar]
- 24.Chen H, Hendricks RL. B7 costimulatory requirements of T cells at an inflammatory site. J Immunol. 1998;160:5045–5052. [PubMed] [Google Scholar]
- 25.Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Q, Chen W, Hendricks RL. Proinflammatory functions of IL-2 in herpes simplex virus corneal infection. J Immunol. 1997;158:1275–1283. [PubMed] [Google Scholar]
- 27.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinnery HR, Humphries T, Clare A, Dixon AE, Howes K, Moran CB, Scott D, Zakrzewski M, Pearlman E, McMenamin PG. Turnover of bone marrow-derived cells in the irradiated mouse cornea. Immunol. 2008;125:541–548. doi: 10.1111/j.1365-2567.2008.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan X-T, Tumpey TM, Kunkel SL, Oakes JE, Lausch RN. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Investigative Ophthalmology & Visual Science. 1998;39:1854–1862. [PubMed] [Google Scholar]
- 30.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264–2271. [PMC free article] [PubMed] [Google Scholar]
- 33.Vollstedt S, O’Keeffe M, Ryf B, Glanzmann B, Hochrein H, Suter M. The long-term but not the short-term antiviral effect of IFN-alpha depends on Flt3 ligand and pDC. Eur. J Immunol. 2006;36:1231–1240. doi: 10.1002/eji.200535759. [DOI] [PubMed] [Google Scholar]
- 34.Hochrein H, Schlatter B, O’Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sapoznikov A, Fischer JA, Zaft T, Krauthgamer R, Dzionek A, Jung S. Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J. Exp. Med. 2007;204:1923–1933. doi: 10.1084/jem.20062373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bukowski JF, Welsh RM. The role of natural killer cells and interferon in resistance to acute infection of mice with herpes simplex virus type 1. J. Immunol. 1986;136:3481–3485. [PubMed] [Google Scholar]
- 37.Sciammas R, Kodukula P, Tang Q, Hendricks RL, Bluestone JA. T cell receptor-gamma/delta cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b) J Exp Med. 1998;188:1841–1848. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourquin C, Schmidt L, Lanz AL, Storch B, Wurzenberger C, Anz D, Sandholzer N, Mocikat R, Berger M, Poeck H, Hartmann G, Hornung V, Endres S. Immunostimulatory RNA oligonucleotides induce an effective antitumoral NK cell response through the TLR7. J. Immunol. 2009;183:6078–6086. doi: 10.4049/jimmunol.0901594. [DOI] [PubMed] [Google Scholar]
- 40.Martinez J, Huang X, Yang Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS. Pathog. 2010;6:e1000811. doi: 10.1371/journal.ppat.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zitvogel L. Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J. Exp. Med. 2002;195:F9–F14. doi: 10.1084/jem.20012040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang Q, Hendricks RL. Interferon gamma regulates platelet endothelial cell adhesion molecule 1 expression and neutrophil infiltration into herpes simplex virus-infected mouse corneas. J Exp Med. 1996;184:1435–1447. doi: 10.1084/jem.184.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito S, Nakano M. Nitric oxide production by peritoneal macrophages of Mycobacterium bovis BCG-infected or non-infected mice: regulatory roles of T lymphocytes and cytokines. J. Leukoc. Biol. 1996;59:908–915. doi: 10.1002/jlb.59.6.908. [DOI] [PubMed] [Google Scholar]
- 44.Molesworth-Kenyon SJ, Yin R, Oakes JE, Lausch RN. IL-17 receptor signaling influences virus-induced corneal inflammation. J. Leukoc. Biol. 2008;83:401–408. doi: 10.1189/jlb.0807571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.