Abstract
Gene therapy holds promise for treating numerous heart diseases. A key premise for the success of cardiac gene therapy is the development of powerful gene transfer vehicles that can achieve highly efficient and persistent gene transfer specifically in the heart. Other features of an ideal vector include negligible toxicity, minimal immunogenicity and easy manufacturing. Rapid progress in the fields of molecular biology and virology has offered great opportunities to engineer various genetic materials for heart gene delivery. Several nonviral vectors (e.g. naked plasmids, plasmid lipid/polymer complexes and oligonucleotides) have been tested. Commonly used viral vectors include lentivirus, adenovirus and adeno-associated virus. Among these, adeno-associated virus has shown many attractive features for pre-clinical experimentation in animal models of heart diseases. We review the history and evolution of these vectors for heart gene transfer.
Keywords: AAV, adenovirus, adeno-associated virus, heart gene delivery, lentivirus, nonviral vector, viral vector
Introduction
Heart disease is a leading health challenge worldwide. Conventional pharmacology, implantable devices and surgery may improve the quality of life and survival of patients with heart diseases. Yet, they cannot completely fulfil clinical needs. Revolutionary breakthroughs in gene transfer technology have now provided exciting new opportunities to address some of the fundamental issues in heart disease treatment. Various gene delivery vectors and heart gene transfer techniques have been developed. Critical proof-of-principle studies have also been conducted in rodents and, more recently, in large animals such as dogs and pigs. Promising results from numerous pre-clinical studies in animal models of heart diseases, as well as from early phase clinical trials, have conclusively demonstrated unique advantages of gene therapy that cannot be matched by traditional therapeutic modalities.
A basic premise of heart gene therapy is the availability of vehicles that can robustly, specifically and persistently deliver therapeutic genetic materials (DNA, RNA, oligonucleotides, etc.) to the heart without generating local and/or systemic toxicity. Broadly speaking, gene delivery vectors can be divided into two categories: nonviral and viral vectors (Figure 1 and Table 1). This review focuses on the history of heart gene delivery vector development.
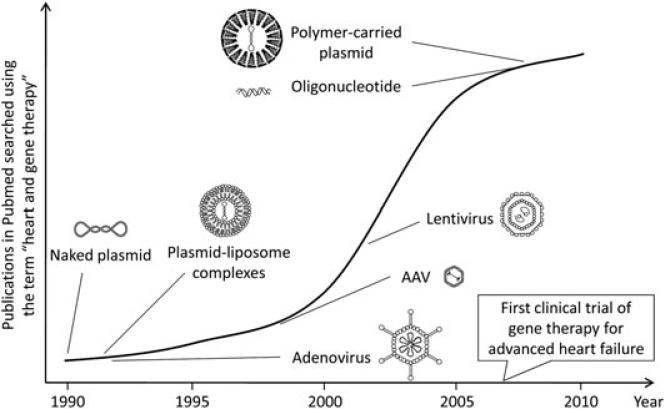
Figure 1.
Activity of heart gene delivery research since its inception. Pubmed is searched using the MeSH term ‘heart and gene therapy’. The number of publications is plotted against the year. The time of introduction of each vector is marked. An explosive increase in the number of heart gene delivery papers is noticed after AAV vector is introduced. The first clinical trial of gene therapy for advanced heart failure was initiated in 2007. AAV vector was used in the trial. Each vector is not drawn to scale.
Table 1.
Gene transfer vectors used in heart gene therapy
| Vector for gene delivery | Delivery methods | Length of gene expression | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Nonviral | |||||
| Plasmids | Intramyocardial | 28–60 days | Low cost, simplicity in design | Restricted transduction, short-term expression | 1–12 |
| DNA–lipid complexes | Intravenous | Ability to deliver systemically | Limited myocardial transduction, off-target expression | 13–17 | |
| Oligonucleotides | Intravenous | 2 weeks to 3 months | Restore expression at mRNA level | Repeated administration, short half-life, patient-specific design | 18–19 |
| Viral | |||||
| Lentivirus | Intramyocardial, coronary arterial | 10 weeks to 6 months | Ability to package up to 8 kb, persistent expression upon integration to the host genome | Myocardial inflammation, insertional mutagenesis | 21–24 |
| Adenovirus | Intramyocardial, intrapericardial, coronary arterial, intravenous | 1–8 weeks | Large packaging capacity (up to 35 kb for gutted adenovirus), efficient cardiomyocyte transduction | Myocardial inflammation, cellular immune responses | 25–41 |
| Adeno-associated virus | Intramyocardial, intrapericardial, coronary arterial, intravenous | 5 weeks to 12 months | Efficient cardiomyocyte transduction, persistent expression | Limited packaging capacity | 42–74 |
Vectors for cardiac gene delivery
Gene delivery using naked plasmids
The ability of the heart to uptake and express naked plasmid DNA after a direct myocardial injection was first shown in 1990 (Figure 1) by Lin et al. [1], who injected 100 μg of a plasmid carrying the β-galactosidase (LacZ) reporter gene to the apex of the rat heart (Figure 2). LacZ positive cardiomyocytes were observed 3 days later [1]. A similar study using additional reporter gene plasmids was reported by Buttrick et al. [2] shortly afterward. Interestingly, the results obtained in these early studies suggest that plasmid-mediated myocardial gene transfer may last up to 28–60 days.
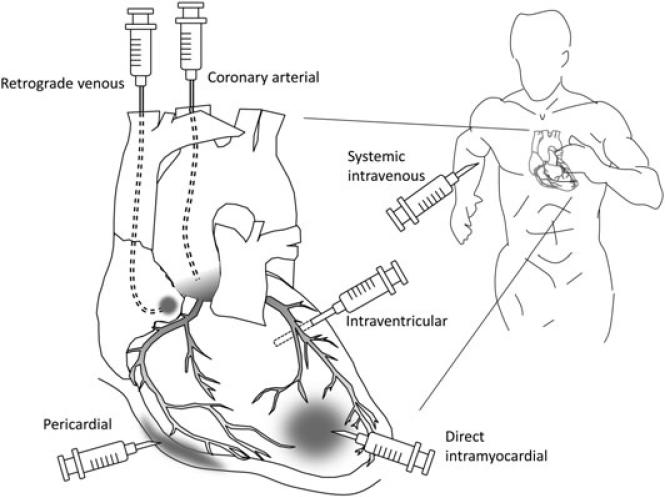
Figure 2.
Methods of heart gene delivery. Many different approaches have been explored to deliver a gene therapy vector to the heart. Most of the early studies involved direct intramyocardial injection. Intrapericardial injection method has also been used in some studies. Coronary perfusion can be achieved with either coronary artery injection or via a retrograde approach. Coronary arterial injection with or without cardiac circulatory isolation may achieve the most efficient and widespread transduction of the heart, although it is moderately invasive. A retrograde venous approach utilizes entry via the coronary sinus. Systemic administration via the peripheral vein is the least invasive method. The vector passes the pulmonary circulation before it reaches the coronary artery. The success of systemic administration is heavily dependent on the cardiac tropism of the vector.
Regulated cardiac gene expression may offer certain therapeutic advantages. To achieve this goal, Fishman et al. [3] applied the tetracycline-controlled transactivation system in plasmid gene transfer. They revealed tight control of gene expression in the heart by subtherapeutic concentrations of tetracycline in a dose-dependent manner.
The demonstration of plasmid gene transfer in the heart opens a whole new world of treating heart diseases with somatic gene therapy. However, plasmid vectors have several important limitations. The transduction efficiency was fairly low and expression was restricted to the vicinity of the injection site. To facilitate gene delivery, plasmid DNA was often mixed with high concentrations of sucrose, which may not be suitable for clinical applications. Collectively, it will be a challenge to apply direct plasmid injection for heart disease gene therapy in patients. Nonetheless, because of its simplicity, direct myocardial plasmid injection remains a useful tool for basic cardiac research. For example, this technique has been used to define in vivo transcriptional regulation of cardiac specific genes (e.g. the myosin heavy chain gene and the cardiac isoform of sarcoplasmic reticulum calcium ATPase, SERCA2a) gene) [4,5].
Despite the difficulties in translating plasmid injection to heart disease treatment, a recent study raises the possibility of plasmid heart gene therapy for certain cardiac conditions [6]. A plasmid was used that expresses the stromal cell-derived factor-1α gene. After direct myocardial injection in a rat model of acute myocardial infarction, increased angiogenesis was observed. Furthermore, heart function was better preserved [6].
Clinical trials have been conducted using vascular endothelial growth factor (VEGF165) plasmid vector to treat symptomatic myocardial ischemia [7–11]. Both direct myocardial injection and percutaneous catheter-based delivery methods were tested. These studies revealed a good safety profile and amelioration of some symptoms (e.g. frequency and severity of angina pectoris) [7–11]. Nevertheless, conclusive evidence of long-term angiogenic benefit has not been established. Recently, a double-blind, placebo-controlled trial was conducted in advanced coronary disease patients using intramyocardial VEGF165 therapy [12]. Unfortunately, no benefit was observed at 3- or 6-month time points [12].
Gene delivery using plasmid–lipid and plasmid–polymer complexes
In late 1980s, it was found that mixing plasmids with cationic lipid could lead to highly efficient and reproducible gene transfer in cultured cells (Figure 1) [13,14]. Because direct injection of plasmids to the heart has only resulted in limited transduction, lipid-mediated plasmid delivery was introduced. In 1992, Stewart et al. [15] tested plasmid–liposome complexes in mice by tail vein injection (Figure 2). Polymerase chain reaction analysis of the plasmid DNA at 9–11 days post-injection showed plasmid transfer in the heart and lung, but not liver and kidney. Subsequently, Hofland et al. [16] developed a strategy of formulating a stable plasmid–lipid complex for in vivo application. Histological evidence of limited myocardium transduction at 24 h after tail vein injection was found. However, expression was also detected in several other tissues, including the lung, spleen and liver and skeletal muscle [16]. Collectively, these studies suggest that experimenting with the ratio and absolute concentrations of the plasmid and lipid, as well as the solution used to form the complex, may lead to detectable myocardial transduction in animals.
In addition to plasmid–lipid complexes, plasmids conjugated with other polymers (such as poly amido amines) have also been investigated (Figure 1) [17]. These newer plasmid–polymer complexes are expected to result in improved cardiac transduction and better pharmacokinetic profiles. Future studies are needed to substantiate the in vivo utility of these newly developed plasmid–polymer complexes.
Delivery of oligonucleotides to the heart
A recent expansion of nonviral gene transfer is to deliver oligonucleotides instead of a functional gene. In these cases, the goal is to edit and/or repair rather than replace the gene. In some inherited diseases, mutation often results in out-of-frame transcripts. Artificial intervention of endogenous splicing sites may offer an opportunity to restore the open reading frame. Specifically designed antisense oligonucleotides (AON) have been used to achieve this goal. Briefly, smaller alternatively spliced transcripts are generated. These transcripts encode an internally truncated but largely functional protein. Initial experimentation with AON resulted in therapeutic level exon skipping in skeletal muscle [18]. However, it was very inefficient in the heart [18]. This hurdle has now been overcome with modified morpholino oligonucleotides using intravascular injection (Figures 1 and 2) [19].
Viral vector for cardiac gene delivery
Viruses are highly infectious simple microorganisms. They have evolved with extraordinary skills to enter our body. It is expected that vectors based on viruses may lead to much more robust gene transfer than plasmid-based non-viral vectors [20]. Several viral vectors have been explored for heart gene delivery. These include lentiviral, adenoviral and adeno-associated virus (AAV) viral vectors (Figure 1).
Lentiviral vectors for heart gene delivery
Lentiviral vectors are originally developed from HIV, a RNA virus [21]. They can package an approximately 8 kb genome. In contrast to traditional retroviral vectors, lentiviral vectors mediate stable gene transfer in terminally differentiated nondividing cells.
Initial studies in cultured cardiomyocytes suggest that lentiviral vectors are extremely powerful. The transduction efficiency reached 70% in adult cardiomyocytes and 100% in neonatal cardimyocytes [22,23]. Using a heterotopic heart transplantation model, Zhao et al. [23] observed robust transduction at 7 days after direct injection of a green fluorescence protein (GFP) lentiviral vector. In a separate study, Fleury et al. [22] showed persistent heart gene transfer up to 10 weeks after direct myocardial injection of the lentiviral GFP vector. Transduction efficiency reached a peak at 3–7 days post-injection but declined thereafter. Additional studies suggested that the drop of gene expression was likely a result of gene transfer-associated myocardial inflammation at the injection site [22].
More recently, Niwano et al. [24] tested whether lentivirus-mediated SERCA2a expression could protect against heart failure in a rat myocardial infarction model. At 21 days after hypothermic intracoronary injection (Figure 2), transduction efficiency reached approximately 40% in the heart but was essentially not detectable in the liver and spleen. Importantly, favorable myocardial remodeling was observed. Echocardiography and a cardiac catheter assay showed significant functional improvement. The survival rate was almost doubled in treated animals when challenged with myocardial infarction 6 months later.
Adenoviral vectors for heart gene delivery
Adenovirues are double-stranded DNA viruses. They carry an approximately 30-kb genome. Adenoviral vectors have been shown to effectively transduce several tissues such as the airway epithelium [25,26]. The use of recombinant adenoviruses for heart gene delivery was initiated in early 1990s (Figure 1). Today, adenoviral vectors have been tested for cardiac gene therapy in rodents, large animals and in humans.
In 1992, Stratford-Perricaudet et al. [27] showed that intravenous injection of an adenoviral LacZ vector to neonatal mice resulted in widespread gene transfer in several tissues, including the heart and skeletal muscle (Figure 2). Sustained LacZ expression was detected for at least 12 months. However, similar injection protocol resulted in minimal LacZ expression at 3 weeks after gene transfer in adult mice [27]. This result suggests that the mammalian heart is amenable to adenovirus transduction but there are important limitations. Several studies published in the subsequent 2 years confirmed the transient nature of adenoviral mediated heart gene transfer [28,29]. Guzman et al. [29] showed that adenoviral vectors were significantly more efficient than plasmids after direct myocardial injection. Robust expression was detected at the injection site during the first week but it diminished to the background level by 30 days. Kass-Eisler et al. [28] obtained similar results with a different adenoviral vector. These results suggest that adenoviral vectors represent an efficient but unstable gene delivery vehicle for the heart.
To further extend the findings in rodents, French et al. [30] explored adenoviral gene transfer in the heart of domestic swine. Adenoviral vectors were injected at multiple locations in the ventricular wall. Similar to that reported in rodents, expression peaked at 7 days and declined thereafter. A clear dose response was also noted. Nevertheless, expression did not spread far from the injection site and, furthermore, a marked leukocytic infiltration was observed near transduced cardiomyocytes [30].
Heart transplantation has become a standard procedure. Ex vivo manipulation of the donor heart by viral gene transfer may further enhance therapeutic outcome. Gojo et al. [31] tested whether a hypothermically preserved heart graft could be transduced by adenovirus. The vector was perfused through the coronary artery of the donor heart at 4 °C before heterotopic transplantation. Interestingly, the heart infected with low-dose virus (1 × 109 plaque forming units, pfu) showed high transduction for 4 weeks. However, strong inflammation was observed around the transduced cardiomyocytes in the heart that was infected with high-dose adenovirus (≥ 1 × 1010 pfu). Furthermore, minimal expression was detected at 2 weeks after operation in the high-dose group [31].
Direct myocardial injections only lead to limited transduction at the injection site. To obtain broader myocardial transduction, Fromes et al. [32] developed an intrapericardial injection strategy (Figure 2). Collagenase and hyaluronidase were injected together with adenovirus to the pericardial sac in rats. This protocol significantly increased adenovirus diffusion into the myocardium. Up to 40% myocardium was efficiently transduced at 7 days post-injection. Nevertheless, expression declined thereafter and became undetectable by 28 days [32].
To achieve global myocardial gene transfer, Hajjar et al. [33] developed a catheter-based delivery method (Figure 2). Briefly, a catheter was inserted through the apex of the left ventricle until it reached just above the root of the aortic valve. The aorta and pulmonary artery were briefly blocked using a clamp while adenovirus was injected. This technique allowed efficient circulation of adenovirus through the coronary arteries. Almost homogeneous cardiac gene transfer was achieved with several different adenoviruses. Importantly, the application of this method to an adenovirus carrying the phospholamban gene resulted in significant change of left ventricular function [33]. Although adenoviral transduction of the lung and liver was also noted, the study demonstrated for the first time that a gene transfer technique could be used to modulate overall heart function. Subsequently, a modified catheter-based technique was investigated in rabbits [34]. To perfuse the coronary beds, Maurice et al. [34] rapidly injected 1.5 ml of adenovirus via a catheter placed in the chamber of the left ventricle. During injection, they transiently clamped the aorta. Diffuse multichamber expression was achieved, with peak expression at 6 days post-injection. Using this system, it was also shown that a therapeutic β-adrenergic receptor adenovirus significantly enhanced heart contractility and hemodynamic performance in rabbits [34].
The first successful demonstration of adenovirus-mediated gene therapy in a cardiomyopathy model was performed by Ikeda et al. [35] 2002 [35]. δ-sarcoglycan deficient hamster is a naturally occurring model of dilated cardiomyopathy. Interestingly, only limited gene transfer (approximately 5%) was achieved when a brief aortic and pulmonary artery occlusion was applied. However, a combination of core temperature reduction, partial cardioplegia and transient aorta/pulmonary artery blocking resulted in an impressive transduction efficiency of approximately 77% [35]. Similar to previous adenovirus studies, inflammation was observed approximately 1 week later and transduction remained short-term. Nevertheless, left ventricular function was significantly improved at 3 weeks after the δ-sarcoglycan adenovirus was delivered.
To achieve persistent adenoviral gene transfer in the heart, Christensen et al. [36] explored embryonic and neonatal intracardiac delivery. Embryonic adenovirus injection appeared to have minimal impact on pregnancy. Adenovirus injection in 1-day-old mice resulted in stable myocardial transduction that lasted for several months. Importantly, there was no inflammatory reaction in the heart of neonatally injected mice. This technique has provided an excellent platform for studying gene function in murine cardiomyocytes through in vivo gene transfer.
Accumulatively, adenovirus-mediated heart gene transfer has been conducted in a relatively large number of human patients by many research groups [37–41]. A particular focus of these studies is to treat coronary artery disease with an adenovirus that expresses VEGF121. In most trials, 4 × 1010 particle units of adenoviral vectors were delivered to the heart. The procedure was well tolerated [37–41]. However, controversy exists regarding the therapeutic efficacy. Although some studies have demonstrated reduced myocardial ischemia during exercise, as well as increased new blood vessel formation [39], others have failed to show an improvement in exercise capacity and myocardial perfusion [41].
Recombinant AAV for heart gene delivery
Basic biology of AAV
One of the greatest challenges in heart gene transfer is to obtain long-term myocardial transduction in adult animals. This apparently impossible task was accomplished with the development of AAV vectors. AAV is a single-stranded DNA virus. It belongs to the dependovirus genera of the parvoviridae family. In the mid 1960s, AAV was discovered as a small contaminating DNA virus in adenovirus preparations (hence, the name adeno-associated virus) [42]. Mature AAV virion is an icosahedrally symmetrical particle approximately 20–25 nm in size. AAV has three overlapping capsid proteins including viral protein (VP) 1, VP2 and VP3. VP3 is the major capsid protein. All three structural proteins are produced from the same open reading frame by alternative splicing and the use of different translation starting codon. AAV is classified into different serotypes based on amino acid sequence of the capsids protein.
Although AAV was initially considered as an integrating virus, recent studies show that both wild-type AAV and recombinant AAV vectors may mainly persist as episomal circular molecules [43,44]. The interest in developing AAV vector originated from a unique biological property of wild type AAV serotype-2 (AAV-2). Most of our current understanding on AAV biology comes from studying AAV-2. Kotin et al. [45] found that AAV-2 genome specifically integrated in a defined region in human chromosome 19. Site-specific integration may significantly enhance the safety profile of a gene therapy vector. Because of the potential safety advantage, and also because the transduction biology of AAV-2 is well studied, early development of AAV vectors has mainly focused on AAV-2.
Pre-clinical studies of AAV-mediated cardiac gene delivery in rodents
Application of AAV vector for heart gene transfer was first demonstrated by Svensson et al. [46] in 1999 (Figure 1). An AAV-2 LacZ vector was delivered to the adult mouse heart either by direct intramyocardial injection or by trans-coronary perfusion before syngeneic transplantation (Figure 2). Minimal expression was seen at the 2-week time point. Unexpectedly, fairly strong expression was noted at 8 weeks after gene transfer. In the case of transcoronary perfusion, 50% of cardiomyocytes were transduced [46]. Li et al. [47] further extended this exciting finding in a hamster heterotopic heart transplantation model. Widespread myocardial transduction was obtained with AAV-2 for more than 1 year via ex vivo trans-coronary perfusion [47].
The success of long-term heart gene transfer with AAV-2 has prompted the testing of this vector in animal models of inherited cardiomyopathy. Hoshijima et al. [48] applied the same hypothermic-cardioplegia technique that was used by Ikeda et al. [35] for adenovirus. Although adenovirus showed transient expression [35], AAV-2 resulted in broad and persistent heart transduction for at least 30 weeks in hamsters [48]. Importantly, expressing a pseudophosphorylated mutant of human phospholamban with AAV-2 improved global cardiac function and suppressed progressive heart failure in the hamster model of dilated cardiomyopathy [48].
The identification and development of novel AAV capsids is one of the greatest advances in AAV gene transfer technology over recent years [49]. Various strategies have been explored to obtain new AAV serotypes that may exhibit unique transduction profiles for specific applications. Several AAV serotypes have subsequently been tested for heart gene delivery. Yue et al. [50] showed that AAV-5 could mediate efficient and persistent myocardial gene transfer in the mdx model of Duchenne muscular dystrophy, the most common lethal muscle disease caused by dystrophin deficiency. Importantly, it was found that a highly miniaturized micro-dystrophin gene could restore the critical dystrophin–glycoprotein complex and improved sarcolemmal integrity in the mdx heart [50].
Novel AAV serotype results in efficient heart gene transfer via peripheral vein injection
Traditionally, heart gene transfer is achieved by direct injection of vectors to the heart (myocardium, cardiac chamber or pericardial sac) or through coronary artery perfusion. These procedures are usually quite invasive and risky. A strategy that allows therapeutic level myocardial transduction via peripheral veins would offer significant advantages (Figure 2). Accordingly, several newly developed AAV serotypes have shown great promise. The first breakthrough was reported by Gregorevic et al. [51], who injected AAV-6 via tail vein to adult mice and found extensive gene transfer in the heart and all skeletal muscles in the body. With co-administration of VEGF, systemic gene delivery was achieved even at lower AAV doses. Despite uniform myocardial transduction, significant CD4-positive cell infiltration was noted in the heart after AAV LacZ infection. Additional studies suggest that this immune response may relate to LacZ expression rather than AAV-6 capsid. Another AAV serotype AAV-8 was also found to cross the vascular barrier effectively [52]. Wang et al. compared AAV-1, 2, 5, 6, 7 and 8 in mice and hamsters via intraperitoneal and/or intravascular injection [52]. Superior heart and skeletal muscle transduction was achieved with AAV-8. Subsequent studies from several independent laboratories included AAV-9 in the comparison [53–56,70]. Pacak et al. [53] and Inagaki et al. [56] showed that AAV-9 was log-fold more robust than AAV-8 in transducing the mouse heart. Their finding was further substantiated by Zincarelli et al. [54] and Bish et al. [55]. Collectively, these results clearly established AAV-9 as a cardiac tropic vector superior to all the other serotypes in rodents. However, AAV-9 also displays a strong tropism for other organs such as the liver. This may result in untoward side-effects. To achieve tissue-specific AAV gene transfer in the heart, Yang et al. [57] constructed a random library by shuffling the capsid genes of AAV-1–9. Subsequent in vivo screening identified AAV-M41 as a heart-specific AAV variant. This new recombinant capsid retained the heart tropism of AAV-9 but showed substantially diminished transduction in skeletal muscle and the liver. A more recent study suggests that random mutagenesis of the original AAV-9 capsid gene may also yield new heart tropic variants with diminished transduction in other tissues [58].
AAV for heart gene delivery in large animals
Experimentation in large animal models is a bottleneck for translating success in rodents to human patients. Kaspar et al. [59] tested direct intracoronary delivery of AAV-2 in pigs. Transduction was observed in most pigs for 8 weeks and no inflammatory response was noted. Raake et al. [60] achieved long-term heart specific expression with pressure-regulated coronary vein retroinfusion using AAV-6. However, AAV genome was also detected in the liver and lung [60]. At the same time, Su et al. [61] reported that AAV-1 was more efficient than AAV-2 in the pig heart after direct myocardial injection. More recently, Hardri et al. [62] demonstrated that intracoronary delivery of AAV-1 SERCA2a significantly improved systolic function and coronary blood flow in a swine model of heart failure.
Dog is another commonly used species in large animal studies. Encouraged by robust gene transfer of AAV-9 in the rodent heart, Yue et al. [63] tested systemic peripheral vein injection of AAV-9 in newborn dogs. Although they observed whole body skeletal muscle transduction, unexpectedly, the heart was minimally transduced. Two groups investigated AAV-6 for canine heart transduction [64,65]. Gregorevic et al. [65] showed that direct infusion of AAV-6 into the external jugular vein of a 2-month-old dog (after a transient immune suppression) resulted in frequent cardiomyocyte transduction. Bish et al. [64] compared AAV-6, 8 and 9 in 2–3-month-old dogs using a percutaneous transendocardial delivery method. This is a relatively non-invasive procedure and a similar protocol has been used in human patients. Consistent with the observations of Yue et al. [63], AAV-9 also performed poorly in young dog hearts. Indeed, both AAV-8 and AAV-9 were ten-fold less efficient than AAV-6 [64]. Nevertheless, the results reported by Pacak et al. [53] suggest that AAV-9 may still hold promise for transducing the heart of nonhuman primates.
Dual vector strategies expand AAV capacity for heart gene therapy
A unique hurdle for AAV-mediated gene transfer is the small viral packaging capacity. A single AAV virion cannot handle a vector genome larger than 5 kb [66]. Several dual vector strategies have been developed to increase AAV packaging capacity. These include cis-activation, trans-splicing, overlapping and hybrid methods [67,68]. The fundamental principle is to split a complete vector genome into two independent virions. Reconstitution is achieved by homologous recombination and/or AAV inverted terminal repeat-mediated viral genome concatamerization. Ghosh et al. [69] and Bostick et al. [70] first showed that trans-splicing AAV-9 vectors could reach the same transduction efficiency as a single intact AAV-9 vector in the heart of normal neonatal mice. Subsequent studies from the same group suggest that trans-splicing AAV-9 vectors were equally effective in the heart of adult dystrophic mice. More recently, Odom et al. [71] reported that overlapping AAV-6 vectors also resulted in robust cardiac transduction in dystrophin-deficient mdx mice.
AAV heart gene therapy yields promising results in early clinical trials
Recent work has greatly advanced AAV-mediated heart gene therapy in human patients [72–74]. Specifically, an AAV-1 SERCA2a vector was delivered to heart failure patients via percutaneous intracoronary delivery. The same vector has been shown to significantly enhance heart function in a swine heart failure model [62]. In an open-label phase 1 study, nine patients received AAV-1 SERCA2a gene therapy at low (1.4 × 1011 DNase resistant particles, DRP), middle (6 × 1011 DRP) and high (3 × 1012 DRP) doses, with three patients in each group [73]. One patient in the medium dose group died for reasons unrelated to gene therapy. No adverse effects were observed in the remaining eight patients. Six patients showed some level of improvement. Two patients (one in the low-dose group and another in the high-dose group) had high pre-existing anti-AAV1 neutralizing antibodies and they did not respond to therapy as expected [73]. Recently, a double-blind placebo-controlled phase 2 trial was conducted [74]. Thirty-nine patients were recruited in the study and AAV1 SERCA2a were delivered at the dose of 6 × 1011 DRP (low-dose), 3 × 1012 DRP (middle dose) and 1 × 1013 DRP (high-dose). Functional studies at 6 months post AAV therapy revealed an improvement in multiple end points (such as 6-min walk test, oxygen consumption, N-terminal pro-B-type natriuretic peptide levels, left ventricular end-systolic volume, New York Heart Association heart failure classification and Minnesota Living with Heart Failure questionnaire). Importantly, no adverse events were observed [74].
Summary and perspectives
The development of heart gene delivery vectors has undergone a fascinating journey. Over a period of 20 years, an apparently distant idea of genetic correction has now become a tangible possibility for the treatment of many heart diseases. Vehicles that can conveniently, efficiently and safely deliver therapeutic genetic material to the heart have been, and will no doubt remain, at the center stage of heart gene therapy. Although a battery of viral and nonviral vectors is now available for different applications, we have yet to shape them for daily clinical practice. Among the many challenges, an imminent issue is the large-scale production of clinical grade vectors. There is no doubt that effort in this direction will greatly accelerate translational gene therapy in heart disease treatment.
Acknowledgements
The research in authors’ laboratory was supported by grants from the National Institutes of Health HL-91883 and AR-49419 (DD), Muscular Dystrophy Association (DD), Parent Project Muscular Dystrophy (DD) and Jesse's Journey: The Foundation for Gene and Cell Therapy (DD). The authors declare that there are no conflicts of interest.
References
- 1.Lin H, Parmacek MS, Morle G, Bollin S, Leiden JM. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990;82:2217–2221. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- 2.Buttrick PM, Kass A, Kitsis RN, Kaplan ML, Leinwand LA. Behavior of genes directly injected into the rat heart in vivo. Circ Res. 1992;70:193–198. doi: 10.1161/01.res.70.1.193. [DOI] [PubMed] [Google Scholar]
- 3.Fishman GI, Kaplan ML. Buttrick PM Tetracycline-regulated cardiac gene expression in vivo. J Clin Invest. 1994;93:1864–1868. doi: 10.1172/JCI117174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buttrick PM, Kaplan ML, Kitsis RN, Leinwand LA. Distinct behavior of cardiac myosin heavy chain gene constructs in vivo. Discordance with in vitro results. Circ Res. 1993;72:1211–1217. doi: 10.1161/01.res.72.6.1211. [DOI] [PubMed] [Google Scholar]
- 5.Fisher SA, Buttrick PM, Sukovich D, Periasamy M. Characterization of promoter elements of the rabbit cardiac sarcoplasmic reticulum Ca(2+)-ATPase gene required for expression in cardiac muscle cells. Circ Res. 1993;73:622–628. doi: 10.1161/01.res.73.4.622. [DOI] [PubMed] [Google Scholar]
- 6.Sundararaman S, Miller TJ, Pastore JM, Kiedrowski M, Aras R, Penn MS. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure. Gene Ther. 2011 doi: 10.1038/gt.2011.18. doi: gt201118 [pii]10.1038/gt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortuin FD, Vale P, Losordo DW, et al. One-year follow-up of direct myocardial gene transfer of vascular endothelial growth factor-2 using naked plasmid deoxyribonucleic acid by way of thoracotomy in no-option patients. Am J Cardiol. 2003;92:436–439. doi: 10.1016/s0002-9149(03)00661-1. [DOI] [PubMed] [Google Scholar]
- 8.Vale PR, Losordo DW, Milliken CE, et al. Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation. 2001;103:2138–2143. doi: 10.1161/01.cir.103.17.2138. [DOI] [PubMed] [Google Scholar]
- 9.Symes JF, Losordo DW, Vale PR, et al. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease. Ann Thorac Surg. 1999;68:830–836. doi: 10.1016/s0003-4975(99)00807-3. [DOI] [PubMed] [Google Scholar]
- 10.Losordo DW, Vale PR, Symes JF, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–2804. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 11.Losordo DW, Vale PR, Hendel RC, et al. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002;105:2012–2018. doi: 10.1161/01.cir.0000015982.70785.b7. [DOI] [PubMed] [Google Scholar]
- 12.Stewart DJ, Kutryk MJ, Fitchett D, et al. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol Ther. 2009;17:1109–1115. doi: 10.1038/mt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang CY, Huang L. Highly efficient DNA delivery mediated by pH-sensitive immunoliposomes. Biochemistry. 1989;28:9508–9514. doi: 10.1021/bi00450a039. [DOI] [PubMed] [Google Scholar]
- 14.Felgner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart MJ, Plautz GE, Del Buono L, et al. Gene transfer in vivo with DNA–liposome complexes: safety and acute toxicity in mice. Hum Gene Ther. 1992;3:267–275. doi: 10.1089/hum.1992.3.3-267. [DOI] [PubMed] [Google Scholar]
- 16.Hofland HE, Nagy D, Liu JJ, et al. In vivo gene transfer by intravenous administration of stable cationic lipid/DNA complex. Pharm Res. 1997;14:742–749. doi: 10.1023/a:1012146305040. [DOI] [PubMed] [Google Scholar]
- 17.Yockman JW, Kastenmeier A, Erickson HM, et al. Novel polymer carriers and gene constructs for treatment of myocardial ischemia and infarction. J Control Release. 2008;132:260–266. doi: 10.1016/j.jconrel.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alter J, Lou F, Rabinowitz A, et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- 19.Wu B, Moulton HM, Iversen PL, et al. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc Natl Acad Sci USA. 2008;105:14814–14819. doi: 10.1073/pnas.0805676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 21.Naldini L, Blomer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 22.Fleury S, Simeoni E, Zuppinger C, et al. Multiply attenuated, self-inactivating lentiviral vectors efficiently deliver and express genes for extended periods of time in adult rat cardiomyocytes in vivo. Circulation. 2003;107:2375–2382. doi: 10.1161/01.CIR.0000065598.46411.EF. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Pettigrew GJ, Thomas J, et al. Lentiviral vectors for delivery of genes into neonatal and adult ventricular cardiac myocytes in vitro and in vivo. Basic Res Cardiol. 2002;97:348–358. doi: 10.1007/s00395-002-0360-0. [DOI] [PubMed] [Google Scholar]
- 24.Niwano K, Arai M, Koitabashi N, et al. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol Ther. 2008;16:1026–1032. doi: 10.1038/mt.2008.61. [DOI] [PubMed] [Google Scholar]
- 25.Rosenfeld MA, Siegfried W, Yoshimura K, et al. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld MA, Yoshimura K, Trapnell BC, et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- 27.Stratford-Perricaudet LD, Makeh I, Perricaudet M, Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kass-Eisler A, Falck-Pedersen E, Alvira M, et al. Quantitative determination of adenovirus-mediated gene delivery to rat cardiac myocytes in vitro and in vivo. Proc Natl Acad Sci USA. 1993;90:11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzman RJ, Lemarchand P, Crystal RG, Epstein SE, Finkel T. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ Res. 1993;73:1202–1207. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- 30.French BA, Mazur W, Geske RS, Bolli R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90:2414–2424. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- 31.Gojo S, Niwaya K, Taniguchi S, Nishizaki K, Kitamura S. Gene transfer into the donor heart during cold preservation for heart transplantation. Ann Thorac Surg. 1998;65:647–652. doi: 10.1016/s0003-4975(97)01295-2. [DOI] [PubMed] [Google Scholar]
- 32.Fromes Y, Salmon A, Wang X, et al. Gene delivery to the myocardium by intrapericardial injection. Gene Ther. 1999;6:683–688. doi: 10.1038/sj.gt.3300853. [DOI] [PubMed] [Google Scholar]
- 33.Hajjar RJ, Schmidt U, Matsui T, et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci USA. 1998;95:5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maurice JP, Hata JA, Shah AS, et al. Enhancement of cardiac function after adenoviral-mediated in vivo intracoronary beta2-adrenergic receptor gene delivery. J Clin Invest. 1999;104:21–29. doi: 10.1172/JCI6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda Y, Gu Y, Iwanaga Y, et al. Restoration of deficient membrane proteins in the cardiomyopathic hamster by in vivo cardiac gene transfer. Circulation. 2002;105:502–508. doi: 10.1161/hc0402.102953. [DOI] [PubMed] [Google Scholar]
- 36.Christensen G, Minamisawa S, Gruber PJ, Wang Y, Chien KR. High-efficiency, long-term cardiac expression of foreign genes in living mouse embryos and neonates. Circulation. 2000;101:178–184. doi: 10.1161/01.cir.101.2.178. [DOI] [PubMed] [Google Scholar]
- 37.Rosengart TK, Lee LY, Patel SR, et al. Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA. Ann Surg. 1999;230:466–470. doi: 10.1097/00000658-199910000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosengart TK, Lee LY, Patel SR, et al. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999;100:468–474. doi: 10.1161/01.cir.100.5.468. [DOI] [PubMed] [Google Scholar]
- 39.Stewart DJ, Hilton JD, Arnold JM, et al. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF (121) (AdVEGF121) versus maximum medical treatment. Gene Ther. 2006;13:1503–1511. doi: 10.1038/sj.gt.3302802. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs S, Dib N, Cohen BM, et al. A randomized, double-blind, placebo-controlled, multicenter, pilot study of the safety and feasibility of catheter-based intramyocardial injection of AdVEGF121 in patients with refractory advanced coronary artery disease. Catheter Cardiovasc Interv. 2006;68:372–378. doi: 10.1002/ccd.20859. [DOI] [PubMed] [Google Scholar]
- 41.Kastrup J, Jorgensen E, Fuchs S, et al. A randomised, double-blind, placebo-controlled, multicentre study of the safety and efficacy of BIOBYPASS (AdGVVEGF121. 10NH) gene therapy in patients with refractory advanced coronary artery disease: the NOVA trial. EuroIntervention. 2011;6:813–818. doi: 10.4244/EIJV6I7A140. [DOI] [PubMed] [Google Scholar]
- 42.Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 43.Duan D, Sharma P, Yang J, et al. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long term episomal persistence in muscle. J Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnepp BC, Jensen RL, Clark KR, Johnson PR. Infectious molecular clones of adeno-associated virus isolated directly from human tissues. J Virol. 2009;83:1456–1464. doi: 10.1128/JVI.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotin RM, Siniscalco M, Samulski RJ, et al. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Svensson EC, Marshall DJ, Woodard K, et al. Efficient and stable transduction of cardiomyocytes after intramyocardial injection or intracoronary perfusion with recombinant adeno-associated virus vectors. Circulation. 1999;99:201–205. doi: 10.1161/01.cir.99.2.201. [DOI] [PubMed] [Google Scholar]
- 47.Li J, Wang D, Qian S, Chen Z, Zhu T, Xiao X. Efficient and long-term intracardiac gene transfer in delta-sarcoglycandeficiency hamster by adeno-associated virus-2 vectors. Gene Ther. 2003;10:1807–1813. doi: 10.1038/sj.gt.3302078. [DOI] [PubMed] [Google Scholar]
- 48.Hoshijima M, Ikeda Y, Iwanaga Y, et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 49.Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- 50.Yue Y, Li Z, Harper SQ, Davisson RL, Chamberlain JS, Duan D. Microdystrophin gene therapy of cardiomyopathy restores dystrophin–glycoprotein complex and improves sarcolemma integrity in the Mdx mouse heart. Circulation. 2003;108:1626–1632. doi: 10.1161/01.CIR.0000089371.11664.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gregorevic P, Blankinship MJ, Allen JM, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Zhu T, Qiao C, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 53.Pacak CA, Mah CS, Thattaliyath BD, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99:e3–9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- 54.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 55.Bish LT, Morine K, Sleeper MM, et al. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum Gene Ther. 2008;19:1359–1368. doi: 10.1089/hum.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inagaki K, Fuess S, Storm TA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang L, Jiang J, Drouin LM, et al. A myocardium tropic adeno-associated virus (AAV) evolved by DNA shuffling and in vivo selection. Proc Natl Acad Sci USA. 2009;106:3946–3951. doi: 10.1073/pnas.0813207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pulicherla N, Shen S, Yadav S, et al. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol Ther. 2011;19:1070–1078. doi: 10.1038/mt.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaspar BK, Roth DM, Lai NC, et al. Myocardial gene transfer and long-term expression following intracoronary delivery of adeno-associated virus. J Gene Med. 2005;7:316–324. doi: 10.1002/jgm.665. [DOI] [PubMed] [Google Scholar]
- 60.Raake PW, Hinkel R, Muller S, et al. Cardio-specific long-term gene expression in a porcine model after selective pressure-regulated retroinfusion of adeno-associated viral (AAV) vectors. Gene Ther. 2008;15:12–17. doi: 10.1038/sj.gt.3303035. [DOI] [PubMed] [Google Scholar]
- 61.Su H, Yeghiazarians Y, Lee A, et al. AAV serotype 1 mediates more efficient gene transfer to pig myocardium than AAV serotype 2 and plasmid. J Gene Med. 2008;10:33–41. doi: 10.1002/jgm.1129. [DOI] [PubMed] [Google Scholar]
- 62.Hadri L, Bobe R, Kawase Y, et al. SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells. Mol Ther. 2010;18:1284–1292. doi: 10.1038/mt.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yue Y, Ghosh A, Long C, et al. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol Ther. 2008;16:1944–52. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bish LT, Sleeper MM, Brainard B, et al. Percutaneous transendocardial delivery of self-complementary adeno-associated virus 6 achieves global cardiac gene transfer in canines. Mol Ther. 2008;16:1953–59. doi: 10.1038/mt.2008.202. [DOI] [PubMed] [Google Scholar]
- 65.Gregorevic P, Schultz BR, Allen JM, et al. Evaluation of vascular delivery methodologies to enhance rAAV6-mediated gene transfer to canine striated musculature. Mol Ther. 2009;17:1427–1433. doi: 10.1038/mt.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lai Y, Yue Y, Duan D. Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome or = 8.2 kb. Mol Ther. 2010;18:75–79. doi: 10.1038/mt.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghosh A, Duan D. Expanding adeno-associated viral vector capacity: a tale of two vectors. Biotechnol Genet Eng Rev. 2007;24:165–177. doi: 10.1080/02648725.2007.10648098. [DOI] [PubMed] [Google Scholar]
- 68.Lai Y, Yue Y, Bostick B, et al. Delivering large therapeutic genes for muscle gene therapy. In: Duan D, editor. Muscle Gene Therapy. Springer Science + Business Media, LLC; New York, NY: 2010. pp. 205–218. [Google Scholar]
- 69.Ghosh A, Yue Y, Long C, Bostick B, Duan D. Efficient whole-body transduction with trans-splicing adeno-associated viral vectors. Mol Ther. 2007;15:750–755. doi: 10.1038/sj.mt.6300081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bostick B, Ghosh A, Yue Y, Long C, Duan D. Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther. 2007;14:1605–1609. doi: 10.1038/sj.gt.3303029. [DOI] [PubMed] [Google Scholar]
- 71.Odom GL, Gregorevic P, Allen JM, Chamberlain JS. Gene therapy of mdx mice with large truncated dystrophins generated by recombination using rAAV6. Mol Ther. 2011;19:36–45. doi: 10.1038/mt.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hajjar RJ, Zsebo K, Deckelbaum L, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008;14:355–367. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 73.Jaski BE, Jessup ML, Mancini DM, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gwathmey JK, Yerevanian AI, Hajjar RJ. Cardiac gene therapy with SERCA2a: from bench to bedside. J Mol Cell Cardiol. 2011;50:803–812. doi: 10.1016/j.yjmcc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]