SUMMARY
ERK1 and ERK2 are widely involved in cell signalling. Using a recombinant approach, it has been shown that exogenous ERK2 is capable of dimerisation and that preventing dimerisation reduces its nuclear accumulation on stimulation. Dimerisation occurs on phosphorylation; the dimer partner of phosphorylated ERK2 may be either phosphorylated or unphosphorylated. It has been assumed that monophosphodimers are hemiactive. Here we show that ERK1 is capable of dimerisation both in vivo and in vitro. Dimerisation of human recombinant ERK1 in vitro requires both ERK1 phosphorylation and cellular cofactor(s); it leads to the formation of a high molecular weight complex that can be dissociated by treatment with β-mercaptoethanol. We demonstrate for the first time in both sea urchin embryos and human cells that native ERK forms dimers and that high ERK kinase activity is largely associated with bisphosphodimers, not with monophosphodimers or phosphorylated monomers: the activity of the bisphosphodimer is about 20-fold higher than that of the phosphorylated monomer in vitro and the bisphosphodimer shows 5 to 7-fold higher in vivo activity than the basal activity attributable to the monophosphodimer. Thus phosphorylation of both partners in the dimer is a hallmark of ERK activation. Judgments made about ERK kinase activity associated with phosphorylated monomers are at best a proxy for ERK activity.
Keywords: MAP kinase, ERK1, homodimerisation, mitotic cell cycle, sea urchin embryos, human cells
INTRODUCTION
The MAP kinase signalling pathway is universal and highly conserved throughout evolution (Pouyssegur and Lenormand, 2003). In budding and fission yeast it controls respectively the pheromone-induced mating response (Choi et al., 1994; Mahanty et al., 1999) and the activation of cdc2 mitotic kinase (King et al., 1994; Nurse, 1990). The same pathway is involved in signal transmission to the nucleus upon mitogenic stimulation of somatic cells (for review see Wilkinson and Millar, 2000). In oocytes it controls meiosis and cell cycle arrest (Abrieu et al., 2001; Ferrell, 1999a; Ferrell, 1999b; Whitaker, 1996). It is also involved in signal transduction during re-entry into the mitotic cell cycle after fertilisation in sea urchin eggs (Philipova et al., 2005a; Philipova et al., 2005b; Philipova and Whitaker, 1998).
Protein dimerisation has been found to be a common signalling motif during the transduction of extracellular signals. For MAP kinase pathways, it has been indirectly shown that phosphorylation of ERK2 facilitates the formation of dimers in vitro. By using equilibrium sedimentation, Khokhlatchev and colleagues have found that phosphorylated ERK2 sediments in vitro primarily as an 84 kDa species while nonphosphorylated ERK2 sediments as a mixture of a lesser amount of the 84 kDa and a greater amount of the 42 kDa species (Khokhlatchev et al., 1998). A model of dimerised kinase has been deduced from the crystal structure of phosphorylated ERK2 which reveals the physical basis for its dimerisation (Canagarajah et al., 1997). Phosphorylation causes conformational changes in the two flexible regions of the protein molecule, the activation loop and the C-terminal extension, and provides the surfaces for homodimerisation. As a result, two ERK2 molecules bind via a hydrophobic zipper complemented by two ion pairs, one on each side of the zipper (for reviews see (Cobb and Goldsmith, 2000; English et al., 1999). It has been suggested that p38, ERK1 and JNK/SAPK also form homodimers (Khokhlatchev et al., 1998). It has also been shown that exogenous recombinant ERK2 dimerises when transfected or microinjected into cells, that dimerisation requires its phosphorylation and that dimerisation is necessary for localization to the nucleus (Khokhlatchev et al., 1998). However despite the structural and cell biological evidence of the importance of dimerisation, the formation of dimers has not been demonstrated biochemically in vivo, nor have the relative kinase activities of phosphodimers and monomers been measured within cells. There has not until now been any evidence of dimer formation in whole-cell extracts, the usual method for determining ERK activity: the status and importance of dimerisation in regulating kinase activity has not been investigated.
Here we use the ready availability of whole-cell extracts from sea urchin embryos to demonstrate that efficient ERK1 phosphodimer formation in vitro requires a soluble co-factor or cofactors. The phosphodimers are components of a high molecular weight protein complex in vivo and in vitro. We use in vitro activation/deactivation reactions to study the formation and disassembly of the ERK1 dimers. We also demonstrate that ERK1 forms homodimers in vivo by determining ways to isolate and detect them. Basal MAP kinase activity in vivo is maintained by homodimers formed by one phosphorylated and one unphosphorylated ERK1 molecule (a monophosphodimer). Peak MAP kinase activity is achieved by the accumulation of dimers formed of two phosphorylated ERK1 molecules (a bisphosphodimer). Free monomers are mainly unphosphorylated and inactive in vivo. We conclude that ERK1 bisphosphodimers are the overwhelmingly predominant form of highly active ERK1 in vivo, that basal ERK1 activity is generated by monophosphodimers and that phosphorylated monomers contribute very little to in vivo ERK1 activity.
MATERIALS AND METHODS
Materials
The polyclonal anti-active ERK and anti-ERK antibodies were obtained from Promega; MBP and the monoclonal anti-dual phosphorylated ERK and MEK antibodies from New England Biolabs; [γ-32P]ATP, ECL and ECL Advance detection kits, the Glutathione Sepharose 4B gel, GSTrap FF and HiTrap Chelating HP columns and the anti-GST AB from Amersham Biosciences; the QuickChange Kit for mutagenesis from Stratagene; EGF from Sigma; all other chemicals from BDH..
Expression vectors and purification of recombinant proteins
ERK1 expression vector encoding full length GST-tagged human ERK1 was a generous gift of Dr Burgering (Utrecht, Holland). ERK1 dimerisation deficient mutant GST-ΔPEHD-ERK1 was created by PEHD deletion situated five residues upstream of the activation loop. Identical ERK2 mutant which lacks the same four amino acids at the dimerisation interface was unable to form dimers when microinjected (Khokhlatchev et al., 1998). Two pRSET constructs encoding constitutively active (His)6-tagged hMEK R4F and (His)6-tagged kinase inactive hMEK1 were kindly provided by Dr J. Ferrell, Jr (Stanford, USA) (Sohaskey and Ferrell, 1999) and originally constructed by Dr N. Ahn (Mansour et al., 1996; Mansour et al., 1994). Bacterial strain Origami (DE3)pLysS (Novagen) was used for transformation with the various constructs described above and the cultures were grown at 20°C to an OD at 600nm of 0,6 in 1l Terrific Broth, 125 μg/ml ampicillin and 25 μg/ml chloramphenicol. After induction of fusion protein synthesis by 0.1 mM IPTG, the bacterial cultures were grown for additional 20 hours. GST-tagged hERK1 and ΔPEHD-ERK1 recombinant proteins were purified on GSTrap FF column following manufacturer protocol. Soluble (His)6-tagged MEK1 proteins were purified on HiTrap Chelating HP column as previously described (Mansour et al., 1994). All pure recombinant proteins were stored at −20°C in PBS, 10 % glycerol for not more than one year. Longer storage results in aggregation, most likely due to their hydrophobic nature. Pure recombinant hCL100 phosphatase was a kind gift of Dr S. Keyse (Dundee, UK).
Cell extracts, Immunoprecipitation and Western Blotting
Cell extracts from sea urchin eggs and early embryos during mitotic cell cycle were prepared as described (Philipova and Whitaker, 1998). In short, eggs/embryos were homogenised on ice, and after repeated centrifugation at 13,000 rpm for 30 minutes the soluble fractions were aliquoted, frozen and stored at −80°C. Extracts from HeLa cells grown in 10% FCS as control or after 10 minutes induction with 100 ng/ml EGF were obtained by washing the cells three times with ice-cold sterile PBS and then lysing and collecting them in lysis buffer (50mM Tris.HCl, pH 7.5, 1% Triton X100, 10mM EDTA, 0.5M NaCl, 10% glycerol, supplemented with phosphatase and protease inhibitors) on ice. High speed supernatants were used for immunoprecipitation and Western Blotting.
Immunoprecipitation was carried out as previously described (Philipova and Whitaker, 1998). Sea urchin or HeLa cell extracts containing 250 μg total cellular protein were used for immunoprecipitation with 1μg of anti-active ERK or anti-ERK antibody. For Western Blotting, the immune complexes or samples were not boiled (unless otherwise stated). Instead, they were left for 20 minutes at room temperature in Laemmli sample buffer and then frozen or used for electrophoresis and blotting. In some of the experiments carried out with recombinant human ERK1, the reducing agent was omitted from the sample buffer, as indicated in the text. When identical samples were probed with different antibodies, they were run in wide wells, then blotted and the blots were cut into strips. Each identical strip was incubated in different antibody; one strip was always used for negative control.
In vitro protein kinase and protein phosphatase assays - In vitro
MEK1 assays were carried out as previously described (Mansour et al., 1996); 0.5 nM of recombinant MEK1 R4F or kinase dead recombinant MEK1 (Mansour et al., 1996; Mansour et al., 1994; Sohaskey and Ferrell, 1999) was used per reaction. Phosphatase assays were carried out in 50 mM Tris.HCl, pH 7.5, 2μM Microcystin in the presence or absence of 2mM Na3VO4 for 1.5 hours at 30°C and 0.007 nM of recombinant human CL100 (Lewis et al., 1995) was used per reaction.
The experiments involving activation/inactivation of the recombinant GST-hERK1 or GST-ΔPEHD-hERK1 protein were carried out as follows. For each sample 6 pM of purified recombinant protein was used. The whole amount of recombinant protein needed for one series of experiments was phosphorylated (see above) for 1 hour at 30°C and then bound to Glutathion-Sepharose (20 μl beads were used per sample) at room temperature for 30 min. The beads were washed twice in washing buffer WB (PBS, 0.1% TX100, 2.5 mM MgCl2) supplemented with phosphatase inhibitors and then samples were aliquated into separate tubes for different treatments. Incubation with cellular extract (15 μg or 75 μg total protein) was carried out for 15 min at room temperature and then beads were washed twice in WB. For phosphatase treatment, the sample was first washed in 50 mM Tris.HCl, pH 7.5, then dephosphorylated for 1.5 hours at 30°C and finally washed in WB. Another sample was washed in MEK1 kinase buffer, left overnight for additional MEK1 activation reaction at 4°C, then washed in WB. At each step a sample was taken and Laemmli buffer without β-mercaptoethanol (β-ME) was added at room temperature for electrophoresis. For kinase activity measurements, the same series of experiments were carried out, samples were washed in MAP kinase buffer (Philipova and Whitaker, 1998) and protein kinase assays were carried out with MBP as substrate. Radioactivities of MBP were analysed by phosphor-imager. In the case of non-radioactive kinase assays (Fig.1, F), dephosphorylated MBP (Upstate Biotech) was used as substrate and phosphorylated MBP was detected by Western Blotting using phospho-specific MBP antibody (Upstate Biotech). A representative of three independent experiments is always shown.
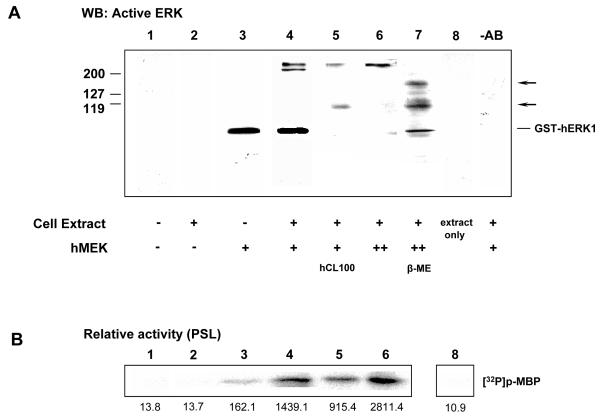
Fig. 1. Human ERK1 activated in vitro dimerises in the presence of cellular co-factors. The highest activity is associated with bisphosphodimers.
A Western blotting of recombinant hERK1 with anti-dualphosphorylated ERK antibody:
Lane 1. unactivated protein.
Lane 2. unactivated protein incubated in cell extract for 15 min.
Lane 3. hERK1 after 1 hour activation by recombinant MEK, not incubated in cell extract.
Lane 4. hERK1 treated as for Lane 3, but with an additional incubation in cell extract for 15 min.
Lane 5. hERK1 treated as for Lane 4, followed by dephosphorylation using hCL100.
Lane 6. hERK1 treated as for Lane 4, followed by additional activation by MEK overnight at 4°C .
Lane 7. hERK1 sample treated identically to the sample in Lane 6, but dissolved in sample buffer containing 5% β-mercaptoethanol.
Lane 8. Control: low ERK1 activity extract (30 min post-fertilisation, 15 μg total cellular protein loaded); active ERK1 is undetectable.
-AB Control: hERK1 sample treated as for Lane 4, but primary antibody was omitted.
The positions of molecular mass markers are shown.
B MAP kinase assays with myelin basic protein (MBP) using samples identical to those in A. Relative protein kinase activity is also shown from densitometry.
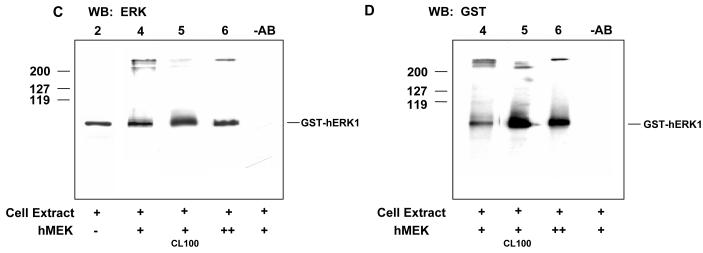
C Western blotting using anti-ERK antibody of samples identical those of lanes 2,4,5,6 and -AB in A. The positions of molecular mass markers are shown.
D Western blotting using anti-GST antibody of samples identical to 4,5,6 and -AB in A. The positions of molecular mass markers are shown.
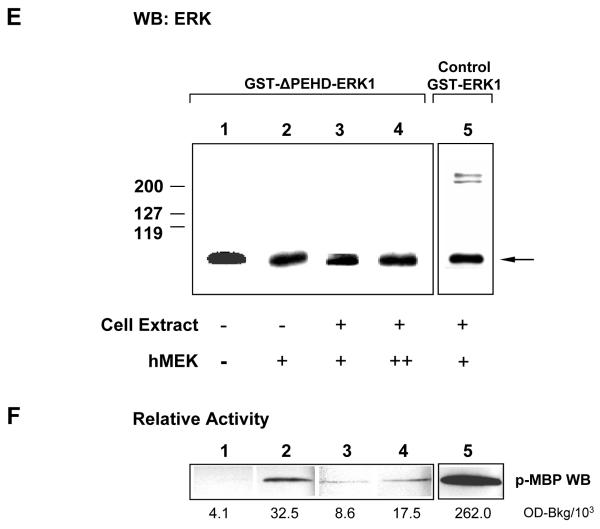
E GST-ΔPEHD-hERK1, a dimerisation deficient mutant, does not show enhanced activity when incubated in whole-cell extract. 6 pM of each ERK1 recombinant protein was used per sample. Western blotting with anti-ERK antibody:
Lane 1. unactivated ΔPEHD-hERK1.
Lane 2. ΔPEHD-hERK1 after 1 hour activation by active MEK not incubated in cell extract.
Lane 3. ΔPEHD-hERK1 treated as for Lane 2, but with an additional incubation in cell extract for 15 min.
Lane 4. ΔPEHD-hERK1 treated as for Lane 3, followed by additional activation by MEK overnight at 4°C.
Lane 5. Equal amount of recombinant control hERK1 treated as for Lane 3.
F MAP kinase assays with MBP using samples identical to those in E. Relative protein kinase activity is also shown from densitometry. Note that the monomeric mutant protein is effectively activated by MEK (eight-fold over baseline), however incubation in cell extract does not result in additional increase in its activity. The same amount of activated control wild type protein forms a complex in cell extract with much higher kinase activity.
RESULTS
Oligomerisation of recombinant human ERK1 in vitro markedly enhances its kinase activity
A previous study has shown that phosphorylated ERK2 dimerises in vitro (Khokhlatchev et al., 1998). To determine whether ERK1 also oligomerises, we used purified monomeric full length human recombinant GST-ERK1 protein in in vitro experiments. The GST tag was used to pull down the protein from the reaction mixture for subsequent analysis using glutathione beads. Purified GST-hERK1 was not active, as judged by western blotting on a non-reducing polyacrylamide gel with an anti-active ERK antibody (Fig.1A, lane 1; the GST chimera has a molecular mass of 71kD). Phosphorylation for 1 hour in a kinase buffer in vitro by active recombinant MEK (Mansour et al., 1996) resulted in activated monomer (Fig. 1A, lane 3). Dimerisation of this pure activated recombinant protein was not observed. However, transfer of the activated recombinant monomer to a sea urchin embryo extract with low ERK1 activity (30 min post-fertilisation; 15 μg total cellular protein; with ERK1 activity undetectable in this assay, lane 8) induced rapid formation within 15 min of two high molecular mass (>200kDa) active protein bands (Fig. 1A, lane 4). The intensity of these bands decreased when an identically treated (as for lane 4) aliquot was then dephosphorylated for 1.5 hours at 30°C using recombinant human CL100 MAP kinase phosphatase (Fig.1A, lane 5). Note that active monomer is preferentially dephosphorylated. A second identical sample was left overnight at 4°C to permit additional MEK phosphorylation. It ran as a single active band of high molecular mass (Fig. 1A, lane 6), suggesting that the highly active complexes were maximally assembled. Addition of Laemmli buffer with 5% β-mercaptoethanol to a sample treated identically to the sample in lane 6 before loading on the gel dissociated the high molecular weight protein complex and gave three active bands (Fig.1A, lane 7, line and arrows) with molecular masses of around 115, 145 and 71 kDa. We judge these to be the GST-hERK1:sea urchin ERK1 dimer (rmm 115 kDa ≡ 71kDa + 44 kDa), the GST-hERK1 homodimer (142 kDa ≡ 2×71 kDa), and the GST-hERK1 monomer (71 kDa). The GST itself tag does not dimerise under these experimental conditions (Armstrong, 1997), as incubation of non-activated GST-hERK1 with sea urchin extract did not result in formation of a complex (Fig.1A, lane2; see also Fig.1C, lane 2). This experiment demonstrates that dimers form between active recombinant hERK1 monomers and between active recombinant hERK1 and sea urchin ERK1 in sea urchin embryo extracts that themselves lack detectable MAP kinase activity.
We also took aliquots treated identically to those of Fig. 1A, but instead of blotting the samples, we washed them into MAP kinase buffer and measured the activities of the GST-hERK1 aliquots against a myelin basic protein (MBP) substrate. The results from one representative experiment are shown in Fig. 1B, beneath the relevant gel lane, together with the quantitated activities. Recombinant GST-hERK1 itself had very little activity (Fig1B, lane 1) that remained the same after incubation in sea urchin extract (Fig.1B, lane 2). When activated with MEK alone for 1 hour, it showed around a ten-fold activation over baseline (Fig. 1B, lane 3). Addition to the sea urchin embryo extract and incubation for 15 min at room temperature elevated the activity of the recombinant kinase a further tenfold (Fig. 1B, lane 4). A five-fold increase in the amount of the cell extract (to 75 μg total protein) did not increase the activity of the aliquot (not shown), indicating that soluble factors were not limiting and that basal cellular kinase activity was not contributing to the ERK1 activity we measured. Partial dephosphorylation of the protein using recombinant CL100 decreased its activity considerably; note that Fig1A, lane 5 indicates that the high molecular weight complex is resistant to dephosphorylation relative to the monomer. Interestingly, additional overnight activation at 4°C by MEK further doubled (Fig. 1B, lane 6) the activity of recombinant hERK1 in this aliquot relative to that activated for 1 hour at 30°C (Fig. 1B, lane 4). Thus, maximal phosphorylation and complex formation under these conditions leads to an MBP kinase activity about 20-fold higher than measured in the aliquot containing only phosphorylated monomeric GST-hERK1.
Two other sets of aliquots, identical to those run on lanes 2, 4, 5 and 6 or lanes 4, 5 and 6 were blotted and probed with anti-ERK (Fig.1C) and anti-GST (Fig. 1D) antibodies respectively. They confirmed that the high molecular mass active protein complexes are indeed ERK1 complexes and that the recombinant GST-hERK1 is one of their components. They also showed that treatment of the highly active aliquot with the dual-specificity CL100 ERK-phosphatase led to loss of GST-hERK1 from the high molecular weight complex and its appearance as monomer (compare lanes 4 and 5 in Fig 1C and D).
These results show that pure recombinant ERK1 can be activated in vitro without dimerisation. However, a short incubation in cellular extract promotes the formation of ERK1 complexes with much higher activity. Dephosphorylation using a MAP kinase phosphatase specific for dual phosphorylated ERK reduces this activity, while additional overnight phosphorylation leads to the exclusive formation of highly active complexes with a further doubling of activity. Addition of reducing agent dissociates the high molecular weight complexes, but leaves the active dimers intact.
A dimerisation deficient mutant, GST-ΔPEHD-hERK1, in which four amino acids were deleted from the sequence of hERK1 (see Methods), was used in control experiments. A similarly-mutated ERK2 recombinant protein has been shown to be unable to dimerise (Khokhlatchev et al., 1998). The mutant protein was successfully activated as a monomer, as shown by its order of magnitude increase (8-fold) in relative activity after activation by MEK in buffer (Fig.1F, lanes 1 and 2), comparable to the 11-fold activation of the unmodified recombinant hERK1 (Fig.1B, lanes 2 and 3). However, it did not cross-react with the anti-dual phosphorylated ERK antibodies, most likely due to an altered configuration as a result of the deletion of four amino acids in close proximity to the activation loop (Khokhlatchev et al., 1998). Unlike the control GST-hERK1 protein (Fig.1E and F, lanes 5), the mutant protein did not show any further increase in activity or dimerisation (Fig.1 E and F, lanes 3) after incubation with low activity sea urchin cell extract. Thus ERK1 dimerisation is crucial for the highly increased kinase activity of the protein complex formed by phosphorylated recombinant hERK1 in cellular extract.
ERK1 activity in vivo resides in two species of homodimer
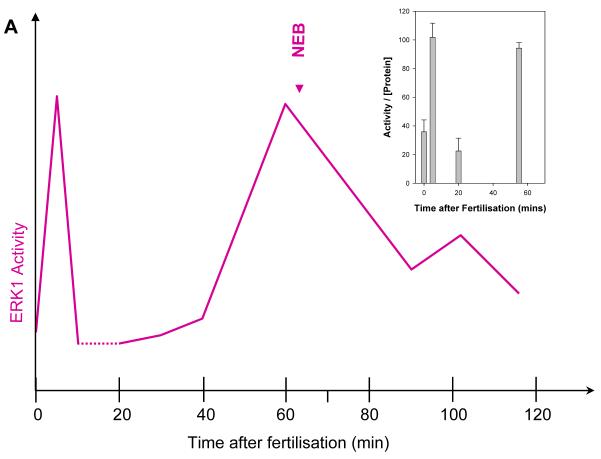
ERK1 activity increases twice during the first mitotic cell cycle after fertilization in the sea urchin, once immediately after fertilization and again during mitosis (Philipova and Whitaker, 1998; Philipova et al.,, 2005a; Philipova et al., 2005b). The timing and extent of these changes is shown schematically (Fig. 2A) for ease of orientation.
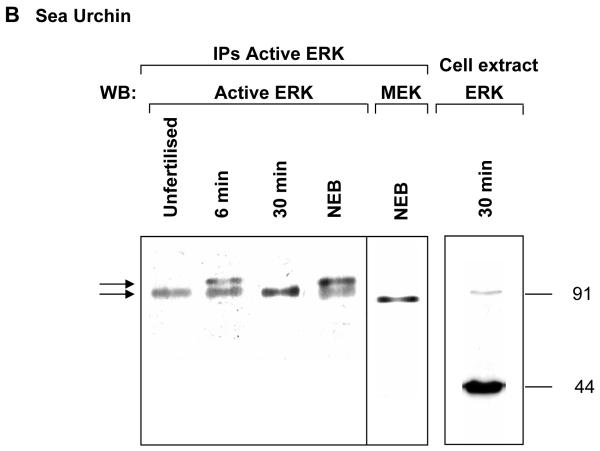
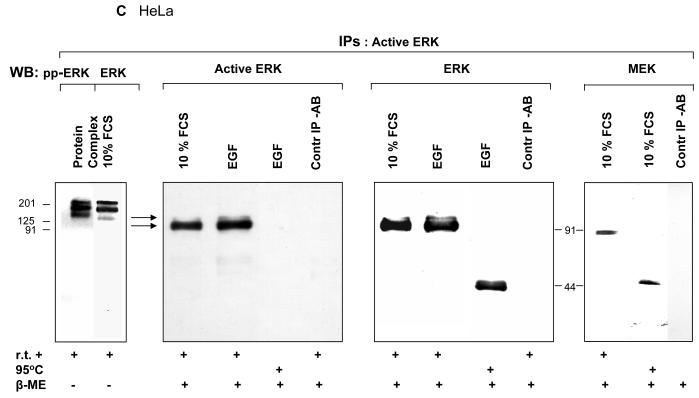
Fig. 2. ERK activity in vivo is associated with its homodimers. Monomers at 44 kDa are largely inactive.
A Schematic diagram of ERK1 activity during the first mitotic cell cycle of sea urchin embryos. An inset with the original data (Philipova and Whitaker, 1998) is also shown.
B Sea urchin embryos Active ERK1 was immunoprecipitated during the first mitotic cell cycle at time points that corresponded to maximum (6min, NEB) and minimum activity (Unfertilised, 30 min) using an anti-dualphosphorylated ERK antibody and detected by Western blotting with a second, different anti-dualphosphorylated ERK antibody or with an anti-MEK antibody (NEB sample). Arrows: active ERK1 dimers. Cell extract: Whole-cell extracts are rich in ERK1 monomers as a Western blot of a 30 min post-fertilisation cell extract detected with anti-ERK antibody demonstrates. It was necessary to load 150 μg total cellular protein in order to obtain a detectable band at 91 kDa for comparison with the anti-dualphosphorylated ERK antibody immunoprecipitate. The positions of molecular mass markers are shown.
C HeLa cells: Active ERK was immunoprecipitated from cell extracts using an anti-dualphosphorylated ERK antibody, and proteins detected on Western blots using a second anti-dualphosphorylated ERK antibody, an anti-ERK antibody or an anti-MEK antibody. The immunoprecipitate was also run in the absence of β-mercaptoethanol (β-ME) before blotting (Protein complex). The positions of molecular mass markers are shown.
To determine the extent of dimer formation we immunoprecipitated active ERK1 from whole cell extracts at various times during the cell cycle using an anti-active ERK polyclonal antibody. The antibody recognizes ERK phosphorylated on both Thr183 and Tyr185 (Canagarajah et al., 1997). Unfertilised eggs showed a single band at about 91 kDa when blotted with a different anti-dual phosphorylated monoclonal antibody (Fig. 2B). At 6 min postfertilization, during the first peak of increased ERK1 activity, a second, shifted band appeared which was absent by 30 min, a time at which ERK1 activity had fallen back to basal levels (Fig 2A, B). The higher, shifted band reappeared at nuclear envelope breakdown (NEB) (Fig 2B: NEB). Immunobloting of an identical sample with anti-MEK antibody revealed that MEK is also a component of the immunoprecipitated active-ERK complex, but it is not a subunit of the ERK dimers (Fig.2B; WB: MEK). It was found in an oligomeric complex (possibly a homodimer) with an apparent molecular mass significantly lower than 91 kDa. It was striking that we detected no active ERK1 monomers in immunoprecipitates from cell extracts using the anti-dual phosphorylated antibody. Yet these whole-cell extracts are rich of ERK1 monomers, as blotting whole extract with an anti-ERK antibody demonstrates, and the ratio of the monomer to the 91 kDa species is very high (Fig. 2B, Cell extract). These data indicate that ERK1 activity in vivo is represented by high molecular weight species twice the molecular weight of the monomer, by homodimers rather then by monomers.
Our previous measurements show that a basal level of ERK1 activity is always detectable throughout the cell cycle; the activity never falls to zero (Philipova and Whitaker, 1998) (Fig 2A), consistent with reports elsewhere (Pouyssegur and Lenormand, 2003). Our immunoprecipitation with the anti-active polyclonal antibody and subsequent blots with the anti-dual phosphorylated monoclonal antibody (Fig 2B) demonstrate that the the 91 kDa protein band contains an active ERK1 molecule phosphorylated on threonine and tyrosine even when MAP kinase activity is at basal levels, at first sight a paradox. Our postulate is that basal ERK1 activity in vivo is manifested by the 91 kDA homodimers as they are consistently detected throughout the cell cycle. These low activity homodimers (monophosphodimers) have the potential to become additionally phosphorylated/activated to form bisphosphodimers at times when peak activity is required for cell cycle progression.
While our experiments with recombinant human ERK1 (Fig. 1) indicate that human ERK1 is capable of forming a complex with sea urchin proteins, it seemed entirely possible that dimerisation to form active ERK1 might be a particular property of sea urchin ERK1. To test this postulate, we immunoprecipitated active ERK from HeLa cell extracts prepared from both normally growing cells and cells hyperstimulated with EGF for 10 minutes (21). We found a result identical to that in sea urchin: stimulation of ERK was associated with an increase in the protein band attributable to an ERK bisphosphodimer (Fig. 2C). Note that the shifted band above 91 kDa cross-reacts with both anti-ERK and anti-active ERK antibodies on immunoblotting and is thus indentified as ERK. Moreover, active ERK was again found in a high molecular weight protein complex under non-reducing condition and MEK again found to be a component of this complex (Fig. 2C, first and fourth panel). Treating samples at 95°C before electrophoresis led to loss of immunoreactivity to the anti-active ERK antibody, while immunoblotting of identical sample with an anti-ERK antibody demonstrated that the human phosphodimers were dissociated under these conditions, in marked contrast to ERK dimers in sea urchin embryos. These data demonstrate that active ERK is found predominantly as dimers in HeLa cells and that heat treatment of dimers leads to dissociation of the dimers and in addition caused a severe reduction in the immunoreactivity of ERK1 towards the anti-active ERK antibody.
In vitro phosphorylation of the 91kDa dimer from basal activity extracts gives rise to a shifted protein band with much higher protein kinase activity
To test the postulate that monophosphodimers represent basal activity and bisphosphodimers activated ERK1, we used anti-ERK polyclonal antibody to immunoprecipitate total ERK from the soluble sea urchin extract made 30 min after fertilisation (when ERK1 shows only basal activity) and treated the immunoprecipitated kinase with either active or kinase dead recombinant MEK. Immunodetection with either an anti-ERK polyclonal antibody or with the anti-dual phosphorylated ERK monoclonal antibody (Fig. 3) showed that active recombinant MEK induced the appearance of a shifted band identical to that seen in cell extracts made at cell cycle stages when in vivo levels of ERK1 activity were high (compare Fig. 3A IP+MEK with the 6 min and NEB time points in Fig. 2B). This shifted band was absent in untreated samples and in those treated with kinase dead MEK (Fig. 3A IP+k.d.MEK). Thus, the shifted band identified as active ERK by the anti-dualphosphorylated antibody can be produced by treatment of basal activity samples with active MEK. This experiment also demonstrates that the anti-ERK antibody preferentially immunoprecipitates the 91kDa species, as it is the predominant species in immunoprecipitates, but undetectable on the blot of whole extract (Fig. 3A, Extr. 30 min). The preference of the anti-ERK antibody for the dimeric forms in immunoprecipitation may be a reflection of the higher affinity conferred by a doubling of the epitope in the dimer.
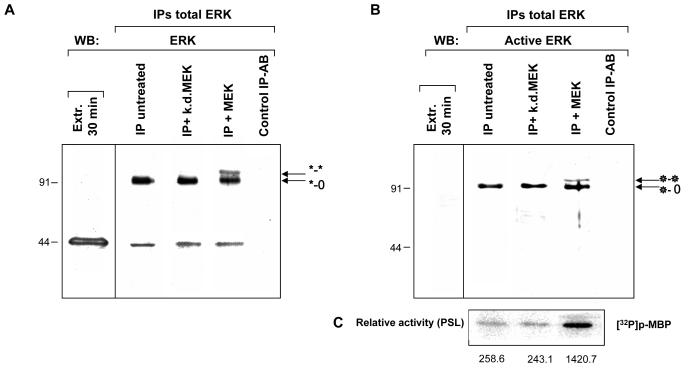
Fig. 3. In vitro phosphorylation by MEK of immunoprecipitated low-activity cellular ERK1 dimer (*-o) results in the appearance of the shifted fully activated dimerised ERK1 (*-*).
Total ERK1 was immunoprecipitated from 30 min extracts from sea urchin embryos using an anti-ERK antibody and then treated in vitro with either active MEK (IP + MEK) or an inactive (kinase dead) mutant of MEK (IP + k.d.MEK); a control IP in the absence of the anti-ERK antibody is also shown (Control IP –AB). A and B show a comparison between Western blots of the same samples with (A) anti-ERK antibody or (B) anti-dualphosphorylated ERK antibody. In both cases, the immunoprecipitates are compared with an untreated aliquot of the 30 min extract used for immunoprecipitation. Note that immunoprecipitates using anti-dualphosphorylated ERK antibody are highly enriched in the 91 kDa dimeric form. (C) Relative activities of samples treated identically as indicated in B, washed in MAP kinase buffer and used in protein kinase assays. The sample activated by MEK shows 6 fold higher activity than those either untreated or treated with kinase dead MEK.
Immunodetection of the same samples with anti-active ERK monoclonal antibody (Fig. 3B) confirmed that the 91kDa species and its shifted counterpart contained active ERK1 and that the monomeric form was inactive. We obtained very similar results with a different anti-active MAP kinase antibody (not shown). These reports support our postulate that the shifted band represents the additionally phosphorylated 91kDa species. It is notable that treatment with MEK does not lead to the appearance of detectable active monomeric ERK1. Either the 91kDa species is a preferential substrate for MEK or any active monomers rapidly dimerise to form additional 91kDa complexes; both may occur.
To test whether the appearance of the shifted band would correspond to a higher MAP kinase activity of the complex, we washed identically treated samples in MAP kinase buffer and used them in kinase assays. As expected, the untreated and kinase dead-treated immunoprecipitated ERK1 complexes showed relatively low activity, while activation by active MEK resulted in much higher kinase activity (Fig. 3C) that correlates with the appearance of the shifted bisphosphodimer band.
Monomers, mono- and bisphosphodimers are present in all fractions of ERK1 purified from sea urchin cell extracts
We previously identified the MAP kinase activity that increases after fertilization and again in mitosis of the first embryonic cell cycle by purifying the activity from mitotic cell extracts using anion exchange and hydrophobic interaction chromatography (Philipova and Whitaker, 1998). We identified the activity using in-gel MBP kinase assays under reducing conditions as a protein of 44 kDa and determined it to ERK1 by peptide sequencing. It was thus important to confirm that the two dimeric forms that we identified here corresponded to the activity we isolated at 44 kDa.
The fractions from the final Phenyl-Sepharose chromatography step were blotted independently with both the anti-ERK and anti-active ERK antibodies. Both dimers and monomers were detected in all active fractions using the anti-ERK antibody, while dimers were detected in all active fractions when probed with the anti-active ERK antibody (not shown). Fraction 23 of the column eluate was close to the peak of ERK1 activity and is shown as an example (Fig. 4A, a and b).
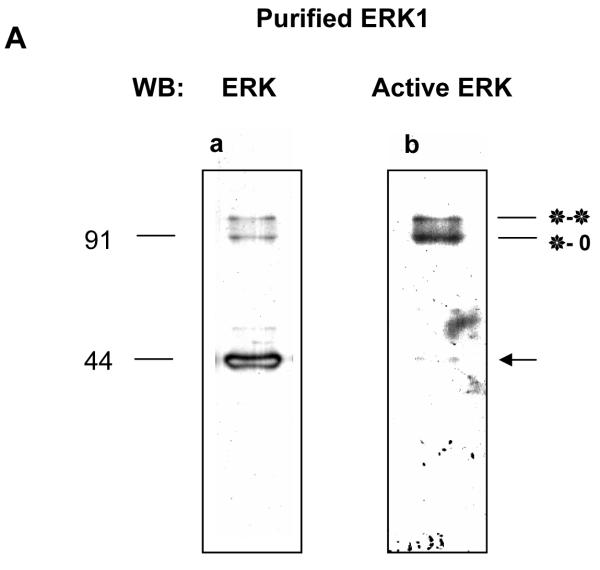
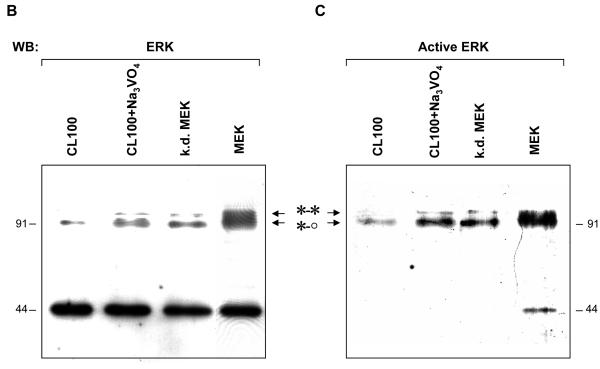
Fig. 4. A. The two ERK1 homodimers (*-o and *-*) co-purify with the ERK1 monomer.
Western blots of a fraction with high MAP kinase activity (Fraction 23) eluted from a Phenyl-Sepharose column during ERK1 purification from mitotic sea urchin embryo extracts probed with (a) an anti-ERK antibody and (b) an anti-dualphosphorylated ERK antibody. 4μl of the fraction loaded for each blot. Blot b was detected using the high-sensitivity ECL Advance Kit: a faint band of active monomer is visible (arrow).
B, C. Phosphorylation by MEK and dephosphorylation using CL100 dual specificity phosphatase cause the predicted gel shifts of the ERK1 dimers in the phenylsepharose column fraction. 0.5 μl of fraction 23 was used for each treatment; samples were blotted and probed with (B) anti-ERK antibody and (C) anti-dualphosphorylated ERK antibody. Samples treated with CL100 in the presence of vanadate to block phosphatase activity (CL100+Na3VO4 or with mutant inactive (kinase dead) MEK (k.d.MEK) serve as controls. Treatment with CL100 led to the loss of the more slowly migrating band, while treatment with MEK enhanced the band. Note that after MEK treatment, dimeric forms accumulate in preference to the monomer.
To demonstrate that the dimerised bands co-eluted with the ERK1 monomer are indeed ERK1 dimers, we carried out in vitro dephosphorylation/phosphorylation of fraction 23. Treatment of the fraction with the recombinant dual-specificity CL100 ERK-phosphatase led to the loss of the shifted dimer band, as judged by western blotting as before (Fig. 4B, C). The loss was prevented by the inclusion of the phosphatase inhibitor vanadate in the incubation buffer (Fig. 4B, C). Treating the fraction with recombinant active MEK dramatically increased the intensity of the shifted band, while kinase-dead MEK was ineffective (Fig. 4B, C). Comparison of the degree of labeling of the dimerised ERK1 in the kinase-dead control with that of the same bands after treatment with active MEK implies recruitment of monomer to dimers; indeed, only a small amount of active monomer is detected under these conditions (Fig.4C, MEK). Thus the previously purified ERK1 shows the same dimerisation behaviour as the MAP kinase studied in cell cycle extracts.
In contrast to immunoprecipitated complexes, ERK1 dimers in whole cell extracts were very resistant to phosphatases (not shown), most likely due to the activity of the upstream activator MEK in the high molecular weight active-ERK protein complex.
Newly-phosphorylated ERK1 accumulates in the active-active bisphosphodimers
As confirmation that the shifted band above 91 kDa represented phosphorylated ERK1, we tracked newly-phosphorylated ERK1 during in vitro radioactive phosphorylation of purified ERK1. In interpreting these results, the reader should note the relative abundance of monomeric ERK: it is far in excess of the 91 kDa band and its shifted counterpart (see Fig.4Aa). We phosphorylated ERK1 using active MEK and took samples at 2, 4 and 6 min (Fig. 5). 32P accumulated only to a very small extent in the 44 kDa monomer. The 120 kDa band (corresponding to the active, shifted band) was most strongly labeled while the 91kDa band showed labeling comparable or in slight excess to the monomer. Radioactivity is also associated with protein complexes and precipitates which do not enter the gel. Similar patterns were seen with both boiled (not shown) and unboiled samples, indicating that sea urchin homodimers do not disassemble when heated under reducing conditions, in contrast to those isolated from HeLa cells (Fig. 2C). Identical experiments using kinase-dead MEK showed no accumulation of 32P in the proteins (not shown).
Fig. 5. Newly phosphorylated ERK1 accumulates as fully active (*-*) homodimers.
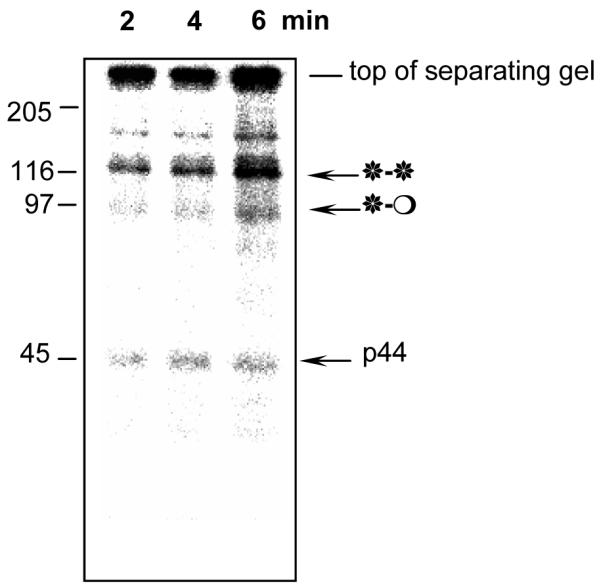
Incorporation of γ-[32P]ATP into purified ERK1 (fraction 23 from the phenylsepharose column) during in vitro phosphorylation by MEK. 0.5μl of the fraction was used per reaction as a substrate for in vitro MEK assays. Kinase reactions were carried out for 2, 4 and 6 min at 30°C, and then terminated by addition of sample buffer. Molecular mass markers are also shown. Note that radioactivity is incorporated predominantly into the band above 116kDa. The radioactivity at the top of the gel represents insoluble material that has not entered the gel.
The pattern of 32P incorporation clearly shows that newly-phosphorylated protein accumulates as the 120 kDa bisphosphodimer. These data confirm that newly-phosphorylated ERK1 does not remain monomeric, but is immediately incorporated into dimers as soon as it is phosphorylated and activated. They also imply that MEK may preferentially phosphorylate the 91 kDa protein rather then the monomer. Both events lead to accumulation of highly labeled bisphosphodimers.
DISCUSSION
ERK1 dimerises within the cell as a consequence of ERK1 phosphorylation
Using a combination of exogenous ERK2 and dysfunctional mutants, it has been demonstrated that ERK2 dimerisation is necessary for nuclear uptake of ERK2 and that a dimer must contain one phosphorylated monomer for uptake to occur (Khokhlatchev et al., 1998); intrinsic kinase activity is not required, as kinase-dead mutants can enter the nucleus as a dimer, provided they are phosphorylated. It was also reported (though not demonstrated) as a preliminary observation (Khokhlatchev et al., 1998) that ERK1 also formed homodimers and that ERK1/ERK2 heterodimers were unstable. This previous study was unable to detect the presence of endogenous ERK2 dimers in cells or cell extracts, though it was convincingly inferred that exogenous ERK2 was able to form dimers with endogenous ERK2. Here we have used a biochemical approach to demonstrate that ERK1 dimerises when phosphorylated. ERK dimers are not easily detectable in cell extracts and there are two obvious reasons for this. First, the dimers form a small fraction of the cellular ERK. Secondly, our finding that sea urchin ERK1 dimers are stable at 95°C, while those of HeLa cells are not, explains why ERK dimers are undetectable by western blotting using boiled samples of mammalian cell extracts.
Evidence for the existence of ERK1 in dimeric form
A summary of our evidence is as follows:
ERK2 has been shown to dimerise in vitro, though not in vivo, and a structure of the dimer has been deduced (Canagarajah et al., 1997; Khokhlatchev et al., 1998).
We have clearly identified MEK-generated GST-hERK1 homodimers and sea urchin ERK1-GST-hERK1 heterodimers.
ERK1 dimers in sea urchin and HeLa cells have a molecular weight consistent with a dimeric form.
Three distinct anti-active ERK antibodies recognize and immunoprecipitate the active, dual phosphorylated form of ERK specifically, and cross react with the 91 kDa species and its shifted congener, as does an anti-ERK antibody.
ERK1 activity tracks with dimers during column chromatography (Philipova and Whitaker, 1998) and this study).
Dephosphorylation of ERK1 with the CL100 phosphatase that removes both Thr183 and Tyr185 phosphates (Canagarajah et al., 1997) diminishes the upper band corresponding to the bisphosphodimer.
Phosphorylation with the dual specificity kinase MEK that phosphorylates Thr183 and Tyr185 enhances the upper band.
MEK-stimulated incorporation of 32P occurs preferentially into the upper band, the bisphosphodimer.
Conjecturing that ERK1 dimers might most readily be identified by immunoprecipitation with anti-active dual-specificity antibodies, we demonstrate that the majority of active ERK1 in vivo is present as dimers; active monomer is not immunoprecipitated.
It is known that GST itself can dimerise (reviewed in Armstrong, 1997). However, the GST tag does not contribute to the dimerisation of the recombinant ERK1 protein under our experimental conditions. The evidence can be summarised as follows: 1/ Pure GST-hERK1 does not oligomerise if stored as indicated in Methods. It is stable as a 71 kDa protein; 2/ it does not oligomerise after incubation with cell extract (Fig. 1 A and C, lanes 2) or after activation with MEK (Fig. 1 A, lane 3); 3/ it forms dimers with the sea urchin endogenous ERK1 which lack GST tags; 4/ Active ERK1, immunoprecipitated or purified from whole cell embryonic or HeLa extracts, is dimerised in the absence of GST-tags. 5/ A GST-tagged dimerisation deficient mutant, GST-ΔPEHD-hERK1, does not dimerise, and moreover, does not show highly increased protein kinase activity after activation and incubation in cell extract, unlike the control GST-hERK1 protein. All these results exclude a role of the GST tag in the dimer-formation and the additional 10 to 20-fold higher activity of the dimerised recombinant hERK1.
ERK1 monophosphodimers manifest basal ERK1 activity, while ERK1 bisphosphodimers are present when ERK1 is fully active
We took advantage of the fact that sea urchin embryos progress very synchronously through the cell cycle and show two distinct episodes of ERK1 activation during the first cell cycle (Philipova and Whitaker, 1998) and compared the behavior of active ERK1 during periods of basal and stimulated ERK1 activity. We infer that the 91kDa active ERK1 monophosphodimer runs anomalously relative to its predicted 88 kDa molecular mass because of a gel shift due to phosphorylation. We found that a proportion of the 91 kDa band that represents the dimer was further shifted during stimulation and used this gel shift to identify the 91 kDa band as an active-inactive monophospho-ERK1 dimer and the shifted band as the active-active bisphospho-ERK1 dimer. Treatment with the MEK inhibitor U0126 in vivo prevents the formation of the bisphosphodimer and leads to abolition of ERK1 activation above the basal level (Philipova et al., 2005a; Philipova et al., 2005b). We conclude that basal ERK1 activity is due to the presence of the monophosphodimer and that further phosphorylation of this species gives rise to the 5-7 fold more active bisphosphodimer. We show that ERK in HeLa cell extracts exhibited identical behaviour.
ERK1 dimers represent a very small proportion of total cell ERK1
By comparing the relative amounts of ERK1 in its three forms (one monomeric and two dimeric) in whole cell extracts without immunoprecipitation, we determined that around 97% of ERK1 is present as the monomer, even when ERK1 activity is at its peak. Around 3% of cell ERK1 is found as the 91 kDa monophosphodimer, while the bisphosphodimer is rarely detectable in whole cell extracts, except by immunoprecipitation. In contrast, the amount of active ERK1 in each dimer was roughly comparable when ERK1 was most active in sea urchin embryos (see Fig. 2B, C). Since each bisphosphodimer contains two dual-phosphorylated ERK1 monomers, this finding implies a ratio of monophosphodimers to bisphosphodimers of 2:1 (if we assume that each antibody binds to a single ERK1 molecule in the bisphosphodimer and that the antibodies have identical affinity for both types of dimers). Thus around a third of the dimers are fully activated during mitosis, when the MBP kinase activity increases five to seven-fold, suggesting that the bisphosphodimers have a 10-20-fold greater kinase activity than the monophosphodimers and in turn represent around 1% of total cell ERK1.
ERK1 dimerises only in the presence of cellular protein(s), it forms a protein complex in vitro and also when isolated from whole-cell extracts
It has been reported on good grounds that dimerisation of ERK2 does not affect its kinase activity: dimerisation defective mutants have a kinase activity comparable to wild type. However, these observations were made on purified ERK2 in vitro; it has been shown to dimerise in simple buffers with a Kd of 7nM (Khokhlatchev et al., 1998). We have not observed dimerisation of activated ERK1 in simple buffers in vitro and therefore tested whether it would occur in the presence of other soluble cellular proteins. We have found that recombinant hERK1 will dimerise in a soluble sea urchin cell extract. At the moment, the only indication of the nature of the co-factor comes from preliminary experiments performed with two types of HeLa whole-cell extracts, obtained either after lysis or after homogenisation. They indicate that the co-factor might be a membrane-associated protein, because extracts from homogenised cells are more efficient in promoting ERK1 homodimerisation in vitro. An interesting and unanswered question is whether the co-factor contributes to the enhanced protein kinase activity. The experiments using a ΔPEHD mutant protein demonstrate that dimerisation is critical for the highly increased activity of the protein complex formed by phosphorylated hERK1 in cellular extract. Further studies are required to understand the sequence of events leading to the formation of semi active and fully active dimers, and to identify the cellular co-factor and its precise role in these events.
Our results show that in vivo the active homodimerised ERK1 is similarly a member of a high molecular weight protein complex that also contains MEK. However, MEK is not associated with the ERK1 dimers that we were able to isolate under reducing conditions. It is known that ERK1 forms complexes in vivo (Tanoue and Nishida, 2002) and there are docking sites for MAP kinases on scaffold proteins, upstream kinases and MAP kinase phosphatases and also on substrates (Tanoue et al., 2000), suggesting that elements of the MAP kinase signaling module may form a molecular machine (Morrison and Davis, 2003) in which ERK dimers play a central role.
CONCLUSION
We demonstrate that active endogenous ERK1 is present in vivo in dimeric form, that monophosphodimers constitute the basal ERK1 activity while peak ERK1 activity is constituted by bisphosphodimers. It is notable that the dimers are good substrates for MEK and that they are resistant to CL100 phosphatase relative to monomeric ERK1. This behaviour is consistent with the idea that activation of ERKs involves strong positive feedback (Xiong and Ferrell, 2003). The dimers make up a very small proportion of total cell ERK1. That it may be energetically and kinetically efficient to maintain low activity dimers that can be highly activated by a single phosphorylation step is evident, but the allosteric basis for the marked activation of the dimer as a consequence of this single phosphorylation step is as yet unexplained.
ACKNOWLEDGMENTS
We should like to thank Dr Burgering for the GST-hERK1 vector, Dr J. Ferrell, Jr. and Dr N. Ahn for the (His)6-tagged hMEK R4F and kinase dead MEK1constructs and Dr S. Keyse for the purified recombinant hCL100. Our thanks go also to Maureen Sinclair for her excellent technical help. This work was supported by a Wellcome Trust Programme Grant.
REFERENCES
- Abrieu A, Doree M, Fisher D. The interplay between cyclin-B-Cdc2 kinase (MPF) and MAP kinase during maturation of oocytes. J Cell Sci. 2001;114:257–67. doi: 10.1242/jcs.114.2.257. [DOI] [PubMed] [Google Scholar]
- Canagarajah B, Khokhlatchev AV, Cobb MH, Goldsmith E. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell. 1997;90:859–869. doi: 10.1016/s0092-8674(00)80351-7. [DOI] [PubMed] [Google Scholar]
- Choi KY, Satterberg B, Lyons DM, Elion EA. Ste5 tethers multiple protein kinases in the MAP kinase cascade required for mating in S. cerevisiae. Cell. 1994;78:499–512. doi: 10.1016/0092-8674(94)90427-8. [DOI] [PubMed] [Google Scholar]
- Cobb MH, Goldsmith E. Dimerization in MAP-kinase signaling. TIBS. 2000;25:7–9. doi: 10.1016/s0968-0004(99)01508-x. [DOI] [PubMed] [Google Scholar]
- English J, Pearson G, Wilsbacher J, Swantek J, Karandikar M, Xu S, Cobb MH. New insights into the control of MAP kinase pathways. Exp Cell Res. 1999;253:255–70. doi: 10.1006/excr.1999.4687. [DOI] [PubMed] [Google Scholar]
- Ferrell J., Jr Building a cellular switch: more lessons from a good egg. BioEssays. 1999a;21:833–842. doi: 10.1002/(SICI)1521-1878(199910)21:10<866::AID-BIES9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ferrell J., Jr Xenopus oocyte maturation: new lessons from a good egg. BioEssays. 1999b;21:866–870. doi: 10.1002/(SICI)1521-1878(199910)21:10<833::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb MH. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–15. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- King R, Jackson P, Kirschner M. Mitosis in transition. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Lewis T, Groom L, Sneddon AA, Smythe A, Keyse SM. XCL100, an inducible nuclear MAP kinase phosphatase from Xenopus laevis: its role in MAP kinase inactivation in differentiated cells and its expression during early development. J. Cell Sci. 1995;108:2885–2896. doi: 10.1242/jcs.108.8.2885. [DOI] [PubMed] [Google Scholar]
- Mahanty SK, Wang Y, Farley FW, Elion EA. Nuclear shuttling of yeast scaffold Ste5 is required for its recruitment to the plasma membrane and activation of the mating MAPK cascade. Cell. 1999;98:501–12. doi: 10.1016/s0092-8674(00)81978-9. [DOI] [PubMed] [Google Scholar]
- Mansour S, Candia J, Matsuura J, Manning M, Ahn N. Interdependent domains controlling the enzymatic activity of mitogen-activated protein kinase kinase 1. Biochemistry. 1996;35:15529–15536. doi: 10.1021/bi961854s. [DOI] [PubMed] [Google Scholar]
- Mansour S, Resing K, Candi J, Heremann A, Gloor J, Herskind K, Wartman M, Davis R, Ahn N. Mitogen-activated protein (MAP) kinase phosphorylation of MAP kinase kinase: determination of phosphorylation sites by mass spectrometry and site-directed mutagenesis. J. Biochem. 1994;116:304–314. doi: 10.1093/oxfordjournals.jbchem.a124524. [DOI] [PubMed] [Google Scholar]
- Morrison D, Davis R. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–8. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Philipova R, Kisielewska J, Lu P, Larman M, Huang J-Y, Whitaker M. ERK1 activation is required for S-phase onset and cell cycle progression after fertilization in sea urchin embryos. Development. 2005a;132:579–89. doi: 10.1242/dev.01607. [DOI] [PubMed] [Google Scholar]
- Philipova R, Larman MG, Leckie CP, Harrison PK, Groigno L, Whitaker M. Inhibiting MAP kinase activity prevents calcium transients and mitosis entry in early sea urchin embryos. J Biol Chem. 2005b doi: 10.1074/jbc.M414437200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipova R, Whitaker M. MAP kinase activity increases during mitosis in early sea urchin embryos. J Cell Sci. 1998;111(Pt 17):2497–505. doi: 10.1242/jcs.111.17.2497. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Eur J Biochem. 2003;270:3291–9. doi: 10.1046/j.1432-1033.2003.03707.x. [DOI] [PubMed] [Google Scholar]
- Sohaskey K, Ferrell J., Jr Distinct, constitutively active MAPK phosphatases function in Xenopus oocytes: implications for p42 MAPK regulation In vivo. Mol Biol. Cell. 1999;10:3729–3743. doi: 10.1091/mbc.10.11.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol. 2000;2:110–6. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Nishida E. Docking interactions in the mitogen-activated protein kinase cascades. Pharmacol Ther. 2002;93:193–202. doi: 10.1016/s0163-7258(02)00188-2. [DOI] [PubMed] [Google Scholar]
- Whitaker M. Control of meiotic arrest. Rev Reprod. 1996;1:127–35. doi: 10.1530/ror.0.0010127. [DOI] [PubMed] [Google Scholar]
- Wilkinson M, Millar J. Control of the eukaryotic cell cycle by MAP kinase signaling pathways. FASEB J. 2000;14 doi: 10.1096/fj.00-0102rev. [DOI] [PubMed] [Google Scholar]
- Xiong W, Ferrell JE. A positive-feedback-based bistable ‘memory module’ that governs a cell fate decision. Nature. 2003;426:460–5. doi: 10.1038/nature02089. [DOI] [PubMed] [Google Scholar]