Abstract
A transient calcium increase triggers nuclear envelope breakdown (mitosis entry) in sea urchin embryos. Cdk1/cyclin B kinase activation is also known to be required for mitosis entry. More recently MAP kinase activity has also been shown to increase during mitosis. In sea urchin embryos both kinases show a similar activation profile, peaking at the time of mitosis entry.
Here we test whether the activity of both kinases is required for mitosis entry and if either kinase controls mitotic calcium signals. We find that reducing the activity of either mitotic kinase prevents nuclear envelope breakdown, this despite the presence of a calcium transient when cdk1/cyclin B kinase activity is alone inhibited. When MAP kinase activity alone is inhibited the calcium signal is absent, suggesting that MAP kinase activity is required to generate the calcium transient that triggers nuclear envelope breakdown. However, increasing intracellular free calcium by microinjection of calcium buffers or InsP3 while MAP kinase is inhibited does not itself induce nuclear envelope breakdown, indicating that additional MAP kinase-regulated events are necessary. After MAP kinase inhibition early in the cell cycle, the early events of the cell cycle (pronuclear migration/fusion and DNA synthesis) are unaffected, but chromosome condensation and spindle assembly are prevented. These data indicate that in sea urchin embryos MAP kinase activity is part of a signaling complex alongside two components previously shown to be essential for entry into mitosis: the calcium transient and the increase in cdk1/cyclinB kinase activity.
Keywords: Calcium, Cell cycle, Mitosis, Sea Urchin, MAP kinase, cdk1/cyclin B kinase, Chromosome condensation
INTRODUCTION
The rapid cycling from interphase to mitosis in early embryos follows the activity of the highly conserved maturation promoting factor (MPF) (1)*. MPF consists of a regulatory subunit, cyclin B, and a catalytic subunit, cdk1 (2-4). Activition of cdk1 is dependent on it binding to its regulatory subunit, cyclin B and subsequent phosphorylation events. Levels of cyclin B begin to rise during G2. Once the cdk1/cyclin B kinase complex is formed it is maintained in an inactive state through phosphorylation of cdk1 at Thr14 and Tyr15 by protein kinases Wee1/Mik1 and Myt1 (5). Activation is a consequence of Thr161 being phosphorylated by the Cdk activating kinase (6,7) and dephosphorylation of Thr14 and Tyr15 by Cdc25C (8-11).
During interphase the complex maintains an overall cytoplasmic localization, but during late prophase as the cell approaches mitosis active cdk1/cyclin B kinase accumulates in the nucleus (12). Nuclear envelope breakdown (NEB) is thought to entail depolymerisation of lamins, which are integral proteins of the nuclear membrane (13). Since cdk1 has been shown to phosphorylate nuclear lamins in vitro (14,15), one possibility is that NEB occurs as the result of lamin phosphorylation by cdk1/cyclin B kinase when it translocates to the nucleus (16). In addition, in sea urchin embryos it has been shown that a second mitotic kinase, MAP kinase, displays a similar activation profile and localization to that of cdk1/cyclin B kinase, prior to NEB (17,18). MAP kinase has also been shown to be active during mitosis in somatic cells in culture and in Xenopus cell free cycling extracts (19,20). MAP kinase and cdk1/cyclin B kinase can interact to activate mutually and rapidly (21,22). The activity increase and re-localisation of MAP kinase to the nucleus just prior to NEB suggest a possible role in mitosis entry (18).
In sea urchin embryos, a cell messenger signalling event is involved in mitosis entry. Increases in intracellular calcium concentration ([Ca2+]i) have been observed just before and during mitosis in oocytes, embryos and mammalian somatic cells (23-26). We have shown that these transients are signals that control entry into mitosis (27) and chromatid disjunction (28) in the early cell cycles of sea urchin embryos. The mitotic Ca2+ signals are generated by activation of phospholipase Cγ which leads to inositol 1,4,5-trisphosphate (InsP3)-induced Ca2+ release from internal stores (29,30). The physiological significance of the mitotic Ca2+ transients in other oocytes and embryos and in mammalian somatic cells is less clear than it is in the sea urchin embryo (31-33), However, there is no doubt that Ca2+ transients and Ca2+ oscillations are frequently detected as the cell approaches and passes through mitosis (34-36). Moreover, stimulation of the activating mitotic phosphatase Cdc25C is dependent on a calmodulin-regulated kinase, CaM Kinase II (37). CaM Kinase II activation is essential for NEB in the sea urchin embryo (38).
It has been shown that chromosome condensation and NEB can be caused prematurely in sea urchin embryos by injecting InsP3 and Ca2+-EGTA buffers (39). However, preventing protein (cyclin) synthesis abolishes cell cycle-associated Ca2+ changes and renders the nucleus insensitive to these exogenously generated Ca2+ increases (39), confirming that cdk1/cyclinB activation is downstream of the calcium signal. It is not known how the cell cycle kinase/phosphatase cascades cause the essential increase in [Ca2+]i just before mitosis.
It has been suggested from first principles that incipient activation of cdk1 may modulate Ca2+ release mechanisms to trigger a Ca2+ signal that coordinates and amplifies cdk1 activation (40). This model is consistent with the observation that Cdc25C is in turn activated by the Ca2+-calmodulin-CaM kinase II pathway (37). More recent data show that MAP kinase is also active during mitosis (17,18,20,41). It too may be required for mitosis entry and may modulate Ca2+ release. Here, we determine whether cdk1/cyclin B kinase actvity, MAP kinase activity or both are required for mitosis entry and capable of modulating the Ca2+ transient associated with NEB in early sea urchin embryos.
We find that inhibiting either mitotic kinase prevents NEB. Preventing increases in MAP kinase activity alone abolishes the Ca2+ increase that precedes NEB, indicating that it modulates the Ca2+ release mechanism. The role of MAP kinase during first mitosis in sea urchin embryos extends beyond controlling Ca2+ release, since injection of InsP3 or Ca2+-EGTA buffer after inhibition of MAP kinase did not rescue the NEB block. Monitoring chromatin and microtubules in embryos treated with MAP kinase inhibitors revealed that MAP kinase activity is also required for chromosome condensation and bipolar spindle assembly.
EXPERIMENTAL PROCEDURES
Chemicals and treatments
Unless otherwise stated all chemicals and materials were obtained from Sigma Chemical company (Poole, UK). Chemicals used in the treatment of embryos were dissolved in DMSO so that the final chamber concentration of DMSO (<1%) did not affect the cell cycle (data not shown). U0126 (100 μM; Promega, Southampton, UK) and roscovitine (20 μM) were added to embryos at 12 and 35 minutes after fertilization, respectively. Roscovitine was a kind gift from Dr L.Meijer.
Gamete handling and fertilization
Eggs from the sea urchin Lytechinus Pictus (Marinus, CA, USA) were obtained by intracoelomic injection of 0.5 M KCl and collected in artificial sea water (ASW: 435 mM NaCl, 40 mM MgCl2, 15 mM MgSO4, 11 mM CaCl2, 10 mM KCl, 25 mM NaHCO3, 1 mM EDTA, pH 8.0, osmolarity 950-1000 mosmol). All chemicals were of analytical grade and came from BDH (Poole, UK). Sperm was collected ‘dry’ and stored at 4°C for no more than two days. Eggs were dejellied by passage through a Nitex mesh and attached to glass coverslips with polylysine, as previously described (42). For experiments on fertilized eggs; dejellied eggs were fertilized, stripped of their fertilization envelopes (by Nitex mesh) and then attached to coverslips (as above). Nuclear envelope breakdown was scored using DIC microscopy. Time to NEB in controls was normalized to 70 minutes in all experiments in which intracellular Ca2+ concentration was monitored.
Expression and purification of His-tagged XCL-100 fusion protein and p13suc1 protein
Plasmid DNA encoding XCL100 was obtained from Dr. X. Wang (43). The fragment was excised from the original construct and subcloned into pBAD/gIII (Invitrogen, CA) before being used to transform LMG194 bacterial cells for expression of the his-tagged fusion protein. An overnight culture of bacteria was diluted 1:10 and grown to an OD600 of 0.5 before being induced with a final concentration of 0.2% arabinose for 5 hours. After harvest and lysis, purification was carried out on a nickel column (ProBond™ Resin, Invitrogen, CA) according to the manufacturers protocol. The majority of the XCL100 was eluted with 100 mM imidazole. Following purification, the imidazole elution buffer was exchanged with PBS and the proteins were concentrated using Microcon® 30 microconcentrators (Amicon, Inc. MA) to 20-25 mg/ml. Protein concentrations were determined using the BCA reagent assay (Pierce Chemical Co. IL). The construct was injected to a volume of 0.5-0.8% egg volume with a pipette concentration of 20-25 mg/ml, resulting in a cytoplasmic concentration of ≥ 100 μg/ml.
The S. pombe p13suc1 expression vector pET21b.p13suc1 was a kind gift of Dr T. Hunt (ICRF Clare Hall Laboratories, UK). After purification by gel filtration the protein was coupled to CNBr-Sepharose (Amersham Pharmacia, UK) according to the manufacturers protocol. The final beads contained about 8 mg p13/ml gel.
Microinjection experiments
All compounds injected were made up in microinjection buffer (0.5 M KCl, 20 mM PIPES and 1 mM EGTA, pH 6.8). Cytoplasmic concentrations were determined by evaluating cytoplasmic displacement (27) (0.1-1.0% pipette concentration).
The microinjection procedure was performed using drawn borosilicate glass micropipettes (Clark Electromedical Instruments, GCF150F-10), which were manipulated with a hydraulic micromanipulator (MO-103, Narishige Instruments, Japan) and a piezoelectric advance (Märzhäuser Wetzlar, Germany). Solutions were then injected using gas pressure and the micropipette withdrawn. The borosilicate glass capillaries with inner filament were pulled by a standard vertical puller (BioScience, Sheerness, Kent, UK) and backfilled using a Hamilton syringe.
Ratiometric measurement of [Ca2+]I
Fura dextran pentapotassium salt (Fura, 10,000 MW; Molecular Probes, Oregon) was used to avoid internalization of the dye into intracellular compartments. The dye was introduced into the egg by microinjection (final concentration ≈ 10 μM). Two interference band pass filters (350 and 380 nm, bandwidth of 10.3 nm and 11.3 nm, respectively; Ealing Optics, Watford, U.K.) were used to illuminate the eggs with light emitted from a 75 W Xenon short arc lamp (Cairn Research, Faversham, U.K.). Controlling the 350 and 380 nm filters was a filter wheel (Cairn Research) set to sample every 4 seconds by acquisition software (UMANS) provided by Dr. C. Regan of Bio-Rad Ltd (Hemel Hempstead, UK). Fluorescence emission was collected from a 400 nm dichroic mirror and filtered through a 510 nm long pass filter and detected on a PMT (Thorn EMI, Middlesex, UK). The [Ca2+]i concentration was determined using ratiometric analysis (44). Confocal images were acquired using the Leica TCS4D system, as previously described (27). Briefly, a mix of calcium green and TMR dextrans (10,000 MW; Molecular Probes) were microinjected to a final concentration of 10 μM. Images were acquired every 15 seconds using a 16 scanline average. Topographical representations were generated using IDL software (Research Systems) on an Indigo2 workstation (Silicon Graphics). All experiments were undertaken at 17°C
In vitro protein kinase assays, Western blotting and immunoprecipitations
Preparation of sea urchin cell lysates, MAP kinase and hH1 kinase assays, Western blotting and immunoprecipitation to determine kinase activity in embryos at various times after fertilization were carried out as previously described (18). Immunoprecipitation of active MAP kinase was carried out by using an anti-active MAPk antibody (Promega). The immune complexes were not boiled. Instead, they were left for 15 min at room temperature in sample buffer and then frozen or loaded on gel for electrophoresis and blotting. The blotted complexes were probed with another, monoclonal anti-dual phosphorylated MAPk antibody (New England Biolabs).
Cdk1 activity was measured also after isolation of cdk1/cyclin B kinase from sea urchin extracts using p13suc1-Sepharose binding (45). The isolated cdk1/cyclin B complexes were used in hH1 kinase assays.
RESULTS
Manipulation of MAP kinase and cdk1/cyclin B kinase activities and their effect on NEB
It was initially thought that there was no increase in MAP kinase activity during mitosis (46-49). More recently an ERK1-like MAP kinase activity has been detected during mitotic divisions in sea urchin embryos (17,18), mammalian cell lines (20) and cycling Xenopus extracts (41). The MAP kinase activity peak correlates closely with the timing of NEB (mitosis entry) in sea urchin embryos (18). We blocked MAP kinase activation by microinjecting a specific recombinant inhibitor (MAP kinase phosphatase: XCL100). We also used a small molecule-based approach (MEK inhibitor: U0126). The usefulness of such soluble, fast-acting, small molecule inhibitors to examine dynamic cellular pathways has been demonstrated recently in dissecting the spatial and temporal control of cytokinesis (Straight et al., 2003). We have recently used XCL100 and U0126 to show that the rapid, transient increase in MAP kinase activity at fertilization controls the onset of DNA synthesis (50). Here, we find that inhibiting MAP kinase activation later in the cell cycle with either treatment prevented NEB.
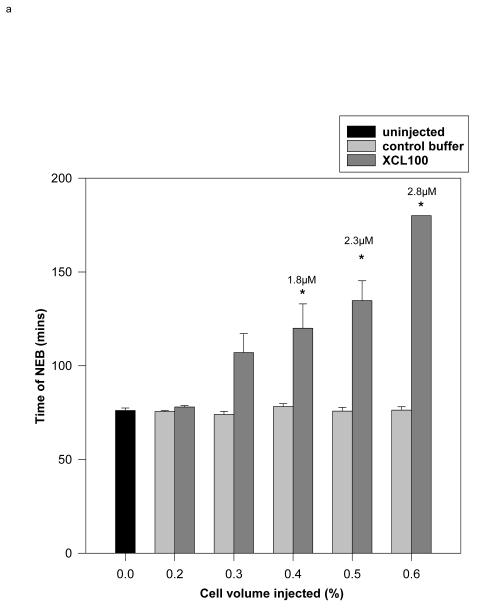
The MAP kinase activity identified in intact sea urchin embryos contains a peptide sequence with 92% identity to ERK1 (18). We used a recombinant MAP kinase-specific phosphatase, Xenopus MAP kinase phosphatase-1 (XCL100) to prevent MAP kinase activation. XCL100 inactivates MAP kinases by dephosphorylating the phosphotyrosine and phosphothreonine regulatory sites and is highly specific for ERK1 and ERK2 (43,51-53). After the first sharp peak that follows fertilization, the activity of ERK1 quickly subsides. At 10 – 15 min after fertilisation, it reaches its lowest level. At about 20-25 min it slowly increases again to reach its second maximum at NEB (18). XCL100 protein was microinjected 12-20 minutes after fertilization to block this second, NEB-associated increase in MAP kinase activity, while sparing the first. A final intracellular concentration of 1.8 μM delayed NEB significantly; ≥ 2.8 μM caused a complete block over a 3 hr observation period and was used in subsequent experiments (Fig. 1a).
Fig. 1.
- Recombinant XCL100 (pipette concentration: 20-25mg/ml) or control vehicle buffer (0.5x PBS) were microinjected into sea urchin embryos 12-20 minutes after fertilization. Experiments were terminated after 180 minutes. Cell volume injections of 0.4, 0.5 and 0.6% give final concentrations of approximately 1.8, 2.3 and 2.8 μM, respectively. * statistically significant at 5%, Student’s t-test between control and XCL100-injected embryos. 5-15 embryos were injected for each treatment.
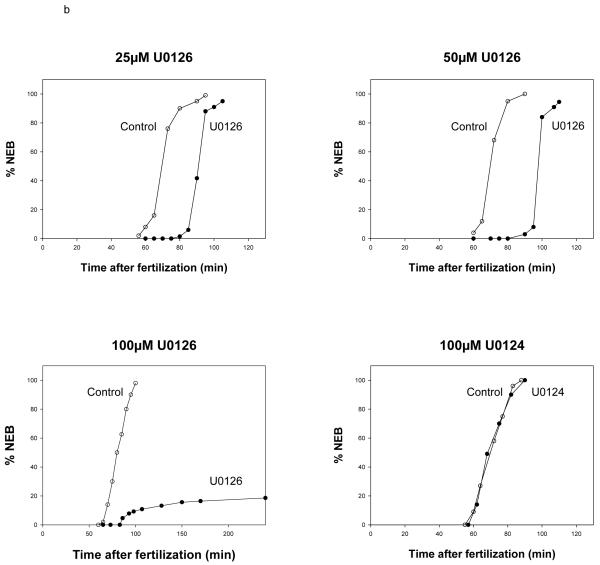
- Control and U0126-treated embryos. Varying concentrations of U0126 were added to sea urchin embryos 12 minutes after fertilization; a comparison of the effect of 100 μM U0126 and its inactive analogue (U0124) showed U0124 to have no effect on NEB. 50 embryos were counted for each point. Embryos were scored for NEB using DIC optics.
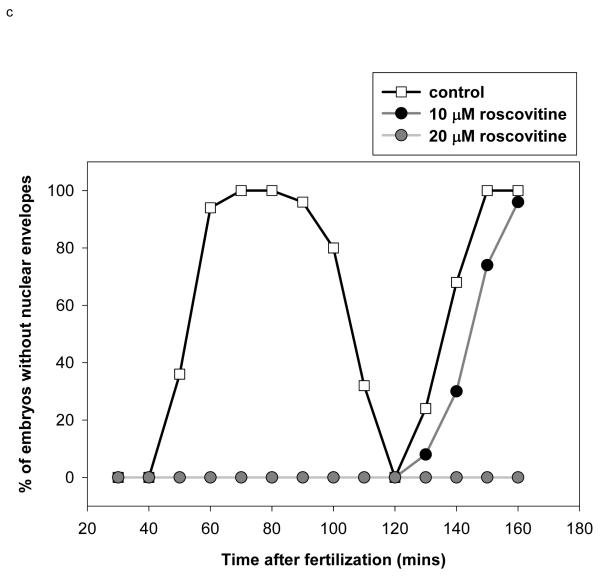
- Blocking cdk1/cyclin B kinase activity with 20 μM roscovitine prevents NEB Roscovitine was added to sea urchin embryos 35 minutes after fertilization (57). Embryos were scored for NEB using DIC optics. 50 embryos were counted for each time point. Note that 10 μM roscovitine substantially delays but does not block NEB.
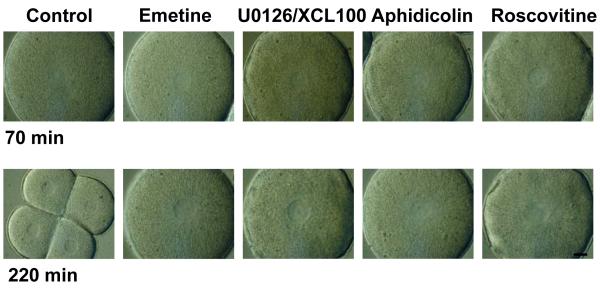
- Images of control and treated embryos with DIC optics at 70 and 220 minutes after fertilization. Blocking MAP kinase activity with U0126 or cdk1/cyclin B activity with roscovitin prevented NEB. Scale is indicated by the image which is 100 μm across.
U0126 is a compound that inhibits the upstream activator of ERK, MEK. U0126 acts as a non-competitive inhibitor for the MEK1 and MEK2 ATP binding sites (54,55). The inhibitor was added at 12 min, again to spare the fertilization-associated MAP kinase increase (we found that addition at later times before mitosis did not produce consistent inhibition). Three different concentrations of U0126 were tested (Fig. 1b): 25μM and 50μM concentrations delayed NEB in all embryos by 20 and 30 min, respectively. A concentration of 100μM blocked the cell cycle in 75 to 85% of the embryos and delayed NEB by 1 hour in the rest of them (data from 16 experiments). Treatment with 100μM U0124, the negative control for U0126, at 12 min did not inhibit NEB and the embryos proceeded through the cell cycle normally. These data with U0126 confirm the results obtained with the recombinant XCL100 protein and further demonstrate that MEK inhibition also prevents entry into mitosis.
As with MAP kinase there are two peaks of cdk1/cyclin B kinase activity following fertilization (18). The first is very small and may represent G1 activation of other cdks. The second increase begins around 30-40 minutes post-fertilisation and peaks during mitosis. The requirement for an increase in cdk1/cyclin B kinase activity in sea urchin embryos has already been shown using roscovitine, a specific cdk inhibitor (56,57) that reduces cdk1/cyclin B kinase activity by blocking the ATP binding pocket of cdk1. Roscovitine was added at 35 minutes post-fertilisation to spare the minor post-fertilization increase but inhibit the second increase in cdk1/cyclin B kinase activity (it was effective when added up to 60 min after fertilization (56 and our unpublished observations). 20 μM was required to maintain a block of NEB for a time period greater than one mitotic cycle in controls, when added at 35 minutes after fertilization (Fig. 1c). This is similar to a concentration previously reported to block NEB in the sea urchin species S. granularis (56).
Figure 1d shows images of embryos taken with DIC optics at 70 and 220 minutes after fertilization. At 70 minutes NEB has just occurred in control embryos. The mitotic asters can be seen at either end of the disappearing nuclear envelope. By 220 minutes the control embryos have undergone two cell divisions, whereas treated embryos are arrested at the 1-cell stage with their nuclear envelopes intact. These data show that activation of both MAP kinase and cdk1/cyclin B kinase is necessary for mitosis to occur.
U0126 and roscovitine inhibit the activity levels of their respective kinases independently
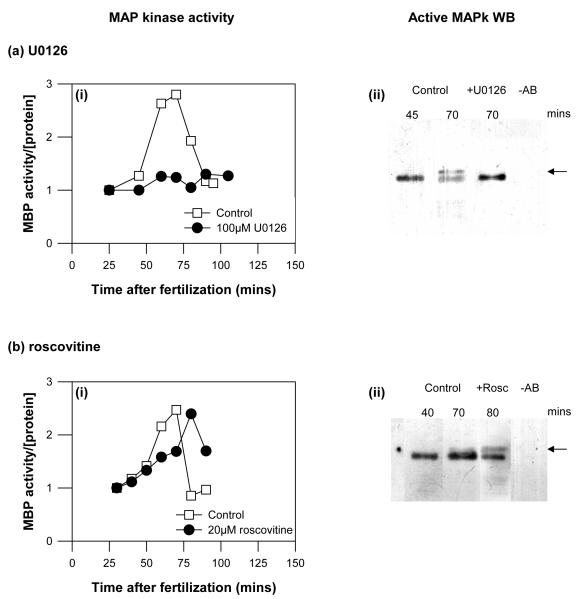
We used two independent methods to confirm that treating sea urchin embryos at 12 minutes post fertilisation inhibited the increase in MAP kinase activity that normally follows pronuclear fusion and peaks at NEB. We monitored MAP kinase activity in intact embryos using myelin basic protein (MBP) as a substrate (18). Figure 2ai shows MBP kinase activity in sea urchin embryo lysates, made at successive time points as the control embryos first entered then completed mitosis. U0126 completely abolished the increase in MAP kinase activity. Secondly, by carrying out immunoprecipitations and probing the blotted complexes with a monoclonal anti-dual phosphorylated MAP kinase antibody (see Methods), we confirmed that U0126 prevents the activation (mobility shift) that is observed during mitosis in control immunoprecipitations (Fig.2aii). The mobility shift correlates with activation of MAP kinase immediately after fertilization and during mitosis (18)
Fig. 2.
Mitotic kinase assays
a,b) (i) MAP kinase activities in cell lysates isolated at the time points shown from intact control and treated embryos undergoing the cell cycle were measured with MBP as a substrate. (ii) To confirm these results, we carried out immunoprecipitations, followed by Western blotting using two different anti-dual phosphorylated MAP kinase antibodies (see Methods). The active MAP kinase was precipitated from the same extracts used in the kinase assays. The time points for immunoprecipitated samples corresponded to the peak of MAP kinase activities obtained in total cell extracts. MAP kinase activation leads to the appearance of a gel-shifted band (arrows). MAP kinase activation and the gel-shift were prevented by 100 μM U0126 (a), but not by 20 μM roscovitine (b).
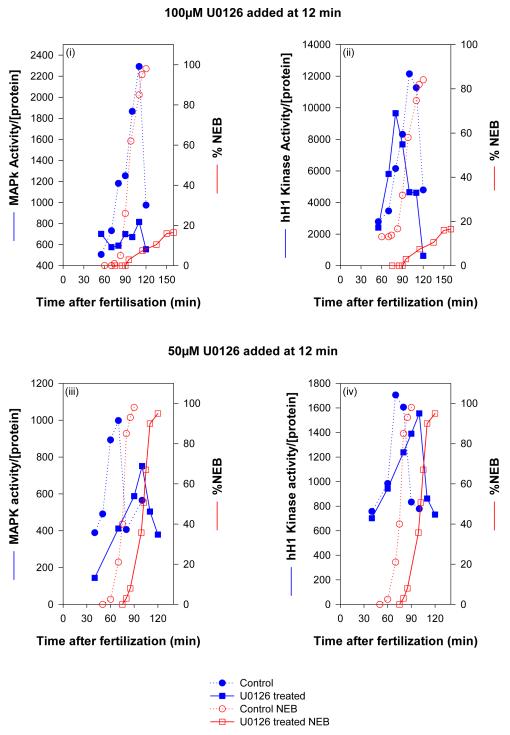
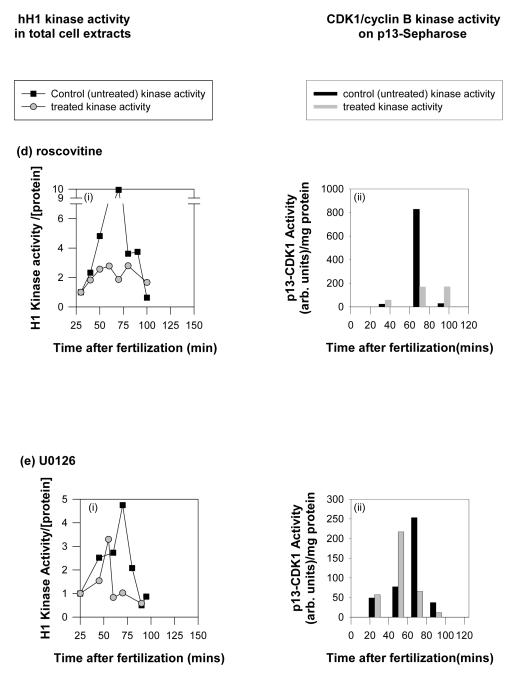
c) Simultaneous measurement of MAP kinase (blue lines and symbols), histone H1 kinase (blue lines and symbols) and NEB (red lines and symbols) in embryos treated with U0126. At a concentration of U0126 (100 μM) that completely inhibits NEB, MAP kinase activity was absent, while histone H1 kinase activity was slightly diminished and advanced slightly in timing. At a concentration of U0126 that substantially delayed NEB, both MAP kinase activation and histone H1 kinase activation were similarly delayed. d,e) Cdk1/cyclin B kinase activities in cell extracts from control and treated embryos undergoing cell cycle were measured using hH1 as a substrate. Additionally, cdk1/cyclin B kinase was isolated from the same extracts using p13suc1-Sepharose beads and assayed for histone H1 kinase activity. 20 μM roscovitine effectively abolished cdk1/cyclin B kinase activity as measured by both methods, while 100 μM U0126 led to a small decrease and an advance in cdk1/cyclin B kinase activation.
U0126 is known to inhibit cdk1/cyclinB activity in some circumstances. It was thus important to ensure that treating sea urchin embryos with U0126 (at 12 minutes post-fertilisation) does not inhibit cdk1/cyclin B kinase activation. Histone H1 was used as a substrate in embryo extracts (Fig. 2bi) and after isolation of cdk1/cyclin B kinase using p13suc1-sepharose binding (Fig. 2bii). Both methods show that U0126 does not inhibit cdk1/cyclin B kinase activation, though interestingly, the timing of the peak in activity does appear to precede slightly that observed in control embryos (Figure 2c,e; we did not investigate this further). The maximal activity in treated embryos was slightly and significantly lower (86.8±1.1% mean ± s.e.m. of control activity from three independent experiments).
We also investigated the effects of lower concentrations of U0126 that did not block but delayed NEB (Fig. 2c). MAP kinase and cdk1/cyclin B activities were measured in time-point lysates from intact embryos treated with 50μM U0126 at 12 min after fertilisation (Fig.2ci-ii). NEB was delayed by 30 min: control embryos showed 98% NEB at 90 min and inhibited embryos showed 95% NEB at 120 min. MAP kinase activation was not abolished. It occurred, but with 30 min delay and with a reduced activity. Interestingly, cdk1/cyclin B was activated to the same extent as in controls, but the peak activity was also delayed by 30 min, suggesting that MAP kinase is upstream of cdk1/cyclinB in this signaling pathway.
These results demonstrate that NEB does not occur if MAP kinase activity is abolished and there is a strong indication that the timing of cdk1/cyclin B activation at mitosis in sea urchin embryos is MAP kinase-dependent (see discussion).
Using the methods described above we confirmed that roscovitine inhibited cdk1/cyclin B kinase activity in intact embryos (Fig. 2di-ii), and that cdk1/cyclin B kinase activity was unaffected by treatment with 100 μM U0126 (Fig. 2ei-ii).
The NEB-associated [Ca2+]i transient requires MAP kinase activation
We have previously described small (50-100 nM) calcium transients that occur just before NEB and anaphase onset (29) and that are absolutely required for mitosis entry (27) and anaphase onset (28) to occur. Here we determine whether these transients depend on activation of the mitotic kinases. Embryos were injected with the calcium indicator dye Fura2 dextran between 25-35 minutes after fertilization (27,44).
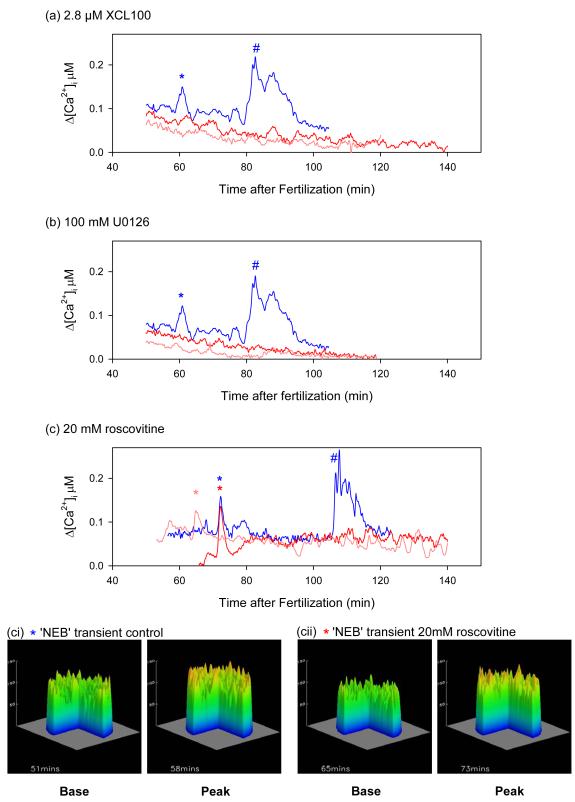
Microinjecting the recombinant MAP kinase inhibitor XCL100 (2.8 μM final concentration at 12-20 min after fertilization) completely suppressed the mitotic Ca2+ transients (Fig. 3a). Treatment of sea urchin embryos with 100 μM U0126 12 minutes after fertilization also suppressed the mitotic Ca2+ signals in 6/7 embryos (Fig. 3b). The one embryo that did show a transient also showed a 37 minute delay to NEB, with the Ca2+ increase occurring just before NEB (not shown).
Fig. 3.
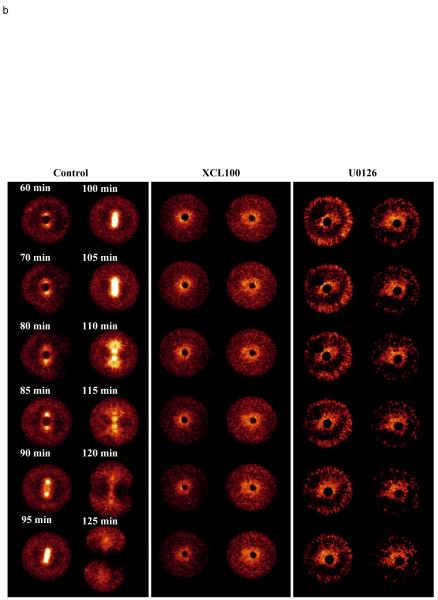
The transient [Ca2+]i increase associated with NEB is inhibited by XCL100 and U0126. Ratiometric [Ca2+]i measurements in control sea urchin embryos and in embryos treated with (a) 2.8 μM XCL100 12-20 minutes (b) 100 μM U0126 12 minutes (c) 20 μM roscovitine 35 minutes. Controls (blue traces) show two peaks of calcium just before NEB (*) and at anaphase (#). Both these peaks are absent in embryos treated with U0126 or XCL100 (a,b). A peak with the same magnitude and timing as the NEB peak is seen in roscovitine embryos (*), but the anaphase peak (#) is absent. Embryos were injected with Fura2 dextran (10 μM) 25-35 minutes after fertilization. Representative experiments are shown from experiments on 4-7 eggs in each case. (ci-cii) topographical representations of confocal images taken at the base and peak of the Ca2+ increase (* in (c)) for both control, and roscovitine treated embryos, showing spatial information. The embryo is 100 μm in diameter.
We then tested the effects of inhibiting cdk1/cyclin B kinase activity with 20 μM roscovitine (added at 35 min after fertilization) on the mitotic Ca2+ transients. In 5/7 embryos a Ca2+ signal comparable to those seen in parallel control embryos just before entry into mitosis was observed, while the anaphase calcium signal (28,29) was abolished (Fig. 3c). In five roscovitine-treated embryos the Ca2+ transient (magnitude: 56.6 ±11.7 nM; mean and s.e.m) occurred 67.1 ±1.8 minutes after fertilization while in five control embryos the transient (magnitude: 43.3 ±7.5 nM) was seen 70.0 ±4.5 minutes after fertilization (Fig. 3c). Neither of these parameters is significantly different at the 20% level between groups. We also confirmed that the Ca2+ transient in roscovitine-treated embryos had the same spatial distribution to that observed in control embryos. A confocal imaging system and a calcium green dextran/rhodamine dextran ratiometric method (28) was used to monitor the Ca2+ transient in embryos treated with roscovitine. Figure 3ci,ii show a topographical representation of the Ca2+ levels at the base and peak of the Ca2+ rise. In cdk-inhibited embryos (Fig. 3cii) as in control embryos (Fig. 3ci) the Ca2+ increase is global. These data demonstrate that roscovitine does not interfere with the NEB Ca2+ signalling mechanism either through blocking cdk1 or in its own right. We confirmed this by measuring the Ca2+ transients induced by InsP3 microinjection in the presence of 20 μM roscovitine. In six roscovitine-treated embryos, the magnitude and rise times in response to 50 μM InsP3 were comparable to controls (magnitudes: 1.3 ± 0.1 μM, 1.3 ± 0.2 μM; t50%: 17.3 ± 0.8 s, 17.33 ± 4.0 s, respectively). NEB did not take place after microinjection of InsP3 (see Table 1), as predicted if cdk1/cyclin B kinase activation is downstream of the calcium signal.
Table 1.
Number of embryos that underwent NEB in response to either InsP3 or Ca2+-EGTA buffer in the presence of various inhibitors of MAP kinase and cdk1.
Embryos were either injected with 10 mM InsP3 or 1μM free Ca2+ (pipette concentration of Ca/EGTA buffer 50 – 80mM) after being treated with 100 μM U0126, 60 μM aphidicolin, 20 μM roscovitine or 150 μM emetine. NEB was scored using DIC optics.
| Number of embryos that underwent NEB in response to: | ||
|---|---|---|
| Treatment | InsP3 | Ca2+-EGTA buffer |
| Control | 5/5 | 5/5 |
| U0126 | 0/10 | 0/18 |
| Roscovitine | 0/13 | 0/15 |
These experiments indicate that the mitosis entry Ca2+ signal that occurs just before NEB is dependent on activation of MAP kinase, but does not require activation of cdk1/cyclin B kinase.
Neither InsP3 or Ca2+-EGTA buffer injections rescue blocks to NEB
Injection of InsP3 or Ca2+-EGTA buffers within 20 minutes of normal mitosis entry can trigger premature NEB in sea urchin embryos (39), indicating that a Ca2+ increase alone is sufficient to trigger NEB as mitosis approaches. We determined whether InsP3 or Ca2+-EGTA buffer injection triggered NEB when mitosis entry is inhibited. Table 1 shows that under conditions where MAP kinase activity is inhibited, neither InsP3-(10 μM final cytosolic concentration) nor Ca2+ buffer-injection (Ca2+-EGTA buffer containing 1 μM free calcium as previously described (39)), results in NEB. Increasing calcium did not overcome the inhibition of NEB caused by abrogating cdk1/cyclin B kinase activity with roscovitine (Table 1),as expected from its position downstream of calcium in the signaling pathway. This result is consistent with the finding that a normal NEB-related Ca2+ increase was observed in roscovitine-treated embryos (Fig. 3c) and that InsP3-triggered Ca2+ release was unaffected (see above). These data indicate that for mitosis entry to occur additional cell cycle events besides the calcium increase require activation of MAP kinase.
MAP kinase inhibition affects DNA condensation, but not sperm DNA decondensation or DNA synthesis
At the time at which MAP kinase activity begins to rise again to its second, mitotic peak (the time at which we added MAP kinase inhibitors), several cell cycle events that precede entry into mitosis had yet to occur. It was instructive to observe which additional cell cycle events were dependent on the pre-mitosis MAP kinase peak.
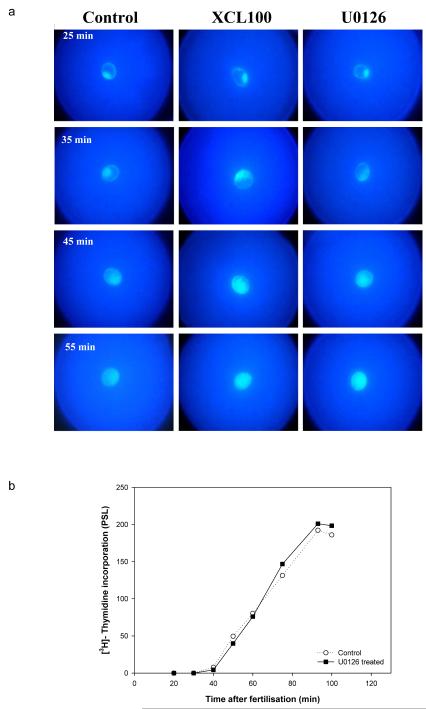
At fertilisation the sperm enters the egg and the male nucleus, containing highly condensed DNA, is released into the female cytoplasm. A process of pronuclear migration begins. Microtubules elongate to form the female aster and bring the female nucleus to the centre of the egg (58). The male nucleus migrates along the female astral microtubules to reach the egg pronucleus about 20 minutes after fertilisation. At this stage the two nuclei fuse. The nucleus of the egg contains the homogeneous, decondensed female chromatin (Fig. 4a, Control, 25 min). As soon as the highly packed sperm chromatin merges with the female nucleus, it undergoes rapid decondensation: at 45 to 50 min after fertilisation the male chromosomes in controls are completely uncoiled and DNA replication has already begun. DNA staining with Hoechst 33342 dye revealed that decondensation of sperm chromatin was not blocked by either XCL100 or U0126 (Fig. 4a).
Fig. 4.
- Chromatin stained with the DNA dye H33342. Sperm chromatin is relatively condensed within the zygote nucleus immediately after fusion of male and female pronuclei. It then gradually disperses within the zygote nucleus. This process is unaffected by treatment with XCL100 or U0126. The embryo is 100 μm in diameter.
- DNA synthesis measured by incorporation of [3H]-thymidine into DNA is unaffected by 100 μM U0126.
Figure 4b shows that blocking MAP kinase activation with U0126 does not interfere with DNA synthesis. Both treated and untreated cells incorporate thymidine to the same extent (Fig. 4b, Control and U0126 at 12 min). This experiment shows that DNA replication was initiated and DNA synthesis occurred when the inhibitor was added as the second increase in MAP kinase activity began and, indeed, before the onset of DNA synthesis itself. These early cell cycle events are thus independent of mid-cycle MAP kinase activation. We have previously determined that they are dependent on the early rapid and transient increase in MAP kinase activity immediately after fertilization (50).
MAP kinase inhibition prevents NEB and chromatin condensation and affects bipolar spindle assembly
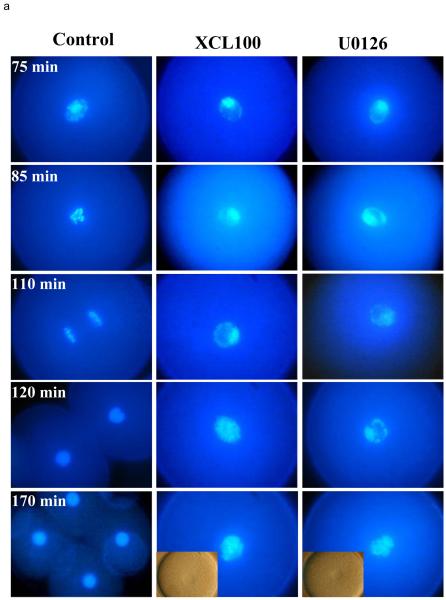
We then looked at the events of mitosis. Control embryos underwent NEB at 80 min after fertilisation (Fig. 5), followed by condensation of mitotic chromosomes, metaphase at 100 min and anaphase at 110 min. By 120 min after fertilization, cytokinesis and reformation of the two daughter nuclei was completed. Both the MAP kinase phosphatase and the MEK inhibitor caused an identical cell cycle block. XCL100-microinjected and U0126-treated embryos were arrested with partly and improperly condensed DNA and also with intact nuclear envelopes as long as 170 min after fertilisation. By this time the control cells were already at the 4-cells stage (Fig. 5a).
Fig. 5.
- Chromatin stained with the DNA dye H 33342. While control embryos are undergoing mitosis with chromatin condensation and chromosome separation, XCL100 and U0126 treated embryos show only partial chromatin condensation. One cell embryo is 100 μm in diameter.
- Microtubule organization at the time of mitosis revealed by microinjection of rhodamine tubulin and confocal microscopy. XCL100 and U0126 treated embryos fail to form a bipolar spindle. One cell embryo is 100 μm in diameter.
- Three signals contribute independently to mitosis entry. The three signals may interact through positive and negative feedback to form a mitotic switch. The advance of cdk1/cyclin B activation when MAP kinase activity is inhibited implies an inhibitory role for ERK1 on cdk1, relieved by the calcium transient generated by MAP kinase activity.
In addition, inhibiting MAP kinase with XCL100 and U0126 appeared under DIC optics to prevent the formation of a bipolar spindle. ERK1 has been previously shown to be localised on the mitotic apparatus in sea urchin embryos (18). To confirm an effect on spindle function, rhodamine-labelled tubulin (50μg/ml) was microinjected 25 min post-fertilisation in control and in embryos treated with 100μM U0126. A third group of embryos was microinjected with XCL100 phosphatase (100μg/ml) mixed with rhodamine-labelled tubulin (Fig. 5b). Control embryos duplicated their centrosomes, which were found at 60 min at both sides of the nucleus (see Control panel). These embryos also formed mitotic spindles at 90 min. Neither XCL100- nor U0126-inhibited embryos were able to form bipolar spindles. Tubulin was found disorganised around the nucleus. MEK/ERK1 activity is therefore also needed for the proper organisation of the microtubules and formation of functional spindle before entry into mitosis.
DISCUSSION
We have reported that an ERK1 MAP kinase activity increases during mitosis in sea urchin embryos (18). Here we show that blocking MAP kinase activation either directly using the recombinant MAP kinase-specific phosphatase XCL100, or indirectly by inhibiting the upstream activator MEK using U0126 prevents mitosis entry. These manoeuvres also abolish the Ca2+ transient that precedes and triggers NEB in the first cell cycle of sea urchin embryos (27). Inhibition of MAP kinase activity also leads to incomplete chromatin condensation and the absence of a bipolar spindle.
MAP kinase activation and cdk1/cyclinB activity
A significant finding is that MAP kinase activation an cdk1/cyclinB activation can be separately inhibited in early sea urchin embryos and that both kinases are independently required for mitosis entry. Two inhibitors with quite distinct modes of action on the MAP kinase pathway produce identical inhibitory phenotypes while not affecting activation of cdk1/cyclin B activity. The cdk inhibitor roscovitine blocks cdk/cyclinB activation without affecting MAP kinase. Thus, the effects that we report on calcium signaling, NEB, chromatin condensation and spindle assembly can be ascribed to MAP kinase, not direct or indirect inhibition of cdk1/cyclinB (56).
We also found, however, at concentrations of MAP kinase inhibitor that delayed rather than blocked NEB, that activation of cdk1/cyclinB was similarly delayed. This observation suggests a complex interaction between MAP kinase and cdk1/cyclinB that we are now investigating. It implies the possibility that cdk1/cyclinB activation is controlled by MAP kinase (59). It is notable that the transition from delay with activation to complete block occurred over a very narrow concentration range of both MAP kinase inhibitors; this and the observation that the MAP kinase activity was sensitive to the MEK inhibitor only at the very onset of mid-cycle MAP kinase activity suggests strong positive feedback during MAP kinase activation (59).
MAP kinase, not cdk1/cyclinB controls the NEB-associated calcium signal
An a priori model of the interaction between cell cycle calcium signals and the cell cycle kinase/phosphatase regulatory network suggested that the NEB calcium signal might be activated by a threshold level of cdk1/cyclinB activity; in this model the role of the calcium signal was to amplify the rise of cdk1/cyclinB activity by feed-forward onto cdc-25 (40). Our data are consistent with the latter postulate, but not the former. Since the NEB calcium signal is blocked by inhibition of the MAP kinase pathway, but not by a cdk inhibitor, it is clear that cdk plays no direct part in generating the signal. The calcium signal is instead under the control of MAP kinase. On the other hand, our observation that microinjection of calcium or InsP3 cannot induce NEB in the presence of a cdk inhibitor confirms the supposition that cdk1/cyclinB acts downstream of the calcium signal. The small reduction of cdk1/cyclin B kinase activation in the absence of MAP kinase activation is consistent with the idea that calcium contributes to cdk1/cyclin B activation (37,40).
The link between MAP kinase activation and the Ca2+ signal is not known. One obvious possibility is that MAP kinase directly or indirectly activates PLCγ, whose activity is known to be required for NEB in sea urchin embryos (30). The PLCγ would in turn produce the transient rise in InsP3 production that is observed at mitosis entry (29).
The requirement for MAP kinase at mitosis entry clearly extends beyond triggering the Ca2+ release mechanism, since injection of InsP3 or Ca2+-EGTA buffers was unable to release the NEB block induced by MAP kinase inhibition. We conclude that all three events are required for entry into mitosis: MAP kinase activation, the calcium signal and cdk1/cyclinB activation.
Condensation of mitotic chromosomes and formation of a bipolar spindle are blocked if the MEK/ERK pathway is inactive
MAP kinase immunoreactivity is concentrated in the nucleus just before NEB (18). We show here that in the absence of high MAP kinase activity chromosome clustering in the intact nucleus eventually occurs, but condensed (super-coiled) chromosomes are not formed.
Two key processes during chromatin condensation are the elimination of catenation of the sister DNA strands by topoisomerases and the packaging of the strands into nucleosomes and thus chromatids by histones (60); for reviews: (61,62). Topoisomerases become highly phosphorylated during G2 and M phases and histone H3 is phosphorylated during the final stages of chromatin condensation (63), a phosphorylation essential for complete chromatin condensation (64). It has been shown that histone H3 is phosphorylated by the downstream activation of the MAP kinase pathway, p90Rsk (65,66). It has also been found that topoisomerase IIα is activated by MAP kinase (in this case an ERK2: (67). These observations indicate that MAP kinase plays an important part in the molecular mechanisms of chromatin condensation.
The bipolarity of the mitotic spindle in sea urchins is fully developed at the time of time of NEB, with spindle poles well separated. We find here that in the absence of active MAP kinase, microtubules become disorganised around the intact nucleus. This is not a simple consequence of inhibition of NEB, since embryos treated with the DNA synthesis inhibitor aphidicolin form a functional bipolar spindle though failing to undergo NEB (68,69). MAP kinase inhibitors have been shown to affect microtubule organization. Potentiation of a taxol-induced increase in tubulin polymerization has been reported in vitro, along with the production of microtubule bundles in vivo, in response to inhibition of MAP kinase activity (70). Inhibiting activation of MEK in Xenopus extracts through immunodepletion or PD98059 (41) also induces the formation of large asters. Adding active MAP kinase to Xenopus egg extracts during interphase causes microtubule shortening and organisation that resembles mitotic arrays (71). Two proteins involved in microtubule depolymerisation, tau, and stathmin/Op18, are both phosphorylated during mitosis at sites that can be targeted by ERK (72-78).
Dual roles for MAP kinase activation during the first cell cycle of sea urchin embryos
We have reported that fertilization results in two peaks of MAP kinase (ERK) activity, one immediately after fertilization and the other at mitosis (18). The first peak of activity has been shown to be responsible for the initiation of DNA synthesis and for decondensation of the sperm chromatin (50), in contrast to earlier reports that suggested that a fall in MAP kinase was involved (79,80). We show here that the second increase in activity co-ordinates events necessary for entry into mitosis.
CONCLUSION
This study shows that MAP kinase plays a key role in triggering mitosis entry in early sea urchin embryos by triggering the appropriate calcium signals and by its promotion of spindle assembly and chromatin condensation. Building on previous work (27) it also shows that the calcium signal, ERK1 activation and cdk1/cyclin B kinase activation are signals that are independently required for mitosis entry. Our data also suggest that these three signals are interdependent (Figure 5c); these interactions bear further investigation.
ACKNOWLEDGEMENTS
This work was supported by the Wellcome Trust. We would like to thank L. Meijer for the kind gift of roscovitine and X. Wang for generously providing the XCL100 construct. We are also grateful to M. Sinclair for assistance with protein purification and Michael Aitchison for preparing the figures.
Footnotes
Abbreviations used in the text MPF maturation promoting factor, NEB nuclear envelope breakdown, [Ca2+]i intracellular calcium concentration, InsP3 inositol 1,4,5-trisphosphate, CaM calmodulin, ASW artificial sea water, XCL100 Xenopus MAP kinase phosphatase-1.
REFERENCES
- 1.Murray AW, Kirschner MW. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- 2.Dunphy WG, Brizuela L, Beach D, Newport J. Cell. 1988;54:423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- 3.Labbe JC, Capony J-P, Caput D, Cavadore J-C, Derancourt J, Kaghad M, Lelias JM, Picard A, Doree M. EMBO J. 1989;8:3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL. Cell. 1990;60:487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- 5.Lew DJ, Kornbluth S. Curr. Opin. Cell Biol. 1996;8:795–804. doi: 10.1016/s0955-0674(96)80080-9. [DOI] [PubMed] [Google Scholar]
- 6.Docommun B, Brambilla P, Felix MA, F. NR, Jr., Karsenti E, Draetta G. EMBO J. 1991;11:3311–3319. doi: 10.1002/j.1460-2075.1991.tb04895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon MJ, Lee T, Kirschner MW. Mol. Biol. Cell. 1992;1:13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunphy WG, Kumagai A. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- 9.Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- 10.Dunphy WG. Trends Cell Biol. 1994;4:202–207. doi: 10.1016/0962-8924(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 11.Takizawa CG, Morgan DO. 2000;12 doi: 10.1016/s0955-0674(00)00149-6. [DOI] [PubMed] [Google Scholar]
- 12.Hagting A, Karlsson C, Clute P, Jackman M, Pines J. EMBO J. 1998;17:4127–4138. doi: 10.1093/emboj/17.14.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newport J, Spann T. Cell. 1987;48:219–230. doi: 10.1016/0092-8674(87)90425-9. [DOI] [PubMed] [Google Scholar]
- 14.Peter M, Nakagawa J, Doree M, Labbe JC, Nigg EA. Cell. 1990;61:591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- 15.Peter M, Heitlinger E, Haner M, Aebi U, Nigg EA. EMBO J. 1991;10:1535–1544. doi: 10.1002/j.1460-2075.1991.tb07673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nigg EA. Curr. Opin. Cell Biol. 1992;4:105–109. doi: 10.1016/0955-0674(92)90066-l. [DOI] [PubMed] [Google Scholar]
- 17.Chiri S, De Nadai C, Ciapa B. J. Cell Sci. 1998;111:2519–2527. doi: 10.1242/jcs.111.17.2519. [DOI] [PubMed] [Google Scholar]
- 18.Philipova R, Whitaker M. J. Cell Sci. 1998;111:2497–2505. doi: 10.1242/jcs.111.17.2497. [DOI] [PubMed] [Google Scholar]
- 19.Guadagno TM, Ferrell JE. Science. 1998;282:1312–1315. doi: 10.1126/science.282.5392.1312. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro PA, Vaisberg E, Hunt AJ, Tolwinski NS, Whaken AM, McIntosh JR, Ahn NG. J. Cell Sci. 1998 doi: 10.1083/jcb.142.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrell JE, Machleder EM. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- 22.Yue J, James E. Ferrell. Current biology - CB. 2004;14:1581–1586. doi: 10.1016/j.cub.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 23.Whitaker M, Patel R. Development. 1990;108:525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]
- 24.Hepler PK. Cell Calcium. 1994;16:322–330. doi: 10.1016/0143-4160(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 25.Berridge MJ. Bioessays. 1995;17:491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- 26.Santella L. Biochemical and biophysical research communications. 1998;244:317–324. doi: 10.1006/bbrc.1998.8086. [DOI] [PubMed] [Google Scholar]
- 27.Wilding M, Wright EM, Patel R, Ellis-Davies G, Whitaker M. J. Cell Biol. 1996;135:191–199. doi: 10.1083/jcb.135.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groigno L, Whitaker M. Cell. 1998;92:193–204. doi: 10.1016/s0092-8674(00)80914-9. [DOI] [PubMed] [Google Scholar]
- 29.Ciapa B, Pesando D, Wilding M, Whitaker M. Nature. 1994;368:875–878. doi: 10.1038/368875a0. [DOI] [PubMed] [Google Scholar]
- 30.Shearer J, De Nadai C, Emily-Fenouil F, Gache C, Whitaker M, Ciapa B. Development. 1999;126:2273–2284. doi: 10.1242/dev.126.10.2273. [DOI] [PubMed] [Google Scholar]
- 31.Tombes RM, Borisy GG. J. Cell Biol. 1989;109:627–636. doi: 10.1083/jcb.109.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tombes RM, Simerly C, Borisy GG, Schatten G. J. Cell Biol. 1992;117:799–811. doi: 10.1083/jcb.117.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitaker M, Larman M. Seminars in Cell and Developmental Biology. 2001;12:53–58. doi: 10.1006/scdb.2000.0217. [DOI] [PubMed] [Google Scholar]
- 34.Steinhardt RA, Alderton J. Nature. 1988;332:364–366. doi: 10.1038/332364a0. [DOI] [PubMed] [Google Scholar]
- 35.Kao JPY, Alderton J, Tsien RY, Steinhardt RA. 1990;111 doi: 10.1083/jcb.111.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kono T, Jones KT, Bos-Mikich A, Whittingham DG, Carroll J. J. Cell Biol. 1996;132:915–923. doi: 10.1083/jcb.132.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel R, Holt M, Philipova R, Moss S, Hidaka H, Whitaker M. J. Biol. Chem. 1999;274:7958–7968. doi: 10.1074/jbc.274.12.7958. [DOI] [PubMed] [Google Scholar]
- 38.Baitinger C, Alderton J, Poenie M, Schulman H, Steinhardt RA. J. Cell Biol. 1990;111:1763–1773. doi: 10.1083/jcb.111.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Twigg J, Patel R, Whitaker M. Nature. 1988;332:366–369. doi: 10.1038/332366a0. [DOI] [PubMed] [Google Scholar]
- 40.Swanson CA, Arkin AP, Ross J. Proc. Natl. Acad. Sci. USA. 1997;94:1194–1199. doi: 10.1073/pnas.94.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guadagno TM, Ferrell JJE. Science. 1998;282:1312–1315. doi: 10.1126/science.282.5392.1312. [DOI] [PubMed] [Google Scholar]
- 42.Becchetti A, Whitaker M. Development. 1997;124:1099–1107. doi: 10.1242/dev.124.6.1099. [DOI] [PubMed] [Google Scholar]
- 43.Wang XM, Zhai Y, Ferrel JE., Jr. J. Cell Biol. 1997;137 doi: 10.1083/jcb.137.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swann K, Whitaker M. J. Cell. Biol. 1986;103:2333–2342. doi: 10.1083/jcb.103.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edgecombe M, Patel R, Whitaker M. EMBO J. 1991;10:3769–3775. doi: 10.1002/j.1460-2075.1991.tb04946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrell JE, Wu M, Gerhaart JC, Martin GS. Mol. Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamemoto H, Kadowaki T, Tobe K, Ueki K, Izumi T, Chatani Y, Kohno M, Kasuga M, Yazaki Y, Akanuma Y. J. Biol. Chem. 1992;267:20293–20297. [PubMed] [Google Scholar]
- 48.Verlhac MH, Kubiak JZ, Clarke HJ, Maro B. Development. 1994;120:1017–1025. doi: 10.1242/dev.120.4.1017. [DOI] [PubMed] [Google Scholar]
- 49.Edelmann W, Cohen PE, Kane M, Lau K, Morrow B, Bennett S, Umar A, Kunkel T, Cattoretti G, Chaganti R, Pollard JW, Kolodner RD, Kucherlapati R. Cell. 1996;85:1125–1134. doi: 10.1016/s0092-8674(00)81312-4. [DOI] [PubMed] [Google Scholar]
- 50.Philipova R, Kisielewska J, Lu P, Larman M, Huang J-Y, Whitaker MJ. Development. 2005;132:579–589. doi: 10.1242/dev.01607. [DOI] [PubMed] [Google Scholar]
- 51.Alessi DR, Smythe C, Keyse SM. Oncogene. 1993;8:2015–2020. [PubMed] [Google Scholar]
- 52.Sun H, Charles CH, Lau LF, Tonks NK. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 53.Lewis T, Groom LA, Sneddon AA, Smythe C, Keyse SM. J. Cell Sci. 1995;108:2885–2896. doi: 10.1242/jcs.108.8.2885. [DOI] [PubMed] [Google Scholar]
- 54.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Vandyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. J. Biol. Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 55.DeSilva DR, Jones EA, Favata MF, Jaffe BD, Magolda RL, Trzaskos JM, Scherle PA. J. Immunol. 1998;160:4175–4181. [PubMed] [Google Scholar]
- 56.Meijer L, Borgne A, Mulner O, Chong JPJ, Blow JJ, Inagaki N, Inagaki M, Delcors J-G, Moulinoux J-P. Cur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 57.Alessi F, Quarta S, Sario M, Riva F, Rossi L, Stivala LA, Scovassi AI, Meijer L, Prosperi E. Exp. Cell Res. 1998;245:8–18. doi: 10.1006/excr.1998.4216. [DOI] [PubMed] [Google Scholar]
- 58.Schatten G, Schatten H, Bestor TH, Balczon R. J. Cell. Biol. 1982;94:455–465. doi: 10.1083/jcb.94.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiong W, Ferrell James E. Nature. 2003;426:460–465. doi: 10.1038/nature02089. [DOI] [PubMed] [Google Scholar]
- 60.Downes CS, Clarke DJ, Mullinger AM, Gimernez-Abian JF, Creighton AM, Johnson RT. Nature. 1994;372:467–470. doi: 10.1038/372467a0. [DOI] [PubMed] [Google Scholar]
- 61.Koshland D, Strunnikov A. Annu. Rev. Cell Dev. Biol. 1996;12:305–333. doi: 10.1146/annurev.cellbio.12.1.305. [DOI] [PubMed] [Google Scholar]
- 62.Moudrianakis EN, Arents G. Cold Spring Harb. Symp. Quant. Biol. 1993;58:273–279. doi: 10.1101/sqb.1993.058.01.032. [DOI] [PubMed] [Google Scholar]
- 63.Bradbury EM. Bioessays. 1992;14:9–16. doi: 10.1002/bies.950140103. [DOI] [PubMed] [Google Scholar]
- 64.Guo XW, Th’ng JP, Swank RA, Anderson HJ, Tudan C, Bradbury EM, Roberge M. EMBO J. 1995;14:976–985. doi: 10.1002/j.1460-2075.1995.tb07078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sassone-Corsi P, Mizzen CA, Cheung P, Crosio C, Monaco L, Jacquot S, Hanauer A, Allis CD. Science. 1999;285:886–891. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- 66.Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Mol. Cell. Biol. 2000;5:905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- 67.Shapiro PS, Whalen AM, Tolwinski NS, Wilsbacher J, Froelich-Ammon SJ, Garcia M, Osheroff N, Ahn NG. Mol. Biol. Cell. 1999;19:3551–3560. doi: 10.1128/mcb.19.5.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geneviere-Garrigues A-M, Barakat A, Doree M, Moreau J-L, Picard A. J. Cell Sci. 1995;108:2693–2703. doi: 10.1242/jcs.108.7.2693. [DOI] [PubMed] [Google Scholar]
- 69.Nishioka D, Balczon R, Schatten G. Cell. Biol. Int. Rep. 1984;8:337–346. doi: 10.1016/0309-1651(84)90161-9. [DOI] [PubMed] [Google Scholar]
- 70.Olsen MK, Reszka AA, Abraham I. J. Cell Biol. 1998;176:525–536. doi: 10.1002/(SICI)1097-4652(199809)176:3<525::AID-JCP9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 71.Gotoh Y, Nishida E, Matsuda S, Shiina N, Kosako H, Shiokawa K, Akiyama T, Ohta K, Sakai H. Nature. 1991;349:251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- 72.Shiina N, Moriguchi T, Ohta K, Gotoh Y, Nishida E. EMBO J. 1992;11:3977–3984. doi: 10.1002/j.1460-2075.1992.tb05491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoshi M, Ohta K, Gotoh Y, Mori A, Murofushi H, Sakai H, Nishida E. Eur. J. Biochem. 1992;203:43–52. doi: 10.1111/j.1432-1033.1992.tb19825.x. [DOI] [PubMed] [Google Scholar]
- 74.Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Mol. Biol. Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marklund U, Brattsand G, Osterman O, Ohlsson PI, Gullberg M. j. Biol. Chem. 1993;268:25671–25680. [PubMed] [Google Scholar]
- 76.Brattsand G, Marklund U, Nylander K, Roos G, Gullberg M. Eur. J. Biochem. 1994;220:359–368. doi: 10.1111/j.1432-1033.1994.tb18632.x. [DOI] [PubMed] [Google Scholar]
- 77.Larsson N, Melander H, Marklund U, Osterman O, Gullberg M. 1995;270:14174–14183. doi: 10.1074/jbc.270.23.14175. [DOI] [PubMed] [Google Scholar]
- 78.Belmont LD, Mitchison TJ. 1996;84:623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- 79.Kumano M, Carroll DJ, Denu JM, Foltz KR. Developmental biology. 2001;236:244–257. doi: 10.1006/dbio.2001.0328. [DOI] [PubMed] [Google Scholar]
- 80.Carroll DJ, Albay DT, Hoang KM, O’Neill FJ, Kumano M, Foltz KR. Developmental biology. 2000;217:179–191. doi: 10.1006/dbio.1999.9526. [DOI] [PubMed] [Google Scholar]