Abstract
The extracellular matrix (ECM) distinctly modulates membrane type 1-matrix metalloproteinase (MT1-MMP) in human endothelial cells (ECs). Herein, ECM-dependent RhoA activation is shown to regulate MT1-MMP localization and activity as well as clathrin-independent internalization in confluent ECs. In this regard, caveolae are revealed as the major MT1-MMP endocytic pathway in human ECs. Thus, MT1-MMP is present at caveolae with caveolin-1 and both proteins together with αvβ3 integrin colocalize at endothelial motility-associated extensions. Remarkably, caveolae traffic is required for proper MT1-MMP localization, activity, and function in migratory ECs as demonstrated by both treatment with caveolae-disrupting agents or selective targeting caveolin-1 expression by interference RNA. Thus, caveolae-mediated traffic constitutes a novel mechanism for MT1-MMP regulation in ECs during angiogenesis.
INTRODUCTION
Membrane-type 1 matrix metalloproteinase (MT1-MMP) is a matrix metalloproteinase (MMP) known to degrade various components of the extracellular matrix (ECM) and to process membrane proteins such as CD44, αv integrins, or tissue transglutaminase (Werb, 1997; Kajita et al., 2001; McCawley and Matrisian, 2001; Ratnikov et al., 2002). MT1-MMP can be found at either specialized membrane protrusions such as invadopodia and lamellipodia for pericellular proteolysis or at cell-cell contacts, and it is important for angiogenesis and tumor invasion (Sato et al., 1994; Nabeshima et al., 2000; Gálvez et al., 2001, 2002; Seiki, 2002).
ECM can regulate the localization and internalization of MT1-MMP, although the underlying mechanism is unknown yet (Gálvez et al., 2002). ECM provides an array of ligands to interact with multiple cell surface integrins, which generate biochemical phosphorylation cascades involving mediators such as FAK, Raf, Ras, mitogen-activated protein kinases (extracellular signal-regulated kinases), or Rho GTPases (Fukata et al., 1999; Martin et al., 2002). This induced signaling modulates cell shape, migration, and invasion by acting on distinct effector proteins. Thus, Rho GTPases are involved in regulating integrin-mediated cell-substratum adhesion, cadherin-mediated cell-cell adhesion, actin cytoskeleton and cell motility, and endocytic traffic (Hall, 1998; Ellis and Mellor, 2000; Fukata and Kaibuchi, 2001; Etienne-Manneville and Hall, 2002).
Subcellular compartmentalization and traffic of MT1-MMP seem to be important for its function because MT1-MMP internalization via clathrin-coated pits regulates its activity (Jiang et al., 2001; Uekita et al., 2001). MT1-MMP has also been found at caveolae in tumor and EC cell lines (Annabi et al., 2001; Puyraimond et al., 2001; Remacle et al., 2003). Caveolae are cholesterol-rich microdomains involved in clathrin-independent internalization of receptors, recycling of molecules to the Golgi complex, and signal transduction (Parton et al., 1994; Conrad et al., 1995; Stahlhut and van Deurs, 2000). Caveolae are abundant in ECs, and their endocytosis can be regulated by actin cytoskeleton and phosphorylation (Parton et al., 1994; Smart et al., 1999; Stahlhut and van Deurs, 2000; Schlegel et al., 2001). They are mainly composed of cholesterol and sphingolipids and are characterized by the presence of a 22-kDa protein termed caveolin-1. Caveolin-1 interacts with a variety of signaling molecules and surface receptors regulating their localization and function (Okamoto et al., 1998; Kurzchalia and Parton, 1999). Thus, caveolin-1 can associate to β1 integrins and regulate anchorage-dependent cell growth or uPAR-dependent cell functions (Wary et al., 1998; Wei et al., 1999). Caveolin-1 may also interact with RhoA-modulating cell shape (Gingras et al., 1998).
In the present study, we have determined that MT1-MMP is present at endothelial caveolae with caveolin-1 and that caveolae constitute a novel pathway for MT1-MMP internalization in human ECs. Moreover, caveolae-mediated MT1-MMP traffic regulates protease localization, activity, and function in ECs during angiogenesis.
MATERIALS AND METHODS
Antibodies and Reagents
Monoclonal antibodies (mAb) anti-β1 integrins TS2/16 and LIA1/2, anti-αv integrins ABA 6D1, anti-VE-cadherin TEA1/31, anti-αvβ3 integrin LM609, anti-transferrin receptor FG2/12, and anti-MT1-MMP LEM-2/15 and LEM-2/63 have been described previously (Gálvez et al., 2001). Anti-caveolin-1 mAb (clone 2234) was purchased from BD Transduction Laboratories (Lexington, KY), anti-RhoA mAb (clone 26C4) was from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-GST mAb was from Amersham Pharmacia AB (Uppsala, Sweden).
Anti-tubulin mAb, type IV gelatin (GEL), β-methyl-cyclodextrin (cdx), filipin, okadaic acid, sphingosine-1-phosphate (SPP), and cholesterol were from Sigma-Aldrich (St. Louis, MO). Collagen type I (COL I) was from ICN Biomedicals (Costa Mesa, CA). Human plasma plasminogen-depleted fibrinogen and C3 ADP-ribosylating exoenzyme were from Calbiochem-Novabiochem (Darmstadt, Germany).
Cells and Cell Cultures
Human ECs from umbilical vein were obtained and cultured up to the third passage as described previously (Gálvez et al., 2001). ECs were seeded on dishes coated with 1% GEL or 10 μg/ml COL I and changed to serum-free medium HE-SFM from Invitrogen (Karlsruhe, Germany) before performing the functional assays.
Flow Cytometry and Analysis of Receptor Internalization
ECs were incubated with the primary mAb at room temperature previously for 20 min, exhaustively washed with phosphate-buffered saline (PBS), and transferred to 37°C in the presence or not of different agents, 10 μg/ml C3, 1 μM SPP, 10 μM okadaic acid, 10 μg/ml filipin, or 1 μM cdx, for 6 h to allow receptor internalization. Half of the cells were then fixed and permeabilized for 10 min at 4°C with lysis buffer from BD Biosciences and all processed for flow cytometry as described previously (Gálvez et al., 2002).
Immunofluorescence Microscopy
Subconfluent ECs on glass coverslips coated with GEL or COL I were incubated or not with different agents, 10 μg/ml C3, 1 μM SPP, or 1 μM cdx for 6 h. Cells were first incubated with the primary antibody (anti-caveolin-1, anti-αvβ3 LM609, or anti-β1 integrin TS2/16 mAbs) and labeled with a secondary rhodamine X-conjugated goat anti-mouse. After saturating with mouse serum, cells were labeled with the anti-MT1-MMP LEM-2/15 biotinylated mAb, and streptavidin-Alexa 488. Samples were examined in a Leica DMR photomicroscope with a 63× oil immersion objective and images recorded using a charge-coupled device camera from Leica (Wetzlar, Germany).
Western Blot Assays
ECs were washed twice with PBS and directly lysed in Laemmli buffer on ice. Lysates were analyzed by Western blot as described previously (Gálvez et al., 2001).
Rho Small GTPase Activity Assays
GST-C21, which recognizes active RhoA, was prepared and pull-down experiments performed as described previously (Sander et al., 1999). Additionally, glutathione S-transferase (GST)-C21 or GST as control were used to stain ECs by immunofluorescence. Briefly, cells fixed in 4% paraformaldehyde were incubated with 100 ng of GST-C21 or GST for 30 min at 37°C. After washing with PBS, cells were stained with the anti-GST mAb and an Alexa 488-anti-mouse secondary antibody.
Zymography Assays
ECs were grown on GEL or COL I and stimulated or not to migrate in serum-free medium in the absence or presence of 10 μg/ml C3, 1 μM SPP, 1 μM cdx, or 10 μg/ml filipin. Cell lysates were analyzed by zymography as described previously (Gálvez et al., 2001). Densitometric analysis was performed on scanned images with the Multi Analist Software from Bio-Rad (Hercules, CA) and specific activity estimated (active MT1-MMP/total MT1-MMP).
Lipid Raft Isolation
Confluent ECs on GEL or COL I were lysed in buffer 25 mM MES, 0.15 mM NaCl, 0.5% Triton X-100 and after ultracentrifugation in a discontinuous sucrose gradient (40-30-5%) distinct fractions were recovered. Precipitated proteins were run in SDS-PAGE and analyzed by Western blot as described previously (Gálvez et al., 2001).
Cell Transmigration, Collagen Gel Invasion, and In Vitro Angiogenesis Assays
Functional assays were performed as described previously (Gálvez et al., 2001), but cells were pretreated or not with 1 μM cdx or 10 μg/ml filipin for 12 h. In transmigration assays, cholesterol replenishment was achieved by adding or not 1 mg/ml cholesterol for 1 h before the assay.
Interference RNA
Sequence AAATGAATTAAAGTGGACCAA of human caveolin-1 mRNA was targeted by designing and synthesizing specific (cav-1) or scrambled (sc cav-1) small interfering RNA (siRNA) according to manufacturer's instructions (Ambion, Austin, TX). siRNAs were transfected in primary ECs by using oligofectAMINE (Invitrogen). Transfected ECs were used for Western blot, immunofluorescence staining, receptor internalization, fibrinogen zymography, or functional assays as described.
Statistical Analysis
Tested and control samples in the functional assays were compared for statistical significance by using the Student's t test.
RESULTS
ECM Regulates MT1-MMP through RhoA Activation in Confluent ECs
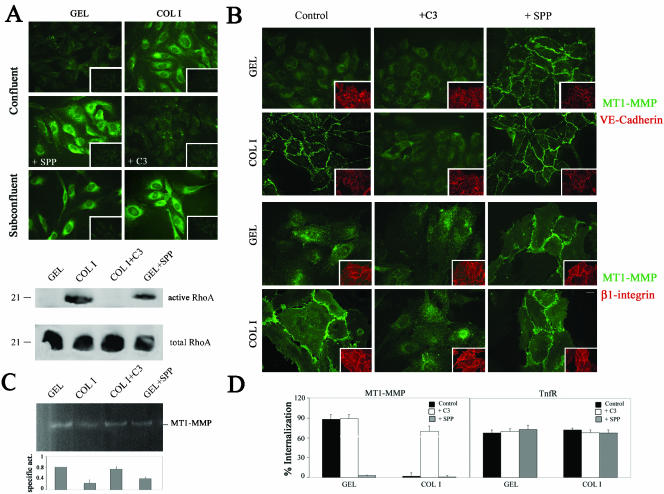
ECM modulates MT1-MMP localization and internalization in human ECs. Thus, in confluent ECs, MT1-MMP is diffuse and constitutively internalized on GEL but localized at cell-cell contacts and noninternalized on COL I (Gálvez et al., 2002). Because the family of Rho GTPases is activated by ECM signaling and regulates cell-cell contact architecture and endocytic traffic, the role of this pathway was first investigated (Ellis and Mellor, 2000; Etienne-Manneville and Hall, 2002). As shown in Figure 1A, top, immunofluorescence staining with the GST-C21 fusion protein that binds active RhoA showed GST-C21 binding to confluent ECs on COL I but not on GEL and also binding to subconfluent ECs regardless the ECM in comparison with the absence of signal observed with the control GST. Moreover, in confluent ECs, the activator SPP induced GST-C21 binding on GEL and the Rho inhibitor C3 inhibited its binding on COL I. The striking regulation of RhoA activation by ECM in confluent ECs was subsequently confirmed by pull-down experiments. Thus, interaction of confluent ECs with COL I but not GEL induced RhoA activation similarly to SPP on GEL, and COL I-induced RhoA activation was blocked by C3 (Figure 1A, bottom). Interestingly, in patches of confluent ECs, SPP relocalized MT1-MMP but not αvβ3 integrin at the cell-cell contacts on GEL, whereas C3 impaired MT1-MMP intercellular localization on COL I without affecting the presence of the junctional marker VE-cadherin or β1 integrins at these sites; moreover, C3 did not interfere with the clustered localization of MT1-MMP at elongated extensions of subconfluent ECs (Figure 1B; our unpublished data). Additionally, SPP-induced MT1-MMP relocalization correlated with a decrease in its activity in confluent ECs on GEL, whereas a restoration of protease activity was achieved by C3 treatment of ECs on COL I (Figure 1C). Finally, in confluent ECs, SPP also blocked constitutive MT1-MMP recycling on GEL, whereas C3 restored MT1-MMP internalization on COL I but did not have any effect on its internalization in subconfluent ECs regardless the ECM (Figure 1D; our unpublished data). Thus, activation of RhoA in confluent ECs by SPP or by COL I but not by GEL would result in the recruitment of MT1-MMP to intercellular contacts, in the blockade of its traffic, and in the decrease of its activity.
Figure 1.
ECM-induced RhoA activation modulates MT1-MMP in confluent ECs. (A) RhoA activation was analyzed by immunofluorescence staining with GST-C21 of confluent ECs on GEL or COL I and treated or not with 1 μM SPP or 10 μg/ml C3 for 12 h, respectively, or of subconfluent ECs on GEL or COL I (top). GST binding is also included as negative control (insets). Additionally, pull-down assays with GST-C21 were performed on lysates of confluent ECs grown on GEL or COL I and incubated or not with 1 μM SPP or 10 μg/ml C3 for 12 h, respectively (bottom). A representative experiment of three performed is shown. Bar, 20 μm. (B) MT1-MMP subcellular distribution (green) was analyzed in ECs grown on GEL or COL I and treated or not with 10 μg/ml C3 or 1 μM SPP for 6 h. VE-cadherin and β1 integrin staining (red) is shown as control of monolayer integrity (insets). Bar, 20 μm. (C) Lysates of confluent ECs grown on GEL or COL I and incubated with 1 μM SPP or 10 μg/ml C3 for 12 h, respectively, were analyzed by fibrinogen zymography. The arithmetic mean and S.D. of the estimated MT1-MMP specific activity (enzymatic activity/amount of protein) from two independent experiments are shown. (D) Internalization of MT1-MMP and TfnR was quantitated by flow cytometry in confluent ECs grown on GEL or COL I and pretreated or not with 10 μg/ml C3 or 1 μM SPP for 6 h. The arithmetic mean and S.D. of the internalization percentages obtained in three independent experiments are shown.
Caveolae Mediate MT1-MMP Traffic in Human ECs
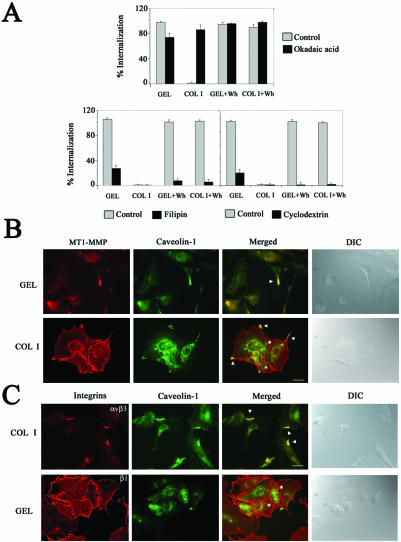
Because SPP and C3 modulated MT1-MMP internalization in confluent ECs without affecting clathrin-dependent transferrin receptor (TnfR) endocytosis (Figure 1D), alternative pathways for MT1-MMP recycling were investigated by using different agents (Smart et al., 1999). The protein phosphatase inhibitor okadaic acid, which stimulates caveolae-mediated endocytosis and inhibits the formation of clathrin-coated vesicles, induced MT1-MMP internalization in confluent ECs on COL I and slightly reduced MT1-MMP internalization on GEL without affecting wound healing-induced internalization regardless the ECM (Figure 2A, top). In contrast, the sterol binding agent filipin, which inhibits the internalization of caveolae, and cdx, which sequesters cholesterol thus disrupting caveolae formation, almost blocked MT1-MMP internalization in all conditions being the effects particularly drastic in migratory ECs in which the blockade was close to 100% (Figure 2A, bottom). EC viability was 98, 95, and 93% after 6 h of treatment with okadaic acid, filipin, or cdx, respectively. Moreover, clathrin-dependent TnfR endocytosis was abolished by okadaic acid and unaffected by filipin or cdx treatment at the low doses used. Thus, caveolae seem to be the major MT1-MMP internalization pathway in migratory ECs; in confluent ECs, caveolae constitute the main endocytic route on GEL but this pathway is blocked on COL I. In addition, MT1-MMP was shown to colocalize with caveolin-1 but not with clathrin in vesicles and clusters along elongated extensions of migratory ECs regardless the ECM but not at cell-cell contacts in confluent ECs on COL I (Figure 2B; our unpublished data). Finally, caveolin-1 also colocalized with αvβ3 integrin at lamellipodia but not with β1 integrins at the cell-cell contacts of subconfluent ECs (Figure 2C).
Figure 2.
MT1-MMP is internalized through caveolae and colocalizes with caveolin-1 at motility-associated extensions in ECs. (A) MT1-MMP internalization was quantitated by flow cytometry on ECs grown on GEL or COL I and induced or not to migrate. ECs were pretreated or not for 6 h with 10 μM okadaic acid, 10 μg/ml filipin, or 1 μM cdx. The arithmetic mean and S.D. of the internalization percentages obtained in three independent experiments are shown. (B and C) Double staining of MT1-MMP (red) and caveolin-1 (green) (B), or of β1 or αvβ3 integrins (red) and caveolin-1 (green) (C) of subconfluent ECs on GEL or COL I was performed. Merged images are shown (yellow) and colocalization is marked (arrowheads). Asterisks highlight intercellular contacts were no caveolin-1 colocalization is found. Differential interference contrast images are also shown. Bar, 20 μm.
MT1-MMP Is Present at Caveolae Domains
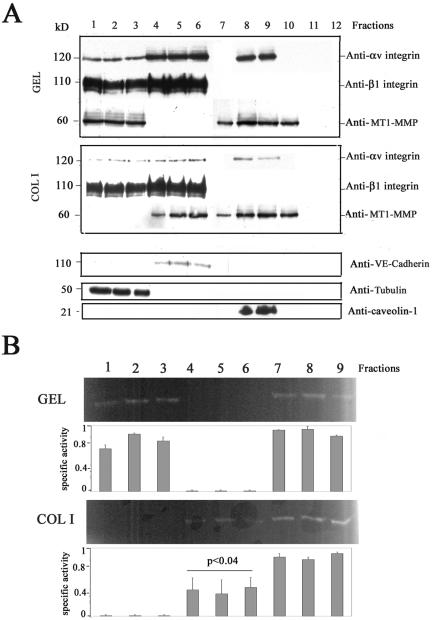
MT1-MMP colocalization with caveolin-1 prompted us to directly assess whether these two proteins were present at the same subcellular compartments by performing lipid raft isolation. As shown in Figure 3A, MT1-MMP was found at caveolae (caveolin-1-positive fractions) in confluent ECs on either GEL or COL I. Interestingly, however, a shift in the presence of MT1-MMP but not of αv or β1 integrins in noncaveolae fractions was observed in ECs on COL I compared with GEL. Thus, MT1-MMP was found at fractions corresponding to plasma membrane (VE-cadherin-positive fractions) in confluent ECs on COL I as opposed to the presence of MT1-MMP at fractions corresponding to cytosol (tubulin-positive fractions) on GEL in accordance with the differential MT1-MMP subcellular localization in confluent ECs at either membrane cell-cell contacts on COL I or diffuse on GEL (Figure 3A; Gálvez et al., 2002).
Figure 3.
MT1-MMP is present at caveolae domains. (A) Fractions 1-12 from subcellular fractioning on sucrose gradient of lysates of confluent ECs grown on GEL or COL I were analyzed by Western blot with anti-MT1-MMP and anti-αv or anti-β1 integrin mAbs. Anti-VE-cadherin, anti-tubulin, and anti-caveolin-1 mAbs were used for characterization of the fractions. (B) Selected fractions from (A) were analyzed by fibrinogen zymography. The arithmetic mean and S.D. of the estimated MT1-MMP-specific activity (enzymatic activity/amount of protein) from two independent experiments are shown. p value is indicated.
Because caveolin-1 can modulate the activity of different proteins (Okamoto et al., 1998; Kurzchalia and Parton, 1999), we took advantage of the presence of MT1-MMP at caveolin-1-positive and -negative fractions to check its activity at distinct subcellular compartments by fibrinogen zymography. MT1-MMP proteolytic activity was detected in both caveolae and noncaveolae (cytosolic and membrane) fractions in confluent ECs regardless the ECM; MT1-MMP specific activity was optimal at caveolae and cytosolic fractions, but it was significantly lower at plasma membrane fractions of confluent ECs on COL I in accordance to our previous data with total cell lysates (Figure 3B; Gálvez et al., 2002).
Caveolae Integrity Is Required for MT1-MMP Function in Migratory ECs
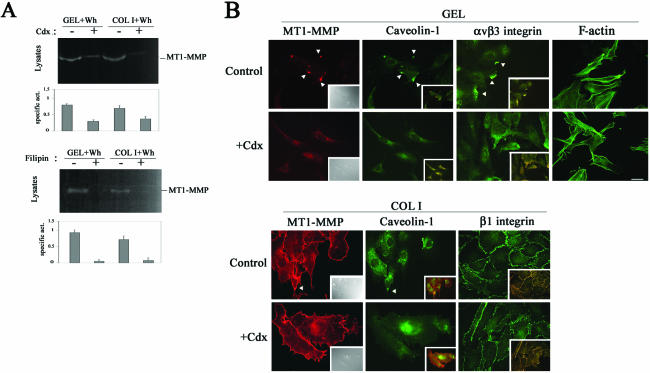
The relevance of caveolae traffic in regulating MT1-MMP activity during endothelial migration was then analyzed. Besides impairing MT1-MMP internalization (Figure 2A, bottom), both cdx or filipin pretreatment of migratory ECs resulted in the decrease of MT1-MMP activity without affecting total protease expression (Figure 4A; our unpublished data). Because cdx disrupts caveolae and filipin mainly affects caveolae traffic, our data point to caveolae-mediated MT1-MMP traffic as relevant for protease activity regulation in migratory ECs. In addition, both cdx and filipin induced the loss of MT1-MMP, caveolin-1, and αvβ3 integrin from motility-associated extensions of migratory ECs without interfering with MT1-MMP/β1 integrin presence at cell-cell contacts on COL I nor with EC morphology assessed by F-actin staining (Figure 4B; our unpublished data). Thus, the presence of caveolin-1 together with MT1-MMP and αvβ3 integrin at caveolae might be involved in the mobilization of this complex to motility-associated structures, thus regulating proteolytic activity during endothelial migration.
Figure 4.
Caveolae-mediated traffic is required for proper MT1-MMP activity and localization in migrating ECs. (A) Lysates from wound healing-stimulated ECs grown on GEL or COL I and pretreated or not with 1 μM cdx or 10 μg/ml filipin for 6 h were analyzed by fibrinogen zymography. The arithmetic mean and S.D. of the estimated MT1-MMP specific activity (enzymatic activity/amount of protein) from two independent experiments are shown. (B) Double staining of MT1-MMP (red) and caveolin-1 (green), or of caveolin-1 (red) and αvβ3 or β1 integrins (green) in subconfluent ECs on GEL or COL I pretreated or not with 1 μM cdx for 6 h was performed. Merged images are shown in the insets (yellow) and colocalization is marked (arrowheads). Differential interference contrast images (insets) and F-actin staining (right) of subconfluent ECs on GEL treated or not with cdx are also shown as control of cell morphology. Bar, 20 μm.
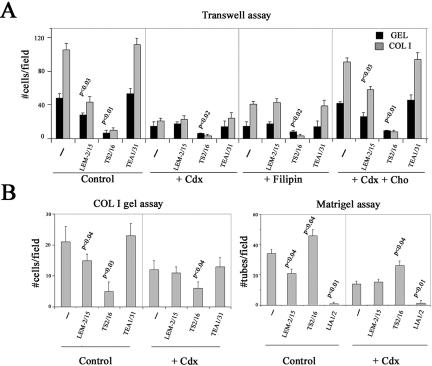
The requirement of caveolae traffic for MT1-MMP-dependent functions during angiogenesis was then analyzed. cdx or filipin per se decreased endothelial migration, COL I gel invasion, and formation of capillary tubes without affecting EC viability (Figure 5, A and B; see above). Remarkably, the inhibitory anti-MT1-MMP mAb LEM-2/15 that significantly impaired EC migration, COL I gel invasion, and capillary formation in control cells, lost its inhibitory ability upon pretreatment of ECs with cdx or filipin (Figure 5, A and B). Cholesterol replenishment restored, however, the anti-MT1-MMP mAb inhibitory ability on endothelial transmigration (Figure 5A). In contrast, the functional effects of the activatory anti-β1 integrin mAb TS2/16 and the neutralizing anti-β1 integrin mAb LIA1/2 were significant in both untreated and cdx- or filipin-treated ECs (Figure 5, A and B). These data point to an important requirement of caveolae traffic for proper regulation of MT1-MMP function in ECs.
Figure 5.
Caveolae-mediated traffic is required for proper MT1-MMP function during angiogenesis. (A) Migration on GEL and COL I of ECs treated or not with 1 μM cdx or 10 μg/ml filipin for 12 h was analyzed by transwell assays (top). Cholesterol replenishment was performed after cdx treatment. The effect of anti-MT1-MMP LEM-2/15, anti-β1 integrin TS2/16, or anti-VE-cadherin TEA1/31 mAbs was quantitated at 5 h. The arithmetic mean and S.D. of a representative out of three independent experiments run in duplicate are shown. (B) ECs treated or not with 1 μM cdx for 12 h were grown on top of COL I gels (left) or on Matrigel (right), and their ability to invade or to form capillary tubes was quantitated in the presence or not of anti-MT1-MMP LEM-2/15, anti-β1 integrin TS2/16 or LIA1/2, or anti-VE-cadherin TEA1/31 mAbs. The arithmetic mean and S.D. of a representative out of three independent experiments run in duplicate are shown. p values are indicated.
Targeting Caveolin-1 Expression Impairs MT1-MMP Function in Human ECs
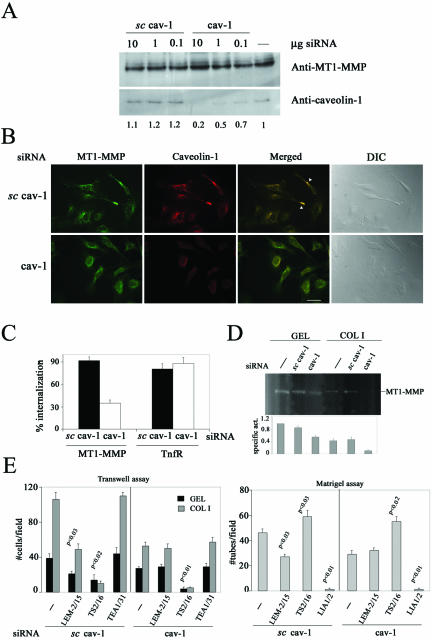
The functional relevance of caveolae traffic in regulating MT1-MMP function was additionally investigated by specifically targeting caveolin-1 expression by interference RNA. Caveolin-1 but not MT1-MMP protein level decreased in a dose-dependent manner by transfection of specific but not scrambled cav-1 siRNA in human ECs (Figure 6A). No MT1-MMP localization at clusters and only a diffuse perinuclear pattern of both MT1-MMP and remaining caveolin-1 similar to cdx-treated ECs (Figure 4C) was observed in subconfluent ECs transfected with specific cav-1 siRNA in contrast to abundant presence of MT1-MMP at clusters in ECs transfected with scrambled cav-1 siRNA (Figure 6B). Interestingly, MT1-MMP internalization and activity were also decreased by transfection of specific cav-1 siRNA compared with scrambled siRNA in subconfluent ECs (Figure 6, C and D). Finally, MT1-MMP function in angiogenesis was tested by the ability of the inhibitory anti-MT1-MMP mAb LEM-2/15 of impairing endothelial transmigration and formation of capillary tubes. As shown in Figure 6E, the anti-MT1-MMP LEM-2/15 mAb significantly decreased both processes in ECs transfected with the scrambled but not the specific cav-1 siRNA in contrast to the significant effects of the anti-β1 integrin mAbs regardless the siRNA transfected. Thus, the presence of caveolin-1 is required for proper regulation of MT1-MMP traffic and function in migratory ECs.
Figure 6.
Decrease in caveolin-1 expression impairs MT1-MMP localization, internalization, activity, and function in human ECs. (A) Lysates from ECs transfected with different doses of specific or scrambled cav-1 siRNA were analyzed by Western blot with anti-caveolin-1 or anti-MT1-MMP mAbs. Densitometric values of caveolin-1 expression are shown. (B) MT1-MMP (green) and caveolin-1 (red) subcellular localization was analyzed in subconfluent ECs on GEL transfected with 1 μg of specific or scrambled cav-1 siRNA. Merged images are shown (yellow) and colocalization is marked (arrowheads). Differential interference contrast images are also displayed. Bar, 20 μm. (C) MT1-MMP internalization was quantitated by flow cytometry in subconfluent ECs on GEL transfected with 1 μg of specific or scrambled cav-1 siRNA. The arithmetic mean and S.D. of two independent experiments are shown. (D) Lysates of subconfluent ECs on GEL or COL I transfected with 1 μg of specific or scrambled cav-1 siRNA were analyzed by fibrinogen zymography. The arithmetic mean and S.D. of the estimated MT1-MMP specific activity (enzymatic activity/amount of protein) from two independent experiments are shown. (E) ECs transfected with 1 μg of specific or scrambled cav-1 siRNA were assayed for transmigration (left) and formation of capillaries (right) in the presence or not of anti-MT1-MMP LEM-2/15, anti-β1 integrin TS2/16 or LIA1/2, or anti-VE-cadherin TEA1/31 mAbs. The arithmetic mean and S.D. of two independent experiments run in duplicate are shown. p values are indicated.
DISCUSSION
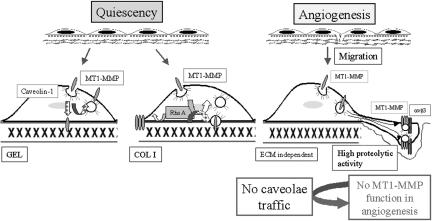
In this work, we characterize caveolae as a novel pathway for MT1-MMP traffic that is required for proper localization, activity, and function of the protease during endothelial migration, invasion, and formation of capillary tubes (see Figure 7 for details).
Figure 7.
Model for MT1-MMP regulation in human ECs during angiogenesis. Role of caveolae-mediated traffic. MT1-MMP function is regulated in ECs during angiogenesis depending on the migratory state and the ECM. Herein, we have characterized the role of caveolae-mediated traffic in regulating MT1-MMP function during this process. During quiescence, MT1-MMP traffic through caveolae is constitutive in ECs on GEL, but it is blocked on COL I in a RhoA activation-dependent manner. During the angiogenic response, ECs become migratory and MT1-MMP seems to be mostly internalized through caveolae independently of the ECM. In this case, MT1-MMP is clustered together with caveolin-1 and αvβ3 integrin at motility-associated structures resulting in an increased proteolytic activity. Disturbing caveolae traffic by inhibitors or by siRNA for caveolin-1 blocks MT1-MMP function during endothelial migration, invasion, and formation of capillary tubes. Discontinuous arrows indicate putative not yet demonstrated pathways.
MT1-MMP was previously found to be distinctly modulated by the ECM (Gálvez et al., 2002). Herein, we have identified the small GTPase RhoA like a mediator of such effect. Although the role of these GTPases in regulating cellular morphology and migration has been well established (Etienne-Manneville and Hall, 2002; Martin et al., 2002) and no differences in RhoA activation could be observed in migrating ECs, we have demonstrated that RhoA is distinctly activated in confluent nonmigratory ECs in an ECM-dependent manner. This differential RhoA activation by specific ECM-cellular interactions has also been reported during cell spreading on fibronectin by binding to α5β1 or αvβ3 integrins (Danen et al., 2002). Interestingly, RhoA activation in confluent ECs accounts for recruitment of MT1-MMP to cell-cell contacts as previously shown for endothelial junction components such as VE-cadherin or β-catenins (Lee et al., 1999; Charrasse et al., 2002) as well as for blockade of its traffic. RhoA-induced MT1-MMP relocalization to the junctions seemed to be independent of actin polymerization because it was still observed in the presence of cytochalasin D (our unpublished data). Our data support that ECM-mediated RhoA activation might regulate MT1-MMP in two different ways: regulation might be direct in confluent ECs by favoring the recruitment of MT1-MMP to the cell-cell contacts on COL I but indirect if any in subconfluent ECs likely by modulating cell morphology and migration. Thus, a novel functional link between ECM, integrins, RhoA, and MT1-MMP in primary human ECs is now established.
It has recently been reported that MT1-MMP can be internalized via clathrin-coated pits in transformed cells (Jiang et al., 2001; Uekita et al., 2001). The blockade of MT1-MMP internalization by RhoA activation without apparently affecting clathrin-mediated TnfR endocytosis in confluent ECs led us to analyze alternative pathways for MT1-MMP traffic. Herein, we have demonstrated that MT1-MMP internalization in ECs takes place through caveolae. This pathway has first been demonstrated by treatment with agents that interfere with caveolae traffic such as filipin or cdx. Although cdx has been reported to also impair clathrin-dependent endocytosis, no effect on TnfR internalization was observed at 1 μM dose of cdx, much lower than the usual 1 mM reported, thus reinforcing the specificity of cdx effects on caveolae in our system (Rodal et al., 1999; Subtil et al., 1999). Furthermore, to avoid any putative unspecific or toxic effects of these inhibitors (although EC viability was unaffected), targeting of caveolin-1 expression by interference RNA was performed in human primary ECs; this approach was very efficient and confirmed all the functional effects observed with the inhibitors. Caveolae are herein demonstrated to be key for MT1-MMP traffic in ECs during wound healing-induced migration and important in confluent ECs on GEL; a partial contribution of the classic endocytic pathway during MT1-MMP internalization in confluent ECs on GEL is not ruled out because okadaic acid slightly inhibited this process. This dual contribution is in agreement with a recent report about MT1-MMP traffic in tumor cells (Remacle et al., 2003). The apparent contrast of our data with the previous unique role assigned to clathrin-dependent endocytosis in MT1-MMP internalization might be related to the use of transformed cells, which contain few or none caveolae, as well as of a dynamin mutant that can also interfere with caveolae traffic (Koleske et al., 1995; Schnitzer et al., 1996).
Caveolae involvement on MT1-MMP traffic is further supported by the colocalization of MT1-MMP and caveolin-1 in ECs. Although this colocalization had previously been proposed (Puyraimond et al., 2001), here it is demonstrated that the two proteins colocalize at clusters in extensions of migratory human ECs. This is in accordance with the presence of caveolin-1 reported at the leading edge of ECs and at membrane ruffles of melanoma cells together with uPA (Isshiki et al., 1998; Stahl and Mueller, 1995). Moreover, we observed an increase in caveolin-1 expression upon induction of endothelial migration (our unpublished results). Caveolin-1 together with MT1-MMP also colocalized with αvβ3 integrin that is involved in endothelial migration and has been preferentially found in lipid microdomains from carcinoma and melanoma cells (Green et al., 1999). In contrast, MT1-MMP at endothelial cell-cell contacts together with β1 integrins does not colocalize with caveolin-1, which is absent from these sites in ECs, although other groups have reported caveolin-1 intercellular staining on adipocytes and on human vascular smooth muscle cells (Parton et al., 1994; Wei et al., 1999). Caveolae disruption by cdx or filipin or by caveolin-1 siRNA results in the disappearance of MT1-MMP, caveolin-1, and αvβ3 integrin from motility-associated structures but not of β1 integrins from cell-cell contacts indicating a role for caveolae in receptor compartmentalization. Therefore, caveolae might constitute a mechanism for MT1-MMP mobilization to cellular migratory sites.
Direct assessment of MT1-MMP compartmentalization in lipid microdomains has also been addressed. Thus, MT1-MMP is demonstrated to be present at caveolae in confluent ECs on GEL or COL I, as recently reported in tumor cells (Annabi et al., 2001; Remacle et al., 2003). In confluent ECs on COL I, MT1-MMP might be associated to ventral caveolae in contrast to mobile caveolae throughout the cell on GEL as suggested by the internalization data as well as by the staining of proteins associated to the ECM after mild cell solubilization (our unpublished results). Coimmunoprecipitation assays suggested an indirect if any association of MT1-MMP and caveolin-1 through either lipid domains or other proteins (our unpublished results). In this regard, αv but not β1 integrins are present at caveolin-positive domains in human ECs in accordance with the colocalization data.
Caveolin-1 can modulate the activity and function of different proteins (Stahl and Mueller, 1995; Okamoto et al., 1998). In this regard, MT1-MMP at caveolae and at noncaveolae cytosolic fractions seemed to display optimal specific activity compared with MT1-MMP at membrane fractions in confluent ECs on COL I, pointing to protease subcellular compartmentalization as a relevant mechanism for controlling protease activity. In this regard, the MT1-MMP 63-kDa mature form present at caveolae of fibrosarcoma cells had previously been shown to be processed to a 43-kDa MT1-MMP form after concanavalin A stimulation (Annabi et al., 2001). The requirement of caveolae traffic for MT1-MMP proper activity in migratory ECs was additionally demonstrated by both pretreatment with caveolae disrupting agents as well as by targeting caveolin-1 with siRNA. Caveolae-mediated internalization seems to be the requirement for regulating MT1-MMP activity because filipin, which mainly affects caveolae traffic, interfered with MT1-MMP activity. This is in accordance with the requirement of clathrin-mediated endocytosis for proper MT1-MMP activity reported previously (Jiang et al., 2001). Mobilization of caveolae to migratory sites might facilitate MT1-MMP clustering and oligomerization known to be important for its activation (Itoh et al., 2001; Lehti et al., 2002). In this regard, clustering of uPAR/uPA in caveolae has been shown to promote efficient cell surface plasminogen activation (Stahl and Mueller, 1995). On the other hand, internalization might be a mechanism for recycling and removal of already inactive forms of the protease as proposed (Uekita et al., 2001).
MT1-MMP plays an active role in endothelial cell migration, invasion, and formation of capillary tubes (Gálvez et al., 2001). This ability of MT1-MMP is, however, lost in the absence of caveolae traffic achieved by pretreatment with cdx or filipin or by selectively targeting caveolin-1 expression. The function of other receptors like β1 integrins is however preserved under these conditions. Defects in caveolin-1 might interfere with MT1-MMP/αvβ3 integrin association and activity but preserve β1 integrin function because no caveolin-1/β1 integrin colocalization is detected in ECs. cdx or filipin treatment per se reduced endothelial motility but the restoration of MT1-MMP-mediated endothelial functions upon cholesterol replenishment pointed to a specific effect in this proteolytic pathway that is reinforced by data obtained with siRNA. In contrast, some studies have shown that the overexpression of caveolin-1 resulted in a loss of MT1-MMP-dependent migration in MT1-MMP-transfected COS7 cells (Annabi et al., 2001). Primary and tumor cells might be differently regulated in this respect because tumor cells often deprived of caveolae would lack of such regulatory mechanism (Koleske et al., 1995; Annabi et al., 2001). Therefore, caveolae and caveolin-1 are important for endothelial migration, invasion, and capillary formation because they might allow a regulated MT1-MMP endocytosis necessary for these functions. In this regard, impaired internalization of cytosolic-truncated MT1-MMP also resulted in inhibition of migration and invasion of transfected-Chinese hamster ovary cells (Uekita et al., 2001).
The relevance of caveolae in pathology is highlighted by the development of lung and muscle disease in animal models deficient in different caveolins and so in caveolae as well as by the presence of caveolin-1 mutations in invasive human breast carcinomas (Razani and Lisanti, 2001). Moreover, caveolin-1 knockout mice show an increase in matrix deposition in the lungs that might be related to an impaired ECM remodeling due to the absence of regulated MT1-MMP activity (Holmbeck et al., 1999; Drab et al., 2001; Razani et al., 2001). This could be relevant for the design of novel therapeutic approaches interfering specifically with the presence of MT1-MMP at caveolae thus selectively targeting active MT1-MMP in disease.
In summary, we have reported caveolae-mediated traffic as a novel mechanism regulating MT1-MMP localization, activity, and function in human ECs. Moreover, the ECM via a RhoA-dependent signaling can modulate this pathway in quiescent ECs. Therefore, there is a delicate communication between ECM and MT1-MMP allowing its proper activity and function during angiogenesis.
Acknowledgments
We thank Dr. C. Martínez-A. (Centro Nacional de Biotecnología, Madrid) and S. Vilaró (Universidad de Barcelona, Barcelona) for the gift of anti-αv and anti-αvβ3 integrin mAbs, Dr. John Collard (The Netherlands Cancer Institute, Amsterdam) for the GST-C21 construction, and Dr. M.A. Alonso for critical comments. We are extremely grateful to E. López and C. Zaragoza for help and advice with caveolae isolation and iRNA technique. B.G.G. is a predoctoral fellow from the Comunidad Autónoma de Madrid. This work was supported by Ayuda de Investigación Básica Juan March 2002 to F.S.-M. and grants FIS00/0114 from Fondo de Investigaciones Sanitarias, CAM 08.3/0015.1/2001 from Comunidad Autónoma de Madrid, and SAF2002-00068 from Ministerio de Ciencia y Tecnología to A.G.A.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-07-0516. Article and publication date are available at www.molbiolcell.org/cgi/doi/mbc.E03-07-0516.
Abbreviations used: cdx, cyclodextrin; COL I, collagen type I; EC, endothelial cell; ECM, extracellular matrix; GEL, gelatin; MMP, matrix metalloproteinase; MT1-MMP, membrane-type 1 matrix metalloproteinase; TnfR, transferrin receptor.
References
- Annabi, B., Lachambre, M., Bousquet-Gagnon, N., Page, M., Gingras, D., and Beliveau, R. (2001). Localization of membrane-type 1 matrix metalloproteinase in caveolae membrane domains. Biochem. J. 353, 547-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, P.A., Smart, E.J., Ying, Y.S., Anderson, R.G., and Bloom, G.S. (1995). Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J. Cell Biol. 131, 1421-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrasse, S., Meriane, M., Comunale, F., Blangy, A., and Gauthier-Rouviere, C. (2002). N-cadherin-dependent cell-cell contact regulates Rho GTPases and β-catenin localization in mouse C2C12 myoblasts. J. Cell Biol. 158, 953-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen, E.H., Sonneveld, P., Brakebusch, C., Fassler, R., and Sonnenberg, A. (2002). The fibronectin-binding integrins α5β1 and αvβ3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J. Cell Biol. 159, 1071-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drab, M., et al. (2001). Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293, 2449-2452. [DOI] [PubMed] [Google Scholar]
- Ellis, S., and Mellor, H. (2000). Regulation of endocytic traffic by rho family GTPases. Trends Cell Biol. 10, 85-88. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and Hall, A. (2002). Rho GTPases in cell biology. Nature 420, 629-635. [DOI] [PubMed] [Google Scholar]
- Fukata, M., and Kaibuchi, K. (2001). Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat. Rev. Mol. Cell. Biol. 2, 887-897. [DOI] [PubMed] [Google Scholar]
- Fukata, M., Nakagawa, M., Kuroda, S., and Kaibuchi, K. (1999). Cell adhesion and Rho small GTPases. J. Cell Sci. 112, 4491-4500. [DOI] [PubMed] [Google Scholar]
- Gálvez, B.G., Matías-Román, S., Albar, J.P., Sánchez-Madrid, F., and Arroyo, A.G. (2001). Membrane type 1-matrix metalloproteinase is activated during migration of human endothelial cells and modulates endothelial motility and matrix remodeling. J. Biol. Chem. 276, 37491-37500. [DOI] [PubMed] [Google Scholar]
- Gálvez, B.G., Matías-Román, S., Yáñez-Mó, M., Sánchez-Madrid, F., and Arroyo, A.G. (2002). ECM regulates MT1-MMP localization with β1 or αvβ3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J. Cell Biol. 159, 509-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras, D., Gauthier, F., Lamy, S., Desrosiers, R.R., and Beliveau, R. (1998). Localization of RhoA GTPase to endothelial caveolae-enriched membrane domains. Biochem. Biophys. Res. Commun. 247, 888-893. [DOI] [PubMed] [Google Scholar]
- Green, J.M., Zhelesnyak, A., Chung, J., Lindberg, F.P., Sarfati, M., Frazier, W.A., and Brown, E.J. (1999). Role of cholesterol in formation and function of a signaling complex involving αvβ3, integrin-associated protein (CD47), and heterotrimeric G proteins. J. Cell Biol. 146, 673-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, A. (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509-514. [DOI] [PubMed] [Google Scholar]
- Holmbeck, K., et al. (1999). MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99, 81-92. [DOI] [PubMed] [Google Scholar]
- Isshiki, M., Ando, J., Korenaga, R., Kogo, H., Fujimoto, T., Fujita, T., and Kamiya, A. (1998). Endothelial Ca2+ waves preferentially originate at specific loci in caveolin-rich cell edges. Proc. Natl. Acad. Sci. USA 95, 5009-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, Y., Takamura, A., Ito, N., Maru, Y., Sato, H., Suenaga, N., Aoki, T., and Seiki, M. (2001). Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 20, 4782-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, A., Lehti, K., Wang, X., Weiss, S.J., Keski-Oja, J., and Pei, D. (2001). Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc. Natl. Acad. Sci. USA 98, 13693-13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajita, M., Itoh, Y., Chiba, T., Mori, H., Okada, A., Kinoh, H., and Seiki, M. (2001). Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J. Cell Biol. 153, 893-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleske, A.J., Baltimore, D., and Lisanti, M.P. (1995). Reduction of caveolin and caveolae in oncogenically transformed cells. Proc. Natl. Acad. Sci. USA 92, 1381-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia, T.V., and Parton, R.G. (1999). Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 11, 424-431. [DOI] [PubMed] [Google Scholar]
- Lee, M.J., Thangada, S., Claffey, K.P., Ancellin, N., Liu, C.H., Kluk, M., Volpi, M., Sha'afi, R.I., and Hla, T. (1999). Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99, 301-312. [DOI] [PubMed] [Google Scholar]
- Lehti, K., Lohi, J., Juntunen, M., Pei, D., and Keski-Oja, J. (2002). Oligomerization through hemopexin and cytoplasmic domains regulates the activity and turnover of membrane-type 1 matrix metalloproteinase (MT1-MMP). J. Biol. Chem. 277, 8440-8448. [DOI] [PubMed] [Google Scholar]
- Martin, K.H., Slack, J.K., Boerner, S.A., Martin, C.C., and Parsons, J.T. (2002). Integrin connections map: to infinity and beyond. Science 296, 1652-1653. [DOI] [PubMed] [Google Scholar]
- McCawley, L.J., and Matrisian, L.M. (2001). Matrix metalloproteinases: they're not just for matrix anymore! Curr. Opin. Cell Biol. 13, 534-540. [DOI] [PubMed] [Google Scholar]
- Nabeshima, K., Inoue, T., Shimao, Y., Okada, Y., Itoh, Y., Seiki, M., and Koono, M. (2000). Front-cell-specific expression of membrane-type 1 matrix metalloproteinase and gelatinase A during cohort migration of colon carcinoma cells induced by hepatocyte growth factor/scatter factor. Cancer Res. 60, 3363-3369. [PubMed] [Google Scholar]
- Okamoto, T., Schlegel, A., Scherer, P.E., and Lisanti, M.P. (1998). Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. 273, 5419-5422. [DOI] [PubMed] [Google Scholar]
- Parton, R.G., Joggerst, B., and Simons, K. (1994). Regulated internalization of caveolae. J. Cell Biol. 127, 1199-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puyraimond, A., Fridman, R., Lemesle, M., Arbeille, B., and Menashi, S. (2001). MMP-2 colocalizes with caveolae on the surface of endothelial cells. Exp. Cell Res. 262, 28-36. [DOI] [PubMed] [Google Scholar]
- Ratnikov, B.I., Rozanov, D.V., Postnova, T.I., Baciu, P.G., Zhang, H., DiScipio, R.G., Chestukhina, G.G., Smith, J.W., Deryugina, E.I., and Strongin, A.Y. (2002). An alternative processing of integrin αv subunit in tumor cells by membrane type-1 matrix metalloproteinase. J. Biol. Chem. 277, 7377-7385. [DOI] [PubMed] [Google Scholar]
- Razani, B., et al. (2001). Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem. 276, 38121-38138. [DOI] [PubMed] [Google Scholar]
- Razani, B., and Lisanti, M.P. (2001). Caveolin-deficient mice: insights into caveolae function and human disease. J. Clin. Investig. 108, 1553-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle, A., Murphy, G., and Roghi, C. (2003). Membrane type 1-matrix metalloproteinase (MT1-MMP) is internalised by two different pathways and is recycled to the cell surface. J. Cell Sci. 116, 3905-3916. [DOI] [PubMed] [Google Scholar]
- Rodal, S.K., Skretting, G., Garred, O., Vilhardt, F., van Deurs, B., and Sandvig, K. (1999). Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10, 961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander, E.E., ten Klooster, J.P., van Delft, S., van der Kammen, R.A., and Collard, J.G. (1999). Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 147, 1009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, H., Takino, T., Okada, Y., Cao, J., Shinagawa, A., Yamamoto, E., and Seiki, M. (1994). A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370, 61-65. [DOI] [PubMed] [Google Scholar]
- Schlegel, A., Arvan, P., and Lisanti, M.P. (2001). Caveolin-1 binding to endoplasmic reticulum membranes and entry into the regulated secretory pathway are regulated by serine phosphorylation. Protein sorting at the level of the endoplasmic reticulum. J. Biol. Chem. 276, 4398-4408. [DOI] [PubMed] [Google Scholar]
- Schnitzer, J.E., Oh, P., and McIntosh, D.P. (1996). Role of GTP hydrolysis in fission of caveolae directly from plasma membranes. Science 274, 239-242. [DOI] [PubMed] [Google Scholar]
- Seiki, M. (2002). The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr. Opin. Cell Biol. 14, 624-632. [DOI] [PubMed] [Google Scholar]
- Smart, E.J., Graf, G.A., McNiven, M.A., Sessa, W.C., Engelman, J.A., Scherer, P.E., Okamoto, T., and Lisanti, M.P. (1999). Caveolins, liquid-ordered domains, and signal transduction. Mol. Cell. Biol. 19, 7289-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, A., and Mueller, B.M. (1995). The urokinase-type plasminogen activator receptor, a GPI-linked protein, is localized in caveolae. J. Cell Biol. 129, 335-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut, M., and van Deurs, B. (2000). Identification of filamin as a novel ligand for caveolin-1, evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol. Biol. Cell 11, 325-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil, A., Gaidarov, I., Kobylarz, K., Lampson, M.A., Keen, J.H., and McGraw, T.E. (1999). Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. USA 96, 6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uekita, T., Itoh, Y., Yana, I., Ohno, H., and Seiki, M. (2001). Cytoplasmic tail-dependent internalization of membrane-type 1 matrix metalloproteinase is important for its invasion-promoting activity. J. Cell Biol. 155, 1345-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wary, K.K., Mariotti, A., Zurzolo, C., and Giancotti, F.G. (1998). A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94, 625-634. [DOI] [PubMed] [Google Scholar]
- Wei, Y., Yang, X., Liu, Q., Wilkins, J.A., and Chapman, H.A. (1999). A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 144, 1285-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb, Z. (1997). ECM and cell surface proteolysis: regulating cellular ecology. Cell 91, 439-442. [DOI] [PubMed] [Google Scholar]