Abstract
The ε4 allele of apolipoprotein E (APOE) is currently the major genetic risk factor identified for Alzheimer’s disease (AD). Previous in vivo data from our laboratory has demonstrated that amyloid-β (Aβ) is rapidly removed from the plasma by the liver and kidney and that the rate of its clearance is affected by ApoE in C57BL/6J and APOE−/− mice. To expand upon these findings, we assessed the peripheral clearance of human synthetic Aβ42 in APOE ε2, ε3, and ε4 knock-in and APOE knock-out mice injected with lipidated recombinant apoE2, E3, and E4 protein. Our results show that APOE does influence the rate at which the mice are able to clear Aβ42 from their bloodstream. Both APOE ε4 mice and APOE knock-out mice treated with lipidated recombinant apoE4 demonstrated increased retention of plasma Aβ42 over time compared to APOE ε2/APOE knock-out rE2 and APOE ε3/APOE knock-out rE3 mice. These findings suggest that the peripheral clearance of Aβ42 is significantly altered by APOE genotype. Given that APOE ε4 is a risk factor for AD, then these novel findings provide some insight into the role of ApoE isoforms on the peripheral clearance of Aβ which may impact on clearance from the brain.
Keywords: Alzheimer’s disease, amyloid-β, APOE genotype, peripheral sink hypothesis
INTRODUCTION
The physiological fate of amyloid-β (Aβ), a key component of AD, is currently poorly understood although its production is being extensively studied. The mechanisms of action with respect to the clearance of Aβ are still under contention, though one of the most accepted hypotheses of Aβ clearance is the so-called “peripheral sink” hypothesis [1]. Themain basis of this hypothesis is that Aβ is transported out of the brain, into the periphery where proteins in the circulation are thought to bind and sequester Aβ thereby preventing it from exerting its toxic effects. For this “Aβ sink” to function properly, however, the body must have a way of removing the Aβ from the periphery. Our laboratory [2–4] and others [5–7] have provided in vitro evidence that apolipoprotein E (ApoE) binds Aβ, in an isoform specific manner. Previous in vivo data from our laboratory examining the peripheral clearance of Aβ42 in C57BL/6J and APOE knock-out mice has demonstrated that Aβ is rapidly removed from the plasma by murine peripheral tissues (liver and kidney) and that ApoE influences the rate of its clearance [2]. Additionally, under in vitro conditions the E4 isoform of ApoE has also been associated with poor binding of Aβ, compared with the other common isoforms, ApoE2 and ApoE3 [4,5]. ApoE has been shown to enhance the uptake of Aβ in CHO [3], fibroblast, and hepatoma [8] cell lines suggesting the ApoE-mediated receptor pathways to be a major route of Aβ clearance, with the liver as primary site of this activity.
To expand upon these previous findings and in order to definitively establish whether APOE regulates Aβ clearance in an isoform specific manner in vivo, we assessed the peripheral clearance of Aβ42 in human APOE ε2, ε3, and ε4 knock-in and APOE knock-out mice injected with lipidated recombinant ApoE2, E3, and E4 protein.
METHODS
Animals
Our colony of APOE knock-in mice homozygous for human APOE ε2, ε3, and ε4, as described previously [9–12], were derived from animals sourced from Taconic (Germantown, NY, USA). APOE knock-out mice (B6.129P2 ApoE−/−, were originally obtained from the Jackson Laboratory, Bar Harbor, Maine). All mice were bred and maintained at the Animal Resources Centre (ARC, Perth, Western Australia). Mice were housed 5–6 per cage in a controlled environment at 22°C on a 12 h day/night cycle (light from 0700 to 1900 h). A standard laboratory chow diet (Rat and Mouse Cubes, Specialty Feeds Glen Forrest, WA, Australia) and water were consumed ad libitum. This study was conducted in accordance with the Australian code of practice for the care and use of animals for scientific purposes as specified by the National Health and Medical Research Council (NHMRC). The experimental protocols were approved by the University of Western Australia Animal Ethics Committee.
Preparation of Aβ peptides and lipid emulsions
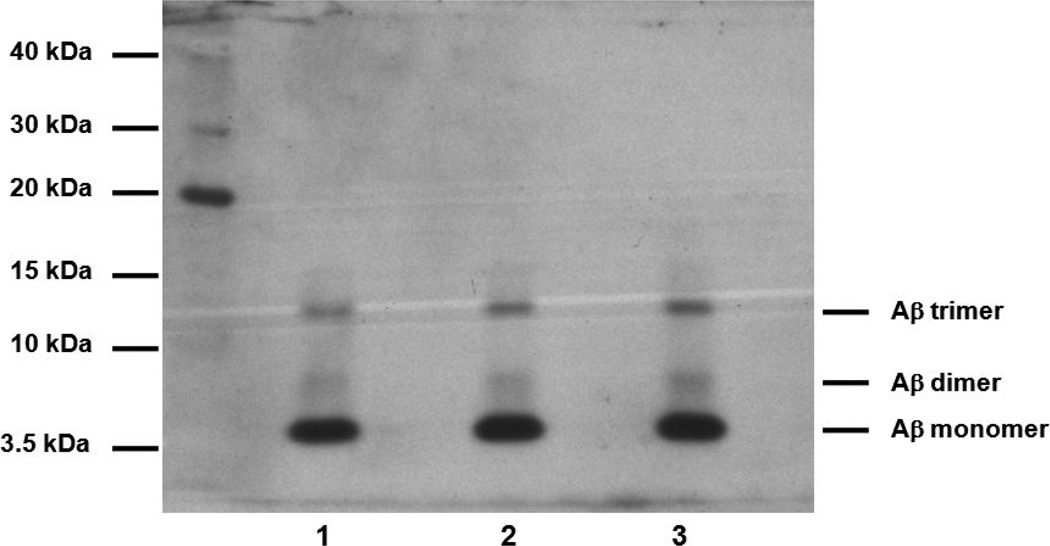
Human synthetic Aβ42 peptide was purchased from the W.M. Keck Foundation Biotechnology Resource Laboratory (Yale University, New Haven, CT). Stock Aβ42 was prepared by dissolving the Aβ42 peptide in 10% Dimethyl sulfoxide (DMSO) to a concentration of 1 mg/ml. The stock was diluted in sterile isotonic saline solution immediately before experimentation to a concentration of 20 µg in 50 µL. This preparation using our method yields a consistently predominantly monomeric Aβ42 preparation (Fig. 1).
Fig. 1.
Aβ peptide preparations were run on a 4–12% NUPAGE Novex Bis-Tris gels, lanes 1–3 represent Aβ42 preparations.
The composition of the remnant-like emulsions was (%by mass n =5) triolein 45.8%±3.2%, total cholesterol and cholesterol oleate 21.5% ± 3.2% and egg yolk phosphatidylcholine 32.7% ± 2.5%. The remnant like emulsion particles had a mean diameter of 133 nm ± 17.6 nm (mean ± SD) as measured by laser light scattering using the Malvern Instruments particle Zetasizer (Malvern Instruments, Worcestershire, United Kingdom). Partially lipidated human recombinant ApoE2, E3, and E4 (Invitrogen, Madison, WI, USA) were freeze dried, resuspended in isotonic saline and then lipidated by incorporation into lipid emulsion particles that were prepared by sonication and purified by ultracentrifugation as described previously [2,13].
Antibodies
Monoclonal WO2 antibody raised against amino acid residues 5 to 8 of the Aβ domain was generously provided by Professor Konrad Beyreuther (University of Heidelberg, Heidelberg, Germany).
Sampling of plasma Aβ levels
To examine if there may be any ApoE-isoform dependent effects in the peripheral clearance of Aβ, 12monthold human APOE ε2, ε3, and ε4 knock-in mice and APOE knock-out mice were anaesthetized with an intraperitoneal injection of Ketamine/Xylazine (75/10 mg/kg). APOE knock-in mice were injected with Aβ42 peptide (20 µg/50 µL) via the lateral tail vein. APOE knock-out mice were injected with Aβ42 (20 µg/50 µl) plus lipidated recombinant apoE (75 µg of rE2, rE3, rE4 or lipidated particle only). Blood was collected over a 60 min period. Blood samples were taken from the retro-orbital sinus using 1.0 mm diameter heparinised haematocrit tubes at 2.5, 5, 10, 15, 30, and 60 min post-injection for Aβ analysis. Plasma samples collected were stored at −80°C for subsequent analysis of Aβ levels.
Analysis of plasma Aβ42 content
Plasma samples (1 µl) were loaded onto 4–12% Bis/Tris NuPAGE® Novex® Mini Gels (Invitrogen, USA) with MES buffer and separated for 2.5 h at 90 V. The proteins were then transferred to nitrocellulose membranes using the iBlot™ Dry Blotting System (Invitrogen, USA) for 8 min at 20 V and immunoblotted. WO2 antibody (1:2,000 dilution), was incubated with membranes for 2 h at room temperature in Tris-buffered saline Tween-20 (TBST), pH 7.4 with 0.5% (w/v) skim milk. HRP-linked goat anti-mouse IgG (1:5,000 dilution) was incubated with membranes for 1 h at room temperature in TBST, pH7.4with 0.5%(w/v) skim milk. Protein visualization was achieved using enhanced chemiluminescence (ECL) western blotting detection reagents and exposure to hyperfilm-ECL film (GE Healthcare Bio-Sciences, Rydalmere, NSW, Australia). The ECL films were then scanned for densito-metric analysis.
Statistical analyses
Means and standard deviations were calculated for all variables using conventional methods. A repeated measures design and one-way ANOVA was used to evaluate significant differences amongst the genotypes. A criterion alpha level of P < 0.05 was used for all statistical comparisons. All data were analyzed using SPSS version 15.0 (SPSS, Chicago, IL, USA).
RESULTS
Peripheral clearance of Aβ42 from the plasma is reduced in APOE ε4 knock-in mice
To assess if APOE genotype affected the peripheral clearance of Aβ42 from the plasma, ε2, ε3, and ε4 knock-in mice were injected with Aβ42 and blood was collected over a 60 min period (Fig. 2). There was no detectable Aβ42 in the plasma of mice prior to injection (data not shown). The levels of plasma Aβ42 show a gradual decrease from 2.5 min post injection, to nearly undetectable levels at 60 min post injection. Western blot analysis of the blood demonstrated a prolonged retention of plasma Aβ42 across the time points for APOE ε4 animals (Fig. 2). Analysis of the data using a repeated measures design showed that there was a significant effect over time and between the different genotypes. In APOE ε4mice, plasma Aβ42 levels were estimated by densitometric analysis to be over 3 times the levels of Aβ42 in APOE ε2 and APOE ε3 mice at 2.5 min.
Fig. 2.
Clearance of Aβ42 from the plasma of 12-month old male human APOE ε2, ε3, and ε4 knock-in mice following tail vein injection of 20 µg/50 µl Aβ42 determined by Western blot quantification. Values are mean ± SEM of 8 animals. *P < 0.05 versus APOE ε2 2.5 min; #P < 0.05 vs. APOE ε3 2.5 min.
Peripheral clearance of Aβ42 from the plasma is also reduced in APOE knock-out mice injected with lipidated ApoE4
There was no detectable Aβ42 in the plasma of mice prior to injection (data not shown). The levels of plasma Aβ42 in the APOE knock-out mice showed a clear ApoE isoform and time effect consistent with results in APOE knock-in mice. APOE knock-out mice injected with lipidated rE2 and rE3 isoforms showed a rapid clearance of plasma Aβ42 over the 60 min post-injection (Fig. 3). Western blot analysis of the blood demonstrated a prolonged retention of plasma Aβ42 across the time points for rE4 injected animals and also control animals injected only with the lipidated particle (Fig. 3). In rE4 mice and lipidated particle only mice, plasma Aβ42 levels were estimated by densitometric analysis to be over 3 times the levels ofAβ42 in rE2 and rE3 mice at 2.5 min. There was a gradual decline in the plasma levels of Aβ in the rE4 and lipidated mice and after 15 min post injection, there were no significant differences detected via western blot analysis between any of the groups through to 60 min post injection.
Fig. 3.
Clearance of Aβ42 from the plasma of 12-month old male APOE KO mice, following tail vein co-injection of 75 µg recombinant ApoE (E2, E3, or E4) and 20 µg/50 µl h Aβ42 determined by Western blot quantification. Values are mean ± SEM of 12 animals. *P < 0.05 between corresponding groups; #P < 0.05 versus rE2 and rE3 groups.
DISCUSSION
Plasma Aβ levels in the periphery are typically low [14], indicating that a metabolic process facilitates rapid clearance of this protein in the periphery. Previous work from our laboratories [2,15] showed that the presence or absence of APOE affects the clearance of Aβ from the periphery in mice. While the mechanisms for the clearance/uptake of Aβ in the periphery are still poorly understood, we have demonstrated previously that the bulk of the Aβ is sequestered by the liver and kidney, but the liver is the major organ responsible for the uptake and degradation/excretion ofAβ peptides [2, 15].
In the current study, we have extended these findings and have observed that the clearance of Aβ42 appears to be dependent upon the expression of human ApoE isoforms. In particular, the plasma of APOE ε4 knock-in mice was found to have higher levels of Aβ42 over time than their ε2 and ε3 counterparts. However, a confounding factor when interpreting the results in the APOE knock-in mice is the higher plasma ApoE levels observed in ε2 mice, which can be up to approximately 15 times greater than ε3 and ε4mice [9,11]. Therefore, to verify these in vivo findings and to control the level of plasma ApoE, we also evaluated the peripheral clearance of Aβ42 in an APOE knock-out mouse model utilizing lipidated recombinant ApoE isoforms to control for the levels of plasma ApoE. Similar to the findings in the APOE knock-in mice, we observed a significant delay in the clearance ofAβ42 in APOE knock-out mice injected with lipidated recombinant ApoE4. We also demonstrated as in previous work [2], that the APOE knock-out mice injected with only lipidated vehicle deficient in ApoE, show the longest plasma retention of Aβ42. The implications of this observation suggests that humans carrying APOE ε4 might have a reduced ability to clear Aβ from their plasma and might possess higher levels of plasma Aβ. One possible reason for this is the different preferences of ApoE isoforms for particular lipoproteins [16]. In fact, poorly lipidated ApoE can contribute to the reduced clearance of Aβ and eventually increase its deposition [17].
The uptake of ApoE-Aβ complexes has been shown to be promoted by ApoE3 but not ApoE4 isoforms [3]. Aβ can also influence the binding and uptake of lipoproteins carrying ApoE isoforms [8]. Results from our current study indicate that Aβ42 exhibited a significantly reduced plasma clearance in both APOE ε4mice and APOE KO mice injected with lipidated recombinant ApoE4, compared to their counterparts. The differential clearance/uptake ofAβ by these APOE knock-in and APOE knock-out mice may be accounted for by the varied affinity of the ApoE isoforms for Aβ, as has been demonstrated in previous in vitro work [3,4, 6,7]. This increased binding of ApoE2 and E3 to Aβ may enhance the clearance of Aβ and also prevent the conversion of Aβ into neurotoxic species [6]. In vitro studies have shown that lipidated ApoE3 binds Aβ with a 20-fold greater affinity thanApoE4 [6,7]. Therefore, ApoE4 may be less functional in the peripheral clearance of Aβ owing to this weaker affinity to Aβ.
It is still unclear whether increased peripheral sequestration and degradation of Aβ may enhance the efflux of Aβ from the brain to the plasma, although, earlier studies by DeMattos and colleagues [1,18] showed that Aβ can be cleared to the plasma from the brain. Additionally, work by Matsuoka et al. [19] also showed that peripheral treatment with an agent having a high affinity for Aβ reduced brain levels of Aβ. Given that it is well established that APOE ε4 is a risk factor for the development of sporadic AD, then our novel findings will provide insight into the role of ApoE isoforms on the peripheral clearance of Aβ which in turn may impact on clearance from the brain. Identifying differences in the peripheral clearance of Aβ peptides across APOE genotypes has important pathological considerations when targeting therapeutic interventions in APOE ε4 carriers. This has the potential for the development of therapeutic agents aimed at increasing peripheral clearance of Aβ peptides in APOE ε4 carriers.
The main conclusion that may be drawn from this data is that the periphery has highly efficient pathways for the clearance of Aβ and that this is likely to be an ApoE dependent process. The rapid clearance of Aβ42 from the plasma of the APOE knock-in mice and APOE knock-out mice injected with lipidated recombinant ApoE and indicates a highly efficient system for Aβ clearance and possibly metabolism. From our data, it can therefore be concluded that expression of the APOE ε4 gene results in the reduced efficiency of Aβ clearance from the periphery. However, there was no difference in detectable levels of Aβ42 near the endpoint of the experiment suggesting the involvement of an alternative pathway which needs to be investigated by further study. Further work will be needed to study any products of Aβ42 metabolism in order to elucidate the main mechanism of its plasma/tissue clearance and degradation. These findings will provide insight into the role of ApoE isoforms on the peripheral clearance of Aβ, which in turn may impact on clearance from the brain.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institute of Health (AG10491to RNM, SG, JG, and JDB).
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=371).
REFERENCES
- 1.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-Abeta antibody alters CNS and plasma Abeta clearance and decreases brain Abeta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hone E, Martins IJ, Fonte J, Martins RN. Apolipoprotein E influences amyloid-beta clearance from the murine periphery. J Alzheimers Dis. 2003;5:1–8. doi: 10.3233/jad-2003-5101. [DOI] [PubMed] [Google Scholar]
- 3.Yang DS, Small DH, Seydel U, Smith JD, Hallmayer J, Gandy SE, Martins RN. Apolipoprotein E promotes the binding and uptake of beta-amyloid into Chinese hamster ovary cells in an isoform-specific manner. Neuroscience. 1999;90:1217–1226. doi: 10.1016/s0306-4522(98)00561-2. [DOI] [PubMed] [Google Scholar]
- 4.Yang DS, Smith JD, Zhou Z, Gandy SE, Martins RN. Characterization of the binding of amyloid-beta peptide to cell culture-derived native apolipoprotein E2, E3, and E4 isoforms and to isoforms from human plasma. J Neurochem. 1997;68:721–725. doi: 10.1046/j.1471-4159.1997.68020721.x. [DOI] [PubMed] [Google Scholar]
- 5.LaDu MJ, Lukens JR, Reardon CA, Getz GS. Association of human, rat, rabbit apolipoprotein E with beta-amyloid. J Neurosci Res. 1997;49:9–18. [PubMed] [Google Scholar]
- 6.LaDu MJ, Pederson TM, Frail DE, Reardon CA, Getz GS, Falduto MT. Purification of apolipoprotein E attenuates isoform-specific binding to beta-amyloid. J Biol Chem. 1995;270:9039–9042. doi: 10.1074/jbc.270.16.9039. [DOI] [PubMed] [Google Scholar]
- 7.LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to beta-amyloid. J Biol Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- 8.Hone E, Martins IJ, Jeoung M, Ji TH, Gandy SE, Martins RN. Alzheimer’s disease amyloid-beta peptide modulates apolipoprotein E isoform specific receptor binding. J Alzheimers Dis. 2005;7:303–314. doi: 10.3233/jad-2005-7406. [DOI] [PubMed] [Google Scholar]
- 9.Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, Sullivan PM, Maeda N. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest. 1999;103:1579–1586. doi: 10.1172/JCI6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan PM, Mezdour H, Quarfordt SH, Maeda N. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe*2. J Clin Invest. 1998;102:130–135. doi: 10.1172/JCI2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martins IJ, Vilcheze C, Mortimer BC, Bittman R, Redgrave TG. Sterol side chain length and structure affect the clearance of chylomicron-like lipid emulsions in rats and mice. J Lipid Res. 1998;39:302–312. [PubMed] [Google Scholar]
- 14.Gandy S, Almeida OP, Fonte J, Lim D, Waterrus A, Spry N, Flicker L, Martins RN. Chemical andropause and amyloid-beta peptide. JAMA. 2001;285:2195–2196. doi: 10.1001/jama.285.17.2195-a. [DOI] [PubMed] [Google Scholar]
- 15.Ghiso J, Shayo M, Calero M, Ng D, Tomidokoro Y, Gandy S, Rostagno A, Frangione B. Systemic catabolism of Alzheimer’s Abeta40 and Abeta42. J Biol Chem. 279:45897–45908. doi: 10.1074/jbc.M407668200. [DOI] [PubMed] [Google Scholar]
- 16.Weisgraber KH. Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J Lipid Res. 1990;31:1503–1511. [PubMed] [Google Scholar]
- 17.Wahrle SE, Jiang H, Parsadanian M, Hartman RE, Bales KR, Paul SM, Holtzman DM. Deletion of Abca1 increases Abeta deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem. 2005;280:43236–43242. doi: 10.1074/jbc.M508780200. [DOI] [PubMed] [Google Scholar]
- 18.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka Y, Saito M, LaFrancois J, Saito M, Gaynor K, Olm V, Wang L, Casey E, Lu Y, Shiratori C, Lemere C, Duff K. Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to beta-amyloid. J Neurosci. 2003;23:29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]