Abstract
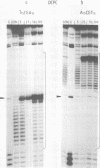
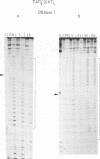
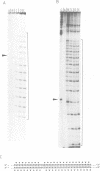
We have prepared DNA fragments containing the sequences A15CGT15, T15CGA15 and T(AT)8CG(AT)15 cloned within the SmaI site of the pUC19 polylinker. These have been used as substrates in footprinting experiments with DNase I and diethylpyrocarbonate probing the effects of echinomycin, binding to the central CG, on the structure of the surrounding sequences. No clear DNase I footprints are seen with T15CGA15 though alterations in the nuclease susceptibility of surrounding regions suggest that the ligand is binding, albeit weakly at this site. All the other fragments show the expected footprints around the CG site. Regions of An and Tn are rendered much more reactive to DNase I and adenines on the 3'-side of the CG become hyperreactive to diethylpyrocarbonate. Regions of alternating AT show unusual changes in the presence of the ligand. At low concentrations (5 microM) cleavage of TpA is enhanced, whereas at higher concentrations a cleavage pattern with a four base pair repeat is evident. A similar pattern is seen with micrococcal nuclease. Modification by diethylpyrocarbonate is strongest at alternate adenines which are staggered in the 5'-direction across the two strands. We interpret these changes by suggesting secondary drug binding within regions of alternating AT, possibly to the dinucleotide ApT. DNase I footprinting experiments performed at 4 degrees C revealed neither enhancements nor footprints for flanking regions of homopolymeric A and T suggesting that the conformational changes are necessary consequence of drug binding.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Hall I. H., Puigjaner L. C. Heteronomous DNA. Nucleic Acids Res. 1983 Jun 25;11(12):4141–4155. doi: 10.1093/nar/11.12.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymami J., Coll M., Frederick C. A., Wang A. H., Rich A. The propeller DNA conformation of poly(dA).poly(dT). Nucleic Acids Res. 1989 Apr 25;17(8):3229–3245. doi: 10.1093/nar/17.8.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresloff J. L., Crothers D. M. Equilibrium studies of ethidium--polynucleotide interactions. Biochemistry. 1981 Jun 9;20(12):3547–3553. doi: 10.1021/bi00515a038. [DOI] [PubMed] [Google Scholar]
- Fox K. R., Kentebe E. Echinomycin binding to the sequence CG(AT)nCG alters the structure of the central AT region. Nucleic Acids Res. 1990 Apr 25;18(8):1957–1963. doi: 10.1093/nar/18.8.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. R., Kentebe E. Footprinting studies on the effect of echinomycin on the structure of a bent DNA fragment. Biochem J. 1990 Jul 1;269(1):217–221. doi: 10.1042/bj2690217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. R., Wakelin L. P., Waring M. J. Kinetics of the interaction between echinomycin and deoxyribonucleic acid. Biochemistry. 1981 Sep 29;20(20):5768–5779. doi: 10.1021/bi00523a020. [DOI] [PubMed] [Google Scholar]
- Fox K. R., Waring M. J. Footprinting at low temperatures: evidence that ethidium and other simple intercalators can discriminate between different nucleotide sequences. Nucleic Acids Res. 1987 Jan 26;15(2):491–507. doi: 10.1093/nar/15.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. R., Waring M. J. The use of micrococcal nuclease as a probe for drug-binding sites on DNA. Biochim Biophys Acta. 1987 Jul 14;909(2):145–155. doi: 10.1016/0167-4781(87)90036-4. [DOI] [PubMed] [Google Scholar]
- Gao X. L., Patel D. J. NMR studies of echinomycin bisintercalation complexes with d(A1-C2-G3-T4) and d(T1-C2-G3-A4) duplexes in aqueous solution: sequence-dependent formation of Hoogsteen A1.T4 and Watson--Crick T1.A4 base pairs flanking the bisintercalation site. Biochemistry. 1988 Mar 8;27(5):1744–1751. doi: 10.1021/bi00405a054. [DOI] [PubMed] [Google Scholar]
- Gilbert D. E., van der Marel G. A., van Boom J. H., Feigon J. Unstable Hoogsteen base pairs adjacent to echinomycin binding sites within a DNA duplex. Proc Natl Acad Sci U S A. 1989 May;86(9):3006–3010. doi: 10.1073/pnas.86.9.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A., Jack A., Viswamitra M. A., Kennard O., Shakked Z., Steitz T. A. A hypothesis on a specific sequence-dependent conformation of DNA and its relation to the binding of the lac-repressor protein. J Mol Biol. 1979 Jul 15;131(4):669–680. doi: 10.1016/0022-2836(79)90196-7. [DOI] [PubMed] [Google Scholar]
- Low C. M., Drew H. R., Waring M. J. Sequence-specific binding of echinomycin to DNA: evidence for conformational changes affecting flanking sequences. Nucleic Acids Res. 1984 Jun 25;12(12):4865–4879. doi: 10.1093/nar/12.12.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan J. A., Palecek E., Lilley D. M. (A-T)n tracts embedded in random sequence DNA--formation of a structure which is chemically reactive and torsionally deformable. Nucleic Acids Res. 1986 Dec 9;14(23):9291–9309. doi: 10.1093/nar/14.23.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M. J., Waring M. J. Chemical probes reveal no evidence of Hoogsteen base pairing in complexes formed between echinomycin and DNA in solution. J Mol Recognit. 1988 Jun;1(3):138–151. doi: 10.1002/jmr.300010307. [DOI] [PubMed] [Google Scholar]
- Mendel D., Dervan P. B. Hoogsteen base pairs proximal and distal to echinomycin binding sites on DNA. Proc Natl Acad Sci U S A. 1987 Feb;84(4):910–914. doi: 10.1073/pnas.84.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal J., Fox K. R., McLean M. J., Richenberg J. L., Waring M. J. Diethyl pyrocarbonate can detect a modified DNA structure induced by the binding of quinoxaline antibiotics. Nucleic Acids Res. 1988 May 11;16(9):3655–3670. doi: 10.1093/nar/16.9.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs J. W., Wagner R. W. Nuclease recognition of an alternating structure in a d(AT)14 plasmid insert. Nucleic Acids Res. 1986 May 12;14(9):3703–3716. doi: 10.1093/nar/14.9.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ughetto G., Wang A. H., Quigley G. J., van der Marel G. A., van Boom J. H., Rich A. A comparison of the structure of echinomycin and triostin A complexed to a DNA fragment. Nucleic Acids Res. 1985 Apr 11;13(7):2305–2323. doi: 10.1093/nar/13.7.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke M. M., Dervan P. B. Echinomycin binding sites on DNA. Science. 1984 Sep 14;225(4667):1122–1127. doi: 10.1126/science.6089341. [DOI] [PubMed] [Google Scholar]
- Wakelin S. P., Waring M. J. The binding of echinomycin to deoxyribonucleic acid. Biochem J. 1976 Sep 1;157(3):721–740. doi: 10.1042/bj1570721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Ughetto G., Quigley G. J., Hakoshima T., van der Marel G. A., van Boom J. H., Rich A. The molecular structure of a DNA-triostin A complex. Science. 1984 Sep 14;225(4667):1115–1121. doi: 10.1126/science.6474168. [DOI] [PubMed] [Google Scholar]
- Ward B., Rehfuss R., Goodisman J., Dabrowiak J. C. Determination of netropsin-DNA binding constants from footprinting data. Biochemistry. 1988 Feb 23;27(4):1198–1205. doi: 10.1021/bi00404a020. [DOI] [PubMed] [Google Scholar]
- Ward B., Rehfuss R., Goodisman J., Dabrowiak J. C. Rate enhancements in the DNase I footprinting experiment. Nucleic Acids Res. 1988 Feb 25;16(4):1359–1369. doi: 10.1093/nar/16.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M. J., Wakelin L. P. Echinomycin: a bifunctional intercalating antibiotic. Nature. 1974 Dec 20;252(5485):653–657. doi: 10.1038/252653a0. [DOI] [PubMed] [Google Scholar]
- Waterloh K., Fox K. R. The effects of actinomycin on the structure of dAn.dTn and (dA-dT)n regions surrounding its GC binding site. A footprinting study. J Biol Chem. 1991 Apr 5;266(10):6381–6388. [PubMed] [Google Scholar]