Abstract
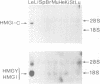
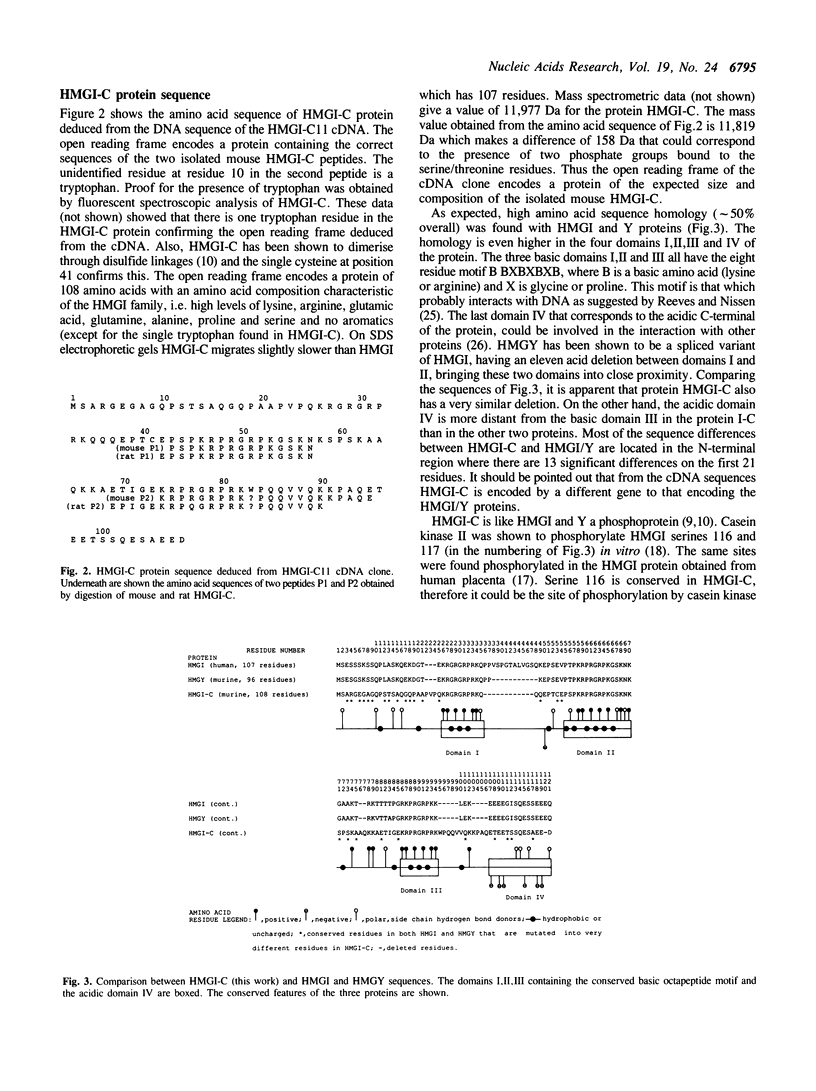
The HMGI-C protein is a nuclear phosphoprotein expressed at high levels in transformed cells. The cDNA encoding the mouse protein has been isolated and the sequence of the encoded protein shows that it is related to the HMGY and I proteins, proteins which bind in the minor groove of DNA containing stretches of A and T. The HMGI-C protein has three short highly basic domains, an acidic C-terminal domain, and potential CDC2/p34 and casein kinase II phosphorylation sites. Analysis of mRNA levels demonstrate that the HMGI-C gene is not expressed in a variety of mouse tissues but is expressed in Lewis lung carcinoma cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. T., Pendleton C. G., Jennings W. W., Saxena A., Glover C. V. Isolation and sequencing of cDNA clones encoding Drosophila chromosomal protein D1. A repeating motif in proteins which recognize at DNA. J Biol Chem. 1989 May 15;264(14):8394–8401. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Devlin C., Tice-Baldwin K., Shore D., Arndt K. T. RAP1 is required for BAS1/BAS2- and GCN4-dependent transcription of the yeast HIS4 gene. Mol Cell Biol. 1991 Jul;11(7):3642–3651. doi: 10.1128/mcb.11.7.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney J. E., Johnson K. R., Magnuson N. S., Sylvester S. R., Reeves R. High-mobility group protein HMG-I localizes to G/Q- and C-bands of human and mouse chromosomes. J Cell Biol. 1989 Nov;109(5):1975–1982. doi: 10.1083/jcb.109.5.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorssers L., Postmes A. M. A simplified, orientation-specific cDNA cloning strategy. Nucleic Acids Res. 1987 Apr 24;15(8):3629–3629. doi: 10.1093/nar/15.8.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G. Cell cycle control in eukaryotes: molecular mechanisms of cdc2 activation. Trends Biochem Sci. 1990 Oct;15(10):378–383. doi: 10.1016/0968-0004(90)90235-4. [DOI] [PubMed] [Google Scholar]
- Giancotti V., Bandiera A., Buratti E., Fusco A., Marzari R., Coles B., Goodwin G. H. Comparison of multiple forms of the high mobility group I proteins in rodent and human cells. Identification of the human high mobility group I-C protein. Eur J Biochem. 1991 May 23;198(1):211–216. doi: 10.1111/j.1432-1033.1991.tb16003.x. [DOI] [PubMed] [Google Scholar]
- Giancotti V., Berlingieri M. T., DiFiore P. P., Fusco A., Vecchio G., Crane-Robinson C. Changes in nuclear proteins on transformation of rat epithelial thyroid cells by a murine sarcoma retrovirus. Cancer Res. 1985 Dec;45(12 Pt 1):6051–6057. [PubMed] [Google Scholar]
- Giancotti V., Buratti E., Perissin L., Zorzet S., Balmain A., Portella G., Fusco A., Goodwin G. H. Analysis of the HMGI nuclear proteins in mouse neoplastic cells induced by different procedures. Exp Cell Res. 1989 Oct;184(2):538–545. doi: 10.1016/0014-4827(89)90352-2. [DOI] [PubMed] [Google Scholar]
- Giancotti V., Pani B., D'Andrea P., Berlingieri M. T., Di Fiore P. P., Fusco A., Vecchio G., Philp R., Crane-Robinson C., Nicolas R. H. Elevated levels of a specific class of nuclear phosphoproteins in cells transformed with v-ras and v-mos oncogenes and by cotransfection with c-myc and polyoma middle T genes. EMBO J. 1987 Jul;6(7):1981–1987. doi: 10.1002/j.1460-2075.1987.tb02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. H., Cockerill P. N., Kellam S., Wright C. A. Fractionation by high-performance liquid chromatography of the low-molecular-mass high-mobility-group (HMG) chromosomal proteins present in proliferating rat cells and an investigation of the HMG proteins present in virus transformed cells. Eur J Biochem. 1985 May 15;149(1):47–51. doi: 10.1111/j.1432-1033.1985.tb08891.x. [DOI] [PubMed] [Google Scholar]
- Grimaldi G., Manfioletti G., Schneider C. A lambda vector for directional cDNA cloning and in vitro transcription. Nucleic Acids Res. 1987 Nov 25;15(22):9608–9608. doi: 10.1093/nar/15.22.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen H. M., Admon A., Bell S. P., Tjian R. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990 Apr 26;344(6269):830–836. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- Johnson K. R., Lehn D. A., Elton T. S., Barr P. J., Reeves R. Complete murine cDNA sequence, genomic structure, and tissue expression of the high mobility group protein HMG-I(Y). J Biol Chem. 1988 Dec 5;263(34):18338–18342. [PubMed] [Google Scholar]
- Johnson K. R., Lehn D. A., Reeves R. Alternative processing of mRNAs encoding mammalian chromosomal high-mobility-group proteins HMG-I and HMG-Y. Mol Cell Biol. 1989 May;9(5):2114–2123. doi: 10.1128/mcb.9.5.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lund T., Holtlund J., Fredriksen M., Laland S. G. On the presence of two new high mobility group-like proteins in HeLa S3 cells. FEBS Lett. 1983 Feb 21;152(2):163–167. doi: 10.1016/0014-5793(83)80370-6. [DOI] [PubMed] [Google Scholar]
- Lund T., Holtlund J., Laland S. G. On the phosphorylation of low molecular mass HMG (high mobility group) proteins in Ehrlich ascites cells. FEBS Lett. 1985 Jan 28;180(2):275–279. doi: 10.1016/0014-5793(85)81085-1. [DOI] [PubMed] [Google Scholar]
- Lund T., Laland S. G. The metaphase specific phosphorylation of HMG I. Biochem Biophys Res Commun. 1990 Aug 31;171(1):342–347. doi: 10.1016/0006-291x(90)91399-d. [DOI] [PubMed] [Google Scholar]
- Lund T., Laland S. G. The metaphase specific phosphorylation of HMG I. Biochem Biophys Res Commun. 1990 Aug 31;171(1):342–347. doi: 10.1016/0006-291x(90)91399-d. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Christenson E., Litchfield D. W., Krebs E. G., Eisenman R. N. Myb DNA binding inhibited by phosphorylation at a site deleted during oncogenic activation. Nature. 1990 Apr 5;344(6266):517–522. doi: 10.1038/344517a0. [DOI] [PubMed] [Google Scholar]
- Palvimo J., Linnala-Kankkunen A. Identification of sites on chromosomal protein HMG-I phosphorylated by casein kinase II. FEBS Lett. 1989 Oct 23;257(1):101–104. doi: 10.1016/0014-5793(89)81796-x. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Reeves R., Elton T. S., Nissen M. S., Lehn D., Johnson K. R. Posttranscriptional gene regulation and specific binding of the nonhistone protein HMG-I by the 3' untranslated region of bovine interleukin 2 cDNA. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6531–6535. doi: 10.1073/pnas.84.18.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Langan T. A., Nissen M. S. Phosphorylation of the DNA-binding domain of nonhistone high-mobility group I protein by cdc2 kinase: reduction of binding affinity. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1671–1675. doi: 10.1073/pnas.88.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Nissen M. S. The A.T-DNA-binding domain of mammalian high mobility group I chromosomal proteins. A novel peptide motif for recognizing DNA structure. J Biol Chem. 1990 May 25;265(15):8573–8582. [PubMed] [Google Scholar]
- Singh J., Dixon G. H. High mobility group proteins 1 and 2 function as general class II transcription factors. Biochemistry. 1990 Jul 3;29(26):6295–6302. doi: 10.1021/bi00478a026. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Strauss F., Varshavsky A. A mammalian high mobility group protein recognizes any stretch of six A.T base pairs in duplex DNA. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1276–1280. doi: 10.1073/pnas.83.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss F., Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984 Jul;37(3):889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Vartiainen E., Palvimo J., Mahonen A., Linnala-Kankkunen A., Mäenpä P. H. Selective decrease in low-Mr HMG proteins HMG I and HMG Y during differentiation of mouse teratocarcinoma cells. FEBS Lett. 1988 Feb 8;228(1):45–48. doi: 10.1016/0014-5793(88)80581-7. [DOI] [PubMed] [Google Scholar]
- Watt F., Molloy P. L. High mobility group proteins 1 and 2 stimulate binding of a specific transcription factor to the adenovirus major late promoter. Nucleic Acids Res. 1988 Feb 25;16(4):1471–1486. doi: 10.1093/nar/16.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Yen H. F., Rothblum L. I. Purification and characterization of a high-mobility-group-like DNA-binding protein that stimulates rRNA synthesis in vitro. Mol Cell Biol. 1988 Aug;8(8):3406–3414. doi: 10.1128/mcb.8.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]