Abstract
Loss of plasma membrane integrity is a feature of acute cellular injury/death in vitro and in vivo. Plasmalemma-resealing agents are protective in acute central nervous system injury models, but their ability to reseal cell membranes in vivo has not been reported. Using a mouse controlled cortical impact (CCI) model, we found that propidium iodide-positive (PI+) cells pulse labeled at 6, 24, or 48 hours maintained a degenerative phenotype and disappeared from the injured brain by 7 days, suggesting that plasmalemma permeability is a biomarker of fatal cellular injury after CCI. Intravenous or intracerebroventricular administration of Kollidon VA64, poloxamer P188, or polyethylene glycol 8000 resealed injured cell membranes in vivo (P<0.05 versus vehicle or poloxamer P407). Kollidon VA64 (1 mmol/L, 500 μL) administered intravenously to mice 1 hour after CCI significantly reduced acute cellular degeneration, chronic brain tissue damage, brain edema, blood–brain barrier damage, and postinjury motor deficits (all P<0.05 versus vehicle). However, VA64 did not rescue pulse-labeled PI+ cells from eventual demise. We conclude that PI permeability within 48 hours of CCI is a biomarker of eventual cell death/loss. Kollidon VA64 reduces secondary damage after CCI by mechanisms other than or in addition to resealing permeable cells.
Keywords: blood–brain barrier, edema, Kollidon VA64, mice, plasmalemma, traumatic brain injury
Introduction
Traumatic brain injury (TBI) triggers delayed and progressive loss of plasmalemma integrity in cells within vulnerable brain regions (Singleton and Povlishock, 2004; Whalen et al, 2008). Plasma membrane permeability may induce loss of cellular homeostasis and is implicated as an initiating event in the pathogenesis of cell death in vitro (Geddes et al, 2003a,2003b; Gennarelli et al, 1989; Prado et al, 2005; Serbest et al, 2006) and in central nervous system insults (Curry et al, 2004; Kilinc et al, 2007; Marks et al, 2001; Singleton and Povlishock, 2004; Whalen et al, 2008). Plasmalemma damage may also occur secondarily as a result of oxidative stress and activation of intrinsic cell death programs.
Using an in vivo propidium iodide (PI) pulse-labeling protocol, we previously reported that plasmalemma permeability occurs early after controlled cortical impact (CCI) in mice, and that permeabilized cells remain in the injured brain much longer than previously anticipated (Whalen et al, 2008). In that study, cells that became permeable to PI within 2 hours after injury disappeared from the brain by 7 days, suggesting fatal cell damage. However, whether the same is true for cells which become permeable at later times was not addressed. This is an important issue because cells that exhibit delayed permeability might be more amenable to rescue.
Synthetic polymers such as poloxamer P188 are putative membrane-resealing agents that have cytoprotective activity in vitro (Grindel et al, 2002; Kilinc et al, 2008; LaPlaca and Thibault, 1998; Lee et al, 1992; Marks et al, 2001; Pettus et al, 1994; Serbest et al, 2005, 2006) and in acute central nervous system injury paradigms (Borgens et al, 2004; Cadichon et al, 2007; Curry et al, 2004; Frim et al, 2004). However, resealing of permeable cells in vivo has never been shown by any pharmacological agent. Here, we tested the hypotheses that plasmalemma permeability within 48 hours of CCI is a marker of eventual cellular demise, and that polymers with membrane-resealing activity in vitro restore plasmalemma integrity, reduce histopathology, and improve functional outcome after CCI in mice. We found that Kollidon VA64 significantly reseals permeable cell membranes and reduces secondary damage after CCI, but does not rescue injured (permeable) cells from eventual demise. The data are discussed in the context of possible membrane resealing-dependent and resealing-independent mechanisms of VA64.
Materials and methods
Animals
Studies were performed using 12-week-old adult male CD-1 mice (Charles River, Wilmington, MA, USA) weighing 25 to 35 g. Mice were housed in a pathogen-free environment with 12-hour day–night cycles. All procedures followed protocols approved by the MGH Institution for Animal Care and Use Committee in accordance with the NIH Guide for Care and Use of Laboratory Animals. In all experiments, data were obtained by investigators blinded to the study group.
Mouse Controlled Cortical Impact Model
A CCI model was used as described previously (Bermpohl et al, 2007) with minor modifications (velocity 6 m/sec, depth 0.5 mm, and duration 100 milliseconds). Mice were anesthetized with 4% isoflurane (Anaquest, Memphis, TN, USA) in 70% N2O and 30% O2 using a Fluotec 3 vaporizer (Colonial Medical, Amherst, NH, USA). Blow by anesthesia was maintained with 3.5% isoflurane for the duration of surgery. The head was positioned in a stereotaxic apparatus and a midline scalp incision was made. A 5.0-mm craniotomy was made lateral to the sagittal suture between the bregma and the lambda using a portable drill and trephine. Craniotomy was carefully removed without damaging the underlying dura, and the cortex was injured using a pneumatically controlled impactor device with a 3.0-mm diameter flat-tip impounder. After injury, the scalp was sutured closed and mice were returned to their cages to recover from anesthesia. Sham-injured mice received isoflurane anesthesia and craniotomy, but not CCI.
Preparation of Brain Tissue for Histochemistry
Mice were deeply anesthetized with isoflurane and the brains removed and frozen in liquid nitrogen vapor and stored at −80°C. The brains were sectioned (12 μm) in the coronal plane on a cryostat. Sections 150 to 200 μm apart from the anterior to the posterior hippocampus (bregma −1.90 to −2.70) were collected on poly--lysine-coated glass slides and stored at −80°C.
Assessment of Degeneration in Pulse-Labeled Propidium Iodide-Positive Cells
Mice were subjected to CCI, administered PI (1 μg/g, intravenous) at 6, 24, or 48 hours, and killed 24 hours later at 30, 48, or 72 hours, respectively. Propidium iodide-positive (PI+) cells were photographed using a Nikon Eclipse T300 fluorescence microscope (Nikon, Tokyo, Japan) using excitation/emission wavelengths 568 and 585 nm, respectively. Sections were then counterstained with hematoxylin and eosin (H&E). Care was taken to photograph the same brain regions used for analysis of PI+ cells. Propidium iodide-positive and argyrophilic cells (dark, shrunken nuclei) were counted and randomly chosen from × 400 cortical or hippocampal fields from 2 mice at each time point for a total of 10 fields counted as described previously (Whalen et al, 2008).
Assessment of Disappearance of Pulse-Labeled Propidium Iodide-Positive Cells from the Injured Brain
Mice were administered PI (1 μg/g mouse intravenous) at 6, 24, or 48 hours after CCI and killed 24 hours, 48 hours, or 7 days later. Propidium iodide-positive cells were detected by fluorescence microscopy and quantitated in 6 cortical and in 3 hippocampal × 200 fields randomly chosen from 5 brain sections separated by at least 250 μm, located within the confines of the cavitary lesion (Whalen et al, 2008). Cell count data were averaged per × 200 field for each mouse.
Administration of Polymers and Evaluation of Plasmalemma Resealing
Protocol 1: At 60 minutes after CCI, mice were administered the green fluorescent membrane-impermeant dye YOYO-1 (Molecular Probes; 1 μg/g mouse in 100 μL phosphate-buffered saline (PBS)) intravenously followed 5 to 10 minutes later by VA64 (1 mmol/L, 500 μL, BASF, Florham Park, NJ, USA, except as noted), poloxamer P188 (5 mmol/L, 500 μL, BASF), poloxamer P407 (5 mmol/L, 500 μL, BASF), polyethylene glycol 8000 (PEG 8000, 5 mmol/L, 500 μL, Sigma, St Louis, MO, USA), or PBS pH 7.4 (500 μL). Polymer doses were chosen based on results of pilot studies in which the maximum concentration tolerated by mice was assessed for resealing ability. At 5 to 10 minutes after polymers/PBS, PI (1 μg/g in 100 μL PBS) was administered intravenously. Mice were killed 5 to 10 minutes after PI administration. In alternate experiments, mice were administered all fluorescent compounds and polymers/PBS in both cerebral ventricles (intracerebroventricularly) as follows: YOYO-1 (750 ng in 6 μL), VA64 (1 mmol/L, 10 μL) or PBS (10 μL), PI (15 ng in 6 μL). Cryostat brain sections were photographed using excitation and emission wavelengths for YOYO-1 (490 and 520 nm, respectively) and PI. Injured cells were defined as YOYO-1+. Resealed cells were defined as YOYO-1+/PI−, and permeable cells as YOYO-1+/PI+. Cell counts were performed in 6 cortical and in 4 dentate gyrus × 200 fields randomly chosen from 5 brain sections separated by at least 150 μm, located within the center of the contusion. Cell count data were averaged per × 200 field for each mouse.
In protocol 2, polymers or PBS were administered intravenously 1 hour after CCI. Experimental outcomes were blood–brain barrier (BBB) permeability (1 to 24 hours), brain edema (24 or 48 hours), brain tissue loss (2 weeks), acute cellular degeneration (H&E staining for argyrophilic cells at 6 hours), hippocampal caspase 3/7 activity (48 hours), and motor function (days 1 to 7).
Measurement of Blood–Brain Barrier Permeability
At 1 hour after CCI, mice were coinjected with a mixture of Evans blue (1%, 100 μL) plus polymers (all 5 mmol/L, 500 μL, except VA64 in which 1 mmol/L was used) or PBS (500 μL) for a total injection volume of 600 μL. At 24 hours after CCI, mice were anesthetized and transcardially perfused with at least 25 mL saline. The brain was separated into right (uninjured) and left (injured) hemispheres. The two hemispheres were weighed and placed separately in 3 mL of N,N-dimethylformamide for 5 days. The solvent was then analyzed spectrophotometrically at 620 nm. Blood–brain barrier permeability was quantitated as μg Evans blue/g brain from a standard curve.
Assessment of Brain Edema
Brain edema was assessed by magnetic resonance (MR) imaging and by the (wet−dry)/wet brain weight method. For MR imaging analyses, mice were anesthetized with isoflurane and secured in an MR-compatible headset. Magnetic resonance imaging acquisitions were performed using a horizontal bore 9.4-T magnet (Bruker Biospin, Billerica, MA, USA) with a 21-cm bore fitted with a custom-built mouse head surface radio frequency transmitter and receiver coil. T2 images were acquired using a spin echo sequence with repetition time/echo time=2,500/60 milliseconds. All images were acquired with 150 μm in-plane resolution and 1-mm slice thickness.
Analysis of MR images was performed using OsiriX for manual selection and measurement of regions of interest. Regions of interest (injured cortex and subcortical tissue) were measured three separate times to determine error in lesion volume measurement, with images assessed in a random sequence to diminish systematic selection bias. Statistical analysis of lesion volume was performed using JMP 8 (JMP, Cary, NC, USA).
For the wet–dry weight method, mice were killed at 24 or 48 hours and their brains removed and bisected at the midline to separate the right and left hemispheres. The cerebellum and brain stem were removed. The uninjured brain tissue both anterior and posterior to the contusion was removed by sectioning in the coronal plane. The wet weight of the remaining injured and uninjured hemispheres was obtained. The brain tissue was dried at 99°C for 48 hours and dry hemispheric weights were obtained. Brain water content was expressed as (wet−dry/wet) weight × 100%. Brain edema was expressed as the difference in % brain water content (left–right hemisphere).
Assessment of Brain Tissue Loss
Morphometric image analysis (NIS Elements, Nikon) was used to determine brain tissue loss as described previously (Bermpohl et al, 2007). Coronal brain sections (12 μm) were cut on a cryostat at 0.5-mm intervals from anterior to the posterior and mounted on poly--lysine-coated slides. Sections were stained with hematoxylin, and the area of the lesion was quantitated using image analysis. Hemispheric volume was calculated by obtaining the total volume of brain tissue in the right (uninjured) and left (injured) hemispheres. Lesion volume was the difference between the right and left hemispheres (mm3).
Effect of VA64 on Degenerative Cells Using Hematoxylin and Eosin
Mice were subjected to CCI and administered VA64 or PBS at 1 hour and were killed at 6 hours after injury. Cryostat brain sections were prepared for histochemistry as described above and stained with H&E. Degenerative cells (dark, shrunken nuclei with hypereosinophilic cytoplasm) were quantitated in 6 randomly chosen × 400 fields in the cortex and in 4 randomly chosen hippocampal fields.
Caspase 3/7 Activity Assay
Anesthetized mice were decapitated and the ipsilateral cortex and hippocampus were rapidly dissected on ice and transferred into the Triton lysis buffer (1% Triton, 20 mmol/L Tris-HCL, 150 mmol/L NaCl, 5 mmol/L EGTA, 10 mmol/L EDTA, 10% glycerol) with protease inhibitors (Complete Mini Protease Inhibitor Cocktail tablet, Roche, Indianapolis, IN, USA). Samples were then briefly sonicated and centrifuged at 14,000 r.p.m. for 30 minutes at 4°C. Protein concentration was determined in supernatants (Bio-Rad DC Protein Assay; Bio-Rad, Hercules, CA, USA), and samples were diluted to 1 mg/mL. The Caspase-Glo 3/7 luminescent assay (Promega Corporation, Madison, WI, USA) was used to determine caspase 3/7 activities in 50 μg brain samples using a luciferase-based assay and the DEVD tetrapeptide sequence according to the instructions of the manufacturer.
Evaluation of Motor Performance
Vestibulomotor function was assessed using composite neuroscore and wire grip tests, as described previously (Mbye et al, 2008; You et al, 2008). For the wire grip test, mice were placed on a metal wire (45 cm long) suspended 45 cm above a foam pad and allowed to traverse the wire for 60 seconds. The latency that a mouse remained on the wire within a 60-second interval was measured, and wire grip scores were quantitated using a 5-point scale. For the composite neuroscore, mice were given an integer score from 0 (severely impaired) to 4 (normal) for each of the following indices: limb flexion during suspension by the tail; foot falls on a grid walk; and the ability to resist lateral pulsion towards the left and right (Murai et al, 1998; Nakamura et al, 1999; Raghupathi, 2004; Riess et al, 2007; Saatman et al, 1997).
Effect of VA64 on Rescue of Injured (Propidium Iodide-Positive) Cells
One cohort of mice (n=6 per group) was administered PI at the time of CCI and VA64 or PBS intravenously at 60 minutes after injury. A second cohort of mice (n=6 per group) was administered PI at 6, 24, and 48 hours and VA64 or PBS at 6.5, 24.5, or 48.5 hours after CCI. Mice were killed at 7 days and brain sections analyzed for the presence of PI+ cells in 6 × 200 cortical and 3 × 200 hippocampal fields from at least 6 brain sections within the cavitary lesion separated by at least 250 μm. Propidium iodide-positive cell counts for each mouse were the average of × 200 cortical or hippocampal fields.
Statistical Analyses
Data are mean±s.e.m. Cell count data, caspase activity assay data, edema, BBB, lesion volume, MR imaging T2 volumetric data, and composite neuroscore data were evaluated by ANOVA (analysis of variance), followed by the Student–Newman–Keul or t-test. Correlation between degenerative cells (H&E) and PI+ cells was assessed using Pearson's product moment correlation. Wire grip test data were analyzed by two-factor repeated-measures ANOVA (group × time). For all comparisons, P<0.05 was considered significant.
Results
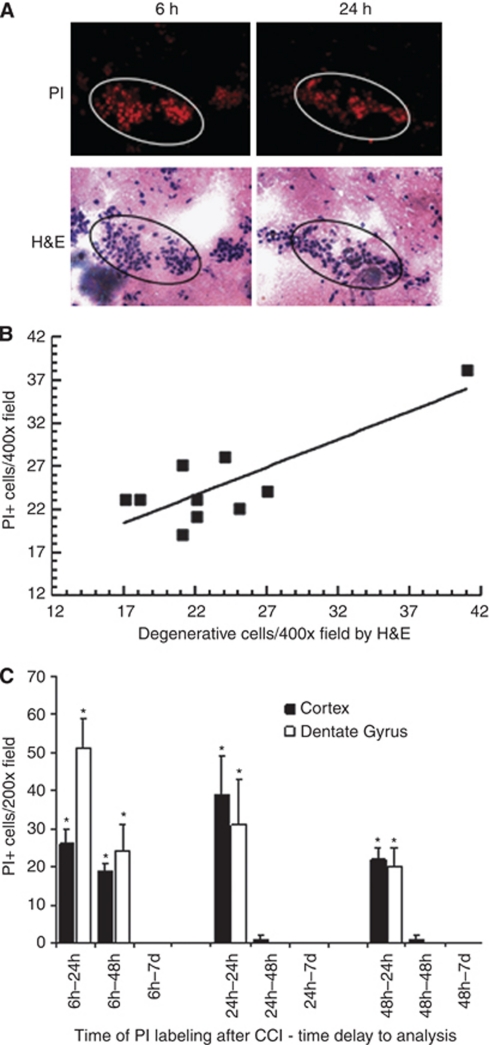
Having previously reported fatal outcome in cells labeled with PI between 0 to 2 hours after CCI (Whalen et al, 2008), we first sought to determine whether PI+ cells labeled at later times after injury might escape degeneration. Figure 1 shows typical results of PI pulse labeling and H&E staining. Nearly all PI+ cells labeled at 6, 24, or 48 hours and allowed to survive for 24 hours were identified as degenerative (argyrophilic) by H&E staining (representative H&E staining of degenerative PI+ cells shown in Figure 1A). Of 247 PI+ cells counted, 240 (97%) were degenerative by H&E criteria (r=0.82, P<0.005, Figure 1B). To determine whether PI+ cells might survive injury and remain in the brain, mice were subjected to CCI and PI+ cells pulse labeled at 6, 24, or 48 hours, and the brains analyzed at various times after pulse labeling for the presence of PI+ cells. Figure 1C shows a time-dependent decrease in PI+ cells labeled at 6, 24, or 48 hours. By 7 days, nearly all PI+ cells labeled at each of the three time points had disappeared from the injured brain, strongly suggesting that plasmalemma damage portends fatal cellular injury.
Figure 1.
Propidium iodide (PI) labeling identifies degenerating cells after controlled cortical impact (CCI). Mice were subjected to CCI and administered PI at the times indicated (6, 24, or 48 hours) and allowed to survive another 24 hours for assessment of cellular degeneration by H&E staining. (A) Representative photomicrographs showing correspondence between PI (top panel) and H&E staining (bottom panel) from the dentate gyrus. Only degenerative cells with shrunken, darkly stained nuclei are defined as positively stained by H&E. (B) Correlation between PI labeling and H&E staining in degenerating cells. The number of PI+ cells strongly correlated with that of argyrophilic/degenerative cells (r=0.82, P<0.005, n=3 to 4 mice per time point). Scale bars=20 μm. (C) Disappearance of PI-positive cells pulse labeled at 6, 24, or 48 hours, after CCI. Mice (n=3 to 6 per time point) were administered PI at 6, 24, or 48 hours after CCI and killed at 24 or 48 hours, or 7 days later. PI-positive cells were quantified in injured cortical and hippocampal (CA-1, CA-3, and DG) regions. Almost no PI+ cells were detected at 7 days after labeling at 6, 24, or 48 hours. CTX: cortex; DG: dentate gyrus. *P<0.05 versus 7 days. H&E, hematoxylin and eosin.
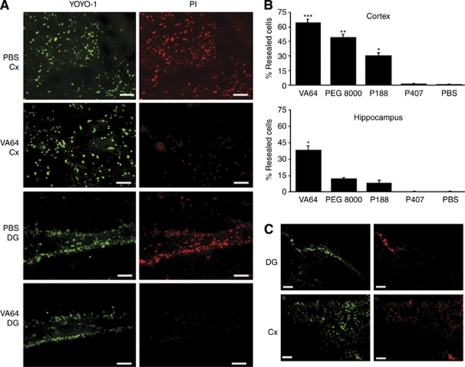
We next performed a set of experiments to determine whether putative membrane-resealing agents might reseal injured (permeable) cells in vivo. Figure 2A shows representative photomicrographs of resealed (YOYO-1+/PI−) cells and non-resealed (YOYO-1+/PI+) cells by VA64 and PBS administration, respectively. Compared with PBS and P407, VA64, P188, and PEG 8000 induced significantly more YOYO-1+/PI− cells in the injured hippocampus, and in the cortex, VA64 resealed significantly more cells compared with all of the other polymers tested (Figures 2B and 2C). In a subset of experiments in which fluorophores and polymers were administered intracerebroventricularly, VA64 again induced robust membrane resealing in all four mice (representative photomicrographs are shown in Figure 2C).
Figure 2.
Evidence of plasma membrane resealing by Kollidon VA64 after controlled cortical impact (CCI). (A) Representative micrographs depicting cells labeled with YOYO-1 (green) and PI (red) in the cortex and DG in PBS-treated or VA64-treated mice (bottom panel). Scale bars=20 μm. (B) Quantitation of YOYO-1+/PI− (resealed) cells in the cortex and hippocampus of PBS- or polymer-treated mice (n=3 to 7 per group). Upper panel: ***P<0.05 versus all other groups; **P<0.05 versus P188, P407, and PBS; *P<0.05 versus P407 and PBS. Lower panel: *P<0.05 versus PBS and P407. (C) Representative photomicrographs showing plasmalemma resealing using intracerebroventricular administration of fluorophores and polymers (n=4 per group). It must be noted that robust membrane resealing is observed using VA64, suggesting a direct effect of VA64 on the plasmalemma of parenchymal cells. Scale bars, 100 μm. DG, dentate gyrus; PBS, phosphate-buffered saline; PI, propidium iodide. The colour reproduction of this figure is available at the Journal of Cerebral Blood Flow & Metabolism journal online.
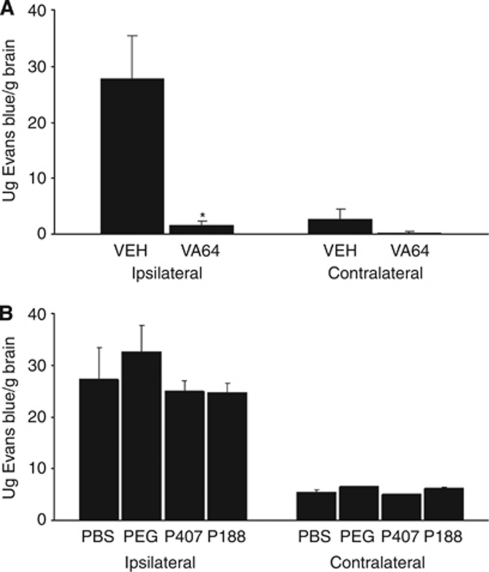
We next assessed the ability of polymers to prevent BBB leakage of Evans blue from 1 to 24 hours after CCI. Blood–brain barrier damage was increased in injured versus uninjured hemispheres of PBS-treated mice (P=0.01), but not in VA64-treated mice (P=0.15, Figure 3A). Compared with PBS treatment, VA64 significantly reduced Evans blue extravasation in injured hemispheres (P<0.01, Figure 3A). None of the other agents tested reduced BBB damage (Figure 3B).
Figure 3.
Reduction of blood–brain barrier damage by VA64 after controlled cortical impact. (A) VA64 significantly reduced Evans blue leakage into the injured (left; L) hemispheres of VA64-treated mice (P<0.01 versus PBS) and uninjured (right; R) hemispheres (*P<0.05 versus PBS, n=6 per group). (B) No differences were observed between the other polymers and PBS (n=5 to 7 per group). PBS, phosphate-buffered saline.
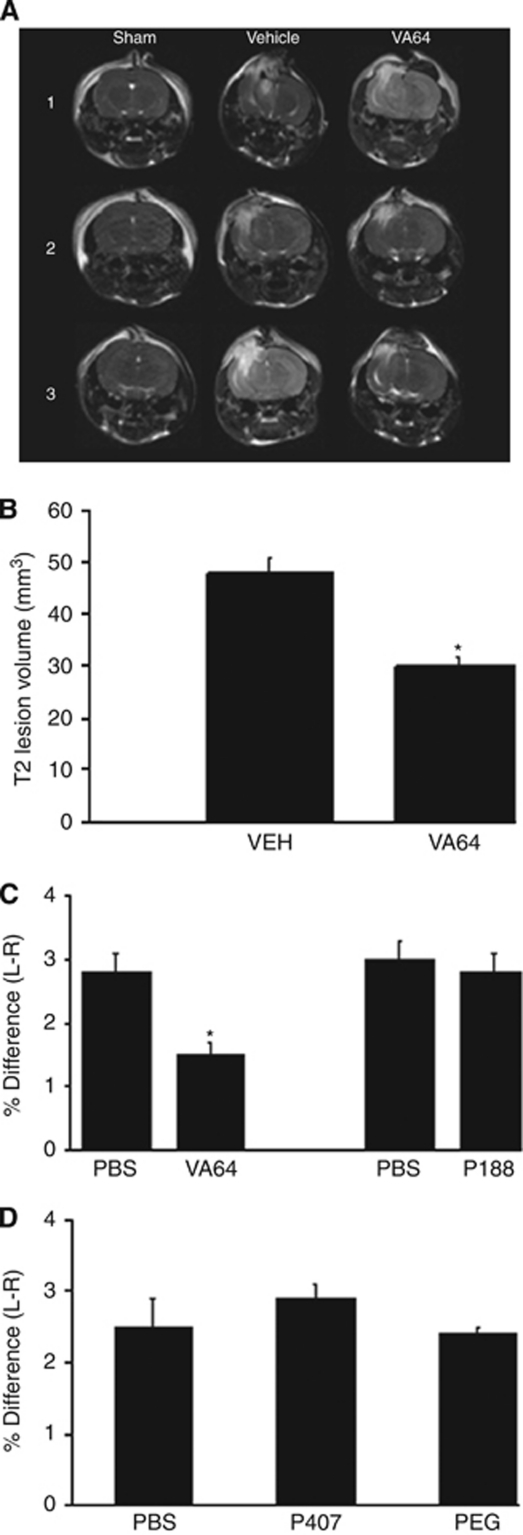
As BBB damage may contribute to brain edema, we next assessed the ability of polymers to reduce posttraumatic brain water content. VA64 significantly reduced T2-weighted lesion volume in injured mice (P<0.01 versus PBS, Figures 4A and 4B). Brain edema assessed by wet to dry weight was reduced by 40% in VA64-treated mice at 24 hours (P<0.01 versus PBS, Figure 4C) but not at 48 hours (VA64, 2.2+0.3% PBS, 3.0+0.3% difference; P=0.067, n=12 per group). Using 250 μL of a 2 mmol/L VA64 solution, brain edema was again reduced by ∼40% (VA64, 1.6+0.2% PBS, 2.3+0.2%, P<0.05, n=6 per group); however, a lower dose of VA64 (1 mmol/L, 200 μL) that reseals injured cortical and hippocampal cells (not shown) had no effect on 24 hours brain edema (VA64, 2.0+0.2% PBS, 1.9+0.2%, n=6 per group), suggesting that membrane resealing alone may not fully explain the reduction in edema by VA64. None of the other polymers tested reduced brain edema after CCI (Figures 4C and 4D).
Figure 4.
Treatment with Kollidon VA64 after controlled cortical impact (CCI) decreases postinjury brain edema. (A) Representative T2-weighted images of sham-injured (1), injured, VA64-treated (2), and injured, PBS-treated (3) mice (n=3 per group). (B) VA64-treated mice had decreased T2 volume than did PBS-treated mice (P<0.01, n=3 per group). (C and D) Bar graphs showing significant reduction in the percentage difference in brain water content (injured–uninjured hemispheres) in VA64- versus PBS-treated mice, but no reduction in brain edema by any of the other polymers tested. L–R, injured–uninjured hemisphere (*P<0.01 versus PBS, n=6 per group). PBS, phosphate-buffered saline.
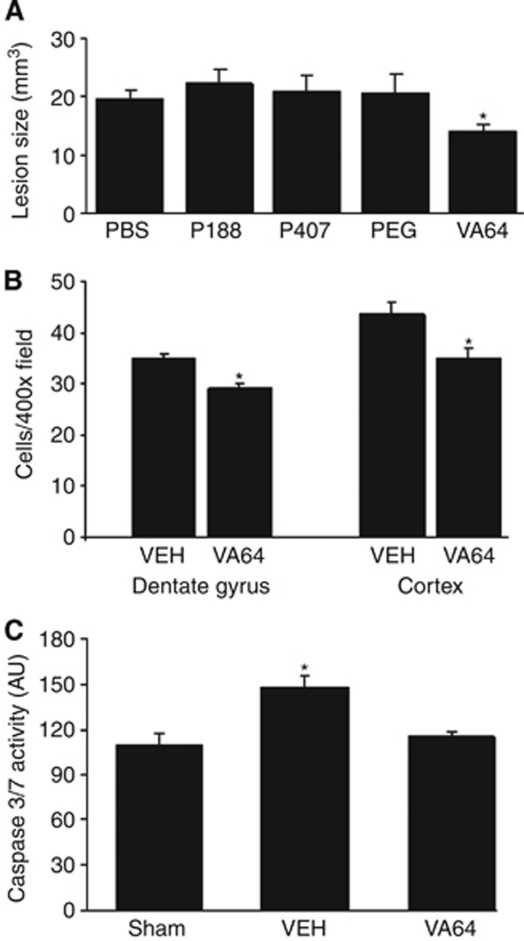
VA64 (but not the other polymers) reduced overall brain tissue damage by 29% (P<0.05 versus PBS; Figure 5A) and modestly reduced argyrophilic cells at 6 hours in the cortex and hippocampus (P<0.05 versus PBS for both regions, Figure 5B). Compared with sham-injured animals, injured PBS-treated mice had significantly increased caspase 3/7 activity in the injured hippocampus (Figure 5C) that was significantly reduced compared with PBS-treated, injured mice (P<0.05 versus PBS) and no different from sham-injured mice (Figure 5C).
Figure 5.
Kollidon VA64 reduces brain lesion volume, cellular degeneration, and caspase activity after controlled cortical impact (CCI). (A) Mice were subjected to CCI, administered polymers or PBS at 1 hour, and killed at various times after injury. Posttraumatic brain tissue loss at 14 days was decreased in VA64-treated mice (*P<0.05 versus PBS treated, n=12 per group for PBS and VA64, n=7 per group for PEG 8000, P407, and n=6 per group for P188). (B) Quantitation of degenerative cells in PBS- or VA64-treated mice in the dentate gyrus and cortex show a modest reduction in degenerative cells by VA64 in both regions (*P<0.05 versus PBS, n=6 per group). (C) Reduced caspase activity by VA64. Caspase 3/7 activity was measured at 48 hours in the injured hippocampus. Marked reduction in caspase 3/7 activity was detected in mice treated with VA64 (*P<0.05 versus sham-injured and injured, VA64-treated mice, n=6 per group). PBS, phosphate-buffered saline.
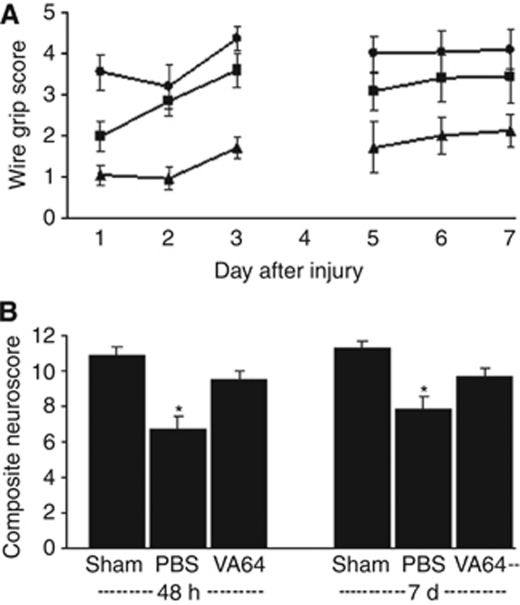
We next assessed the effect of polymers on postinjury motor function. Significant deficits in the wire grip and composite neuroscore tests were observed in injured mice (P<0.05 versus sham injured). VA64 improved wire grip scores to a level similar to that of sham-injured mice (P<0.05 versus PBS, group effect, Figure 6A) and improved composite neuroscores by 20% compared with PBS (P<0.05 for days 2 and 7) (Figure 6B). Wire grip test scores were not improved by any of the other polymers (P>0.05 for group, PEG 8000, P407, and P188; n=7, 7, and 6 per group respectively, data not shown).
Figure 6.
Posttreatment with Kollidon VA64 attenuates motor deficits after controlled cortical impact (CCI). (A) Injured PBS-treated animals (n=8) had impaired wire grip scores compared with sham-injured animals (n=10) (P<0.05 group effect). In contrast, wire grip scores of VA64-treated mice (n=8) did not differ from those of sham injured, and were significantly higher than those of PBS-treated mice (P<0.05 group effect). (B) Composite neuroscores of injured, PBS-treated animals (n=12) were significantly lower than those of sham-injured animals (n=10) at both 48 hours and 7 days. However, composite neuroscores of injured, VA64-treated animals (n=12) were not different from those of sham-injured animals and were significantly higher than those of injured PBS-treated mice at both 48 hours and 7 days (*P<0.05 sham versus injured, PBS; *P<0.05 injured, PBS versus injured, VA64). PBS, phosphate-buffered saline.
Having shown that VA64 reseals injured cell membranes, we sought to determine whether VA64 may rescue injured cells from death at later times (1 week) after CCI. Cells were pulse labeled with PI at various times after CCI, followed by administration of VA64 (‘Materials and methods' section). Rescued cells were PI+ cells remaining in the brain at 7 days. In a single VA64 dose paradigm in which cells were labeled with PI at the time of CCI and VA64 administered at 1 hour, no differences in PI+ cells remaining in the brain at 7 days after CCI was observed between VA64-treated (cortex 0.03+0.03, dentate gyrus 0+0 PI+ cells/ × 200 field) or PBS-treated mice (cortex 0.06+0.06, dentate gyrus 0+0 PI+ cells/ × 200 field). To assess whether VA64 could rescue cells which became permeable to PI at later times after CCI, PI was administered to mice (n=6 per group) at 6, 24, and 48 hours after CCI, followed by PBS or VA64 administration at 6.5, 24.5, and 48.5 hours. Propidium iodide-positive cells remaining in the brain at 7 days also did not differ between VA64-treated (cortex, 0±0, dentate gyrus 0+0 PI+ cells/ × 200 field) and PBS (cortex, 0±0, dentate gyrus 0±0 PI+ cells/ × 200 field, n=6 per group).
Discussion
We present evidence that plasmalemma permeability to PI between 6 and 48 hours after CCI portends eventual cell demise, and that intravenous administration of VA64 reseals injured (permeabilized) cells. VA64 also reduces brain edema, BBB damage, acute cellular degeneration, brain tissue loss, and motor dysfunction by mechanism(s) other than rescue of injured cells, because membrane resealing by VA64 did not rescue PI+ cells from eventual demise by 7 days. These findings confirm and extend our previous report that plasmalemma permeability portends eventual demise of brain cells injured early after CCI (Whalen et al, 2008), and suggest a novel application of VA64 to reduce secondary injury and improve functional outcome after contusion TBI through mechanisms that remain to be elucidated.
We hypothesized that cells that lose plasmalemma integrity at delayed times after CCI (6 to 48 hours) might be more amenable to recovery and membrane repair leading to cell salvage. However, nearly all PI+ cells labeled at 6, 24, or 48 hours after CCI and allowed to survive for 24 hours were degenerative by H&E staining, and almost all PI+ cells pulse labeled between 6 and 48 hours disappeared from the injured brain by 7 days (Figure 1 and Whalen et al (2008)). One explanation for these data is that some neurons might repair their membrane damage, extrude PI, and change their course to become healthy again as suggested in models of neonatal brain damage (Bassanini et al, 2007; Hallene et al, 2006). However, we previously reported that PI labeling is irreversible in our CCI model and portends fatal cellular injury in cells damaged at 0 to 2 hours (Whalen et al, 2008). From this study, we conclude that plasmalemma permeability to PI within the first 48 hours after CCI is a marker of cell death or removal using the methodology described herein.
Previous studies in mild fluid percussion TBI showed that plasmalemma permeability might be reversible because some permeable cells had a normal ultrastructure and spontaneous restoration of plasmalemma integrity (Farkas et al, 2006; Singleton and Povlishock, 2004). However, whether damaged cells eventually died or survived mild FPI was not reported beyond 8 hours (Farkas et al, 2006). It is possible that plasmalemma damage may not necessarily portend cell death in less severe TBI models characterized by infrequent neuronal death (Farkas et al, 2006). The methodology described herein can be used to directly test this hypothesis. This is significant because it is currently unknown whether injured (permeable) cells may undergo spontaneous repair and long-term survival after central nervous system injury of any type (Borgens et al, 2004; Cadichon et al, 2007; Curry et al, 2004; Farkas et al, 2006; Frim et al, 2004; Singleton and Povlishock, 2004; Unal-Cevik and Dalkara, 2003; Whalen et al, 2008).
This study is the first that we know of to document plasmalemma resealing in vivo by synthetic polymers. One caveat to our experimental design is the potential for artifactual cell resealing caused by microvascular plugging by activated platelets, microthrombosis, and/or vasospasm (Schwarzmaier et al, 2010), which might limit the transit of the second fluorophore used to interrogate neuronal and glial plasmalemma integrity. To avoid these possible confounds, we used an experimental paradigm that incorporates rapid sequential administration of fluorescent dyes and resealing agents within a short time epoch, and observed >95% nonresealing after CCI in PBS-treated mice. In contrast, we observed robust membrane resealing with P188, PEG 8000, and VA64 (defined as significantly increased numbers of YOYO-1+/PI− cells). These data could be explained by the resealed endothelium preventing contact of PI with injured neurons and glia. The observation that VA64 resealed brain cells in an intracerebroventricular administration paradigm (Figure 3C) suggests the possibility that intravenous VA64 may also directly restore plasmalemma integrity in injured parenchymal cells.
Within the dose ranges used herein, single-dose VA64 was superior to poloxamer P188, PEG 8000, and P407 in reducing postinjury brain edema, BBB damage, brain tissue loss, and motor deficits. Inadequate dosing of other polymers and/or the presence of impurities might have precluded beneficial effects of industrial-grade P188 and P407. VA64 also had the greatest membrane-resealing activity in the injured cortex assessed by YOYO-1+/PI− cell counts. Membrane resealing might reduce the release of damage-associated molecular pathogens from injured cells, such as high-mobility group box-1 and others, which when released from necrotic cells bind to immune cells and elicit an inflammatory response that may promote secondary damage after TBI (Pisetsky et al, 2011). Alternatively, a mechanism(s) of VA64 unrelated to membrane resealing may be critical for its protective effects in brain edema and other outcome measures. Such mechanisms may include increased blood oncotic pressure, aquaporin channel and/or matrix metalloproteinase inhibition, and rheologic effects to augment cerebral blood flow, among others. Studies aimed at identifying relevant cellular and molecular mechanisms of VA64, as well as its pharmacokinetic and pharmacodynamic profiles are required to better understand how it provides neuroprotection in TBI.
VA64 reduced brain tissue damage by 25%, but only modestly reduced acute cellular degeneration (H&E) and caspase 3/7 activity (Figure 6), and did not rescue injured (PI+) cells from eventual demise after CCI. These data suggest that VA64 may prevent acute cell death rather than rescue injured cells per se, perhaps in part by inhibiting caspases (Figure 6). Alternatively, reduced caspase activation by VA64 may simply be related to less overall tissue damage. The inability of VA64 to rescue PI+ cells from ultimate demise may be attributed to a number of possibilities, including a short in vivo half-life and/or the possibility that plasmalemma permeability to PI is not a mediator but a marker of a ‘point of no return' from injury. It is conceivable that prolonged administration of VA64 (including continuous infusion) might maintain cells resealed and thereby rescue injured cells from death. Alternatively, plasmalemma resealing may not be sufficient for survival of injured cells. From our data, we conclude that mechanisms other than cell rescue mediate the protective effects of VA64 in CCI.
Strikingly, a single dose of VA64 administered at 1 hour after CCI markedly reduced leakage of Evans blue albumin across the damaged BBB for up to 24 hours after CCI. These data suggest a long-lasting effect of VA64 in our mouse CCI model. The effect of VA64 on BBB damage seems to be immediate because we coadministered VA64 with Evans blue in the experimental paradigm, and suggests a possible physical interaction with the endothelium and perhaps other cell types that comprise the BBB. Recent studies have implicated BBB damage as an etiological agent in the pathogenesis of epilepsy from various causes, including TBI (Marchi et al, 2011; Cacheaux et al, 2009; Janigro, 2010). Further studies are required to examine VA64 as a potential therapy to prevent posttraumatic epilepsy in TBI patients (Temkin, 2009).
We found a dose-dependent effect of VA64 on development of brain edema after CCI assessed by two independent methods. The 1 hour time point is clinically relevant for treatment in the field by emergency personnel. The finding that 250 μL of 2 mmol/L VA64 was also effective is important inasmuch as limiting volume administration would reduce the risk of fluid overload in TBI patients given VA64. Our finding that a dose of VA64 that resealed damaged cells was ineffective at reducing edema (data not shown) reinforces the notion that mechanisms other than or in addition to membrane resealing mediate protective effects of VA64. Reduced BBB damage may be one mechanism for reduced edema by VA64. Reduction of posttraumatic brain edema by VA64 is potentially clinically significant because many TBI patients die or have secondary ischemic complications from brain edema and resulting intracranial hypertension.
We conclude that plasmalemma damage is a point of no return from cellular injury that portends a fatal outcome after CCI. Kollidon VA64 restores plasmalemma integrity and improves clinically relevant outcome measures after CCI; however, our data do not conclusively show that plasmalemma resealing is the only, or even the major, mechanism explaining the observed protection. Further studies are required to evaluate additional potential mechanisms of VA64. These uncertainties notwithstanding, Kollidon VA64 has exciting potential as a therapeutic agent to reduce acute and long-term sequelae of TBI, such as brain edema and development of posttraumatic epilepsy. Further studies are required to determine blood pharmacokinetics, brain pharmacodynamics, and optimal dosing of VA64 for possible use in patients with focal TBI associated with BBB damage and intracranial hypertension.
The authors declare no conflict of interest.
Footnotes
This study was supported by NINDS 5RO1NS061255 (MJW) and the Nature Science Foundation of China (LT).
References
- Bassanini S, Hallene K, Battaglia G, Finardi A, Santaguida S, Cipolla M, Janigro D. Early cerebrovascular and parenchymal events following prenatal exposure to the putative neurotoxin methylazoxymethanol. Neurobiol Dis. 2007;26:481–495. doi: 10.1016/j.nbd.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermpohl D, You Z, Lo EH, Kim HH, Whalen MJ. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J Cereb Blood Flow Metab. 2007;27:1806–1818. doi: 10.1038/sj.jcbfm.9600487. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Bohnert D, Duerstock B, Spomar D, Lee RC. Subcutaneous tri-block copolymer produces recovery from spinal cord injury. J Neurosci Res. 2004;76:141–154. doi: 10.1002/jnr.20053. [DOI] [PubMed] [Google Scholar]
- Cacheaux LP, Ivens S, David Y, Lakhter AJ, Bar-Klein G, Shapira M, Heinemann U, Friedman A, Kaufer D. Transcriptome profiling reveals TGF-β signaling involvement in epileptogenesis. J Neurosci. 2009;29:8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadichon SB, Le Hoang M, Wright DA, Curry DJ, Kang U, Frim DM. Neuroprotective effect of the surfactant poloxamer 188 in a model of intracranial hemorrhage in rats. J Neurosurg. 2007;106:36–40. doi: 10.3171/ped.2007.106.1.36. [DOI] [PubMed] [Google Scholar]
- Curry DJ, Wright DA, Lee RC, Kang UJ, Frim DM. Poloxamer 188 volumetrically decreases neuronal loss in the rat in a time-dependent manner. Neurosurgery. 2004;55:943–948. doi: 10.1227/01.neu.0000137890.29862.2c. [DOI] [PubMed] [Google Scholar]
- Farkas O, Lifshitz J, Povlishock JT. Mechanoporation induced by diffuse traumatic brain injury: an irreversible or reversible response to injury. J Neurosci. 2006;36:2130–2140. doi: 10.1523/JNEUROSCI.5119-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frim DM, Wright DA, Curry DJ, Cromie W, Lee R, Kang UJ. The surfactant poloxamer-188 protects against glutamate toxicity in the rat brain. Neuroreport. 2004;15:171–174. doi: 10.1097/00001756-200401190-00033. [DOI] [PubMed] [Google Scholar]
- Geddes DM, Cargill RS, 2nd, LaPlaca MC. Mechanical stretch to neurons results in a strain rate and magnitude-dependent increase in plasma membrane permeability. J Neurotrauma. 2003a;20:1039–1049. doi: 10.1089/089771503770195885. [DOI] [PubMed] [Google Scholar]
- Geddes DM, LaPlaca MC, Cargill RS., 2nd Susceptibility of hippocampal neurons to mechanically induced injury. Exp Neurol. 2003b;184:420–427. doi: 10.1016/s0014-4886(03)00254-1. [DOI] [PubMed] [Google Scholar]
- Gennarelli TA, Thibault LE, Tipperman R, Tomei G, Sergot R, Brown M, Maxwell WL, Graham DI, Adams JH, Irvine A, Gennarelli LM, Duhaime AC, Boock R, Greenberg J. Axonal injury in the optic nerve: a model simulating diffuse axonal injury in the brain. J Neurosurg. 1989;71:244–253. doi: 10.3171/jns.1989.71.2.0244. [DOI] [PubMed] [Google Scholar]
- Grindel JM, Jaworski T, Piraner O, Emanuele RM, Balasubramanian M. Distribution, metabolism, and excretion of a novel surface-active agent, purified poloxamer 188, in rats, dogs, and humans. J Pharm Sci. 2002;91:1936–1947. doi: 10.1002/jps.10190. [DOI] [PubMed] [Google Scholar]
- Hallene KL, Oby E, Lee BJ, Santaguida S, Bassanini S, Cipolla M, Marchi N, Hossain M, Battaglia G, Janigro D. Prenatal exposure to thalidomide, altered vasculogenesis, and CNS malformations. Neuroscience. 2006;142:267–283. doi: 10.1016/j.neuroscience.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janigro D. Not again! The role of blood–brain barrier failure in epileptogenesis: a molecular update. Epilepsy Curr. 2010;10:67–69. doi: 10.1111/j.1535-7511.2010.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilinc D, Gallo G, Barbee K. Poloxamer 188 reduces axonal beading following mechanical trauma to cultured neurons. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:5395–5398. doi: 10.1109/IEMBS.2007.4353562. [DOI] [PubMed] [Google Scholar]
- Kilinc D, Gallo G, Barbee KA. Mechanically-induced membrane poration causes axonal beading and localized cytoskeletal damage. Exp Neurol. 2008;212:422–430. doi: 10.1016/j.expneurol.2008.04.025. [DOI] [PubMed] [Google Scholar]
- LaPlaca MC, Thibault LE. Dynamic mechanical deformation of neurons triggers an acute calcium response and cell injury involving the N-methyl-D-aspartate glutamate receptor. J Neurosci Res. 1998;52:220–229. doi: 10.1002/(SICI)1097-4547(19980415)52:2<220::AID-JNR10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lee RC, River LP, Pan FS, Ji L, Wollmann RL. Surfactant-induced sealing of electropermeabilized skeletal muscle membranes in vivo. Proc Natl Acad Sci USA. 1992;89:4524–4528. doi: 10.1073/pnas.89.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Tierney W, Alexopoulos AV, Puvenna V, Granata T, Janigro D. The etiological role of blood-brain barrier dysfunction in seizure disorders. Cardiovasc Psychiatry Neurol. 2011;2011:1–9. doi: 10.1155/2011/482415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks JD, Pan CY, Bushell T, Cromie W, Lee RC. Amphiphilic, tri-block copolymers provide potent membrane-targeted neuroprotection. FASEB J. 2001;15:1107–1109. doi: 10.1096/fj.00-0547fje. [DOI] [PubMed] [Google Scholar]
- Mbye LH, Singh IN, Carrico KM, Saatman KE, Hall ED. Comparative neuroprotective effects of cyclosporin A and NIM811, a nonimmunosuppressive cyclosporin A analog, following traumatic brain injury. J Cereb Blood Flow Metab. 2008;29:87–97. doi: 10.1038/jcbfm.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai H, Pierce JE, Raghupathi R, Smith DH, Saatman KE, Trojanowski JQ, Lee VM, Loring JF, Eckman C, Younkin S, McIntosh TK. Twofold overexpression of human beta-amyloid precursor proteins in transgenic mice does not affect the neuromotor, cognitive, or neurodegenerative sequelae following experimental brain injury. J Comp Neurol. 1998;392:428–438. doi: 10.1002/(sici)1096-9861(19980323)392:4<428::aid-cne2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Saatman KE, Galvin JE, Scherbel U, Raghupathi R, Trojanowski JQ, McIntosh TK. Increased vulnerability of NFH-LacZ transgenic mouse to traumatic brain injury-induced behavioral deficits and cortical damage. J Cereb Blood Flow Metab. 1999;19:762–770. doi: 10.1097/00004647-199907000-00006. [DOI] [PubMed] [Google Scholar]
- Pettus EH, Christman CW, Giebel ML, Povlishock JT. Traumatically induced altered membrane permeability: its relationship to traumatically induced reactive axonal change. J Neurotrauma. 1994;11:507–522. doi: 10.1089/neu.1994.11.507. [DOI] [PubMed] [Google Scholar]
- Pisetsky DS, Gauley J, Ullal AJ. HMGB1 and microparticles as mediators of the immune response to cell death. Antioxid Redox Signal. 2011;15:2209–2219. doi: 10.1089/ars.2010.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado GR, Ross JD, DeWeerth SP, LaPlaca MC. Mechanical trauma induces immediate changes in neuronal network activity. J Neural Eng. 2005;2:148–158. doi: 10.1088/1741-2560/2/4/011. [DOI] [PubMed] [Google Scholar]
- Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riess P, Molcanyi M, Bentz K, Maegele M, Simanski C, Carlitscheck C, Schneider A, Hescheler J, Bouillon B, Schafer U, Neugebauer E. Embryonic stem cell transplantation after experimental traumatic brain injury dramatically improves neurological outcome, but may cause tumors. J Neurotrauma. 2007;24:216–225. doi: 10.1089/neu.2006.0141. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Contreras PC, Smith DH, Raghupathi R, McDermott KL, Fernandez SC, Sanderson KL, Voddi M, McIntosh TK. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp Neurol. 1997;147:418–427. doi: 10.1006/exnr.1997.6629. [DOI] [PubMed] [Google Scholar]
- Schwarzmaier SM, Kim SW, Trabold R, Plesnila N. Temporal profile of thrombogenesis in the cerebral microcirculation after traumatic brain injury in mice. J Neurotrauma. 2010;27:121–130. doi: 10.1089/neu.2009.1114. [DOI] [PubMed] [Google Scholar]
- Serbest G, Horwitz J, Barbee K. The effect of poloxamer-188 on neuronal cell recovery from mechanical injury. J Neurotrauma. 2005;22:119–132. doi: 10.1089/neu.2005.22.119. [DOI] [PubMed] [Google Scholar]
- Serbest G, Horwitz J, Jost M, Barbee K. Mechanisms of cell death and neuroprotection by poloxamer 188 after mechanical trauma. FASEB J. 2006;20:308–310. doi: 10.1096/fj.05-4024fje. [DOI] [PubMed] [Google Scholar]
- Singleton RH, Povlishock JT. Identification and characterization of heterogeneous neuronal injury and death in regions of diffuse brain injury: evidence for multiple independent injury phenotypes. J Neurosci. 2004;24:3543–3553. doi: 10.1523/JNEUROSCI.5048-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50:10–13. doi: 10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- Unal-Cevik I, Dalkara T. Intravenously administered propidium iodide labels necrotic cells in the intact mouse brain after injury. Cell Death Differ. 2003;10:928–929. doi: 10.1038/sj.cdd.4401250. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Dalkara T, You Z, Qiu J, Bermpohl D, Mehta N, Suter B, Bhide PG, Lo EH, Ericsson M, Moskowitz MA. Acute plasmalemma permeability and protracted clearance of injured cells after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:490–505. doi: 10.1038/sj.jcbfm.9600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z, Savitz SI, Yang J, Degterev A, Yuan J, Cuny GD, Moskowitz MA, Whalen MJ. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:1564–1573. doi: 10.1038/jcbfm.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]