Abstract
N-methyl--aspartate (NMDA) receptors are glutamate-gated cation channels that mediate excitatory neurotransmission in the central nervous system. In addition to glutamate, NMDA receptors are also activated by coagonist binding of the gliotransmitter, -serine. Neuronal NMDA receptors mediate activity-dependent blood flow regulation in the brain. Our objective was to determine whether NMDA receptors expressed by brain endothelial cells can induce vasodilation of isolated brain arteries. Adult mouse middle cerebral arteries (MCAs) were isolated, pressurized, and preconstricted with norepinephrine. N-methyl--aspartate receptor agonists, glutamate and NMDA, significantly dilated MCAs in a concentration-dependent manner in the presence of -serine but not alone. Dilation was significantly inhibited by NMDA receptor antagonists, -2-amino-5-phosphonopentanoate and 5,7-dichlorokynurenic acid, indicating a response dependent on NMDA receptor glutamate and -serine binding sites, respectively. Vasodilation was inhibited by denuding the endothelium and by selective inhibition or genetic knockout of endothelial nitric oxide synthase (eNOS). We also found evidence for expression of the pan-NMDA receptor subunit, NR1, in mouse primary brain endothelial cells, and for the NMDA receptor subunit NR2C in cortical arteries in situ. Overall, we conclude that NMDA receptor coactivation by glutamate and -serine increases lumen diameter in pressurized MCA in an endothelial and eNOS-dependent mechanism.
Keywords: -serine, eNOS, glutamate, middle cerebral artery, NMDA receptor, NR2C
Introduction
Glutamate is a central nervous system excitatory neurotransmitter that can activate both G-protein-coupled (metabotropic) receptors and ligand-gated ion channels, which include AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and N-methyl--aspartate (NMDA) receptors. N-methyl--aspartate receptors are heterotetramers consisting of seven subunits (NR1, NR2A–D, NR3A,B), and are critical regulators of neuronal excitability, synaptic plasticity (Collingridge and Singer, 1990), excitotoxicity (Choi et al, 1988), and cerebral blood flow (Busija et al, 2007). Neuronal NMDA receptors most often consist of two NR1 and two NR2A or NR2B subunits. N-methyl--aspartate receptors consisting of two NR1 and combinations of NR2C, NR2D, NR3A, or NR3B have also been identified and differ functionally from NR2A/B configurations. Most notably, they are resistant to baseline voltage-dependent channel blockade by Mg2+ that is characteristic of NMDA receptors expressing NR2A/B (Chatterton et al, 2002; Monyer et al, 1994). Nonneuronal NMDA receptors with such configurations have been identified in cell types including astrocytes, oligodendrocytes, vascular endothelial cells, lymphocytes, and cardiomyocytes, and mediate diverse functions such as myelination, immune response, and blood–brain barrier permeability (Schipke et al, 2001; Boldyrev et al, 2005; Karadottir et al, 2005; Lalo et al, 2006; Reijerkerk et al, 2010). N-methyl--aspartate receptor activation requires glutamate, which binds NR2 subunits, and binding of a coagonist, glycine or -serine. While glycine and -serine are both NMDA receptor coagonists, -serine binds with greater affinity and dominates as the main endogenous agonist in most of the brain regions (Mothet et al, 2000). -Serine is produced and stored in astrocytes (Schell et al, 1995). These stores are released by a Ca2+ and SNARE protein-dependent mechanism (Martineau et al, 2008; Mothet et al, 2005) and, in turn, influence neuronal excitability and plasticity (Mothet et al, 2000; Yang et al, 2003).
Functional hyperemia is an endogenous regulatory pathway in the brain coupling elevated neuronal activity with vasodilation and increased local cerebral blood flow (Iadecola, 2004). Application of glutamate directly to the brain surface in vivo consistently triggers vasodilation and increased blood flow in several animal models (Busija and Leffler, 1989; Faraci and Breese, 1993; Faraci and Brian, 1995; Meng et al, 1995; Philip and Armstead, 2004). There is broad literature consensus that glutamate can induce vasodilation in two ways. First, neuronal NMDA receptors can be activated, stimulating Ca2+ influx, and nitric oxide (NO) production by neuronal nitric oxide synthase (nNOS) (Bari et al, 1996b; Chi et al, 2003; Faraci and Brian, 1995; Fergus and Lee, 1997; Meng et al, 1995). Second, glutamate can stimulate astrocytic release of vasodilators produced from arachidonic acid, such as epoxyeicosatrienoic acids and prostaglandins (Metea and Newman, 2006; Takano et al, 2006). A third possibility with little support to date is that glutamate mediates vasodilation by acting directly at the microvasculature level. Intriguingly, brain endothelial cells reportedly express NMDA receptors (Krizbai et al, 1998; Reijerkerk et al, 2010; Scott et al, 2007; Sharp et al, 2003), and glutamate has been shown to stimulate endothelial cell production of vasodilators, such as CO (Parfenova et al, 2003) and NO (Scott et al, 2007). There is also at least one report that glutamate dilates isolated cerebral arterioles by an endothelium-dependent mechanism (Fiumana et al, 2003). On the other hand, several groups have argued against such a mechanism based on limited direct effects of glutamate on lumen diameter in isolated artery preparations using wire or pressure myography (Faraci and Breese, 1993; Hardebo et al, 1989; Simandle et al, 2005; Wendling et al, 1996). There are also reports that specifically refute claims that brain endothelial cells express NMDA receptor subunits (Domoki et al, 2008; Morley et al, 1998).
We postulated that the demonstrated failure of isolated, pressurized brain arteries to dilate in response to glutamate may in part be explained by an absence of NMDA receptor coagonist in these experiments. The objectives of the current study were to determine whether coactivation of endothelial NMDA receptors by -serine and glutamate is sufficient to cause dilation of isolated brain arteries, and to determine whether the pan-NMDA receptor subunit NR1 is expressed in the brain endothelial cells. We report that glutamate or NMDA applied to isolated middle cerebral arteries (MCAs) with -serine, but not alone, causes vasodilation dependent on endothelial function and endothelial nitric oxide synthase (eNOS) activity. NR1 expression was also detected in primary brain endothelial cell cultures.
Materials and methods
Pressure Myography
All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA), unless otherwise noted. Animal protocols were approved by the Animal Care Committee at the University of Manitoba. Male CD1 mice (15 weeks old) were anesthetized with 70% CO2/30% O2 and decapitated. The brains were removed and placed in ice-cold Krebs buffer (118 mmol/L NaCl, 4.65 mmol/L KCl, 1.18 mmol/L MgSO4, 1.18 mmol/L KHPO3, 25 mmol/L NaHCO3, 2.5 mmol/L CaCl2, 5.5 mmol/L glucose, 0.026 mmol/L EDTA). A MCA was dissected from the brain, mounted between two glass pipettes in a pressure myograph chamber, and adherent tissue was removed. The vessels were secured in place with suture ties and pressurized with a Servo-controlled peristaltic pump to an intraluminal pressure of 30 mm Hg. Branches from the main artery were closed by electrocauterization or suture ties. The arteries were bathed in Krebs aerated with 5% CO2, 20% O2, and 75% N2 at 37°C throughout the experiments.
Changes in arterial diameter during the drug treatments were measured with a video dimension analyzer (Living Systems Instrumentation, Burlington, VT, USA) attached to a Nikon Eclipse TS100 microscope (Melville, NY, USA). Vessel viability was confirmed before the start of the experiments by ⩾50% constriction to 125 mmol/L KCl. The vessels were equilibrated for 1 hour between treatments. Arteries were preconstricted with norepinephrine (1 μmol/L), and vascular responses were measured in response to incremental doses of glutamate or NMDA with or without -serine.
To assess the role of the endothelium, the endothelial cell layer was removed by pushing a bolus of air through the vessel. Complete denudation of the endothelium was confirmed by failure of the vessel to relax in the presence of acetylcholine (ACh; 10 nmol/L to 10 μmol/L). Viability of the smooth muscle layer was verified by treatment with sodium nitroprusside (SNP; 100 nmol/L to 100 μmol/L), a NO donor.
Phenolic Denervation and Nicotinamide Adenine Dinucleotide Phosphate-Diaphorase Staining
Residual postdissection nerve terminals on MCA smooth muscle surfaces were chemically destroyed by incubating the arteries in 0.15% phenol and 1.35% ethanol for 3 minutes, which was adapted from previous denervation methods (Wang and Bukoski, 1999). Vessel viability was confirmed by dilation to ACh (10 nmol/L to 10 μmol/L) and SNP (100 nmol/L to 100 μmol/L). To verify denervation, arteries were fixed with 4% paraformaldehyde (pH 7.6) for 10 minutes. Nicotinamide adenine dinucleotide phosphate (NADPH)-diaphorase solution, containing nitro blue tetrazolium (5 μmol/L), the reduced form of β-nicotinamide adenine dinucleotide phosphate (10 μmol/L), and 0.2% Triton X-100 in TBS (Tris-buffered saline; 0.05 mol/L; pH 8.0), was applied for 30 minutes at 37°C to detect nNOS-positive neurons.
Brain Slice Preparation and Two-Photon Imaging
The brains from 14- to 19-day-old CD1 mice were cut on a vibrating blade microtome (Vibratome, Bannockburn, IL, USA) into 350-μm thick sections. Slices were loaded with Rhod-2 AM (Invitrogen, Burlington, ON, USA), a calcium indicator preferentially taken up by astrocytes, and isolectin B4 from Griffonia simplicifolia tagged with Alexa Fluor 488 (Invitrogen) to label the brain slice vasculature. Slices were incubated in artificial cerebrospinal fluid aerated with 20% O2, 5% CO2, and 75% N2 while imaged on an upright microscope with scanhead for two-photon imaging (Prairie Technologies, Middleton, WI, USA) attached to a pulsed Ti-sapphire laser (Coherent Inc., Santa Clara, CA, USA) at 800 nm. Glutamate (10 μmol/L) and -serine (10 μmol/L) were bath applied to the slices and the change in arteriolar diameter was recorded over time.
Primary Endothelial Cell Culture
Endothelial cells were isolated from 15-week-old male CD1 mouse brains, as described previously (Jung and Levy, 2005). Briefly, the brains were cut into small pieces with a scalpel and digested with 0.05% collagenase. Digested material was resuspended in 17% Dextran solution and centrifuged at 10,000 g for 30 minutes at 4°C, which resulted in an endothelial cell pellet. Isolated endothelial cells were collected on glass beads and cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum, 2 mmol/L -glutamine, 1% penicillin-streptomycin, and 150 μg/mL endothelial cell growth supplement. Cells were passaged twice before reaching confluence.
Polymerase Chain Reaction
Total RNA was isolated from primary endothelial cell cultures, unpurified brain cultures, or mouse brain using the Qiagen RNeasy Mini Kit (Qiagen, Mississauga, ON, USA), and DNase digested according to the Turbo DNA-free protocol (Applied Biosystems, Carlsbad, CA, USA). Turbo DNA-free RNA was reverse transcribed to cDNA according to the SuperScript III RNase H− Reverse Transcriptase protocol (Invitrogen), and complementary mRNA was digested using RNase H. cDNA was purified according to the PCR Purification Kit (Qiagen).
Mouse GAPDH (glyceraldehyde-3-phosphate dehydrogenase; internal control) and NR1 genes were amplified using the following primers: GAPDH forward=CACGGCAAATTCAACGGCACAGT, GAPDH reverse=TGGGGGCATCGGCAGAAGG; NR1 forward=TGTGCGGGACAACAAGCTCCA, NR1 reverse=ATGCCGATGCCAAAGCCGGA. All gene targets were PCR amplified according to the DreamTaq protocol (Fermentas, Burlington, ON, USA) using 10 ng of cDNA as template. There was an initial denaturation at 95°C for 2 minutes, followed by 40 cycles of 95°C for 30 seconds, 60°C (GAPDH), 63°C (NR1), or 65°C (von Willebrand's factor) for 30 seconds, and 72°C for 30 seconds, followed by 72°C for 5 minutes. These PCR products were visualized on a 1% agarose gel.
Immunofluorescence
Primary endothelial cells were rinsed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 1 hour. Cells were permeabilized for 5 minutes with 0.2% Triton X-100 in PBS and blocked at room temperature with 1% bovine serum albumin in PBS. Goat anti-NR1 polyclonal primary antibody (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) was applied overnight at 4°C. The secondary antibody Alexa Fluor 568 donkey anti-goat IgG (H+L) and endothelial cell marker, Isolectin B4 tagged with Alexa Fluor 488 (Invitrogen), were applied in 1% bovine serum albumin in PBS for 1 hour at room temperature. Staining was visualized with a Zeiss LSM510 laser-scanning microscope (Oberkochen, Germany).
For brain slice immunofluorescence, mouse brains (15 week old) were rinsed with PBS (pH 7.2) and placed in 4% paraformaldehyde for 24 hours. Frozen sagital sections (50 μm) were cut and free-floating sections were exposed to an antigen retrieval step with 10 mmol/L citrate (pH 8.5) at 80°C for 30 minutes. Sections were rinsed in 0.1 mol/L TBS (pH 7.6), treated with a mouse-on-mouse blocking kit (Vector Laboratories, Burlingame, CA, USA) and incubated with rabbit anti-NR2C (Santa Cruz Biotechnology) with either mouse anti-GFAP (glial fibrillary acidic protein) (Sigma) or anti-mouse p-glycoprotein (Mdr-1; Santa Cruz Biotechnology) overnight at 4°C. After washing, the sections were incubated with secondary antibodies for 1 hour at room temperature. Goat anti-rabbit IgG (Alexa Fluor 568) was used to detect NR2C signal, while goat anti-mouse IgG (Alexa Fluor 488) was used to detect GFAP and p-glycoprotein. Stained sections were visualized with a Zeiss LSM510 laser-scanning microscope.
Western Blot
Cultured endothelial cells were scraped in nondenaturing lysis buffer (10% glycerol and 1% Triton X-100 in 20 mmol/L Tris–HCl, 137 mmol/L NaCl, and 2 mmol/L EDTA with protease inhibitors; pH 8) and protein concentrations were determined using a BCA assay. Crude protein was separated on 7.5% polyacrylamide gel and transferred to polyvinylidene difluoride membrane. Membranes were blocked for 60 minutes with 5% skim milk powder in TBS with 20% Tween 20 (TBS-T) and incubated with rabbit polyclonal anti-NR1 (1:300) (Cell Signaling, Danvers, MA, USA) overnight at 4°C. A goat anti-rabbit secondary antibody conjugated with horseradish peroxidase (1:1,000) (Cell Signaling) was added to the membrane for 2 hours (room temperature), and an ECL Plus chemiluminescence kit (GE Life Sciences, Piscataway, NJ, USA) was used to visualize the protein bands with a Fluo-STM MultiImager (Bio-Rad Laboratories, Hercules, CA, USA).
Statistics
For dose–response pressure myography experiments, two-way ANOVA (analysis of variance) for repeated measures were conducted with Bonferroni post-hoc tests. For experiments where arteries were treated with a single concentration of drug, data were analyzed by one-way ANOVA test with Newman–Keuls post-hoc test.
Results
N-Methyl--Aspartate Receptor Agonists Directly Dilate Isolated Middle Cerebral Arteries and Penetrating Cortical Arterioles
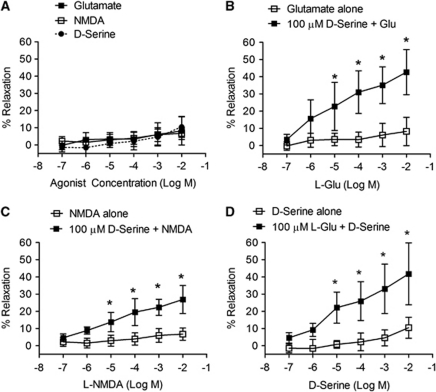
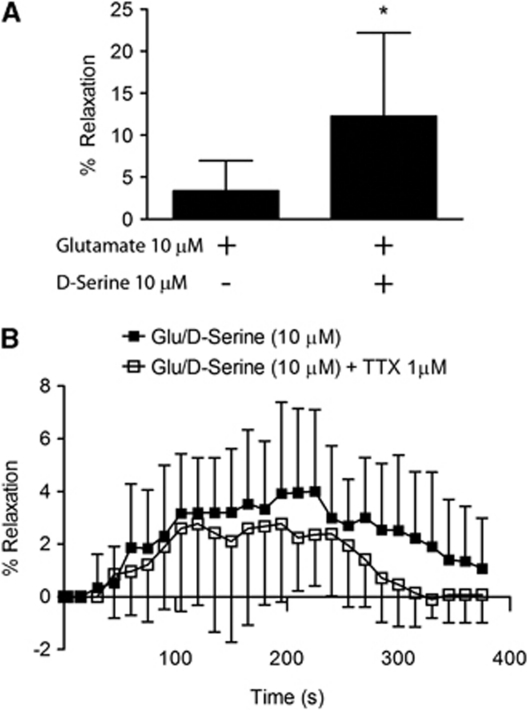
Bath application of increasing concentrations (0.1 μmol/L to 10 mmol/L) of NMDA receptor agonists, glutamate and NMDA, to preconstricted MCAs had no significant effect on lumen diameter (Figure 1A). In contrast, with -serine present (100 μmol/L), incremental concentrations of glutamate (Figure 1B), or NMDA (Figure 1C) produced significant concentration-dependent relaxation of MCAs, which was first statistically detectable (P<0.05) at 10 μmol/L glutamate (22.9%±5.7%) or NMDA (13.8%±2.3%). -Serine concentrations 10-fold lower than this (10 μmol/L) were sufficient to evoke vasodilation in the presence of 100 μmol/L glutamate (Figure 1D). This -serine concentration is consistent with unstimulated forebrain -serine levels analyzed by microdialysis (Hashimoto et al, 1995). In an effort to find the lower range of -serine and glutamate concentrations able to cause MCA dilation, we used both coagonists at 10 μmol/L and found this condition to cause a significant increase in MCA diameter (Figure 2A). -Serine was unable initiate vasodilation when administered with another known vasodilator, ACh (Supplementary Figure 1), indicating a specific effect observed only when given with an NMDA receptor glutamate site agonist. To explore whether the vasodilatory effect of -serine can be extended to arterioles or capillaries in the brain parenchyma, we determined whether the combination of -serine and glutamate dilates cortical arterioles in mouse brain slices maintained at 20% O2. Bath application of glutamate and -serine (each 10 μmol/L) dilated brain slice arterioles (maximum 4.0%±3.1%) over 400 seconds (Figure 2B). Adding tetrodotoxin to block neuronal excitability had no significant effect on dilation (maximum 2.8%±3.0%), thus eliminating the possibility that signaling through neuronal excitation is responsible for this effect of glutamate and -serine.
Figure 1.
Dilation of isolated middle cerebral arteries (MCAs) by N-methyl--aspartate (NMDA) and glutamate requires -serine. (A) Individually, glutamate, NMDA, and -serine failed to significantly affect vessel diameter (n=6). Glutamate (B) and NMDA (C) caused concentration-dependent vasodilation of MCAs in the presence of fixed (100 μmol/L) -serine (n=6). (D) -Serine caused concentration-dependent increases in MCA lumen diameter in the presence of fixed glutamate (100 μmol/L, n=6). Data are presented as mean±s.d.; *P<0.05 compared with other groups using two-way analysis of variance (ANOVA) for repeated measures and Bonferroni post-hoc test.
Figure 2.
Glutamate and -serine increase lumen diameter in the brain slice arterioles. (A) Combinations of glutamate and -serine as low as 10 μmol/L each agonist causes significant middle cerebral artery (MCA) dilation. *P<0.05 compared with glutamate only group using a t-test. (B) The combination of glutamate and -serine causes significant dilation of penetrating cortical arterioles in cortical slices in the presence and absence of the neuronal excitability blocker, tetrodotoxin (TTX). Data are presented as mean values±s.d. of 14 (control) or 18 (TTX group) brain slices and were analyzed by two-way analysis of variance (ANOVA) for repeated measures and Bonferroni post-hoc test.
Glutamate and -Serine-Mediated Vasodilation Is N-Methyl--Aspartate Receptor Dependent
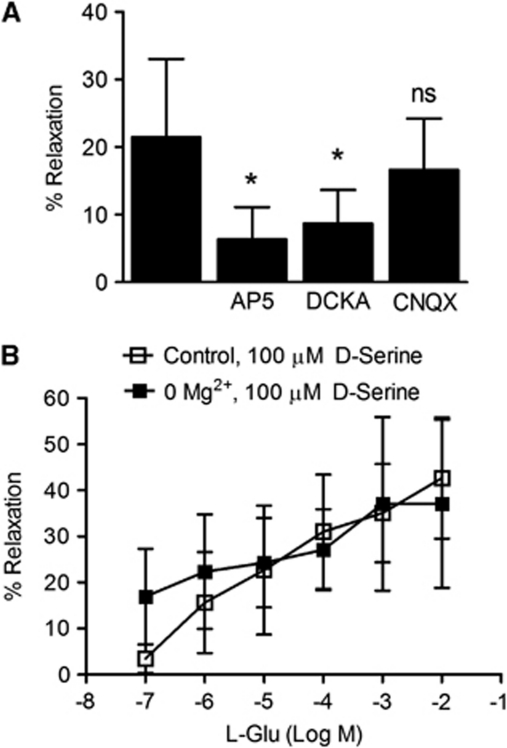
To determine the glutamate receptor subtype involved in MCA dilation by NMDA receptor coagonists, several antagonists were used, including a competitive glutamate binding site NMDA receptor antagonist, AP5 (-2-amino-5-phosphonopentanoate), a glycine/-serine coagonist site NMDA receptor antagonist, DCKA (5,7-dichlorokynurenic acid), and an AMPA/kainate receptor antagonist, CNQX (6-cyano-7-nitroquinoxaline-2,3-dione). -2-Amino-5-phosphonopentanoate (100 nmol/L) and DCKA (300 nmol/L) significantly blocked MCA dilation induced by glutamate and -serine (100 μmol/L each combined) (P<0.05), while CNQX (up to 10 μmol/L) had no significant effect (Figure 3A). This suggests that NMDA receptors, but not AMPA/kainate receptors mediate vasodilation by glutamate and -serine.
Figure 3.
N-methyl--aspartate (NMDA) receptors mediate vasodilation in response to glutamate and -serine. (A) Treatment of middle cerebral arteries (MCAs) with glutamate and -serine together (both 100 μmol/L) caused a significant increase in lumen diameter. NMDA receptor antagonists, AP5 (-2-amino-5-phosphonopentanoate; 100 nmol/L) and DCKA (5,7-dichlorokynurenic acid; 300 nmol/L) attenuated vasodilation (n=6). 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX), an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor antagonist, did not significantly inhibit vasodilation up to 20 μmol/L (n=6). Data are presented as mean±s.d.; *P<0.05 compared with glutamate/-serine control using one-way analysis of variance (ANOVA) with Newman–Keuls post-hoc test. (B) No significant differences in vasodilatory response to incremental glutamate in the presence of -serine (100 μmol/L) were detected between control arteries and arteries incubated in Mg2+-free buffer. Data are presented as mean±s.d. and were analyzed using two-way ANOVA for repeated measures and Bonferroni post-hoc test.
Neuronal NMDA receptors require plasma membrane depolarization to expel channel-bound Mg2+, whereas NMDA receptors identified on nonneuronal cell types, such as astrocytes and oligodendrocytes, have little Mg2+ sensitivity (Karadottir et al, 2005; Lalo et al, 2006). Removal of extracellular Mg2+ did not significantly change vasodilation induced by glutamate and -serine (Figure 3B; P>0.05), indicating low or no sensitivity to Mg2+ block. This is consistent with other nonneuronal NMDA receptors.
Endothelial-Derived Nitric Oxide Mediates N-Methyl--Aspartate Receptor-Mediated Vasodilation
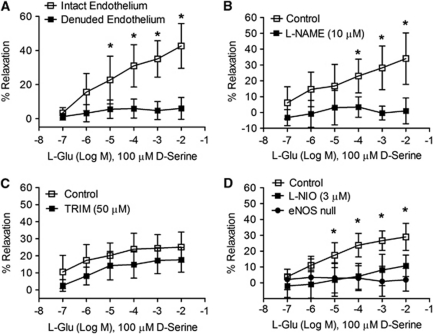
Endothelium was removed from selected MCAs by pushing an air bolus through the lumen. These arteries constricted slightly more strongly than MCAs with intact endothelium (Supplementary Figure 2A), attributable to a loss of baseline NO production, and failed to dilate in response to ACh (10 nmol/L to 10 μmol/L; Supplementary Figure 2B), confirming endothelial destruction. Denuded arteries dilated normally in response to the NO donor, SNP (100 nmol/L to 100 μmol/L; Supplementary Figure 2C). Exposure of denuded arteries to glutamate (0.1 μmol/L to 10 mmol/L) in the presence of -serine (100 μmol/L) produced no significant vasodilatory response (Figure 4A), indicating the endothelial layer is required for NMDA receptor-mediated vasodilation.
Figure 4.
Vasodilation by glutamate and -serine requires intact endothelium and endothelial nitric oxide synthase (eNOS). (A) Removal of the endothelium isolated middle cerebral arteries (MCAs) an air bolus significantly attenuated smooth muscle relaxation caused by incremental glutamate concentrations in the presence of 100 μmol/L -serine (n=6). (B) N-nitro--arginine methyl ester (-NAME; 10 μmol/L) blocked concentration-dependent vasodilation caused by glutamate and -serine (n=6). (C) Glutamate/-serine-induced MCA relaxation is not affected by the neuronal NOS inhibitor, 1-(2-(trifluoromethylphenyl)) imidazole (TRIM; 50 μmol/L). (D) N5-(1-iminoethyl)--ornithine (-NIO; 3 μmol/L), an eNOS-selective antagonist, and eNOS deletion prevented MCA relaxation by incremental glutamate increases in the presence of 100 μmol/L -serine (n=6). Data are presented as mean±s.d.; *P<0.05 using two-way analysis of variance (ANOVA) for repeated measures and Bonferroni post-hoc test.
We next tested whether NO mediates NMDA receptor-induced vasodilation. Treatment of isolated MCAs with the nonselective NOS inhibitor, -NAME (N-nitro--arginine methyl ester; 10 μmol/L), prevented dilation induced by glutamate and -serine together (Figure 4B). The NOS inhibitor, TRIM (1-(2-(trifluoromethylphenyl)) imidazole), at a concentration selective for nNOS (50 μmol/L) (Handy et al, 1995) did not significantly alter vasodilation by glutamate and -serine (P>0.05; Figure 4C). In contrast, the NOS inhibitor, -NIO (N5-(1-iminoethyl)--ornithine dihydrochloride), significantly blocked relaxation induced by glutamate and -serine (P<0.05; Figure 4D) at a concentration selective for eNOS (3 μmol/L) (Chinellato et al, 1998; Rees et al, 1990). Similarly, isolated MCAs from eNOS knockout mice did not significantly dilate in response to glutamate and -serine (P<0.05; Figure 4D). This suggests eNOS, but not nNOS, is involved in the mechanism of vasodilation by glutamate and -serine.
Vascular Nerve Endings do not Contribute to Glutamate/-Serine-Mediated Vasodilation
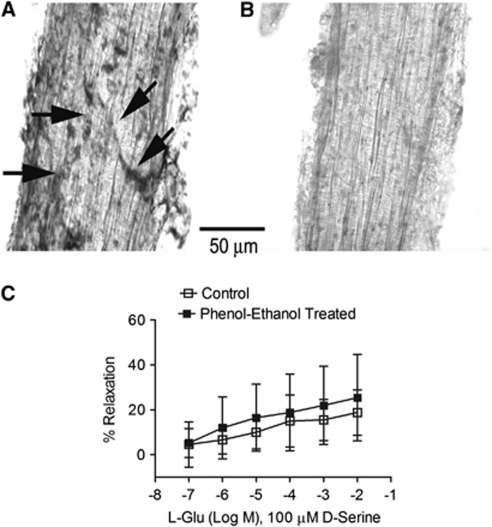
To eliminate the possibility that residual vascular nerve endings expressing NOS and/or NMDA receptors contribute to vasodilation, selected MCAs were chemically denervated and tested for responses to glutamate and -serine. Middle cerebral arteries subjected to nicotinamide adenine dinucleotide phosphate-diaphorase staining displayed NO-positive nerve endings on the smooth muscle surface (Figure 5A). Chemical denervation of arteries in 0.15% phenol and 1.35% ethanol removed the majority of NO-containing terminals (Figure 5B). Denervated arteries were able to constrict normally in response to norepinephrine and dilate in response to ACh (10 nmol/L to 10 μmol/L) and SNP (100 nmol/L to 100 μmol/L; Supplementary Figures 2B and 2C), indicating full functional viability. Chemical denervation did not significantly affect the magnitude of vasodilation caused by glutamate (0.1 μmol/L to 10 mmol/L) in the presence of -serine (100 μmol/L; Figure 5C). This indicates that NOS-containing vascular nerve endings remaining after brain arterial dissections do not contribute to vasodilation by glutamate and -serine.
Figure 5.
N-methyl--aspartate (NMDA) receptor-mediated vasodilation persists following chemical denervation of middle cerebral arteries (MCAs). Isolated mouse MCAs were mechanically stripped and treated with vehicle (A) or 1.35% ethanol with 0.15% phenol for 3 minutes (B) to cause chemical denervation. Vessels were either analyzed for nitric oxide (NO)-containing nerve endings by nicotinamide adenine dinucleotide phosphate (NADPH)-diaphorase staining (A, B) or analyzed for responses to glutamate/-serine by pressure myography (C). (A) Untreated MCA has NADPH-diaphorase-positive nerve fiber tracts (arrows). (B) Vessels treated for 3 minutes showed no indication of NADPH-diaphorase staining and therefore neuronal NO. (C) Both intact and denervated vessels relaxed significantly in a concentration-dependent manner (P<0.05, 0.1 μmol/L versus 10 mmol/L) and there were no significant differences between the groups at any dose. Data are presented as mean values±s.d. for six MCAs. Data were compared using two-way analysis of variance (ANOVA) for repeated measures, followed by the Bonferroni test for selected pairs.
Mouse Brain Endothelial Cells Express N-Methyl--Aspartate Receptor Subunits
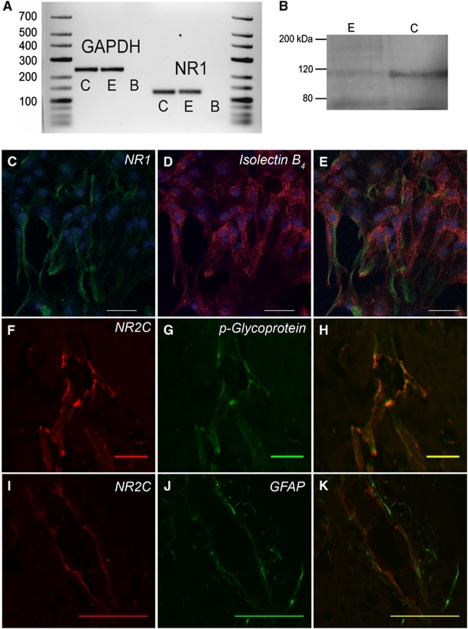
To test whether mouse cerebral endothelial cells express NMDA receptors, PCR, immunocytochemistry and Western blot approaches were employed using primary brain endothelial cell cultures isolated from 15-week-old mice. mRNA for the pan-NMDA receptor subunit, NR1, and endothelial marker, von Willebrand's factor, were identified, indicating the presence of NR1 transcript in the brain endothelial cells (Figure 6A). An immunoreactive product consistent with NR1 (116 kDa) was detected by Western blot of whole-cell lysates from purified mouse brain primary endothelial cells (Figure 6B). This band was also observed in positive control mouse whole brain homogenates. NR1 immunoreactivity was identified in cultured brain endothelial cells that express isolectin B4 (Figures 6C–6E), confirming NR1 protein expression in these cells.
Figure 6.
Brain endothelial cells express N-methyl--aspartate (NMDA) receptor subunits. (A) Purified primary adult mouse brain endothelial cultures expressed mRNA for housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and NR1. C denotes cortex; E denotes endothelial culture; B denotes blank control. (B) Western blot of protein from cortex (C) and endothelial cultures (E) produced a band for NR1 at 116 kDa. (C–E) Immunofluorescence shows colocalization of NR1 (C, green) and the endothelial cell marker, isolectin B4 (D, red), in cultured endothelial cells. Nuclei were stained with ToPro-3. Scale bars for (C–E) are 100 μm. (F–H) Coimmunofluorescence of NR2C (F, red) and p-glycoprotein (G, green) in cortical arteries near the pial surface. (I–K) Immunofluorescence for NR2C (I, red) and astrocyte glial fibrillary acidic protein (GFAP; J, green) does not overlap (K) in cortical arteries near the pial surface. Scale bars for (F–K) are 50 μm.
We also investigated whether NMDA receptor subunits are expressed by brain slice cortical arteries near the pial surface. Vessels with diameters consistent with those of MCAs used in pressure myography experiments (50 to 80 μm) were chosen for analysis. Although apparent vascular NR1 immunoreactivity was detected, it was largely obscured by stronger neuronal NR1 signal (not shown). Immunoreactivity for the NMDA receptor subunit, NR2C, which is largely silent in adult cortical neurons, was identified in cortical arteries of the targeted diameter range. NR2C signal was colocalized with the endothelial cell marker, p-glycoprotein (Figures 6F–6H). In contrast, NR2C signal was not colocalized with perivascular astrocytes that express GFAP (Figures 6I–6K). Overall, these results indicate endothelial expression of NR2C immunoreactivity in cortical arteries in situ.
Discussion
Here, we present evidence supporting vasodilation of mouse brain arteries by endothelial NMDA receptors and eNOS. N-methyl--aspartate receptor coagonists glutamate and -serine stimulated vasodilation of isolated, denervated mouse MCAs in a manner sensitive to NMDA receptor antagonists and eNOS inhibition or deletion. We also found evidence that primary brain endothelial cells and brain arteries in situ express NMDA receptor subunits.
Numerous studies covering a range of species have shown that NMDA or glutamate application topically to pial arteries through a cranial window causes vasodilation (Bari et al, 1996a; Busija and Leffler, 1989; Faraci and Breese, 1993, 1994; Philip and Armstead, 2004). This effect has been widely attributed to activation of neuronal NMDA receptors and nNOS. In apparent agreement with this, several studies have found no effect of exogenous glutamate or NMDA on lumen diameter in isolated cerebral arteries (Faraci and Breese, 1993; Hardebo et al, 1989; Simandle et al, 2005; Wendling et al, 1996). Our results are consistent with these findings by demonstrating that NMDA receptor agonists alone have little effect on isolated cerebral arterial diameter. However, for the first time we found significant vasodilation when -serine was applied with glutamate or NMDA. The question of whether this observation could translate into a physiologically relevant dilatory mechanism in vivo at our experimental glutamate and -serine concentrations is important. Local extracellular glutamate levels can approach low millimolar levels (Clements et al, 1992) and basal -serine levels in vivo have been reported in the 5 to 8 μmol/L range by microdialysis (Hashimoto et al, 1995). We found that glutamate and -serine concentrations in these ranges (100 μmol/L glutamate, 10 μmol/L -serine) were sufficient to cause a 22.2% dilation of MCAs. According to Poiseuille's law, vasodilation of this magnitude would decrease vascular resistance ∼2-fold. We further showed that 10 μmol/L -serine caused vasodilation in combination with glutamate concentrations as low as 10 μmol/L and that this combination induced dilation of penetrating arterioles in mouse cortical slices. Although further work is required to demonstrate that endogenous -serine and glutamate are capable of producing cerebral vasodilation, these observations show that locally attainable concentration ranges of -serine and glutamate dilate isolated brain arteries and, importantly, small arterioles in the more anatomically relevant brain slice model.
Our data support an effect of glutamate and -serine together at NMDA receptors. The fact that -serine is required for vasodilation is suggestive of an NMDA receptor-mediated response to begin with, as no other glutamate receptors with a coagonist requirement have been identified. In addition, competitive NMDA receptor antagonists AP5, which blocks the glutamate binding site, and DCKA, which blocks the -serine/glycine binding site, both significantly inhibited vasodilation induced by combining glutamate and -serine. Lastly, an AMPA/kainate antagonist, CNQX, did not alter vasodilation. All of these observations support the hypothesis that glutamate and -serine together influence vessel diameter by an NMDA receptor-mediated mechanism. Neuronal NMDA receptor configurations consist of NR1 and NR2A/B subunits and are sensitive to blockade of the cation channel by Mg2+ at resting membrane potential. We tested whether Mg2+-free Krebs buffer could enhance vasodilation to gain insight into the similarity of eNMDA (endothelial cell NMDA) receptor subunit configuration and function in comparison with neuronal NMDA receptors. We found that removing Mg2+ had no significant effect on vasodilation induced by glutamate and -serine, suggesting that eNMDA receptors may contain NR2C, NR2D, or NR3 subunits, which confer much lower Mg2+ sensitivity to NMDA receptor function (Chatterton et al, 2002). This is consistent with NMDA receptors identified in other nonneuronal subtypes, including astrocytes and oligodendrocytes, which are not subject to Mg2+ block and appear to consist of NR2C and NR3 subunits (Karadottir et al, 2005; Lalo et al, 2006).
Numerous studies in the brain slices and live animals have suggested NMDA receptor activation stimulates NO production via nNOS activity (Bari et al, 1996b; Chi et al, 2003; Faraci and Breese, 1993; Faraci and Brian, 1995; Fergus and Lee, 1997; Meng et al, 1995). In isolated arteries, we have now identified a potential role of eNOS in NMDA receptor-mediated vasodilation. TRIM, at a concentration relatively selective for nNOS (50 μmol/L) (Handy et al, 1995), did not significantly alter vasodilation by glutamate and -serine. In contrast, dilation was inhibited by the nonselective NOS inhibitor, -NAME, by the NOS inhibitor, -NIO, at a concentration selective for eNOS (3 μmol/L) (Chinellato et al, 1998; Rees et al, 1990), and in eNOS−/− MCAs. These findings strongly indicate eNOS involvement in a direct vascular effect of NMDA receptors. It is not our position that new observations of a potential role for eNOS in glutamate-mediated vasodilation argue against the established role of nNOS in vivo. Rather, our data suggest that eNOS may contribute to glutamate-induced vasodilation alongside or in place of nNOS in certain conditions. Endothelial nitric oxide synthase has a major role in regulation of the brain vascular tone (Iadecola, 1993) and baseline brain blood flow (Ma et al, 1996), and while eNOS does not have a significant role in functional hyperemia evoked by whisker stimulation or hypercapnia (Ayata et al, 1996; Ma et al, 1996), it is a critical mediator of hyperemia resulting from experimental seizures, focal brain trauma (Hlatky et al, 2003), and cerebral ischemia (Endres et al, 2004). These conditions are associated with large-scale elevations of extracellular glutamate and -serine levels; thus, it is tempting to speculate that eNOS-induced hyperemia is linked to eNMDA receptors that require high brain glutamate production for activity.
Glutamate and -serine-mediated dilation was also abolished by removal (denudation) of the endothelium. Thus, our experiments support a link between NMDA receptor activation, endothelial function, and eNOS. Results agree with previous results in a brain endothelial-derived cell line displaying NMDA receptor-dependent eNOS activation (Scott et al, 2007), but our study is the first to demonstrate linkage between vascular NMDA receptors and eNOS in intact arteries. We considered the possibility that neuronal NMDA receptors expressed by remnant nerve endings adhering to the vascular smooth muscle surface mediate endothelial-dependent eNOS activation. To address this, we used two approaches. First, we chemically denervated MCA segments and found that this did not impact MCA dose-dependent dilation to glutamate and -serine. This indicates glutamate and -serine exert direct effects on vascular NMDA receptors. Second, we presented evidence that NMDA receptor subunits are expressed by endothelial cells. NR1 expression was detected in primary cultured endothelial cells from 15-week-old mice by PCR, Western blot, and immunocytochemistry. This agrees with previous studies reporting the presence of NMDA receptor expression in cultured endothelial cells (Chen et al, 2005; Krizbai et al, 1998; Scott et al, 2007; Sharp et al, 2003) and endothelial cells in the brain tissue (Reijerkerk et al, 2010). While this is an important result, the preparation used contains microvascular endothelium as well as arterial endothelium. Since the focus of the current study is on brain arteries, we stained fixed cortical slices for NMDA receptor subunits and selected arteries >50 μm in diameter near the pial surface for analysis. Cortical neurons were positive for NR1, as expected. NR1 signal was also apparent in the brain vasculature but was considerably less intense than surrounding neuronal signal and ambiguous for fine determination of cellular distribution. We surveyed immunoreactivity for other NMDA receptor subunits in situ and found a strong signal for NR2C in cortical arteries but not the majority of cortical neurons. NR2C signal overlapped with the endothelial marker, p-glycoprotein, suggesting an endothelial distribution. NR2C was distinctly more luminal than GFAP, further supporting an endothelial distribution. Other groups have specifically argued against endothelial cell NMDA receptor expression (Domoki et al, 2008; Morley et al, 1998). The reason for disparate results between these two studies and those supporting endothelial NMDA receptor expression are not clear and comprehensive immunochemical analyses of brain endothelium in situ are required to address this further.
This study provides new evidence linking -serine with endothelial NMDA receptor activation and eNOS-mediated dilation in intact brain arteries. Astrocytes mediate activity-dependent changes in the brain blood flow and release both -serine and glutamate in response to neuronal activity (Mothet et al, 2005; Parpura et al, 1994). Thus, it is rational to hypothesize that astrocyte -serine stores contribute to blood flow regulation in hyperamia in certain conditions. The current results lend significant support to this possibility, but further studies in intact brain preparations at the arteriole and capillary level are required to determine the role of -serine in regulating lumen diameter and blood flow in vivo.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by Canadian Institutes of Health Research, Manitoba Health Research Council, Heart and Stroke Foundation of Canada.
Supplementary Material
References
- Ayata C, Ma J, Meng W, Huang P, Moskowitz MA. L-NA-sensitive rCBF augmentation during vibrissal stimulation in type III nitric oxide synthase mutant mice. J Cereb Blood Flow Metab. 1996;16:539–541. doi: 10.1097/00004647-199607000-00002. [DOI] [PubMed] [Google Scholar]
- Bari F, Errico RA, Louis TM, Busija DW.1996aDifferential effects of short-term hypoxia and hypercapnia on N-methyl-D-aspartate-induced cerebral vasodilatation in piglets Stroke 271634–1639.discussion 1639–1640 [DOI] [PubMed] [Google Scholar]
- Bari F, Errico RA, Louis TM, Busija DW. Interaction between ATP-sensitive K+ channels and nitric oxide on pial arterioles in piglets. J Cereb Blood Flow Metab. 1996b;16:1158–1164. doi: 10.1097/00004647-199611000-00010. [DOI] [PubMed] [Google Scholar]
- Boldyrev AA, Carpenter DO, Johnson P. Emerging evidence for a similar role of glutamate receptors in the nervous and immune systems. J Neurochem. 2005;95:913–918. doi: 10.1111/j.1471-4159.2005.03456.x. [DOI] [PubMed] [Google Scholar]
- Busija DW, Bari F, Domoki F, Louis T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res Rev. 2007;56:89–100. doi: 10.1016/j.brainresrev.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busija DW, Leffler CW. Dilator effects of amino acid neurotransmitters on piglet pial arterioles. Am J Physiol. 1989;257:H1200–H1203. doi: 10.1152/ajpheart.1989.257.4.H1200. [DOI] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Chen H, Fitzgerald R, Brown AT, Qureshi I, Breckenridge J, Kazi R, Wang Y, Wu Y, Zhang X, Mukunyadzi P, Eidt J, Moursi MM. Identification of a homocysteine receptor in the peripheral endothelium and its role in proliferation. J Vasc Surg. 2005;41:853–860. doi: 10.1016/j.jvs.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Chi OZ, Liu X, Weiss HR. Effects of inhibition of neuronal nitric oxide synthase on NMDA-induced changes in cerebral blood flow and oxygen consumption. Exp Brain Res. 2003;148:256–260. doi: 10.1007/s00221-002-1310-7. [DOI] [PubMed] [Google Scholar]
- Chinellato A, Froldi G, Caparrotta L, Ragazzi E. Pharmacological characterization of endothelial cell nitric oxide synthase inhibitors in isolated rabbit aorta. Life Sci. 1998;62:479–490. doi: 10.1016/s0024-3205(97)01144-2. [DOI] [PubMed] [Google Scholar]
- Choi DW, Koh JY, Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990;11:290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Domoki F, Kis B, Gaspar T, Bari F, Busija DW. Cerebromicrovascular endothelial cells are resistant to L-glutamate. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1099–R1108. doi: 10.1152/ajpregu.90430.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends Neurosci. 2004;27:283–289. doi: 10.1016/j.tins.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Breese KR. Nitric oxide mediates vasodilatation in response to activation of N-methyl-D-aspartate receptors in brain. Circ Res. 1993;72:476–480. doi: 10.1161/01.res.72.2.476. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Breese KR. Dilatation of cerebral arterioles in response to N-methyl-D-aspartate: role of CGRP and acetylcholine. Brain Res. 1994;640:93–97. doi: 10.1016/0006-8993(94)91860-0. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Brian JE., Jr19957-Nitroindazole inhibits brain nitric oxide synthase and cerebral vasodilatation in response to N-methyl-D-aspartate Stroke 262172–2175.discussion 2176 [DOI] [PubMed] [Google Scholar]
- Fergus A, Lee KS. Regulation of cerebral microvessels by glutamatergic mechanisms. Brain Res. 1997;754:35–45. doi: 10.1016/s0006-8993(97)00040-1. [DOI] [PubMed] [Google Scholar]
- Fiumana E, Parfenova H, Jaggar JH, Leffler CW. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am J Physiol Heart Circ Physiol. 2003;284:H1073–H1079. doi: 10.1152/ajpheart.00881.2002. [DOI] [PubMed] [Google Scholar]
- Handy RL, Wallace P, Gaffen ZA, Whitehead KJ, Moore PK. The antinociceptive effect of 1-(2-trifluoromethylphenyl) imidazole (TRIM), a potent inhibitor of neuronal nitric oxide synthase in vitro, in the mouse. Br J Pharmacol. 1995;116:2349–2350. doi: 10.1111/j.1476-5381.1995.tb15078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardebo JE, Wieloch T, Kahrstrom J. Excitatory amino acids and cerebrovascular tone. Acta Physiol Scand. 1989;136:483–485. doi: 10.1111/j.1748-1716.1989.tb08690.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Oka T, Nishikawa T. Extracellular concentration of endogenous free D-serine in the rat brain as revealed by in vivo microdialysis. Neuroscience. 1995;66:635–643. doi: 10.1016/0306-4522(94)00597-x. [DOI] [PubMed] [Google Scholar]
- Hlatky R, Lui H, Cherian L, Goodman JC, O'Brien WE, Contant CF, Robertson CS. The role of endothelial nitric oxide synthase in the cerebral hemodynamics after controlled cortical impact injury in mice. J Neurotrauma. 2003;20:995–1006. doi: 10.1089/089771503770195849. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Regulation of the cerebral microcirculation during neural activity: is nitric oxide the missing link. Trends Neurosci. 1993;16:206–214. doi: 10.1016/0166-2236(93)90156-g. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Jung SS, Levy E. Murine cerebrovascular cells as a cell culture model for cerebral amyloid angiopathy: isolation of smooth muscle and endothelial cells from mouse brain. Methods Mol Biol. 2005;299:211–219. doi: 10.1385/1-59259-874-9:211. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizbai IA, Deli MA, Pestenacz A, Siklos L, Szabo CA, Andras I, Joo F. Expression of glutamate receptors on cultured cerebral endothelial cells. J Neurosci Res. 1998;54:814–819. doi: 10.1002/(SICI)1097-4547(19981215)54:6<814::AID-JNR9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci. 2006;26:2673–2683. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Meng W, Ayata C, Huang PL, Fishman MC, Moskowitz MA. L-NNA-sensitive regional cerebral blood flow augmentation during hypercapnia in type III NOS mutant mice. Am J Physiol. 1996;271:H1717–H1719. doi: 10.1152/ajpheart.1996.271.4.H1717. [DOI] [PubMed] [Google Scholar]
- Martineau M, Galli T, Baux G, Mothet JP. Confocal imaging and tracking of the exocytotic routes for D-serine-mediated gliotransmission. Glia. 2008;56:1271–1284. doi: 10.1002/glia.20696. [DOI] [PubMed] [Google Scholar]
- Meng W, Tobin JR, Busija DW.1995Glutamate-induced cerebral vasodilation is mediated by nitric oxide through N-methyl-D-aspartate receptors Stroke 26857–862.discussion 863 [DOI] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morley P, Small DL, Murray CL, Mealing GA, Poulter MO, Durkin JP, Stanimirovic DB. Evidence that functional glutamate receptors are not expressed on rat or human cerebromicrovascular endothelial cells. J Cereb Blood Flow Metab. 1998;18:396–406. doi: 10.1097/00004647-199804000-00008. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci USA. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenova H, Fedinec A, Leffler CW. Ionotropic glutamate receptors in cerebral microvascular endothelium are functionally linked to heme oxygenase. J Cereb Blood Flow Metab. 2003;23:190–197. doi: 10.1097/01.WCB.000004823561824.C4. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Philip S, Armstead WM. NMDA dilates pial arteries by KATP and Kca channel activation. Brain Res Bull. 2004;63:127–131. doi: 10.1016/j.brainresbull.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijerkerk A, Kooij G, van der Pol SM, Leyen T, Lakeman K, van Het Hof B, Vivien D, de Vries HE. The NR1 subunit of NMDA receptor regulates monocyte transmigration through the brain endothelial cell barrier. J Neurochem. 2010;113:447–453. doi: 10.1111/j.1471-4159.2010.06598.x. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipke CG, Ohlemeyer C, Matyash M, Nolte C, Kettenmann H, Kirchhoff F. Astrocytes of the mouse neocortex express functional N-methyl-D-aspartate receptors. FASEB J. 2001;15:1270–1272. doi: 10.1096/fj.00-0439fje. [DOI] [PubMed] [Google Scholar]
- Scott GS, Bowman SR, Smith T, Flower RJ, Bolton C. Glutamate-stimulated peroxynitrite production in a brain-derived endothelial cell line is dependent on N-methyl-D-aspartate (NMDA) receptor activation. Biochem Pharmacol. 2007;73:228–236. doi: 10.1016/j.bcp.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp CD, Hines I, Houghton J, Warren A, Jackson TH, Jawahar A, Nanda A, Elrod JW, Long A, Chi A, Minagar A, Alexander JS. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am J Physiol Heart Circ Physiol. 2003;285:H2592–H2598. doi: 10.1152/ajpheart.00520.2003. [DOI] [PubMed] [Google Scholar]
- Simandle SA, Kerr BA, Lacza Z, Eckman DM, Busija DW, Bari F. Piglet pial arteries respond to N-methyl-D-aspartate in vivo but not in vitro. Microvasc Res. 2005;70:76–83. doi: 10.1016/j.mvr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bukoski RD. Use of acute phenolic denervation to show the neuronal dependence of Ca2+-induced relaxation of isolated arteries. Life Sci. 1999;64:887–894. doi: 10.1016/s0024-3205(99)00009-0. [DOI] [PubMed] [Google Scholar]
- Wendling WW, Chen D, Daniels FB, Monteforte MR, Fischer MB, Harakal C, Carlsson C. The effects of N-methyl-D-aspartate agonists and antagonists on isolated bovine cerebral arteries. Anesth Analg. 1996;82:264–268. doi: 10.1097/00000539-199602000-00009. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci USA. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.