Abstract
The role of N-methyl--aspartate (NMDA) receptor-mediated mechanisms in the formation of a blood oxygen level-dependent (BOLD) response was studied using electrical stimulation of the right perforant pathway. Stimulation of this fiber bundle triggered BOLD responses in the right hippocampal formation and in the left entorhinal cortex. The perforant pathway projects to and activates the dentate gyrus monosynaptically, activation in the contralateral entorhinal cortex is multisynaptic and requires forwarding and processing of signals. Application of the NMDA receptor antagonist MK801 during stimulation had no effect on BOLD responses in the right dentate gyrus, but reduced the BOLD responses in the left entorhinal cortex. In contrast, application of MK801 before the first stimulation train reduced the BOLD response in both regions. Electrophysiological recordings revealed that the initial stimulation trains changed the local processing of the incoming signals in the dentate gyrus. This altered electrophysiological response was not further changed by a subsequent application of MK801, which is in agreement with an unchanged BOLD response. When MK801 was present during the first stimulation train, a dissimilar electrophysiological response pattern was observed and corresponds to an altered BOLD response, indicating that NMDA-dependent mechanisms indirectly affect the BOLD response, mainly via modifying local signal processing and subsequent propagation.
Keywords: electrical stimulation, entorhinal cortex, fMRI, hippocampus, medetomidine, MK801, perforant pathway
Introduction
Functional magnetic resonance imaging (fMRI) enables a noninvasive visualization of localized alterations in neuronal activity triggered by specific stimuli. However, in fMRI studies, changed neuronal activities are only indirectly visualized by hemodynamic parameters, such as changes in blood flow/volume and blood oxygen level. Thus, an fMRI signal depends on neuronal-triggered mechanisms that control the vascular system. An understanding of the underlying mechanisms for this neurovascular coupling is needed for any interpretation of fMRI results, especially when seemingly similar stimuli induce different fMRI responses. The activity of the participating neurons in this region is mainly controlled by the quantity and quality of the synaptic inputs they receive from all incoming fibers. Consequently, the overall incoming synaptic activation in a given region should be a crucial factor for the generation of a blood oxygen level-dependent (BOLD) response. Glutamate is the main excitatory transmitter in the brain; and therefore, glutamate receptor mechanisms are likely candidates for mediating BOLD responses. Glutamate acts on ionotropic (AMPA (2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid), Kainate, N-methyl--aspartate (NMDA)) and metabotropic (mGluR1-8) receptors, which may contribute to the generation of a BOLD response. In a previous study, NMDA receptor activation was assumed to be an essential part of the signaling chain generating the BOLD response after neuronal activation (Gsell et al, 2006). This is of particular interest because NMDA receptors are considered to be coincidence detectors and their activation is necessary for various forms of synaptic plasticity. Presence of the NMDA receptor antagonist MK801 during electrical forepaw stimulation induced a clear reduction of the BOLD response in the somatosensory cortex (Gsell et al, 2006). However, based on this approach it is not entirely clear whether the effect of MK801 is caused by influencing the signal propagation/processing on the way to the cortex, or by affecting the local signal processing within the somatosensory cortex. Consequently, to prove a direct contribution of NMDA receptor-dependent mechanisms for the generation of a BOLD response, a more direct approach is required. Previously, we developed an experimental set-up that allows for the visualization of BOLD responses elicited by electrical stimulation of a central fiber system, that is, the right perforant pathway (Angenstein et al, 2007). Using this approach, the right dentate gyrus becomes directly, or monosynaptically activated, and dependent on the activation protocol, the contralateral entorhinal cortex region becomes indirectly, that is, multisynaptically activated. In contrast, the right entorhinal cortex becomes both, monosynaptically, by antidromically stimulation of layer II neurons (Jones, 1994) and multisynaptically via the hippocampal trisynaptic pathway activated during perforant pathway stimulation. In addition, simultaneous electrophysiological recordings in the dentate gyrus will give information about the quality of signal processing in this region. Local signal processing in a region can be roughly defined as relation between input and output activity. The input activity into the dentate gyrus is predefined by the perforant pathway stimulation, whereas the output activity corresponds to the measured action potentials of the granular cells, that is, the population spikes. That means local signal processing is changed whenever an identical stimulation protocol causes an altered population spike pattern. Using the following simultaneous measured parameters: BOLD response in a monosynaptically activated region (i.e., right dentate gyrus), BOLD response in a multisynaptically activated region (i.e., left entorhinal cortex), and the quality of local signal processing (i.e., development of population spike amplitude and latency), we studied the effect of NMDA receptor inhibition on the generated BOLD response.
Should the NMDA receptor activation be an essential component for the generation of activity-triggered BOLD responses, inhibition of this receptor by MK801 should thus similarly affect the BOLD response both in the right dentate gyrus and in the left entorhinal cortex. Alternatively, if NMDA receptor-mediated mechanisms affect the generation of a BOLD response by disrupting the propagation of signals to the measured region, the presence of MK801 should modify the BOLD response in the left entorhinal cortex region in a different way as in the right dentate gyrus. Finally, if the BOLD response depends on NMDA receptor-dependent mechanisms controlling local signal processing, inhibition of this receptor by MK801 should affect the BOLD response whenever an electrophysiological altered response pattern is observed under MK801. By combination of different stimulation paradigms, we present evidences that NMDA receptor inhibition controls the BOLD response mainly by affecting local signaling processes and, as a result, by modifying the signal propagation to multisynaptically connected regions.
Materials and methods
Animals and Surgical Procedure
The experiments were approved by the animal care committee of the State Saxony-Anhalt, Germany (No. 203.h-42502-2-852 IfN).
For electrode implantation, 7- to 8-week-old male Wistar rats were anesthetized with Nembutal (40 mg/kg intraperitoneally) and placed into a stereotactic frame. A bipolar stimulation electrode (114 μm in diameter, made from teflon-coated tungsten wire) was placed into the perforant pathway in the hemisphere at the coordinates anterior–posterior: −6.9 mm, medial–lateral: 4.1 mm from Bregma, dorsal–ventral: 2.5 to 3.0 mm from the dural surface. A monopolar recording electrode was lowered into the granular cell layer of the dentate gyrus anterior–posterior: −2.8 mm, medial–lateral: 1.8 mm from Bregma, dorsal–ventral: 2.8 to 3.2 mm from the dural surface. Monitoring the evoked field potentials during implantation controlled the correct placement, especially with regard to electrode depth. By placement of the recording electrode into the granular cell layer, we can assume that a measured population spike reflects summed action potentials of granular cells, because the electrode is located close to the side of the action potential generation (Frey and Frey, 2009). Because granular cells are the principal cells in the dentate gyrus, observed variations in the population spike amplitude and latency indicate an altered output activity from the dentate gyrus. Grounding and indifferent electrodes (silver wires) were set on the dura through the left side of the cranium, and fixed to the skull with dental cement and plastic screws. After surgery, the animals were housed individually and given 7 days for recovery, with ad libitum food and water.
Magnetic Resonance Imaging Measurements and Stimulation
Rats were initially anesthetized with 1.0% to 1.5% isoflurane (in 50:50 N2:O2; v:v), and connected to the stimulation and recording electrodes. After placing the animal on the stereotactic platform, narcosis was switched to deep sedation by applying medetomidine hydrochloride (Domitor, Pfizer GmbH, Karlsruhe, Germany; bolus of 50 μg/kg subcutaneously and after 15 minutes 100 μg/kg per hour subcutaneously). The animals were fixed using a head holder with a bite bar to reduce motion artifacts. Heating was provided from the ventral side and breathing rate, heart rate, and oxygen saturation were monitored during the whole experiment using an MRI-compatible pulse oxymeter (MouseOx, Starr Life Sciences, Pittsburgh, PA, USA).
Electrophysiological responses were evoked by a stimulus generator (Isolated Pulse Stimulator, Model 2100; Science Products, Hofheim, Germany) and recorded with a Quad Channel Differential Extracellular Amplifier (Ex4-400, Science Products) and transformed by an analogue-to-digital interface (Power1401mkII, fast data acquisition and analysis interface, Science Products). No preprocessing of the electrophysiological data was necessary. For each generated population spike, the population spike amplitude and latency was determined.
To determine the appropriate stimulation intensities for the fMRI experiment, the perforant pathway was first stimulated with increasing intensities (from 30 to 600 μA) and the appropriate field potentials recorded. According to this input/output curve, the intensity required to elicit a population spike and the maximal population spike amplitude could be determined, and the two stimulation intensities for the experiment duly assigned. Initially, a low intensity that was just below the threshold stimulation intensity (around 50 μA) was used, followed by a second high intensity stimulation that elicited 50% of the maximal amplitude population spikes (around 200 μA). Electrical perforant pathway stimulation, both single test stimuli and burst stimuli, caused no eye or head movements that could perturb the fMRI measurement.
Magnetic resonance imaging experiments were performed on a Bruker Biospec 47/20 scanner (Bruker BioSpin GmbH, Ettlingen, Germany) at 4.7 T (free bore of 20 cm), equipped with a BGA09 (400 mT/m) gradient system (Bruker BioSpin GmbH). A 50-mm Litzcage small animal imaging system (Doty Scientific, Columbia, SC, USA) was used for radio frequency (RF) excitation and signal reception. For anatomical images, eight horizontal T2-weighted spin-echo images were obtained simultaneously using an RARE sequence (rapid acquisition relaxation enhanced; Hennig et al, 1986) configured using the following parameters: repetition time 4,000 ms, echo time 15 ms, slice thickness 1 mm, field of view (FOV) 40 × 40 mm, matrix 256 × 256, RARE factor 8, averages 4. The total scanning time was 8 minutes 32 seconds. Functional MRI was performed using an EPI (echo planar imaging) sequence with the following parameters: repetition time 2,000 ms, echo time 24 ms, slice thickness 1 mm, FOV 40 × 40 mm, matrix 64 × 64, total scanning time per frame 2 seconds.
To prove an effect of MK801 on the generated BOLD response in the hippocampal formation, the perforant pathway was stimulated with three consecutive identical stimulation blocks. Immediately after the first stimulation block MK801 (TOCRIS Bioscience, Bristol, UK; 0.5 mg/kgbody weight) was injected intraperitoneally using a plastic catheter (Vasofix Braunüle, B Braun Melsungen AG, Melsungen, Germany); thus, MK801 was present during the second and third stimulation blocks. The third stimulation block started 13 minutes after application of MK801, after a time period in which MK801 should cause an effective NMDA receptor blockade. Control animals were not treated with MK801, but received the same stimulus protocol (see Figure 1A). As soon as the ratio of BOLD responses of the third block to the first block in MK801-treated animals differed from the same ratio observed in control animals, an effect of MK801 on the BOLD response was assumed. In a second experimental approach, MK801 was applied immediately before the stimulation protocol started; thus, MK801 was present during the entire stimulation experiment. During this condition, the second stimulation block started again 13 minutes after application of the NMDA inhibitor, thus in terms of effective MK801 presence, the second block in this approach corresponds to the third block in the first experimental approach (see Figure 1A). Application of MK801, although it can increases locomotion after injection of >0.3 mg/kg (Roberts et al, 2008), did not cause any motion artifacts, probably because medetomidine acts not only as a sedative but also it induces muscle relaxation.
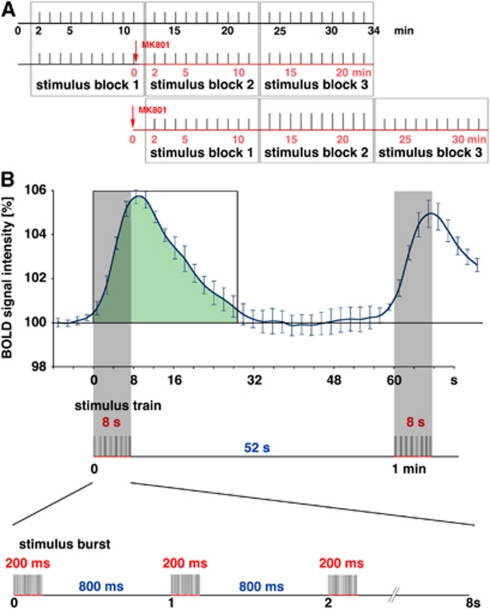
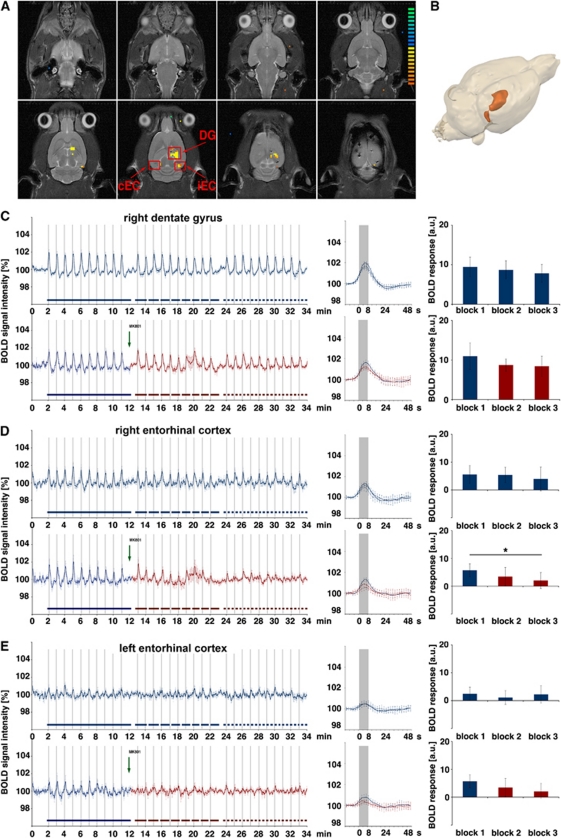
Figure 1.
Schematic overview of the stimulation protocol. (A) The perforant pathway was stimulated with 3 consecutive stimulation blocks containing 10 identical stimulation trains (indicated as gray bars). The N-methyl--aspartate (NMDA) receptor inhibitor MK801 was applied either immediately after the first stimulation block (middle graph) or immediately before the stimulation protocol started (lower graph). The effective time of MK801 is indicated by the red time scale. An effect of MK801 is assumed after a time delay of 8 to 12 minutes (Gsell et al, 2006), thus during the third block when the inhibitor is applied during the stimulation protocol or during the second block when the inhibitor was applied before the stimulation started. (B) Temporal relation between measured blood oxygen level-dependent (BOLD) response and electrical stimulation of the perforant pathway. Every minute one stimulation train was applied that lasted 8 seconds, which corresponds to four frames in the functional magnetic resonance imaging (fMRI) (indicated by the gray bar). One stimulation train consists of 8 bursts of 20 stimuli at 100 Hz. Thus, one burst lasts 200 ms followed by 800 ms rest. For quantification of a BOLD response, the area below the hemodynamic response curve was calculated starting with the stimulus onset end ending 28 seconds later (green area). The colour reproduction of this figure is available on online version.
In general, each block consisted on 10 identical stimulation trains. Each block started with a 2-minute resting period, followed by the 10 stimulation trains. One stimulation train lasted 8 seconds and was followed by 52 seconds rest, thus every minute one stimulation train was applied. One stimulation train, in turn, consisted on eight stimulation bursts starting every second. Each burst contained 20 stimuli (stimulus: square pulse 0.2 ms with the appropriate intensity) given with 10 ms intervals (i.e., 100 Hz) and lasted 200 ms (Figure 1B).
To distinguish the effect caused by low (threshold) and high intensity stimulations (50% of the maximal amplitude population spikes), blocks with low intensity and high intensity stimuli were alternated within the experiment. Unless otherwise stated, each experiment consisted of three blocks leading to a total scanning time of 34 minutes (1,020 frames; Figure 1A).
Data Processing and Analysis
The functional data were loaded and converted into BrainVoyager data format. A standard sequence of preprocessing steps implemented in the BrainVoyager QX software (Brain Innovation, Maastricht, The Netherlands), such as 3D motion correction and temporal filtering (Gaussian filter; FWHM (full width at half maximum) 3 data points) were applied to each data set. Functional activation was analyzed by correlation of the observed signal intensity changes in each voxel with the given stimulus protocol (see above); based on this, the appropriate activation map could be generated. To account for the hemodynamic delay, the stimulus representing block design was modified by a double-gamma hemodynamic response function (onset 0 seconds, time to response peak 5 seconds, time to undershoot peak 15 seconds). To exclude false positive voxels, we considered only voxels with a significance level <10−9 (tmin=6) for the analysis of the size of the activated area. All presented BOLD time series correspond to the average BOLD signal time course of all measured animals, the standard deviations are expressed as ±standard error of the mean.
Event-related BOLD responses were calculated by measuring the signal intensities starting 6 frames (−12 seconds until 0 seconds) before stimulus onset (stimulus presentation was between 0 and 8 seconds, which corresponds to four frames), until 20 frames (8 seconds to 48 seconds) after the stimulus end. To avoid a confounding effect of putative variations in baseline BOLD signal intensities on the calculated BOLD response (i.e., BOLD signalstimulus/BOLD signalbaseline × 100%), each BOLD response was related to BOLD signal intensities of the stimulus preceding 12 seconds. Averaged signal intensities within the appropriate area in the first five frames (−12 seconds until −2 seconds) were set to 100%.
To compare the amount of elicited BOLD responses between consecutive stimulation blocks, the area below the BOLD response corresponding to each stimulation train was calculated for each individual animal, starting with the stimulus train onset and ending 28 seconds later (see Figure 1B). Thereupon, the average BOLD responses were calculated for each of the three blocks. That means only the development of the BOLD response within one experiment were analyzed and tested for significant differences. Because the absolute magnitude of the BOLD response varies between individual animals, absolute BOLD responses measured in different groups were not compared, to avoid false positive or negative results that are based on the compilation of these groups. The variation in the magnitude of the BOLD response generated in the three subsequent stimulation blocks was tested for significant differences using a two-tailed Wilcoxon signed-rank test. At a calculated P-value <0.05, the differences were considered as significant. Because the BOLD signal intensities in all regions remained elevated after the first high intensity stimulation train and affected by that the next elicited BOLD response, we considered for the analysis only the last six stimulation trains in this stimulation block.
Results
To test the effect of MK801 on the magnitude of the BOLD response, the perforant pathway was stimulated with 3 identical blocks of 10 stimulation trains, with MK801 injected between the first and second blocks. Two incongruent intensities were chosen; a high intensity that elicited ∼50% of the maximal population spike amplitude to single test stimuli, and a low intensity that was not sufficient to elicit detectable population spikes to single test stimuli. In a previous study, we showed that during high intensity stimulation, BOLD responses were generated in the dentate gyrus and the two entorhinal cortex regions. However, during low intensity stimulation, BOLD responses were only observed in the ipsilateral hippocampal formation (Angenstein et al, 2009).
Effect of MK801 Applied During Repetitive High Intensity Stimulation Trains
Repetitive stimulation of the perforant pathway with high intensity stimulation trains resulted in a BOLD time series, and is a phenomenon observed in a previous study by (Angenstein et al, 2010). First, in the right dentate gyrus, the BOLD response varied during the first stimulation block (Figures 2A–2C). A strong BOLD response was observed after the first stimulation train, which was followed by a late positive component that was coincident with heavy after-discharges observed by electrophysiological recordings. However, the BOLD response to the second train was concealed by this baseline elevation in the BOLD signal (Figure 2C, upper graph). Thereafter, the baseline BOLD signal intensity normalized, and starting around the fourth train, consistent BOLD responses were generated and no further after-discharges generated. During the second and third stimulation blocks, all elicited BOLD responses were similar, though at a significantly lower magnitude than the first block. A comparable time course was also observed in the right and left entorhinal cortex regions, although only in the contralateral entorhinal cortex a significant reduction was observed in the last block, compared with the first block (Figures 2D and 2E, upper graphs). Application of MK801 after the first stimulation block did not obviously affect the magnitude of BOLD responses in the right dentate gyrus (Figure 2C, lower graph). Again, the magnitude of the BOLD response decreased significantly after the first stimulation blocks and remained similar between blocks 2 and 3. In contrast, when NMDA receptors were blocked by MK801 during stimulation, the magnitude of BOLD responses decreased significantly in the right entorhinal cortex and, even more pronounced, in the left entorhinal cortex region, both from the first to the second and from the second to the third block (Figures 2D and 2E, lower graphs). Consequently, presence of MK801 significantly affected the BOLD responses in the right and left entorhinal cortices but not in the right dentate gyrus. Simultaneous electrophysiological recordings revealed an effect of MK801 on the evoked field potentials (Figure 3). During the first block, the pattern of field potentials changed as previously described by Angenstein et al (2010), whereas in the subsequent stimulation blocks the pattern remained stable (Figure 3, left side). Application of MK801 after the first stimulation block did not alter the general pattern of field potentials but modified the strength of variations within each train. In particular, the augmentation of the population spike amplitude during each train was reduced and the differences in the latency between the first and last population spike within a train were smaller (Figure 3, right side). When the generated BOLD responses were related to the simultaneously elicited electrophysiological measured responses, a significant separation of the electrophysiological responses was observed in the third block, whereas the BOLD responses remained similar (Figure 4). Consequently, presence of MK801 had an effect on the neuronal activity without changing the resulting BOLD signal in the dentate gyrus.
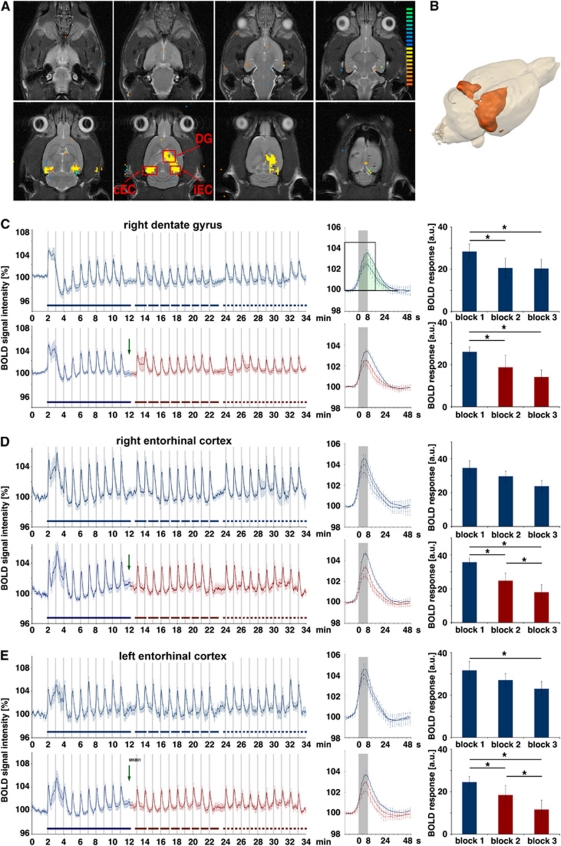
Figure 2.
Development of the blood oxygen level-dependent (BOLD) response during application of MK801. The perforant pathway was stimulated with 3 consecutive stimulation blocks containing 10 high intensity stimulation trains. In the test group, MK801 was applied after the first stimulation block (green arrow) whereas the control group remained untreated. (A) BOLD response pattern caused by the stimulation and the measured regions: DG, ipsilateral (right) dentate gyrus; iEC, ipsilateral entorhinal cortex; cEC, contralateral (left) entorhinal cortex. (B) 3D visualization of significant BOLD responses induced by repetitive high intensity stimulation trains. (C) Averaged BOLD time series in the dentate gyrus during control (upper graph) and test conditions (lower graph). The presence of MK801 is indicated by the red color. The averaged BOLD responses measured during each block are depicted in the middle part. The solid lines represent the averaged BOLD responses of the first block (only the last six trains were considered), the dashed line the BOLD responses of the second block, and the dotted line the BOLD responses of the third block. Comparing the BOLD time series revealed no difference whether MK801 was added or not. The amount of BOLD responses was calculated by measuring the area below each BOLD response in the entire signal time course between stimulus onset and 14 frames later (for the first stimulation block indicated by the green area in the black rectangle in the middle part). Both under control and test condition, the BOLD responses in the second and third block stimulation blocks were significantly decreased; thus, MK801 had no significant effect on the BOLD response in the dentate gyrus. (D) BOLD time series measured simultaneously in the ipsilateral entorhinal cortex. In this region, the magnitude of the BOLD response decreased significantly in the second and third blocks after MK801 was added. (E) The development of the simultaneous BOLD response in the contralateral entorhinal cortex. In this region, the magnitude of the BOLD response was also significantly reduced in the second and third stimulation blocks after MK801 was injected. In contrast, in the control group only a significant difference was found between the first and last stimulation blocks. Therefore, presence of MK801 only reduced the elicited BOLD signals in the left and right entorhinal cortices (control group: n=6, test group: n=6; *P<0.05, Wilcoxon's test).
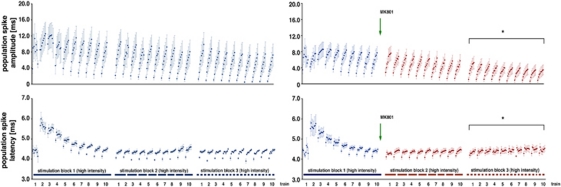
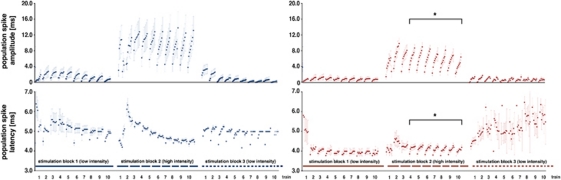
Figure 3.
Development of the population spike amplitude and latency in the right dentate gyrus during application of MK801. Electrophysiological recordings were performed during acquisition of the functional magnetic resonance imaging (fMRI) data; thus, these data correspond to the blood oxygen level-dependent (BOLD) responses shown in Figure 2C. During the first stimulation block, the population spike latency varied considerably; after an initial increase during the second train they decrease to reach a stable value at the end of the first block. During the next two stimulation blocks, the average latencies remained stable and no effect on the average latency was observed when MK801 was added after the first stimulation block (application indicated by the green arrow and presence indicated by the red color). During the second and third stimulation blocks, an augmentation of the population spike amplitude occurred during each train, which was significantly reduced by MK801. Similarly, the difference in the population spike latency between the first and last stimulus within one train was significantly reduced in the third stimulation block when MK801 was present (comparison with the control condition, *P<0.05, Student's t-test).
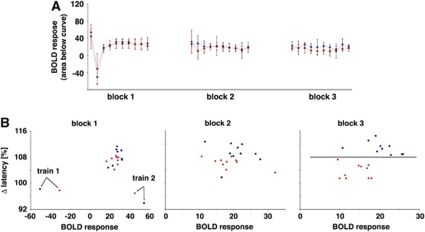
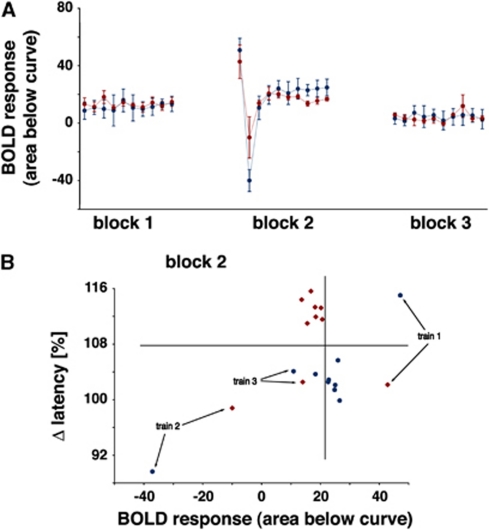
Figure 4.
Effect of MK801 on the blood oxygen level-dependent (BOLD) response and evoked field potentials in the dentate gyrus during repetitive stimulation of the perforant pathway. (A) Development of the BOLD responses (area below the BOLD signal time course, see Figure 2C) under control (blue line) and test (application of MK801 after the first stimulus train, red line) condition. The development of BOLD responses did not differ significantly between both conditions. (B) Plotting the individual BOLD responses (abscissa) and the corresponding variations in the population spike latency (last population spike/first population spike (in %), ordinate) revealed an effect of MK801 only on the electrophysiological parameter. A clear separation of the Δ latency was observed in the third stimulation block, whereas the BOLD responses remained similar.
Effect of MK801 Applied During Repetitive Low Intensity Stimulation Trains
Stimulation of the perforant pathway with three blocks of low intensity stimulation trains resulted in a more uniform BOLD response during the entire experiment. Within the right dentate gyrus, constant BOLD responses were elicited during all three successive stimulation blocks (Figures 5A–5C, upper graph). During low intensity stimulation, the magnitude of the BOLD response was not significantly affected in the dentate gyrus after MK801 was applied, although, at the same time, the BOLD response decreased significantly in the ipsilateral entorhinal cortex (Figure 5D, lower graph). No consistent BOLD responses were triggered in the contralateral entorhinal cortex; thus, any effects of MK801 were not measurable (Figure 5E).
Figure 5.
Development of the blood oxygen level-dependent (BOLD) response during application of MK801. The perforant pathway was stimulated with 3 consecutive stimulation blocks containing 10 low intensity stimulation trains. MK801 was again applied after the first stimulation block (green arrow), whereas the control group remained untreated. (A) BOLD response pattern caused by the stimulation and the measured regions: DG, ipsilateral (right) dentate gyrus; iEC, ipsilateral entorhinal cortex; cEC contralateral (left) entorhinal cortex. (B) 3D visualization of significant BOLD responses induced by repetitive low intensity stimulation trains. (C–E) The elicited BOLD responses in these areas are shown again as in Figure 2. Again MK801 did not change significantly the BOLD response in the dentate gyrus, whereas the magnitude of the BOLD response in the right entorhinal cortex decreased significantly during the third stimulation block. No clear BOLD signals are elicited in the contralateral entorhinal cortex; therefore, no conclusions can be made (control group: n=6, test group: n=6, *P<0.05, Wilcoxon's test).
Effect of MK801 Applied Immediately Before Repetitive Stimulation Trains
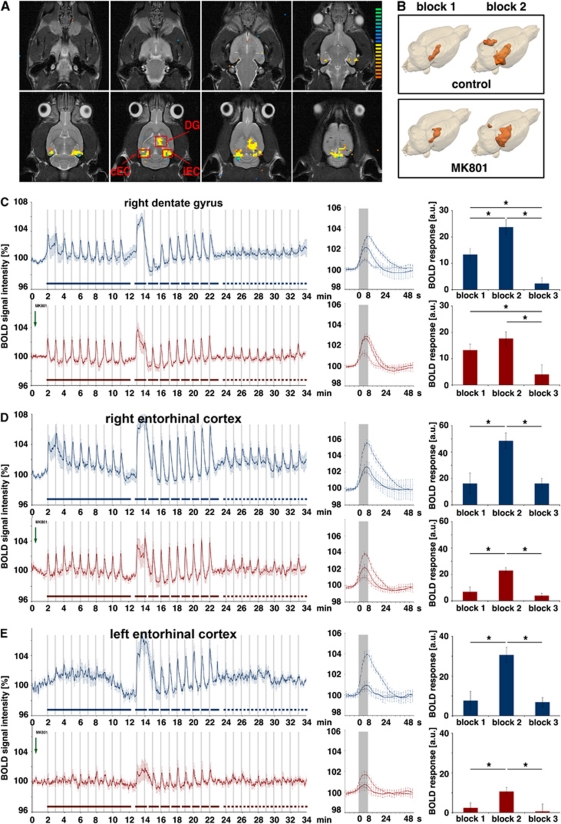
During high intensity stimulation, the first stimulation train caused a functional reorganization within the dentate neuronal network leading to a difference in the processing of incoming signals during the second and third stimulation blocks (Figures 2C and 3). Under this changed functional state, MK801 had only an effect on the recorded field potentials, and no effect on the BOLD response. To test whether MK801 might have an effect on the BOLD response when applied before the first high intensity stimulation train, two groups of animals were used. The first group received MK801 before the initial high intensity stimulation, whereas the second (the control group) remained untreated (Figure 1A). However, a direct comparison of BOLD responses between the two groups is problematic, principally owing to the variability in the magnitude of individual BOLD responses between different animals. Therefore, a relative comparison is needed. As shown above, the BOLD responses in the dentate gyrus to repetitive low intensity stimulations remain similar over time and are not affected by MK801, suggesting that no reorganization of the local dentate gyrus network occurs during low intensity stimulation (Figure 5C). Therefore, the magnitude of the BOLD response to these low intensity stimulation trains can be used as a comparative parameter for the BOLD response to subsequent high intensity stimulation trains. As described previously, BOLD responses to low intensity stimulation trains become reduced when high intensity stimulation trains are inserted (Angenstein et al, 2010). To check whether this effect depends on NMDA-dependent mechanisms, we added a third stimulation block containing identical low intensity stimulation trains. In the right dentate gyrus, this stimulation paradigm generated BOLD time series with uniform BOLD responses to low intensity stimulation trains during the first block, variable BOLD responses to high intensity stimulation trains during the second block, and as seen previously, only small or even absent BOLD responses to the low intensity stimulation trains during the third block (Figure 6C, upper graph). The average magnitude of BOLD responses during the second, high intensity stimulation train was significantly increased, when compared with the first, low intensity stimulation block. When the same experiment was performed in the presence of MK801, the BOLD time series differed during the second block. First, the average magnitude of the BOLD response did not increase significantly during in the second block, when compared with the first block (Figure 6C, lower graph). Consequently, the BOLD responses in the dentate gyrus to high intensity stimulation trains were reduced when MK801 was already present during the first high intensity stimulation train. Second, the development of the BOLD signal intensities after the first high intensity stimulation train differed considerably. Under control conditions, the BOLD signal intensity further increased after cessation of the first high intensity train. When MK801 was present, the BOLD signal intensity decreased, enabling the generation of a small positive BOLD response to the second stimulation train. This may indicate that the presence of MK801 modified the elicited neuronal activation pattern during and immediately after the first stimulation train.
Figure 6.
Development of the blood oxygen level-dependent (BOLD) response after MK801 was applied. (A) The perforant pathway was again stimulated with three consecutive stimulation blocks, starting with one block containing low intensity stimulation trains, followed by one block containing high intensity stimulation trains, and again a block with low intensity stimulation trains. (B) 3D visualization of significant BOLD responses induced by repetitive low intensity stimulation trains. During the first stimulation block, the BOLD response is restricted to the right hippocampus. Increasing the stimulation intensity during the second block caused a spreading of the BOLD response in the right hippocampal formation both in control and in MK801-treated animals. In MK801-treated animals, the spatial extend of the BOLD response was only reduced in the left entorhinal cortex region, consequently in the right hippocampus only the magnitude of the BOLD response is affected by MK801 but not the size of the activated region. (C–E) The average magnitude of the BOLD response generated during the first block (solid line) was used as a comparative value for the elicited BOLD response generated during the second block (dashed line) and third block (dotted line). In the dentate gyrus of the control group (blue graphs in panel C), the average BOLD response during high intensity stimulation increased significantly, whereas in the test group (red graphs in panel C) this increase was absent. In both groups, the BOLD responses during the third stimulation block were significantly decreased compared with the first stimulation train. In the two other regions, the BOLD responses elicited in presence of MK801 were also reduced (D, E). In these regions, no significant changes between the first and last stimulation blocks were observed (control group: n=6, test group: n=6; *P<0.05, Wilcoxon's test).
During the last stimulation block, the magnitude of the BOLD response was also significantly reduced when MK801 was present. Thus, the previously observed changes in the BOLD response between the first and last stimulation blocks are not NMDA receptor dependent. Similarly to the effects during three high intensity stimulation blocks, the presence of MK801 significantly reduced the magnitude of the BOLD response in the right region, and was more pronounced in the left entorhinal cortex region (Figures 6D and 6E).
Consistently with the observed changes in the BOLD response during the high intensity stimulation block presence of MK801 also affected the elicited field potentials in the dentate gyrus. In agreement with the concurrent observed differences in the BOLD signal time courses, the most obvious difference appeared at the beginning. The typical strong increase in the latency during the second stimulation train was absent; thus, the population spike latencies were similar during the entire high intensity stimulation block. In addition, the augmentation of the population spike amplitude during each train was again significantly reduced (Figure 7). Comparing the BOLD responses with the pattern of elicited field potentials revealed a separation of the two groups, after the forth stimulus train and is in accordance with the latency development as well as to the size of the BOLD signal (Figure 8).
Figure 7.
Development of the population spike amplitude and latency in the right dentate gyrus when MK801 was applied before stimulation. Electrophysiological recordings were performed during acquisition of the functional magnetic resonance imaging (fMRI) data; thus, these data correspond to the blood oxygen level-dependent (BOLD) responses shown in Figure 6. When MK801 was already present before the stimulation starts an effect on the development of the population spike latency during the high intensity stimulation block was observed. The effect is clearest during the second high intensity stimulation train where the strong increase in the latency was almost absent, so that the latencies were almost similar during the entire second block. This was again paralleled with a smaller augmentation of the population spike amplitude during each high intensity stimulation train, however also with a stronger increase of the population spike latency within one stimulation train (comparison with the control condition, *P<0.05, Student's t-test).
Figure 8.
Effect of MK801 on the blood oxygen level-dependent (BOLD) response and evoked field potentials in the dentate gyrus during repetitive stimulation of the perforant pathway. (A) Development of the BOLD responses (area below the BOLD signal time course, see Figure 1) under control (blue line) and test (application of MK801 before the first stimulus train, red line) condition. During the first low intensity stimulation block, no differences in the generated BOLD responses were observed. (B) During the high intensity stimulation block, the BOLD responses differed between both groups and a clear separation (indicated by the vertical and horizontal line) of BOLD responses and the development of the population spike latency was observed after the fourth stimulation train.
Discussion
In this study, we measured the BOLD response elicited by defined electrical stimulation of the right perforant pathway, in the presence or absence of the NMDA receptor antagonist MK801. Our data indicate that (1) in the dentate gyrus, NMDA receptor-dependent mechanisms are not essential for the generation of a BOLD response, although they can control the magnitude of the BOLD response via modification of the functional state of the dentate neuronal circuits, that is, the current interplay of principal cells and interneurons that controls the quality of the signal processing and by that propagation of incoming stimuli; (2) in the contralateral entorhinal cortex, a region that is not monosynaptically activated by perforant pathway stimulation, inhibition of NMDA receptors reduces the BOLD response though at the same time the BOLD response in the dentate gyrus remained unchanged.
N-Methyl--Aspartate Receptor Inhibition Reduces the Blood Oxygen Level-Dependent Responses in Regions that Become Multisynaptically Activated by Perforant Pathway Stimulation
In fMRI studies, changes in neuronal activity are only indirectly visualized by hemodynamic parameters, such as changes in blood flow/volume and blood oxygen levels. Thus, an fMRI signal depends on neuronal-triggered mechanisms that control the vascular system. This so-called neurovascular coupling is thought to be mediated by vasoactive factors, such as nitric oxide or arachidonic acid derivates (Cholet et al, 1997; Lecrux et al, 2011; Pepicelli et al, 2005; Stefanovic et al, 2006, 2007) and specific transmitter systems, such as dopamine, GABA, or glutamate (Busija et al, 2007; Cauli et al, 2004; Choi et al, 2006; Kocharyan et al, 2008). The main problem in the study of the particular role of a transmitter system in the generation of a BOLD response is that the applied receptor agonists or antagonists may have diverse points of action.
Primarily, the drug used could modify the signal propagation from the stimulation site to the region where the BOLD response is measured. This is a major confounding factor, in particular when a peripheral stimulus is presented, e.g., visual, auditory or tactile, and the BOLD response is measured in the appropriate cortical area. Under these circumstances, all signals that arrive in the cortex have already been processed in the subcortical structures. Should the pharmacologically modified transmitter system be essential for signal processing in subcortical structures, an altered activation pattern may reach the structure in which the BOLD response is measured. Consequently, a qualitatively different BOLD response may be triggered, although the modified transmitter system may not be involved in the local generation of the BOLD response in the cortex. To avoid any putative effects of the drug on signal processing and propagation on the way to the region where the BOLD response is monitored, a central fiber bundle that projects directly to this region can be stimulated. Therefore, we stimulated the main input system directly into the hippocampal formation, via the perforant pathway. This pathway projects monosynaptically to the granular cells and interneurons in the dentate gyrus, as well as to other subregions of the hippocampal formation such as CA3, CA1, and the subiculum. In addition, activated granular cells stimulate neurons in the CA3 region, and these cells in turn project to the CA1 regions. Consequently, these regions receive both monosynaptic inputs from the perforant pathway stimulation, and in addition, more or less processed inputs from other regions of the hippocampal formation. Thus, within the entire hippocampal formation, only the input activity to right dentate gyrus is directly controlled by the applied stimulation parameters and cannot be confounded by the applied drug. Especially in this region, no effect of MK801 was noted, whereas in the ipsilateral and more pronounced in the contralateral entorhinal cortex regions, a significant impact of MK801 was seen. Consequently, MK801 affects the BOLD response by modifying the signal propagation to the appropriate region. Alternatively, the formation of a BOLD response in cortical regions could be more strongly depend on NMDA-dependent mechanisms such as the BOLD response in the dentate gyrus. Although this possibility cannot be completely excluded, the gradual differences in the effects of MK801 on the magnitude of the BOLD response in the right and left entorhinal cortex region argue against this possibility. To further confirm that the reduced BOLD response in the entorhinal cortex is in fact mediated by an altered signal processing and signal propagation in the dentate gyrus and hippocampus proper a local application of an NMDA receptor inhibition in the right hippocampus and, in a different experiment, in the left entorhinal cortex is inevitable. Such an approach would also enable the use of various NMDA receptor inhibitors with different form of actions.
N-Methyl--Aspartate Receptor Inhibition Modifies the Blood Oxygen Level-Dependent Response in the Dentate Gyrus by Affecting Local Signaling Processing
Second, the applied receptor agonist/antagonist may change the general properties of participating local networks, e.g., by a selective increase or decrease of excitatory or inhibitory components, changing the intrinsic activities of the participating neurons, or modifying the efficacy of synaptic transmissions. This is of particular importance, because both the subset of activated neurons in a given region (Enager et al, 2009) and the quality of local processing of the incoming activity (Angenstein et al, 2009; Attwell and Iadecola, 2002; Ekstrom, 2010; Harris et al, 2010; Logothetis, 2003) defines the magnitude of the BOLD response. Thus, an observed altered BOLD response, although clearly related to the applied drug, is still not unambiguous proof that the receptor system is directly involved in the generation of a BOLD response. Our results indicate that in the dentate gyrus, MK801 did not affect the BOLD response during ongoing low intensity stimulation when the drug was applied after the first stimulation block. Therefore, under this condition NMDA receptor activation is dispensable for the formation of a BOLD response. Whereas during low intensity stimulation, the time courses of BOLD responses were similar between all three stimulation blocks, the time courses varied during high intensity stimulation (Figure 2C). As shown previously, the functional properties of dentate neuronal circuits change during the first high intensity stimulation trains (Angenstein et al, 2010). Consequently, the conditions that lead to BOLD responses in the second and third stimulation blocks are highly representative of this altered state. Again, under this condition, inhibition of NMDA receptors by MK801 had no obvious effect on the magnitude of the BOLD response. One reason could be that the applied concentration of MK801 (i.e., 0.5 mg/kgbody weight) was not sufficient enough to inhibit NMDA-dependent processes, at least during the beginning until distribution processes are completed. In the previous paper by Gsell et al (2006), an effect of MK801 on generated BOLD responses was observed with a delay of 8 to 12 minutes, indicating that in our approach an effect of MK801 should also occur during the second block after application. During the third stimulation block, we observed a significant change of the population spike augmentation and development of the population spike latency, indicating that at this time the applied concentration of MK801 was efficient enough to modify the neuronal activities (Figures 3 and 4). In contrast, when MK801 was already applied before the first high intensity stimulation train, it affected the generated BOLD responses both between the first stimulation trains (i.e., trains 1 to 3), and later stimulation trains (i.e., trains 6 to 10) (Figures 6C and 8). MK801 mainly affected the time course of the BOLD response to the first high intensity stimulation train, rather than the magnitude of the BOLD response. In the presence of MK801, the delayed increase in the intensity of the BOLD signal after cessation of the stimulation was absent, and instead a slow decline in the BOLD signal intensity was observed. A simple decrease in the excitability of the neurons by NMDA receptor inhibition seems unlikely because in the dentate gyrus, no changes in the BOLD response were observed when MK801 was applied after the first high intensity stimulation block. The altered shape of the BOLD response may indicate that in the presence of MK801, the first high intensity stimulation train modifies the dentate neuronal circuits differently when under control conditions, with the assumption supported by the simultaneous electrophysiological recordings. This, however, could be the result of a reduced excitability of the participating neurons during the first stimulus train when MK801 was present. This could explain a different modification of dentate neuronal circuits, as it would be induced during normal excitability of the cells. During the first high intensity stimulation trains, MK801 particularly affected the processing of incoming stimuli. Under control conditions, the latencies of the population spike trains increase considerably during the second train, though this change was not observed in the presence of MK801. However, to what extent this is related to the smaller augmentation of the population spike amplitudes during later stimulation trains is not clear. Altered electrophysiological responses to similar stimuli could explain the different average magnitudes of the BOLD response to later stimulation trains (i.e., trains 6 to 10; Figure 8). Under MK801, the average magnitude of the BOLD response is nearly identical to low intensity stimulation, whereas under control conditions, a clear increase was found (Figure 6). Thus, the only effect of MK801 on the BOLD response in the dentate gyrus occurred when it was applied before the first high intensity stimulation train, with only the processing of the incoming stimuli being qualitatively affected. Therefore, we conclude that in our experiments, the effect of NMDA receptor inhibition on the BOLD response reflects a changed functional state in the participating neuronal circuits.
MK801 Induced Reduction of the Blood Oxygen Level-Dependent Response During High-Frequency Burst Stimulation Is Not Mediated by Direct Effects on the Neurovascular Coupling
Finally, MK801 could modify mechanisms that directly control neurovascular coupling; if this were the only action of the drug, then neuronal activity should ideally remain unchanged, whereas the BOLD response to the same stimulus should differ after application of the drug. NMDA receptor activation has been shown to mediate an increase in cerebral blood flow, probably via nitric oxide and the cGMP effector kinase protein kinase G (Garthwaite, 2008; Girouard et al, 2009; Iadecola and Nedergaard, 2007) or via COX2-derived vasodilatory prostanoids (Lecrux et al, 2011; Pepicelli et al, 2005). In our experiment, no effects of MK801 on the BOLD response were observed when electrophysiological conditions remained similar. The application of three blocks of low intensity stimulation did not change the properties of the dentate neuronal circuits and the resulting BOLD responses independent of whether MK801 was applied or not (Figure 5). This indicates that, at least during this chosen stimulation condition, direct effects of MK801 on neurovascular coupling are negligible and other mechanisms control the neurovascular coupling. However, it remains elusive, whether during the low intensity stimulation condition substantial NMDA receptor activation occurred. Even during successive high intensity stimulation trains, no significant changes in the BOLD response were observed in the dentate gyrus when MK801 was added. Under this condition, the electrophysiological recordings indicated an altered response behavior of the granular cells in the dentate gyrus but, nevertheless, the BOLD responses remained unchanged. That means under these conditions NMDA-dependent mechanisms are not controlling the BOLD response or the neurovascular coupling in the dentate gyrus but affecting the neuronal firing pattern in response to the direct stimulation of the perforant pathway. This does not exclude the possibility that during other stimulation conditions NMDA receptor inhibition may affect the BOLD response by directly interfering with mechanisms of neurovascular coupling. In the rat barrel cortex, vibrissal (i.e., peripheral) stimulation induced a sharp increase in the local cerebral blood flow, measured by laser Doppler flowmetry, that was significantly reduced in presence of intracisternally injected MK801. This inhibition was not further enhanced by a cyclooxygenase-2 inhibitor, although cyclooxygenase-2 inhibition itself already reduced the enhanced cerebral blood flow during whisker stimulation (Lecrux et al, 2011). These results indicate that in the barrel cortex NMDA receptor-dependent mechanisms can control the local blood flow via release of cyclooxygenase-2 generated metabolites. Similar effects of NMDA receptor inhibition on local hemodynamic parameters were also observed in the somatosensory cortex during electrical stimulation of forepaw stimulation (Gsell et al, 2006) or the ramus infraorbitalis of the trigeminal nerve (Norup Nielsen and Lauritzen, 2001) and, as shown in the current fMRI study, in the entorhinal cortex during electrical perforant pathway stimulation. Consequently, NMDA receptor-dependent mechanisms may be more important for the generation of a BOLD response in cortical areas, or, more likely, the direct perforant pathway stimulation is efficient enough to trigger hemodynamic responses via additional mechanisms. In fact, the two laser Doppler flowmetry studies indicate the presence of additional pathways that are involved in activity-related changes in cerebral blood flow, namely AMPA receptor-mediated mechanisms (Norup Nielsen and Lauritzen, 2001) and GABA-A receptor-mediated mechanisms (Lecrux et al, 2011).
In our stimulation approach, the variation of the electrophysiological responses was rather quantitatively than qualitatively, indicating that not every variation in the local signal processing can be detected by BOLD-fMRI measurements. This means, although variations in the BOLD response are always paralleled with altered local signal processing, the opposite, namely variations in signal processing, are not necessarily paralleled with an altered BOLD response. Consequently, the relationship between quality of signal processing and BOLD response is not biunique.
In summary, our results indicate that the magnitude of a BOLD response is not always directly controlled by NMDA receptor-mediated mechanisms, but can be modified by affecting the local processing of incoming signals. This may occur on the way to the activated region as well as within the regions displaying a BOLD response. These effects are more important than possible direct effects of NMDA receptor-triggered mechanisms on the vascular system. Thus, studying transmitter receptor-mediated effects on the formation of a BOLD response should distinguish between direct effects controlling the neurovascular coupling, and indirect effects that modify local signal processing and signal propagation. Thus, the approach presented here to induce neuronal activity and BOLD responses by a defined stimulus, both monosynaptically (within the dentate gyrus) and multisynaptically (within the contralateral entorhinal cortex) can now address this problem.
Acknowledgments
The authors would like to thank Dr Jonathan Lovell for critical reading of the manuscript.
The authors declare no conflict of interest.
Footnotes
FA is supported by the Deutsche Forschungsgemeinschaft (DFG) AN200-6.
References
- Angenstein F, Kammerer E, Niessen HG, Frey JU, Scheich H, Frey S. Frequency-dependent activation pattern in the rat hippocampus, a simultaneous electrophysiological and fMRI study. Neuroimage. 2007;38:150–163. doi: 10.1016/j.neuroimage.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Angenstein F, Kammerer E, Scheich H. The BOLD response in the rat hippocampus depends rather on local processing of signals than on the input or output activity. A combined functional MRI and electrophysiological study. J Neurosci. 2009;29:2428–2439. doi: 10.1523/JNEUROSCI.5015-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angenstein F, Krautwald K, Scheich H. The current functional state of local neuronal circuits controls the magnitude of a BOLD response to incoming stimuli. Neuroimage. 2010;50:1364–1375. doi: 10.1016/j.neuroimage.2010.01.070. [DOI] [PubMed] [Google Scholar]
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Busija DW, Bari F, Domoki F, Louis T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res Rev. 2007;56:89–100. doi: 10.1016/j.brainresrev.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Cholet N, Seylaz J, Lacombe P, Bonvento G. Local uncoupling of the cerebrovascular and metabolic responses to somatosensory stimulation after neuronal nitric oxide synthase inhibition. J Cereb Blood Flow Metab. 1997;17:1191–1201. doi: 10.1097/00004647-199711000-00008. [DOI] [PubMed] [Google Scholar]
- Ekstrom A. How and when the fMRI BOLD signal relates to underlying neural activity: the danger in dissociation. Brain Res Rev. 2010;62:233–244. doi: 10.1016/j.brainresrev.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enager P, Piilgaard H, Offenhauser N, Kocharyan A, Fernandes P, Hamel E, Lauritzen M. Pathway-specific variations in neurovascular and neurometabolic coupling in rat primary somatosensory cortex. J Cereb Blood Flow Metab. 2009;29:976–986. doi: 10.1038/jcbfm.2009.23. [DOI] [PubMed] [Google Scholar]
- Frey S, Frey JU. Synaptic plasticity and the analysis of the field-EPSP as well as the population spike using separate recording electrodes in the dentate gyrus in freely moving rats. J Neurosci Methods. 2009;184:79–87. doi: 10.1016/j.jneumeth.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Wang G, Gallo EF, Anrather J, Zhou P, Pickel VM, Iadecola C. NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci. 2009;29:2545–2552. doi: 10.1523/JNEUROSCI.0133-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gsell W, Burke M, Wiedermann D, Bonvento G, Silva AC, Dauphin F, Buhrle C, Hoehn M, Schwindt W. Differential effects of NMDA and AMPA glutamate receptors on functional magnetic resonance imaging signals and evoked neuronal activity during forepaw stimulation of the rat. J Neurosci. 2006;26:8409–8416. doi: 10.1523/JNEUROSCI.4615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S, Jones M, Zheng Y, Berwick J. Does neural input or processing play a greater role in the magnitude of neuroimaging signals. Front Neuroenergetics. 2010;2:pii: 15. doi: 10.3389/fnene.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig J, Nauerth A, Friedburg H. RARE imaging: a fast imaging method for clinical MR. Magn Reson Med. 1986;3:823–833. doi: 10.1002/mrm.1910030602. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Jones RS. Synaptic and intrinsic properties of neurons of origin of the perforant path in layer II of the rat entorhinal cortex in vitro. Hippocampus. 1994;4:335–353. doi: 10.1002/hipo.450040317. [DOI] [PubMed] [Google Scholar]
- Kocharyan A, Fernandes P, Tong XK, Vaucher E, Hamel E. Specific subtypes of cortical GABA interneurons contribute to the neurovascular coupling response to basal forebrain stimulation. J Cereb Blood Flow Metab. 2008;28:221–231. doi: 10.1038/sj.jcbfm.9600558. [DOI] [PubMed] [Google Scholar]
- Lecrux C, Toussay X, Kocharyan A, Fernandes P, Neupane S, Levesque M, Plaisier F, Shmuel A, Cauli B, Hamel E. Pyramidal neurons are ‘neurogenic hubs' in the neurovascular coupling response to whisker stimulation. J Neurosci. 2011;31:9836–9847. doi: 10.1523/JNEUROSCI.4943-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norup Nielsen A, Lauritzen M. Coupling and uncoupling of activity-dependent increases of neuronal activity and blood flow in rat somatosensory cortex. J Physiol. 2001;533:773–785. doi: 10.1111/j.1469-7793.2001.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepicelli O, Fedele E, Berardi M, Raiteri M, Levi G, Greco A, Ajmone-Cat MA, Minghetti L. Cyclo-oxygenase-1 and -2 differently contribute to prostaglandin E2 synthesis and lipid peroxidation after in vivo activation of N-methyl-D-aspartate receptors in rat hippocampus. J Neurochem. 2005;93:1561–1567. doi: 10.1111/j.1471-4159.2005.03150.x. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Williams SC, Modo M. A pharmacological MRI assessment of dizocilpine (MK-801) in the 3-nitroproprionic acid-lesioned rat. Neurosci Lett. 2008;444:42–47. doi: 10.1016/j.neulet.2008.07.090. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Bosetti F, Silva AC. Modulatory role of cyclooxygenase-2 in cerebrovascular coupling. Neuroimage. 2006;32:23–32. doi: 10.1016/j.neuroimage.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Schwindt W, Hoehn M, Silva AC. Functional uncoupling of hemodynamic from neuronal response by inhibition of neuronal nitric oxide synthase. J Cereb Blood Flow Metab. 2007;27:741–754. doi: 10.1038/sj.jcbfm.9600377. [DOI] [PubMed] [Google Scholar]