Abstract
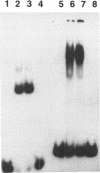
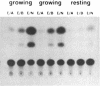
The intron 2 of the murine thymidine kinase (TK) gene was observed to contain two DNase hypersensitive site. In vitro footprinting experiments indicated specific binding sites for nuclear proteins which were characterized within the sequence of intron 2. Two GC boxes (binding sites for transcription factor SP1) and two new protein binding regions, one at the promoter proximal end of intron 2, the other one close to the border to exon 3 were found. Oligonucleotides were synthesized comprising the two new binding sites and were shown in gel mobility shift experiments to be capable of forming specific complexes with nuclear proteins. These proteins are present in growing as well as in quiescent cells suggesting that the sites described here do not contribute to growth regulation of TK expression. That they might play a role in upregulation of TK expression is, however, indicated by the results of CAT assays in which inclusion of downstream sequences of the TK gene containing parts or all of intron 2 were found to positively modulate the activity of the TK promoter.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcot S. S., Flemington E. K., Deininger P. L. The human thymidine kinase gene promoter. Deletion analysis and specific protein binding. J Biol Chem. 1989 Feb 5;264(4):2343–2349. [PubMed] [Google Scholar]
- Aubin R. J., Weinfeld M., Paterson M. C. Factors influencing efficiency and reproducibility of polybrene-assisted gene transfer. Somat Cell Mol Genet. 1988 Mar;14(2):155–167. doi: 10.1007/BF01534401. [DOI] [PubMed] [Google Scholar]
- Bello L. J. Regulation of thymidine kinase synthesis in human cells. Exp Cell Res. 1974 Dec;89(2):263–274. doi: 10.1016/0014-4827(74)90790-3. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. Sequence requirements for premature termination of transcription in the human c-myc gene. Cell. 1988 Apr 22;53(2):245–256. doi: 10.1016/0092-8674(88)90386-8. [DOI] [PubMed] [Google Scholar]
- Chen Z., Harless M. L., Wright D. A., Kellems R. E. Identification and characterization of transcriptional arrest sites in exon 1 of the human adenosine deaminase gene. Mol Cell Biol. 1990 Sep;10(9):4555–4564. doi: 10.1128/mcb.10.9.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppock D. L., Pardee A. B. Control of thymidine kinase mRNA during the cell cycle. Mol Cell Biol. 1987 Aug;7(8):2925–2932. doi: 10.1128/mcb.7.8.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng T. L., Li Y., Johnson L. F. Thymidylate synthase gene expression is stimulated by some (but not all) introns. Nucleic Acids Res. 1989 Jan 25;17(2):645–658. doi: 10.1093/nar/17.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Q. P., Fridovich-Keil J. L., Pardee A. B. Inducible proteins binding to the murine thymidine kinase promoter in late G1/S phase. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1157–1161. doi: 10.1073/pnas.88.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D., Bornkamm G. W. Transcriptional arrest within the first exon is a fast control mechanism in c-myc gene expression. Nucleic Acids Res. 1986 Nov 11;14(21):8331–8346. doi: 10.1093/nar/14.21.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnham P. J., Means A. L. Sequences downstream of the transcription initiation site modulate the activity of the murine dihydrofolate reductase promoter. Mol Cell Biol. 1990 Apr;10(4):1390–1398. doi: 10.1128/mcb.10.4.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin G. C., Donovan M., Adam G. I., Holmgren L., Pfeifer-Ohlsson S., Ohlsson R. Expression of the human PDGF-B gene is regulated by both positively and negatively acting cell type-specific regulatory elements located in the first intron. EMBO J. 1991 Jun;10(6):1365–1373. doi: 10.1002/j.1460-2075.1991.tb07656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich-Keil J. L., Gudas J. M., Dou Q. P., Bouvard I., Pardee A. B. Growth-responsive expression from the murine thymidine kinase promoter: genetic analysis of DNA sequences. Cell Growth Differ. 1991 Feb;2(2):67–76. [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gross M. K., Kainz M. S., Merrill G. F. Introns are inconsequential to efficient formation of cellular thymidine kinase mRNA in mouse L cells. Mol Cell Biol. 1987 Dec;7(12):4576–4581. doi: 10.1128/mcb.7.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M. K., Merrill G. F. Regulation of thymidine kinase protein levels during myogenic withdrawal from the cell cycle is independent of mRNA regulation. Nucleic Acids Res. 1988 Dec 23;16(24):11625–11643. doi: 10.1093/nar/16.24.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Casimir C. Post-transcriptional regulation of the chicken thymidine kinase gene. Nucleic Acids Res. 1984 Feb 10;12(3):1427–1446. doi: 10.1093/nar/12.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas J. M., Knight G. B., Pardee A. B. Nuclear posttranscriptional processing of thymidine kinase mRNA at the onset of DNA synthesis. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4705–4709. doi: 10.1073/pnas.85.13.4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas J. M., Knight G. B., Pardee A. B. Ordered splicing of thymidine kinase pre-mRNA during the S phase of the cell cycle. Mol Cell Biol. 1990 Oct;10(10):5591–5595. doi: 10.1128/mcb.10.10.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer R., Müllner E., Seiser C., Wintersberger E. Cell cycle regulated synthesis of stable mouse thymidine kinase mRNA is mediated by a sequence within the cDNA. Nucleic Acids Res. 1987 Jan 26;15(2):741–752. doi: 10.1093/nar/15.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtig E., Loncarević I. F., Büscher M., Herrlich P., Rahmsdorf H. J. A new cAMP response element in the transcribed region of the human c-fos gene. Nucleic Acids Res. 1991 Aug 11;19(15):4153–4159. doi: 10.1093/nar/19.15.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Conrad S. E. Independent regulation of thymidine kinase mRNA and enzyme levels in serum-stimulated cells. J Biol Chem. 1990 Apr 25;265(12):6954–6960. [PubMed] [Google Scholar]
- Johnson L. F., Rao L. G., Muench A. J. Regulation of thymidine kinase enzyme level in serum-stimulated mouse 3T6 fibroblasts. Exp Cell Res. 1982 Mar;138(1):79–85. doi: 10.1016/0014-4827(82)90093-3. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., FREARSON P. M. DECLINE OF THYMIDINE KINASE ACTIVITY IN STATIONARY PHASE MOUSE FIBROBLAST CELLS. J Biol Chem. 1965 Jun;240:2565–2573. [PubMed] [Google Scholar]
- Kauffman M. G., Kelly T. J. Cell cycle regulation of thymidine kinase: residues near the carboxyl terminus are essential for the specific degradation of the enzyme at mitosis. Mol Cell Biol. 1991 May;11(5):2538–2546. doi: 10.1128/mcb.11.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman M. G., Rose P. A., Kelly T. J. Mutations in the thymidine kinase gene that allow expression of the enzyme in quiescent (G0) cells. Oncogene. 1991 Aug;6(8):1427–1435. [PubMed] [Google Scholar]
- Kim Y. K., Lee A. S. Identification of a 70-base-pair cell cycle regulatory unit within the promoter of the human thymidine kinase gene and its interaction with cellular factors. Mol Cell Biol. 1991 Apr;11(4):2296–2302. doi: 10.1128/mcb.11.4.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Tourkine N., Jeanteur P., Blanchard J. M. Demonstration in living cells of an intragenic negative regulatory element within the rodent c-fos gene. Cell. 1990 May 4;61(3):485–496. doi: 10.1016/0092-8674(90)90530-r. [DOI] [PubMed] [Google Scholar]
- Levy-Wilson B., Fortier C., Blackhart B. D., McCarthy B. J. DNase I- and micrococcal nuclease-hypersensitive sites in the human apolipoprotein B gene are tissue specific. Mol Cell Biol. 1988 Jan;8(1):71–80. doi: 10.1128/mcb.8.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Matkovich D. A. Genetic determinants of growth phase-dependent and adenovirus 5-responsive expression of the Chinese hamster thymidine kinase gene are contained within thymidine kinase mRNA sequences. Mol Cell Biol. 1986 Jun;6(6):2262–2266. doi: 10.1128/mcb.6.6.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowndes N. F., Bushel P., Mendelsohn L., Wu J., Yen M. Y., Allan M. A short, highly repetitive element in intron -1 of the human c-Ha-ras gene acts as a block to transcriptional readthrough by a viral promoter. Mol Cell Biol. 1990 Sep;10(9):4990–4995. doi: 10.1128/mcb.10.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottavio L., Chang C. D., Rizzo M. G., Travali S., Casadevall C., Baserga R. Importance of introns in the growth regulation of mRNA levels of the proliferating cell nuclear antigen gene. Mol Cell Biol. 1990 Jan;10(1):303–309. doi: 10.1128/mcb.10.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehl H. H., Conrad S. E. Identification of a G1-S-phase-regulated region in the human thymidine kinase gene promoter. Mol Cell Biol. 1990 Jul;10(7):3834–3837. doi: 10.1128/mcb.10.7.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. E., Owen R. A., Merrill G. F. An intragenic region downstream from the dihydrofolate reductase promoter is required for replication-dependent expression. J Biol Chem. 1990 Oct 15;265(29):17397–17400. [PubMed] [Google Scholar]
- Seiser C., Knöfler M., Rudelstorfer I., Haas R., Wintersberger E. Mouse thymidine kinase: the promoter sequence and the gene and pseudogene structures in normal cells and in thymidine kinase deficient mutants. Nucleic Acids Res. 1989 Jan 11;17(1):185–195. doi: 10.1093/nar/17.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherley J. L., Kelly T. J. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988 Jun 15;263(17):8350–8358. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stewart C. J., Ito M., Conrad S. E. Evidence for transcriptional and post-transcriptional control of the cellular thymidine kinase gene. Mol Cell Biol. 1987 Mar;7(3):1156–1163. doi: 10.1128/mcb.7.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travali S., Lipson K. E., Jaskulski D., Lauret E., Baserga R. Role of the promoter in the regulation of the thymidine kinase gene. Mol Cell Biol. 1988 Apr;8(4):1551–1557. doi: 10.1128/mcb.8.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichselbraun I., Ogris E., Wintersberger E. Bidirectional promoter activity of the 5' flanking region of the mouse thymidine kinase gene. FEBS Lett. 1990 Nov 26;275(1-2):49–52. doi: 10.1016/0014-5793(90)81436-r. [DOI] [PubMed] [Google Scholar]