Abstract
Objective
During the early phase of evaluation of a new intervention, data exist for present practice. The authors propose a method of constructing a fair comparator group using these data. In this case study, the authors use the example of external aortic root support, a novel alternative to aortic root replacement.
Design
A matched comparison group, of similar age, aortic size and aortic valve function to those having the novel intervention, was constructed, by minimization, from among patients having conventional aortic root replacement in other hospitals during the same time frame.
Setting
Three cardiac surgical units in England.
Patients
The first 20 patients, aged 16–58 years with aortic root diameters of 40–54 mm, having external support surgery were compared with 20 patients, aged 18–63 years and aortic root diameters of 38–58 mm, who had conventional aortic root replacement, between May 2004 and December 2009.
Interventions
A pliant external mesh sleeve, customised by computer-aided design, encloses the whole of the ascending aorta. The comparator group had conventional aortic root replacement, 16 valve-sparing and four with composite valved grafts.
Main outcome measures
Duration of cardiopulmonary bypass (CPB), myocardial ischaemic time, blood loss and transfusion of blood, platelets and clotting factors.
Results
Comparing total root replacement and customised aortic root support surgery: CPB (median (range)) was 134 (52–316) versus 0 (0–20) min; myocardial ischaemia 114 (41–250) versus 0 (0–0) min; 4 h blood loss was 218 (85–735) versus 50 (25–400) ml; and 9/18 had blood transfusion, 9/18 platelets and 12/18 fresh frozen plasma after root replacement versus 1/20, 0/20 and 0/20, respectively, for the novel surgery.
Conclusions
Avoidance or large reductions in CPB, myocardial ischaemia and blood product usage were achieved with the novel surgery. These data are of use in decision analysis and health economic evaluation and are available early in evaluation before randomised trial data are available.
Article summary
Article focus
A novel comparative research method used existing data to derive a comparison group in the pre-randomised controlled trial phase of evaluation of a new technology.
A case study in aortic root surgery is described. Comparison was made of the perioperative burden of care between root replacement and a novel tissue conserving approach to reducing the risk of dissection in Marfan syndrome.
Key messages
Existing data, where available, should be used in decision analysis and health economic evaluation.
Before randomised trials can be completed and reported, there may be available data to allow reliable estimates of differences between surgical approaches to the same clinical problem.
Strengths and limitations of this study
Limitations are that the allocation was not randomised and the comparison data were not collected prospectively.
A strength is that available clinical data acquired while different surgical strategies were employed at different institutions during the same time frame allow rigorous and timely comparison to be made at an early stage in the introduction of a novel technology.
Introduction
As new technologies are proposed, they are tested for efficacy and safety.1 Where there is a pre-existing technology, comparative evaluation should be routine so that when a new technology is demonstrably superior, in terms of effectiveness and cost-effectiveness, it should replace existing practice. It appears self-evident that the competing technologies should be subjected to a ‘fair test’,2 and the fairest test is unbiased allocation in a randomised controlled trial (RCT). RCTs have been infrequently used in evaluation of surgery to the point that some dismiss them as just too difficult to countenance and thus inappropriate—a ‘square peg in a round hole’.3 Too often this attitude provides an excuse to rely solely on case series, analysed retrospectively, and riven with flaws.4 UK surgeons are aware of the need to recruit into surgical trials5 but when it is randomisation that appears to be the insurmountable obstacle it is important not to forget the second word in RCT: ‘controlled’. To have some comparative data is surely better than having none in evaluating technology.
The case in point is the evaluation of a new technology to reduce the risk of aortic dissection in people with Marfan syndrome, which is by far the most important life-threatening consequence of the disease.6–8 The new technology involves the use of modern digital imaging and computer-aided design (called CAD modelling) to manufacture a pliant but adequately strong external support, made of a porous mesh, custom made to fit the individual's ascending aorta from the left ventricle to the proximal aortic arch.9 It is available as an alternative to total aortic root replacement.10 11
Total replacement of the ascending aorta and the aortic valve, a major, radical and ablative operation, became established practice from about 25 years ago.12 Based on comparison with historical life expectancy data, it was convincingly effective in increasing the lifespan of people with Marfan syndrome.13 As the surgical risk reduced over time, root replacement became the routine pre-emptive strategy,14 but while the operative risk diminished, mandatory life long anticoagulation, sometimes from a young age, became a major concern. Incorporation of a tissue (rather than a mechanical) valve removed the need for anticoagulation15 but introduced the problem of failing tissue valves, typically by about 15 years.
Technically exacting methods of conserving the patient's own valve leaflets were the next development.16 17 Valve replacement and valve conserving strategies have been compared in a systematic review of 1385 patients in 11 surgical cohort studies published from 1999 to 2009.18 More than a quarter of patients had further aortic valve surgery within 20 years of a valve-sparing operation and more than half had further complex aortic root surgery during their lifetimes. The overall likelihood of a valve-related event (further aortic surgery, thromboembolism or endocarditis) for all forms of root replacement was 1.5% per annum.18 These patients are operated on at an average age of 35 and might hope to live at least as long afterwards. By then the cumulative lifetime risk of serious valve-related events associated with aortic root replacement reaches 50%—that is to say ‘even odds’. It is easy to overlook the real implication of data provided in low percentage rates per annum.19
The non-ablative option in which all of the aorta, the aortic valve and the functioning blood/endothelial interface are conserved, employing modern technology, is now available,9 but there have been to date real obstacles to a randomised trial.
The detrimental events following aortic root replacement with a composite mechanical valved graft, tissue valve replacement and valve-sparing surgery occur over a long time frame and are manifest at different points in time.
Marfan syndrome has a prevalence of 2–3 patients per 100 000.20 Any prospective study might take an unconscionable time to accrue sufficient patients into an adequately powered trial.
Ablative root replacement is usually deferred until there is proven progression but the conservative external support operation is intended to halt the local process of dilatation. Relatively few patients are similarly eligible for both operations at the same time point so a trial design might involve the indirect comparison of external support versus a continued watch and wait policy.
Existing practice is regarded as conventional management, while external support is framed clearly as an innovation.11 There is as yet no sense of a level playing field for evaluation, so the criterion of equipoise, required for a randomised trial, is not attained.
It is worth noting that it is not generally the innovators who are reluctant to subject their innovation to a randomised trial and the reason is a simple and self-evident one. The innovator stands to gain up to a 50% share of practice during evaluation in an RCT, while the established method can only lose ‘market share’. Accrual of evidence will inevitably take many years and many operations, irrespective of whether the evidence comes from an RCT or a cohort study.11
Meanwhile, there are important question that can be answered from existing data.
Cardiopulmonary bypass (CPB) is essential in all aortic root replacement operations but is routinely avoided with the tissue conserving external aortic root support (EARS) operation. This difference can be quantified and its clinical and cost implications can be estimated from existing data.
Transfusion of blood and blood products is common with aortic root replacement due to the nature of the surgery and the detrimental effects of CPB on the coagulation system. The difference in use of blood products can be quantified from existing data and the clinical consequences estimated.
The coronary arteries must be disconnected for a period of an hour or more for root replacement operations.21 The deleterious effects are ameliorated by cooling and hyperkalaemic arrest but it is a hazard that is routinely avoided with EARS.
Opening the aorta provides an opportunity for air and particulate embolisation and a consequent risk of stroke. In the aortic root support surgery, the aorta is never opened.
All four are differences by intent between the root replacement and root conserving strategies. It does not require an RCT to discover that these are different; it is an evident effect of the surgical approach. Putting bounds on how great or small the differences might be and their consequences can be estimated on existing data provided careful comparison of like with like is made.
Methods
Surgical units known to have expertise in aortic root surgery for Marfan syndrome were invited to collaborate in this study. The National Research Ethics Service advised that the study did not require ethical approval under the NHS Research Governance Arrangements (letter dated: 30 June 2009). The project was registered with the local audit departments, and all data were collected and verified by a single member of the Cardiac Surgical staff in each hospital from prospectively maintained databases and retrospective notes review. The centres were asked to identify all patients undergoing elective aortic root replacement for Marfan syndrome from May 2004 to December 2009, which was the period during which the first 20 patients received an EARS at the Royal Brompton Hospital.21
The centres were asked to record on a standardised case report form (CRF) for the patients who had undergone elective aortic root replacement only preoperative characteristics, namely date of birth, sex, date of operation, operation performed (composite valved graft aortic root replacement or valve-sparing aortic root replacement), the latest preoperative aortic root dimension, whether echocardiography, CT or MRI were used for this measurement, and the grade of any aortic regurgitation. No intraoperative or outcome data were requested at this stage. The CRFs were sent to the Clinical Trials and Evaluation Unit (CTEU) at the Royal Brompton Hospital without patient identifiers and were checked (BL) to ensure that there were no missing data and that there was no outcome information recorded.
The date of birth, sex, preoperative aortic root diameter, degree of any aortic regurgitation and the date of elective surgery for the first 20 patients having the novel operation of aortic root support at the Royal Brompton Hospital were obtained by the CTEU. In order to select a group of 20 patients that matched them, with respect to age, sex, aortic root size and degree of aortic regurgitation, we applied the principles of minimisation.22–24
Only after this process was completed were the intraoperative and immediate postoperative details obtained: date of discharge from hospital, operative start and end times, bypass time, ischaemic time, chest tube drainage up to 4 and 12 h, blood product usage (including red cells, platelets and fresh frozen plasma) and oral anticoagulant usage for the elective aortic root replacement cohort (AR and RA) and for the EARS cohort (KMJC). The details were recorded on a standardised CRF. Again no patient identifiers were recorded or sent to the CTEU. The CTEU checked that there were no missing data prior to analysis. Data analysis was then performed in a research unit (CORU) remote from any of the three clinical sites (SC and TT).
Data analysis
The analysis plan was prepared (TT and SC) prior to any data being received or analysed. The elective aortic root replacement cohort were compared with the EARS cohort with respect to age at operation, preoperative aortic diameter, operation time, bypass time, cross-clamp time, postoperative days in hospital, chest tube drainage at 4 and 12 h postoperative and type and amount of blood products and oral anticoagulants given.
The purpose of the analysis was to quantify the burden of the interventions in the different operative strategies. It was not appropriate to apply hypothesis testing statistical methods to bypass or ischaemic time since these were inherent in the surgical strategy. Hospital stay, blood loss and blood product usage were the outcomes of these different strategies and these can be compared meaningfully.
Results
Comparator case selection
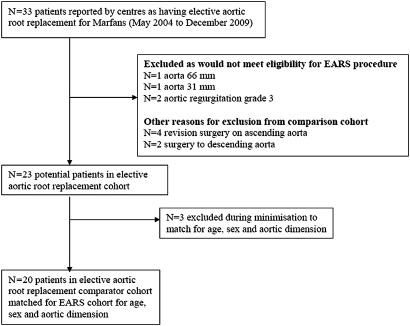
Queen Elizabeth Hospital, Birmingham, UK, and Guys and St Thomas' Hospital, London, UK, collaborated in the study and a team member at each (AR in Birmingham and RA at St Thomas' Hospital) were the only point of contact with the CTEU (BL). Data on a total of 33 anonymous patients, identified as having root replacement for Marfan syndrome during the specified time frame, were sent by the two other cardiac units (figure 1). Four patients had repeat surgery and in two, surgery involved the arch or descending aorta; they would not have been candidates for EARS and were excluded. The external root support patients, by protocol, had aortic root diameters of 4–5.5 cm and no more than grade 1 (trivial) aortic regurgitation. Four patients who clearly fell outside those criteria were excluded. For 23 remaining patients, the age range was 16–73 years and aortic diameters of 24–66 mm. To minimise imbalance22 between the groups in terms of age and aortic root dimension, three further exclusions were made so that 20 patients remained who provided the best match with the 20 patients in the EARS cohort with regard to age, aortic dimension and aortic regurgitation.
Figure 1.
Flow chart for control group. EARS, external aortic root support.
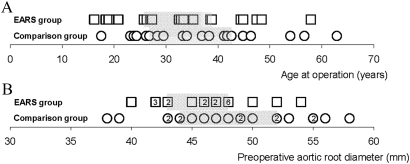
Of these 20 patients, four had composite valved grafts (ages 30, 46, 53 and 63 years) and 16 had valve-sparing operations. There was a poor match for sex: 14 males in the novel surgery group and eight males in the comparison group. There were insufficient patients to improve further on matching for sex so the method was only partially successful due (at least in part) to an insufficiently large pool of patients from which to draw an ideal comparison group. The age and preoperative aortic dimension of each cohort are shown for all individuals in figure 2 and summary statistics are in table 1.
Figure 2.
Comparison between the external aortic root support (EARS) patients (boxes) and comparison group (circles) for (A) age at operation and (B) aortic diameter preoperation. The vertical bars denote the medians and the boxes indicate the IQRs.
Table 1.
Comparison of the external aortic root support (EARS) patients (N=20) and the comparison group (N=20) with respect to age at operation and aortic diameter preoperation (millimetres)
| Mean | Median | IQR | Range | |
| Age at operation (years) | ||||
| EARS | 33 | 33 | 26–39 | 16–58 |
| Comparison | 37 | 35 | 27–43 | 18–63 |
| Aortic diameter (mm) | ||||
| EARS | 46 | 47 | 43–48 | 40–54 |
| Comparison | 48 | 48.5 | 44–52 | 38–58 |
Operative data
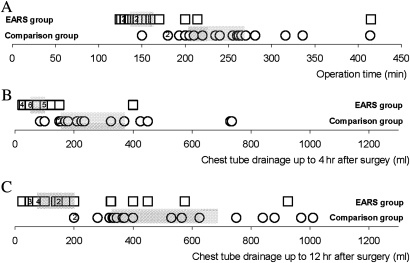
The operation time, bypass time, ischaemic time, postoperative days in hospital and chest tube drainage up to 4 and 12 h after surgery are shown in table 2 for both groups of patients. The median operation time for the novel surgery group (table 2 and figure 3) was 2.5 h compared with 4 h for root replacement patients. In the novel surgery group, the first patient had a brief period (20 min) of CPB but thereafter bypass was not used in any patient and all were operated upon without any myocardial ischaemia. In comparison, the median time on CPB for root replacement patients was 134 min and their median ischaemic time was 114 min. Postoperative stay in hospital was similar in both groups (table 2) and when tested was not significantly different (Mann–Whitney U test).
Table 2.
Comparison of the external aortic root support (EARS) patients and the comparison group with respect to procedural data
| Median | IQR | Range | |
| Operation time (min) | |||
| EARS (N=20) | 148 | 136–163 | 125–415 |
| Comparison (N=19*) | 240 | 204–269 | 150–414 |
| Bypass time (min) | |||
| EARS (N=20) | 0 | 0–0 | 0–20† |
| Comparison (N=20) | 134 | 117–146 | 52–316 |
| Ischaemic time (min) | |||
| EARS (N=20) | 0 | 0–0 | 0–0 |
| Comparison (N=20) | 114 | 91–127 | 41–250 |
| Postoperative days in hospital | |||
| EARS (N=20) | 6 | 5–7 | 4–16 |
| Comparison (N=20) | 7 | 6–8 | 4–17 |
| Chest tube drainage up to 4 h after surgery (ml) | |||
| EARS (N=20) | 50 | 50–100 | 25–400 |
| Comparison (N=18*) | 230 | 155–370 | 85–735 |
| Chest tube drainage up to 12 h after surgery (ml) | |||
| EARS (N=20) | 120 | 75–200 | 25–925 |
| Comparison (N=18*) | 385 | 326–688 | 200–1010 |
Missing data for one or more patients.
Note that for the EARS group, cardiopulmonary bypass was used for 20 min in the first patient only and none had any interruption to coronary blood flow.
Figure 3.
Comparison between the external aortic root support (EARS) patients (boxes) and comparison group (circles) of (A) duration of surgery in minutes, (B) blood loss in the first 4 h postoperation in millilitres and (C) blood loss in the first 12 h postoperation in millilitres. The grey boxes indicate the interquartile range.
The chest tube drainage for conservative surgery was less than that for root replacement (table 2 and figure 3) (p<0.02, Mann–Whitney U test). Table 3 shows the number of patients and the nature of blood products. One of the patients in the novel surgery group was given a single unit red cell transfusion but they received no other blood products. In the comparison group, 9/18 patients (two not ascertained) were given red cell transfusions (p=0.002, Fisher's test), 9/18 platelets and 12/18 fresh frozen plasma (p<0.001, Fisher's test) (see table 3 and legend). Five of the patients having root replacement were prescribed oral anticoagulants, which will be mandatory for life for those with a mechanical heart valve.
Table 3.
Comparison of how many external aortic root support (EARS) patients and how many patients in the comparison group had a red cell, platelet or fresh frozen plasma (FFP) transfusion
| Transfusion product | Number of patients |
|
| EARS group (N=20) | Comparison group (N=18)* | |
| Red cell | 1 | 9 |
| Platelet | 0 | 9 |
| FFP | 0 | 12 |
Note that of the EARS group, 1/20 patients received a single unit red cell transfusion. Of the comparison group (*data missing for two patients), 9/18 were recorded as receiving red cell transfusions (mean 2.0 units per transfused patient), 9/18 received platelet transfusions (mean 1.6 units per transfused patient) and 12/18 received FFP transfusions (mean 4.8 units per transfused patient).
Discussion
There are two distinct elements to this discussion. First, we must consider the validity, limitations and lessons learnt about the method, which to the best of our knowledge has a degree of innovation. Only then is it useful to consider the reliability and value of what has been learnt in comparing the new technology of aortic root conservation and support with the established aortic root replacement surgery.
Considering its limitations, this novel method of comparison lacks two of the essential elements of an RCT: patients were not allocated by randomisation and the study was not prospective. On the other hand, in RCTs of surgery, blinding is nearly always impossible so bias in the delivery of potentially compensating forms of care, and bias in collection and recording of data, cannot be excluded and so a real difference in outcome may be diminished or not evident. In the present study, the 20 patients who had root replacement were among a very large number of patients having all forms of cardiac surgery. The teams could not know that their routinely collected clinical data would be used for a future comparison with a competing technology. Thus, the use of previously obtained patient data to derive a group for comparison has the incidental advantage that those recording those data had no knowledge of the research question and this may confer a useful advantage.
Our objective in this study was to find a contemporaneous group of patients, operated on by surgeons performing conventional root replacement, uncontaminated by involvement in the development of the new customised external root support. The construction of the comparison group employed the concept of minimisation as a means of ensuring that important variables were as similar as possible in the two groups. Critics of minimisation argue that the opportunity for bias cannot be excluded because the likely allocation of the next patient may be predictable from knowledge of existing mismatches between the groups.24 This problem does not exist, at least not in this precise form, when patients are selected from existing data but the opportunity certainly does for the investigators to select patients in whom the outcome is already known. For that reason, we fastidiously avoided any knowledge of the outcome of these patients being accessible to those involved in the process of deriving the comparison group.
A further limitation in the present study was that the pool of patients for comparison was not large enough to match as completely as we would have liked and failed to achieve balance on sex mix. Also the root replacement group included both those whose root replacement spared or replaced the valve. An ideal study would have representative numbers of these two operations for comparison (composite root replacement with a tissue valve is likely to be infrequent in this age group). We had approached other teams who declined sharing data on this occasion. However, the differences in the outcomes were so large that in this instance we do not believe further matching would have altered the conclusion.
The method includes another useful feature. It shares the principles of an expertise-based study.25 In drug trials, the patient is handed identically labelled packs of pills and no therapeutic expertise is involved but the expertise of the deliverer of care is an important consideration in non-pharmacological interventions.26 This applies in all technical interventions, in physical interventions such as physiotherapy and in psychotherapy; in all these examples, the person providing care is an important element in the potential effectiveness of that care. In the present study, surgeons were not asked to perform operations by random allocation but had already performed their preferred operation in which they were practiced and confident, a feature shared with an expertise-based randomised trial.25
There is a recognised problem in achieving sufficient numbers for conventional study designs in rare diseases.27 It is made more difficult if important outcome measures and adverse events accrue over many years. For example, native valve failure in valve-sparing surgery is important even after 10–15 years of perfect function. A devastating thromboembolic event in the first year after surgery, with residual disability, will dominate the outcome even if there are no subsequent events. Patients will weigh these risks differently as they make choices and they are influenced by both the weight put on them by doctors giving advice and whether they appear imminent or remote.28 Marfan syndrome is uncommon and the number of patients meeting the clinical criteria, such that they are candidates for either operation, is small but, in uncommon diseases, ‘some unbiased evidence is clearly better than none’.27
We believe that this approach to evaluation of a new technology may be generalisable. New interventional procedures are first introduced in one or a few centres while existing practice continues elsewhere. Many of these patients would have been candidates for the new as well the existing procedure, which they have received. By collecting initially only preoperative variables and by placing this process in the hands of the clinical trials unit (Royal Brompton Hospital, CTEU), we ensured that the selection was made only on explicit pre-stated criteria. To generalise the method, the case selection would need to be from as large a pool as possible and carried out by completely uninvolved and impartial investigators, without access to the outcome data at the time that the case selection is being made. If this method is to be replicated, it should be considered mandatory that the process be put in the hands of entirely neutral methodologists, blind to outcomes and with no vested interest in the answer to the research question.
To turn to what we have learnt about the specific subject of our research, we have previously drawn a comparison between the first 20 patients having EARS so the findings presented here are not completely new.21 However, the comparison in the earlier study was with 28 patients having root replacement in the same hospital, not all with Marfans syndrome and who were not candidates for the EARS surgery. The comparison group reported here are non-overlapping, operated on during the same time frame, in other hospitals where external support was not available. All 40 patients were eligible for replacement or aortic root conserving surgery. Although the findings are not surprising, they provide a much better estimate of real differences than we could achieve in our previous study.21
Avoidance of CPB and reduction in transfusion of blood products have been consistent objectives in reducing the detrimental effects of cardiac surgery. In this study, CPB was used as a safety measure in the first patient having aortic root support29 but was not used thereafter. One patient had a single unit blood transfusion. In addition, operative times were shorter even during the learning period for this new operation. There was no interruption to normal physiological myocardial perfusion through the coronary arteries. In contrast, interrupting coronary perfusion, while the myocardium is protected by hypothermic hyperkalaemic cardiac arrest, is mandatory for root replacement and was used from 40 min to up to 4 h, with a median approaching 2 h. These perioperative benefits were anticipated from a theoretical standpoint, being inherent in the non-ablative nature of the surgery, but they cannot be assumed to be consistently achievable. Formal quantification is essential if the data are to be used in decision analysis and health economic evaluation. There are potentially very large differences in the burden of care, costs of care and the potential for harm.
These differences did not translate into reduced hospital stay in this study. Postoperative stay is likely to be influenced by the chest wall incision, hitherto a signifier of major cardiac surgery. Median sternotomy does not require hospital stay beyond the initial recovery period, say the first night, and much shorter stay is an attainable goal in the future.
None of the existing forms of aortic root surgery, whether they involve mechanical or tissue valve replacement, or valve-sparing surgery, offers a perfect or permanent solution for people with Marfan syndrome.11 30 The first test of effectiveness of this novel technology should be that it is not inferior to the alternatives in terms of the combined lifetime risks of aortic dissection, other Marfan-related cardiovascular events, arterial thromboembolism, anticoagulant-related bleeding, endocarditis and reoperation. Table 4 summarises some of these from a theoretical standpoint and suggests that, for a checklist of preoperative and lifetime hazards, external support has a favourable profile.
Table 4.
The relative merits of four approaches to surgical management of the aortic root in Marfan syndrome to reduce the risk of death due to dissection
| Hazard | Bentall mechanical | Bentall tissue | Valve sparing | External support |
| Cardiopulmonary bypass | + | + | + | − |
| Blood products | + | + | + | − |
| Thrombembolic risk | + | − | − | − |
| Anticoagulation | + | − | − | − |
| Endocarditis | + | + | − | − |
| Reoperation for valve failure | − | + | +/− | ? |
The symbols + or − represent a simple dichotomy where + indicates an inherent hazard whether inevitable such as the need for cardiopulmonary bypass or a serious but uncommon risk such as endocarditis.
The ultimate test of external support is whether it will prevent dissection in the ascending aorta, which is the only achievable objective of surgery. Monitoring of aortic size by echo, and deferring surgery until agreed size criteria are reached, is well-established practice. While the association between dissection and larger aortic size is highly significant, dissection can occur in Marfan syndrome in a relatively small aorta.31 32 While this intimately applied external support might not completely obviate that risk, it seems a reasonable expectation that it will be substantially reduced. It is also probable that if dissection should occur, its consequences will be less severe. There is evidence from an animal study (as yet unpublished) that the mesh of the supporting sleeve becomes incorporated in the aortic adventitia and increases the stress tolerance of the arterial wall. Knowledge of the surgical pathology of aortic dissection encourages us to think that this form of external support will confer these benefits—but nothing other than long-term follow-up will answer those questions.
It should be noted that this computer-designed and customised support, manufactured from a pliant and porous mesh, is very different to wrapping the aorta with the stiff graft material as has previously been proposed.33 34
It has become an entrenched belief that excision of the aorta is an essential component of Marfan root surgery, and reservations about the external support have drawn attention to the fact that the aortic tissue remains and yet the extent of excision in root replacement does not routinely include the entire ascending aorta. For surgeons who perform this surgery without circulatory arrest, the safe placement of an aortic cross-clamp, proximal to the brachiocephalic artery with sufficient clearance for the distal suture line, mandates that at least a couple of centimetres of the ascending aorta remains. Surgeons who routinely perform an ‘open’ distal anastamosis achieve more complete replacement of the aortic root and a more technically satisfactory distal anastamosis, at the price of 10–15 min of total circulatory arrest.35–37 The external mesh supports the aorta to beyond the brachiocephalic artery without the need for any circulatory arrest, obviating this technical debate.
Consideration of these technical details highlights a further consideration in the comparison between valve-sparing root replacement and external support of the existing of aorta and valve. Intraoperative skill and decision making are to a large extent replaced by preoperative measurement and device manufacture, moving the intervention from ‘workmanship of risk’ towards the ‘workmanship of certainty’.38 This concept is well understood in design and production of wooden furniture, which ranges from unique craft made objects to those manufactured to precise and reproducible specifications. While the uniqueness of wooden carving is part of its charm and value, to deliberately retain hazard and uncertainty in a surgical operation, when there is an engineered alternative, would surely be a mistake.
The best evidence for effectiveness and cost-effectiveness would ideally be obtained from direct comparison in a RCT,5 but these first 20 operations were performed by one surgeon in a single institution as part of formal early phase evaluation. Randomisation is not considered possible until the operation is shown to be achieved consistently.1 All 40 patients in this study would have been candidates for either external support or root replacement. Even so, it must be recognised that the cardiologist and/or the patient might have expressed a firm preference for one or the other, thus blocking random allocation. Not all prospective patients who are candidates for root replacement could be randomly allocated to external support or vice versa. The indications for external support and for root replacement overlap but they have important differences. For aortic root replacement, there is no upper size limit and it is not precluded by any severity of aortic regurgitation, whereas at this stage in the evaluation of external support, an already established large aneurysm or aortic regurgitation (more than trivial) would be a contraindication.
An RCT has the potential to provide the definitive answer to one well framed question but there are many other things we need to know in evaluating competing technologies. While considering the data required for health economic evaluation,39 we found that a published decision analysis was severely hampered by lack of data on probability of dissection-free survival of patients with Marfan syndrome.40 Bentall's first patient to have total root replacement as an unplanned procedure had a massive aneurysm, with walls ‘so thin that the blood could be seen eddying within’.41 The most likely natural outcome was death from dissection or rupture of the aortic wall and the ‘number needed to treat’ to save that patient's life was, in all probability, one but the number needed to treat (NNT) to save a single life gets larger as the threshold for any intervention lowers. For example, in carotid endarterectomy, NNT is six for 70%–99% carotid stenosis and rises to 24 for 50%–69% carotid stenosisi based on randomised trials. As surgery has become safer, we have progressively lowered the aortic size at which surgery is performed electively in Marfan syndrome. Some patients, perhaps many, may have lived without progressive dilatation of dissection, and, although how many and who they would have been can never be known, for those patients their operation availed them nothing. Harms become an ever more important part of the equation. Each element of the decision-making process should be updated as practice changes.39 Where contemporary data already exist, without the need for an RCT, it makes sense to use them.
Supplementary Material
Acknowledgments
We are grateful to the following consultant surgeons who agreed to data being included: Robert Bonser, University of Birmingham, School of Clinical and Experimental Medicine and University Hospitals of Birmingham NHS Foundation Trust, Birmingham and David Anderson, Graham Venn, Christopher Young, St Thomas' Hospital, London, UK, and Viv Barnett Database Co-ordinator, Queen Elizabeth Hospital, Birmingham, UK.
Footnotes
To cite: Treasure T, Crowe S, Chan KMJ, et al. A method for early evaluation of a recently introduced technology by deriving a comparative group from existing clinical data: a case study in external support of the Marfan aortic root. BMJ Open 2012;2:e000725. doi:10.1136/bmjopen-2011-000725
Contributors: TT and MU designed the study. BL helped to plan the study, prepared the clinical report forms and was the interface with the centres. KMJC, AR and RA extracted and verified data at the clinical centres. SC did all data analysis and presentation. TG manufactured the customised external aortic root supports (EARS). JP performed all the EARS operations. TT and SC drafted the manuscript. All authors agreed the final version.
Funding: Development costs have been met by Exstent who manufacture the custom made devices for each patient. Costs per device are partly recovered from NHS purchasing.
Competing interests: TG is a shareholder and director of Exstent, which holds the intellectual property rights in the external aortic root support project. TG was the originator of the concept and the first recipient of the device. No other author has any pecuniary interests or any other conflict of interests.
Ethics approval: The National Research Ethics Service advised that the study did not require ethical approval under NHS Research Governance Arrangements (letter dated: 30 June 2009).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: We are willing to share data.
References
- 1.McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105–12 [DOI] [PubMed] [Google Scholar]
- 2.Evans I, Thornton H, Chalmers I, et al. Testing Treatments. 2nd edn London: Pinter & Martin, 2011 [PubMed] [Google Scholar]
- 3.Cooper JD. Randomized clinical trials for new surgical operations: square peg in a round hole? J Thorac Cardiovasc Surg 2010;140:743–6 [DOI] [PubMed] [Google Scholar]
- 4.Treasure T, Utley M. Ten traps for the unwary in surgical series: a case study in mesothelioma reports. J Thorac Cardiovasc Surg 2007;133:1414–18 [DOI] [PubMed] [Google Scholar]
- 5.Morton D, Treasure T. GRIST: growing recruitment into interventional and surgical trials. JRSM 2012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murdoch JL, Walker BA, Halpern BL, et al. Life expectancy and causes of death in the Marfan syndrome. N Engl J Med 1972;286:804–8 [DOI] [PubMed] [Google Scholar]
- 7.Januzzi JL, Marayati F, Mehta RH, et al. Comparison of aortic dissection in patients with and without Marfan's syndrome (results from the International Registry of Aortic Dissection). Am J Cardiol 2004;94:400–2 [DOI] [PubMed] [Google Scholar]
- 8.Ranasinghe A, Strong D, Bonser R. Easily missed? Aortic dissection. Br Med J 2011;343:317–19 [DOI] [PubMed] [Google Scholar]
- 9.Pepper J, Golesworthy T, Utley M, et al. Manufacturing and placing a bespoke support for the Marfan aortic root: description of the method and technical results and status at one year for the first ten patients. Interact Cardiovasc Thorac Surg 2010;10:360–5 [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence (NICE) Understanding NICE Guidance: Information for People Who Use NHS Services, Fitting an External Stent to Support the Aorta in Patients with Marfan Syndrome. National Institute for Health and Clinical Excellence (NICE), 2011. http://guidance.nice.org.uk/IPG394 [Google Scholar]
- 11.Treasure T, Pepper JR. Aortic root surgery in Marfan syndrome. Heart 2011;97:951–2 [DOI] [PubMed] [Google Scholar]
- 12.Treasure T. The evolution of aortic root surgery for Marfan syndrome. Interact Cardiovasc Thorac Surg 2010;10:353–5 [DOI] [PubMed] [Google Scholar]
- 13.Silverman DI, Burton KJ, Gray J, et al. Life expectancy in the Marfan syndrome. Am J Cardiol 1995;75:157–60 [DOI] [PubMed] [Google Scholar]
- 14.Treasure T. Cardiovascular surgery for Marfan syndrome. Heart 2000;84:674–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirsch ME, Ooka T, Zannis K, et al. Bioprosthetic replacement of the ascending thoracic aorta: what are the options? Eur J Cardiothorac Surg 2009;35:77–82 [DOI] [PubMed] [Google Scholar]
- 16.Yacoub MH, Gehle P, Chandrasekaran V, et al. Late results of a valve-preserving operation in patients with aneurysms of the ascending aorta and root. J Thorac Cardiovasc Surg 1998;115:1080–90 [DOI] [PubMed] [Google Scholar]
- 17.David TE, Armstrong S, Ivanov J, et al. Results of aortic valve-sparing operations. J Thorac Cardiovasc Surg 2001;122:39–46 [DOI] [PubMed] [Google Scholar]
- 18.Benedetto U, Melina G, Takkenberg JJ, et al. Surgical management of aortic root disease in Marfan syndrome: a systematic review and meta-analysis. Heart 2011;97:955–8 [DOI] [PubMed] [Google Scholar]
- 19.Treasure W. Diagnosis and Risk Management in Primary Care: Words that Count, Numbers that Speak. London: Radcliffe Publishing, 2011 [Google Scholar]
- 20.Judge DP, Dietz HC. Marfan's syndrome. Lancet 2005;366:1965–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pepper J, John CK, Gavino J, et al. External aortic root support for Marfan syndrome: early clinical results in the first 20 recipients with a bespoke implant. J R Soc Med 2010;103:370–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther 1974;15:443–53 [DOI] [PubMed] [Google Scholar]
- 23.Taves DR. The use of minimization in clinical trials. Contemp Clin Trials 2010;31:180–4 [DOI] [PubMed] [Google Scholar]
- 24.Treasure T, Farewell V. Minimization in interventional trials: great value but residual vulnerability. J Clin Epidemiol 2012;65:7–9 [DOI] [PubMed] [Google Scholar]
- 25.Devereaux PJ, Bhandari M, Clarke M, et al. Need for expertise based randomised controlled trials. BMJ 2005;330:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boutron I, Moher D, Altman DG, et al. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295–309 [DOI] [PubMed] [Google Scholar]
- 27.Lilford RJ, Thornton JG, Braunholtz D. Clinical trials and rare diseases: a way out of a conundrum. BMJ 1995;311:1621–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treasure T, Hasan A, Yacoub M. Is there a risk in avoiding risk for younger patients with aortic valve disease? BMJ 2011;342:d2466. [DOI] [PubMed] [Google Scholar]
- 29.Golesworthy T, Lampérth M, Mohiaddin R, et al. The Tailor of Gloucester: a jacket for the Marfan's aorta. Lancet 2004;364:1582. [DOI] [PubMed] [Google Scholar]
- 30.Treasure T, Pepper J, Golesworthy T, et al. External aortic root support: NICE guidance. Heart 2012;98:65–8 [DOI] [PubMed] [Google Scholar]
- 31.Murgatroyd F, Child A, Poloniecki J, et al. Does routine echocardiographic measurement of the aortic root diameter predict the risk of aortic dissection in the Marfan syndrome. Eur Heart J 1991;12:410 [Google Scholar]
- 32.Murgatroyd F, Child A, Poloniecki J, et al. Echocardiographic and clinical features are inadequate guides to the management of patients with the Marfan syndrome. Br Heart J 1993;68:84–5 [Google Scholar]
- 33.Robicsek F, Thubrikar MJ. Conservative operation in the management of annular dilatation and ascending aortic aneurysm. Ann Thorac Surg 1994;57:1672–4 [DOI] [PubMed] [Google Scholar]
- 34.Robicsek F, Thubrikar M. The mechanism and prevention of aortic dissection in Marfan Syndrome. In: Hetzer R, Gehke P, Ennker J, eds. Cardiovascular Aspects of Marfan Syndrome. Darmstadt: Steinkopff, 1995:61–70 [Google Scholar]
- 35.Treasure T. The Safe Duration of Total Circulatory Arrest with Profound Hypothermia. Thesis: University of London, 1982 [PMC free article] [PubMed] [Google Scholar]
- 36.Treasure T, Naftel DC, Conger KA, et al. The effect of hypothermic circulatory arrest time on cerebral function, morphology, and biochemistry. An experimental study. J Thorac Cardiovasc Surg 1983;86:761–70 [PubMed] [Google Scholar]
- 37.Treasure T. The safe duration of total circulatory arrest with profound hypothermia. Ann R Coll Surg Engl 1984;66:235–40 [PMC free article] [PubMed] [Google Scholar]
- 38.Pye D. The Nature and Art of Workmanship. Huntingdon, UK: Cambridge University Press, 1968 [Google Scholar]
- 39.Treasure T, Golesworthy T, Pepper J, et al. Prophylactic surgery of the aortic root in Marfan Syndrome: reconsideration of the decision making process in the era of customised external aortic root support. J Vasc Endovasc Surg 2011;18:215–23 [Google Scholar]
- 40.Kim SY, Martin N, Hsia EC, et al. Management of aortic disease in Marfan Syndrome: a decision analysis. Arch Intern Med 2005;165:749–55 [DOI] [PubMed] [Google Scholar]
- 41.Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23:338–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.