Abstract
Vernier acuity, a form of visual hyperacuity, is amongst the most precise forms of spatial vision. Under optimal conditions Vernier thresholds are much finer than the inter-photoreceptor distance. Achievement of such high precision is based substantially on cortical computations, most likely in the primary visual cortex. Using stimuli with added positional noise, we show that Vernier processing is reduced with advancing age across a wide range of noise levels. Using an ideal observer model, we are able to characterize the mechanisms underlying age-related loss, and show that the reduction in Vernier acuity can be mainly attributed to the reduction in efficiency of sampling, with no significant change in the level of internal position noise, or spatial distortion, in the visual system.
Many visual functions decline with age1,2,3,4,5,6,7,8,9,10,11. Surprisingly, Vernier acuity, a form of visual hyperacuity, has been reported to remain unchanged with advancing age12,13,14,15. However, more recent work using high resolution displays (especially important when measuring Vernier acuity, where the threshold is as small as a few seconds of arc under optimal stimulus conditions16), showed that Vernier acuity was degraded by as much as a factor of two after the age of 60 years17,18. The stimulus configurations used in these two studies are strongly resistant to optical degradation19. Moreover, Vernier acuity is quite stable with respect to changes in contrast and luminance at suprathreshold levels20,21. Therefore the reduction in Vernier performance should not be attributed to optical changes, but is thought to be due to genuine neural changes. A recent study reports that both spatial and temporal aspects of Vernier processing are affected by aging22. Electrophysiological evidence also supports the notion that Vernier acuity is reduced and that the time taken to process Vernier information is prolonged in the elderly23,24. However the mechanisms underlying the reduction of Vernier acuity with increasing age are not yet entirely clear.
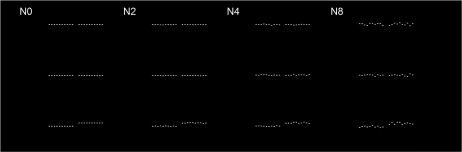
Our present experiment was aimed at exploring the mechanisms underlying age-related deterioration in Vernier hyperacuity. We hypothesized that neural changes during the aging process might introduce spatial noise (distortions) or reduce the efficiency with which visual information is sampled, similar to what we have observed in the disordered visual system25,26. In order to test this hypothesis, we measured Vernier acuity with added positional noise (illustrated in Fig. 1) to mimic spatial noise/distortion in the visual system, and employed an ideal positional averaging model to estimate internal noise and sampling efficiency. Noise was introduced into the Vernier stimulus by perturbing the vertical position of each discrete dot according to a Gaussian probability function, the standard deviation of which determined the amount of scatter. The internal positional noise was estimated by systematically manipulating the amount of external stimulus noise (SD = 0, 0.167, 0.337 & 0.667 arcmin for stimuli N0, N2, N4 & N8 respectively; the number following N indicates the SD in terms of the number of screen pixels, pixel-to-pixel distance = 5 arcsec). The visual task was to determine the average vertical position of each Vernier element and thus identify the misaligned pair among the three choices (3-alternative forced-: upper, middle or lower; all the lower pairs shown in Fig. 1 are misaligned with the right sections higher than those on the left).
Figure 1. Vernier Stimuli in Position Noise.
The Vernier stimulus was comprised of two horizontal segments separated by a small gap (N0). Each segment consisted of ten discrete white rectangular dots against a black background. Noise was introduced by manipulating the position of each dot vertically with a Gaussian probability function; its standard deviation determined the vertical spread of the dots (N2–N8). The visual task was to identify the misaligned pair among the three choices (upper, middle or lower). In the figure, all the lower pairs shown are misaligned - the right segments are higher than those on the left.
Results
Visual acuity
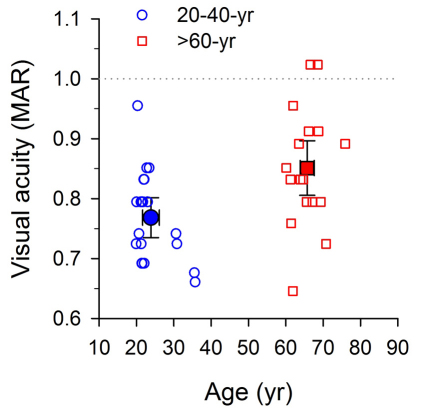
Vernier acuity in positional noise was examined in both younger (n = 20; mean age = 23.9±SD5.0 yrs; range = 20−36 yrs) and older (n = 18; mean age = 65.7±SD4.0 yrs; range = 60−76 yrs) observers. All had normal or corrected-to-normal vision, and were free of any manifest eye disease or abnormality. All participants except two in each group had Snellen acuity of 20/20 (Minimum Angle of Resolution, MAR, of 1 arc min) or better in each eye (Fig. 2, the area below the dotted line). The visual acuity values shown in the figure represent the average of the monocular visual acuities as a function of age for all observers. The mean visual acuities were 0.77±2SE0.033 arcmin and 0.85±2SE0.046 arcmin for the younger and older age groups, respectively (Fig. 2, solid symbols; all error bars throughout the following figures represent ±two SEs, unless otherwise indicated). The acuity difference between the two groups was small - approximately 10% or 2 letters on a standard LogMAR letter chart.
Figure 2. Visual acuity and Age.
Thirty-eight adults in total were recruited in two age groups: 21–40 years (blue circle symbols) and 60–80 years (red square symbols). All but two participants in each group had Snellen acuity of 20/20 (MAR = 1 arc min, the dotted line) or better in both eyes. The acuity difference between the two groups was small (approximately 2 letters on a standard LogMAR letter chart). Note that the visual acuity values reported represent the average of the monocular visual acuities for each observer. In this and the following figures, open symbols and solid symbols are used for showing individual and mean data respectively. Error bars represent ±two SEs, unless otherwise indicated.
Vernier acuity in position noise
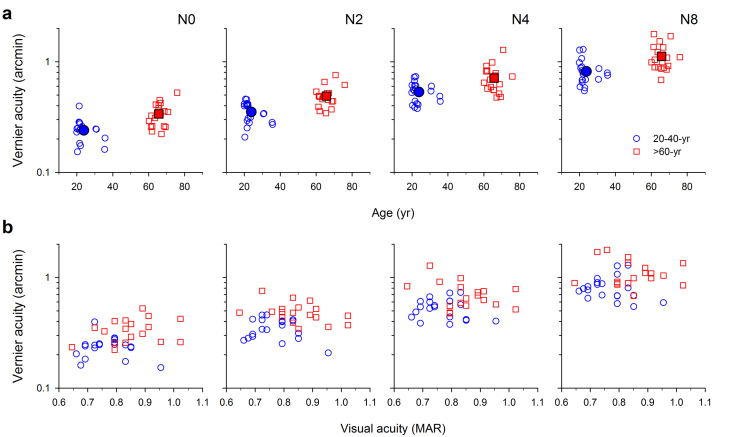
We found that Vernier thresholds were significantly elevated in our elderly observers, on average by 38%, compared with our younger observers at all positional noise levels (Fig. 3a). The data points of the older observers are clustered upwards above those of the younger observers. Two-way repeated measures ANOVA revealed a significant age-related increase in Vernier thresholds in noise (age: F(1,36) = 10.265, p = 0.003). The effect of age was dependent on the amount of noise (there was a significant age x noise interaction: F(3) = 4.588, p<0.005), with Vernier acuity most elevated in the elderly with no noise (N0). The mean thresholds were elevated in the older age group by 41%, 39%, 34% and 37% for N0-8, and the difference was statistically significant for each noise level (Post-hoc test: Holm-Sidak method; N0: t = 3.275, p = 0.001; N2: t = 3.698, p<0.001; N4: t = 4.136, p<0.001; N8: t = 5.426, p<0.001).
Figure 3. Vernier acuity and Age.
(a) Mean Vernier thresholds (solid symbols) were significantly elevated in the elderly group when compared with the younger group at all positional noise levels (N0, N2, N4 and N8), on average by 38% across all noise settings. Standard error bars are omitted in this figure for clarity. Open symbols show the acuity data for individual observers. (b) The individual Vernier acuities are replotted as a function of visual acuity. Within the acuity range between 0.6 and 1 arcmin, no statistically significant correlation was observed between visual acuity and Vernier acuity for any noise level.
The individual Vernier acuities (from Fig. 3a) are replotted as a function of visual acuity in Figure 3b. Within the very limited acuity range of our observers (∼0.65 to 1 arcmin), there was no statistically significant correlation between visual acuity and Vernier acuity, across any of the noise settings (mean r2 = 0.057) and the linear regression slopes were not significantly different from zero in any case (mean slope = −0.27, mean p = 0.43).
Positional averaging model
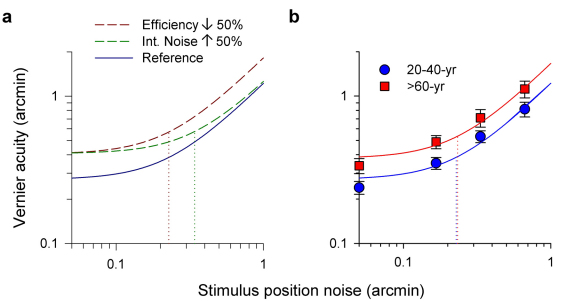
In an attempt to understand the mechanisms underlying the loss of visual performance, the Vernier acuity vs. position noise datasets were fitted with an ideal positional averaging model (see Methods, Equation 1) for individual observers, allowing the measurement of (1) internal positional noise: which reflects the amount of positional noise (in some sense, spatial distortion) which the visual system adds to the visual target, and (2) sampling efficiency: which quantifies how many samples are extracted from the visual target for computing the global position of each Vernier element. Figure 4a depicts two possible hypothetical mechanisms explaining the decrease in visual performance with increasing age: (i) A pure decrease in efficiency of the sampling process, i.e. the ability to extract visual information from the visual stimulus, would shift the threshold-versus-noise (TvN) function up – red curve. (ii) A pure increase in internal positional noise would shift the “knee” point of the function up, and also to the right – green curve. These two putative mechanisms are not necessarily mutually exclusive.
Figure 4. Ideal Positional Averaging Model.
(a) The dashed curves show the effects of (i) decreased sampling efficiency (by 50%; dark red) with fixed internal noise and (ii) increased internal noise (by 50%; dark green) with fixed efficiency in relation to a reference model (dark blue). The dotted lines indicate the magnitude of internal noise in each case. (b) Mean Vernier acuity data from Figure 3a were fitted with an ideal positional averaging model (Equation 1 in Methods; error bars: ±2SE). The TvN curve of the older group was displaced vertically upward relative to that of the younger group. The knee points (dotted lines) of the two curves are similar, indicating roughly the same internal noise levels for the two groups.
The effects of external position noise on mean Vernier acuity are shown in Figure 4b. Each data point is the average threshold of eighteen or twenty observers in each group; the error bars show plus or minus two standard errors of the mean. Note that for display purposes, the two leftmost data points for zero noise (N0) are arbitrarily set to 0.05 arcmin (Log scale), and the curve of the older group was displaced upward relative to that of the younger group. The turning points of the two curves remain roughly the same (see Equation 1 in Methods).
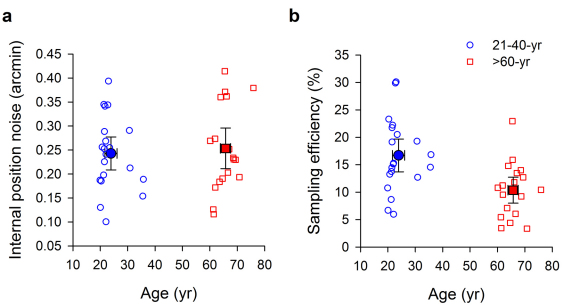
Internal positional noise was very slightly elevated, by 4%, in the older age group (Fig. 5a, younger group: 0.24±2SE0.03 arcmin; older group: 0.25±2SE0.04 arcmin), and the mean difference was not statistically significant (unpaired t = 0.390, df = 36; p = 0.699). In contrast, sampling efficiency was significantly decreased in our older observers by 38% when compared with our younger observers. The mean sampling efficiencies were 16.7±2SE3.0 % and 10.4±2SE2.4 % for the younger and older groups, respectively (Fig. 5b, unpaired t = 3.275, df = 36; p = 0.002).
Figure 5. Internal Position Noise and Sampling Efficiency.
(a) Mean internal positional noise (solid symbols) was slightly, but not significantly, elevated (by 4%), in the older group. (b) Mean sampling efficiency (solid symbols) was significantly decreased in the older group (by 38%) when compared with the younger group. Error bars: ±2SE.
Discussion
We have shown that Vernier acuity in positional noise is reduced with normal aging. It had been generally thought that Vernier acuity was immune to aging effects12,13,14,15. Li et al.17 pointed out the importance of having sufficiently small pixel-size to avoid floor effects in measuring hyperacuities, and reported that Vernier acuity is degraded with advancing age. Our present research extends the previous findings by measuring Vernier acuity in positional noise. The curve relating the Vernier threshold to positional noise for the older observers was found to be displaced upward relative to that for young adults. Using an ideal position averaging model, we are able to attribute the deterioration in visual performance largely to decreased sampling ability, with a very small non-significant increase in internal noise.
All our elderly participants had normal visual acuity. All had clear ocular media and were free of significant lens opacities. Moreover, the Vernier stimulus configuration which we used, with a 4 minute of arc gap between the two Vernier elements is optimally resistant to optical degradation19. Thus the reduced Vernier acuity may be presumed to be due to genuine neural changes, and not to optical changes27,28. It might be argued that reduced retinal illuminance in the aging visual system, resulting from smaller pupil size29 and reduced ocular transmittance30, could potentially cause the degraded Vernier performance. Previous work17 controlling for these factors has shown that reduced retinal illuminance does not cause the decrease in Vernier acuity in the elderly eye. Moreover, our unpublished data also reveal that a large decrease in target contrast, from 100% to 50% causes minimal degradation of Vernier acuity.
It is not surprising that neural changes affect Vernier performance in the aged visual system. It has been shown that, for a range of visual tasks, visual processing is limited by reduced sampling efficiency or increased internal noise, or both in older people5,31,32,33,34,35,36,37,38. Most participants in our study had acuity of 20/20 or better, indicating that a large part of the neural changes are likely to have taken place beyond the retinal level, possibly at the sub-cortical and/or cortical levels.
For separated targets, Vernier acuity shows little dependence on target visibility, but depends mainly on spatial relations. Therefore instead of commonly used luminance noise, we chose to use positional noise, i.e. perturbation of the positions of parts of the stimulus39,40,41,42 in our investigations of the mechanisms underlying the losses incurred with increasing age. For abutting targets, unlike separated targets, Vernier processing relies on the response properties of contrast-sensitive filters43. From this perspective, luminance noise might be appropriate when using abutting targets44.
The Vernier thresholds reported in this study are in general higher than those reported in the Li et al. study17. We note that there are some important methodological differences between the two studies. A much stricter threshold criterion (detectability, d' = 1.1) was adopted in the present study, whereas the thresholds in the previous study corresponded to d' = 0.67. Had we adopted the same criterion as the previous study, our thresholds would have been approximately 40 percent lower. Second, the three-element Vernier stimuli employed previously had two Vernier offset cues to be detected, one at each end of the middle element. Third, the target orientation in this experiment was horizontal, while vertical orientation was examined in the previous study. We are confident that the step size in this study was small enough to obtain an accurate measurement of Vernier acuity, given the fact that the smallest threshold observed among all participants was 9 arcsec, which was approximately twice the minimum step size (5 sec arc).
The loss of neurons in the visual pathways45,46,47,48,49 and the functional changes in their response properties50,51,52 during the aging process might explain the age-related changes in visual perception. Previous work suggests that the number of ganglion cells decrease by ∼1.1 to 4.5 percent per decade over the third to the seventh decade of life45,46,47; this is somewhat less than the rate suggested from psychophysical studies of contrast sensitivity vs. stimulus size (3.4–7.7 percent per decade53). Our results show a reduction of sampling efficiency of about 38% over the roughly forty-two years separating the two groups, a loss of efficiency of about 9 percent per decade, comparable to the estimate of Pearson et al.53. It is important to note that neuronal changes do not necessarily manifest as functional deterioration, for example see Govenlock et al.54.
In summary, the present findings contribute to an understanding of Vernier processing in the aged visual system. A theoretical explanation of reduced Vernier acuity in the aged visual system is presented in terms of sampling efficiency, and to a much lesser extent internal positional noise. The “noisy” Vernier test may have potential clinical value in the detection of pathological conditions of the retina (for example, central serous retinopathy and metamorphopsia, unpublished data) and other neuro-ophthalmological disorders (for example, amblyopia25), and is currently under study in our laboratories.
Methods
Visual stimuli
In this study, we used an experimental set-up similar to that in our previous studies42 to measure Vernier acuity. The Vernier stimulus was comprised of two horizontal segments separated by a 4 arcmin gap (Fig. 1). Each segment consisted of 10 discrete white rectangular dots (1 arcmin x 0.5 arcmin) against a black background, with a dot separation of 1 arcmin. The length of the whole stimulus was 42 arcmin, with each segment subtending 19 arcmin.
Positional noise was produced by distributing the position of each dot vertically according to a Gaussian probability function (Fig. 1: N2-N8), the standard deviation of which determined the vertical spread of the dots. The position of each dot was computed independently and did not affect the others. For zero standard deviation, the Vernier elements were free of external noise (Fig. 1: N0). The jitter of dots was increased by increasing the standard deviation of the distribution of the vertical displacement of the dots. In the Vernier acuity assessment, we tested our observers with four different noise levels: standard deviations 0, 0.167, 0.337 and 0.667 arcmin (N0, 2, 4 & 8: the number indicates the standard deviation in terms of screen pixels).
The visual stimuli were displayed on a 21 inch flat monitor screen (Sony GDM-F520) at 1800 x 1440 resolution with a 90 Hz refresh rate. The screen was viewed binocularly in a mirror, at an optical distance of 8 m. The vertical inter-pixel distance (or step size) was 5 sec of arc. The luminance of the Vernier stimulus was 130 cd/m2 and that of the background was 0.9 cd/m2, Michelson contrast 99%. Light shielding was used to block stray light from the screen. The monitor screen edges were covered with cardboard cut with undulating edges so that observers could not use the straight edges of the monitor as visual cues, i.e. use bisection or spatial interval discrimination, in making Vernier judgments.
Psychophysical methods
A 3-alternative forced-choice psychophysical method (3AFC) was applied to measure Vernier threshold. The observers' task was to identify the one misaligned pair of dot groupings out of three choices (upper, middle or lower). For an example illustrated in Figure 1a, the lower pair is not aligned, with the right segment higher than the left. The paradigm was designed to measure the observer's ability to detect the vertical offset between the two segments. Stimuli remained on the monitor screen until the observer responded. Trial-by-trial feedback was provided. A modified interleaved staircase method was used to control the offset level and to track individual thresholds55. An offset was produced by randomly shifting the right segment up or down. A Weibull analysis was performed to fit the psychometric curve with the response data. The position-discrimination threshold was defined as the offset at which 66% correct responses were obtained (detectability d' = 1.15). A threshold was based on 100 responses for each noise setting (400 in total for all four noise levels). The task was self-paced to allow for the longer reaction time of older observers. A break was given every hundred trials, and whenever an observers tired and requested one.
Positional averaging modeling
The noise model employed was based on the assumption that the Vernier processing system has additive internal noise56. An ideal position averaging model was used to characterize the effects of external positional noise (σe) on observer's positional discrimination threshold (σth):
where σi denotes equivalent input noise, and k denotes the number of stimulus samples extracted for positional averaging. For 66% correct response probability, the detectability (d') was 1.157. By measuring the thresholds in different external noise settings, both σi and k can be estimated by fitting a threshold versus noise curve on the basis of a least squares computation.
Equivalent input noise is the noise that must be added to the stimulus in order to act as the limiting noise in the visual system. It largely reflects the amount of intrinsic noise which the observer's visual system adds to the stimulus and quantifies the spatial distortion of the visual space. When external stimulus noise is small compared with equivalent input noise, threshold is determined mainly by equivalent input noise. As the stimulus noise increases and equals the equivalent input noise in magnitude, the threshold begins to rise in proportion to the stimulus noise level. Sampling efficiency (E) is quantified by the fraction of samples extracted for positional averaging, reflecting how well the human observer making use of the stimulus information.
Observers
Altogether thirty-eight adults with normal vision were recruited in two age groups: 21–40 (n = 20) and 60–75 (n = 18) years. The mean ages of the younger and older groups were 23.9 and 65.7 years respectively (Fig. 2). All observers underwent a thorough eye examination carried out by an experienced optometrist (first author, RWL). The maculae of all observers were assessed as normal; specifically there were no drusen or abnormal pigment changes in an area of about one disc diameter around the macula. All observers had clear ocular media, as assessed by direct ophthalmoscopy, and were free of lens opacities in the natural pupil area. They had no manifest ocular diseases, nor did they have strabismus or amblyopia. All but two participants in each group had visual acuity of 20/20 (MAR = 1 arc min) or better in both eyes (see Fig. 2). Viewing was binocular with full optical corrections for the testing distance of 8 m. The measurements took 60–90 minutes in 1 or 2 sessions. All observers were inexperienced observers regarding Vernier acuity measurements, except one observer (the first author) in the younger age group.
The experimental procedures were approved by the University of California-Berkeley Committee for the Protection of Human Subjects and the Hong Kong Polytechnic University Ethics Committee. The research was conducted according to the principles expressed in the Declaration of Helsinki. The experiments were undertaken with the understanding and written consent of each participant.
Author Contributions
R. L., and B. B. conceived the research. R. L. designed and programmed the Vernier acuity testing system. R. L., C. N., and S. C. conducted the experiment. R. L. performed the data analysis. R. L., B. B., M. E. and D. L. co-wrote the paper. All authors discussed the results and revised the manuscript.
Acknowledgments
This research project was supported by National Eye Institute Grant R01EY01728 and a research grant from the Hong Kong Polytechnic University.
References
- Gardiner S. K., Johnson C. A. & Spry P. G. D. Normal age-related sensitivity loss for a variety of visual functions throughout the visual field. Optom. Vis. Sci. 83, 438–443 (2006). [DOI] [PubMed] [Google Scholar]
- Tang Y. & Zhou Y. Age-related decline of contrast sensitivity for second-order stimuli: earlier onset, but slower progression, than for first-order stimuli. J. Vis. 9, 18 (2009). [DOI] [PubMed] [Google Scholar]
- Sjöstrand J., Laatikainen L., Hirvelä H., Popovic Z. & Jonsson R. The decline in visual acuity in elderly people with healthy eyes or eyes with early age-related maculopathy in two Scandinavian population samples. Acta Ophthalmol. 89, 116–123 (2011). [DOI] [PubMed] [Google Scholar]
- Betts L. R., Sekuler A. B. & Bennett P. J. The effects of aging on orientation discrimination. Vision Res. 47, 1769–1780 (2007). [DOI] [PubMed] [Google Scholar]
- Bennett P. J., Sekuler R. & Sekuler A. B. The effects of aging on motion detection and direction identification. Vision Res. 47, 799–809 (2007). [DOI] [PubMed] [Google Scholar]
- Li R. W. et al. Aging and visual counting. PLoS One 5, e13434 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Viva M. M. & Agostini R. Visual spatial integration in the elderly. Invest. Ophthalmol. Vis. Sci. 48, 2940–2946 (2007). [DOI] [PubMed] [Google Scholar]
- Norton D., McBain R. & Chen Y. Reduced ability to detect facial configuration in middle-aged and elderly individuals: Associations with spatiotemporal visual processing. J. Gerontol. B Psychol. Sci. Soc. Sci. 64B, 328–334 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaroff C. M., Knutelska M. & Frumkes T. E. Variation in stereoacuity: Normative description, fixation disparity, and the roles of aging and gender. Invest. Ophthalmol. Vis. Sci. 44, 891–900 (2003). [DOI] [PubMed] [Google Scholar]
- Owsley C. Aging and vision. Vision Res. 51, 1610–1622 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B., Yap M. K. & Fan W. C. Decrease in stereoacuity in the seventh decade of life. Ophthalmic Physiol. Opt. 13, 138–142 (1993). [DOI] [PubMed] [Google Scholar]
- Odom J. V., Vasquez R. J., Schwartz T. L. & Linberg J. V. Adult Vernier thresholds do not increase with age; Vernier bias does. Invest. Ophthalmol. Vis. Sci. 30, 1004–1008 (1989). [PubMed] [Google Scholar]
- Lakshminarayanan V., Aziz S. & Enoch J. M. Variation of the hyperacuity function with age. Optom. Vis. Sci. 69, 423–426 (1992). [DOI] [PubMed] [Google Scholar]
- Whitaker D., Elliott D. B. & MacVeigh D. Variations in hyperacuity performance with age. Ophthalmic Physiol. Opt. 12, 29–32 (1992). [PubMed] [Google Scholar]
- Kline D. W., Culham J. C., Bartel P. & Lynk L. Aging effects on Vernier hyperacuity: a function of oscillation rate but not target contrast. Optom. Vis. Sci. 78, 676–682 (2001). [DOI] [PubMed] [Google Scholar]
- Westheimer G. & McKee S. P. Spatial configurations for visual hyperacuity. Vision Res. 17, 941–947 (1977). [DOI] [PubMed] [Google Scholar]
- Li R. W., Edwards M. H. & Brown B. Variation in Vernier acuity with age. Vision Res. 40, 3775–3781 (2000). [DOI] [PubMed] [Google Scholar]
- Garcia-Suarez L., Barrett B. T. & Pacey I. A comparison of the effects of ageing upon Vernier and bisection acuity. Vision Res. 44, 1039–1045 (2004). [DOI] [PubMed] [Google Scholar]
- Williams R. A., Enoch J. M. & Essock E. A. The resistance of selected hyperacuity configurations to retinal image degradation. Invest. Ophthalmol. Vis. Sci. 25, 389–399 (1984). [PubMed] [Google Scholar]
- Wehrhahn C. & Westheimer G. How Vernier acuity depends on contrast. Exp. Brain Res. 80, 616–618 (1990). [DOI] [PubMed] [Google Scholar]
- Waugh S. J. & Levi D. M. Visibility, luminance and Vernier acuity. Vision Res. 33, 505–526 (1993). [DOI] [PubMed] [Google Scholar]
- Roinishvili M., Chkonia E., Stroux A., Brand A. & Herzog M. H. Combining Vernier acuity and visual backward masking as a sensitive test for visual temporal deficits in aging research. Vision Res. 51, 417–423 (2011). [DOI] [PubMed] [Google Scholar]
- Li R. W., Edwards M. H. & Brown B. Variation in Vernier evoked cortical potential with Age. Invest. Ophthalmol. Vis. Sci. 42, 1119–1124 (2001). [PubMed] [Google Scholar]
- Plomp G., Kunchulia M. & Herzog M. H. Age-related changes in visually evoked electrical brain activity. Hum Brain Mapp (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. W. & Levi D. M. Characterizing the mechanisms of improvement for position discrimination in adult amblyopia. J. Vis. 6, 476–487 (2004). [DOI] [PubMed] [Google Scholar]
- Li R. W., Klein S. A. & Levi D. M. Prolonged perceptual learning of positional acuity in adult amblyopia: perceptual template retuning dynamics. J. Neurosci. 28, 14223–14229 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao A. et al. Average optical performance of the human eye as a function of age in a normal population. Invest. Ophthalmol. Vis. Sci. 40, 203–213 (1999). [PubMed] [Google Scholar]
- Berrio E., Tabernero J. & Artal P. Optical aberrations and alignment of the eye with age. J. Vis. 10, 34 (2010). [DOI] [PubMed] [Google Scholar]
- Sloane M. E., Owsley C. & Alvarez S. L. Aging, senile miosis and spatial contrast sensitivity at low luminance. Vision Res. 28, 1235–1246 (1988). [DOI] [PubMed] [Google Scholar]
- van de Kraats J. & van Norren D. Optical density of the aging human ocular media in the visible and the UV. J. Opt. Soc. Am. A 24, 1842–1857 (2007). [DOI] [PubMed] [Google Scholar]
- Welford A. T. Signal, noise, performance, and age. Hum. Factors 23, 97–109 (1981). [DOI] [PubMed] [Google Scholar]
- Cremer R. & Zeef E. J. What Kind of Noise Increases With Age? J. Gerontol. 42, 515–518 (1987). [DOI] [PubMed] [Google Scholar]
- Allen P. A., Madden D. J., Groth K. E. & Crozier L. C. Impact of age, redundancy, and perceptual noise on visual search. J. Gerontol. 47, 69–74 (1992). [DOI] [PubMed] [Google Scholar]
- Allen P. A., Weber T. A. & May N. Age Differences in letter and color matching: selective attention or internal noise? J. Gerontol. 48, 69–77 (1993). [DOI] [PubMed] [Google Scholar]
- Speranza F., Moraglia G. & Schneider B. A. Age-related changes in binocular vision: detection of noise-masked targets in young and old observer. J. Gerontol. B Psychol. Sci. Soc. Sci. 50, 114–123 (1995). [DOI] [PubMed] [Google Scholar]
- Pardhan S., Gilchrist J., Elliott D. B. & Beh G. A comparison of sampling efficiency and internal noise level in young and old subjects. Vision Res. 36, 1641–1648 (1996). [DOI] [PubMed] [Google Scholar]
- Bennett P. J., Sekuler A. B. & Ozin L. Effects of aging on calculation efficiency and equivalent noise. J. Opt. Soc. Am. A 16, 654–668 (1999). [DOI] [PubMed] [Google Scholar]
- Speranza F., Moraglia G. & Schneider B. A. Binocular detection of masked patterns in young and old observers. Psychol. Aging 16, 281–292 (2001). [DOI] [PubMed] [Google Scholar]
- Meer P. & Zeevi Y. Y. Importance of global information in Vernier acuity. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 3, 880–884 (1986). [DOI] [PubMed] [Google Scholar]
- Badcock D. R. & Wong T. L. Resistance to positional noise in human vision. Nature 343, 554–555 (1990). [DOI] [PubMed] [Google Scholar]
- Wang H., Levi D. M. & Klein S. A. Intrinsic uncertainty and integration efficiency in bisection acuity. Vision Res. 36, 717–739 (1996). [DOI] [PubMed] [Google Scholar]
- Li R. W., Levi D. M. & Klein S. A. Perceptual learning improves efficiency by re-tuning the "template" for position discrimination. Nat. Neurosci. 7, 178–183 (2004). [DOI] [PubMed] [Google Scholar]
- Levi D. M., Klein S. A. & Carney T. Unmasking the mechanisms for Vernier acuity: evidence for a template model for Vernier acuity. Vision Res. 40, 951–972 (2000). [DOI] [PubMed] [Google Scholar]
- Skoczenski A. M. & Aslin R. N. Assessment of Vernier acuity development using the "equivalent intrinsic blur" paradigm. Vision Res. 35, 1879–1887 (1995). [DOI] [PubMed] [Google Scholar]
- Curcio C. A. & Drucker D. N. Retinal ganglion cells in Alzheimer's disease and aging. Ann. Neurol. 33, 248–257 (1993). [DOI] [PubMed] [Google Scholar]
- Spear P. D. Neural bases of visual deficits during aging. Vision Res. 33, 2589–2609 (1993). [DOI] [PubMed] [Google Scholar]
- Lovasik J. V., Kergoat M. J., Justino L. & Kergoat H. Neuroretinal basis of visual impairment in the very elderly. Graefes Arch. Clin. Exp. Ophthalmol. 241, 48–55 (2003). [DOI] [PubMed] [Google Scholar]
- Devaney K. O. & Johnson H. A. Neuron loss in the aging visual cortex of man. J. Gerontol. 35, 836–841 (1980). [DOI] [PubMed] [Google Scholar]
- Ahmad A. & Spear P. D. Effects of aging on the size, density, and number of rhesus monkey lateral geniculate neurons. J. Comp. Neurol. 334, 631–643 (1993). [DOI] [PubMed] [Google Scholar]
- Zhang J. et al. Spatial and temporal sensitivity degradation of primary visual cortical cells in senescent rhesus monkeys. Eur. J. Neurosci. 28, 201–207 (2008). [DOI] [PubMed] [Google Scholar]
- Yang Y. et al. Aging affects contrast response functions and adaptation of middle temporal visual area neurons in rhesus monkeys. Neuroscience 156, 748–757 (2008). [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhou Y., Ma Y. & Leventhal A. G. Degradation of signal timing in cortical areas V1 and V2 of senescent monkeys. Cereb. Cortex 15, 403–408 (2005). [DOI] [PubMed] [Google Scholar]
- Pearson P. M., Schmidt L. A., Ly-Schroeder E. & Swanson W. H. Ganglion cell loss and age-related visual loss: a cortical pooling analysis. Optom Vis. Sci. 83, 444–454 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govenlock S. W., Taylor C. P., Sekuler A. B. & Bennett P. J. The effect of aging on the orientational selectivity of the human visual system. Vision Res. 49, 164–172 (2009). [DOI] [PubMed] [Google Scholar]
- Carkeet A., Levi D. M. & Manny R. E. Development of Vernier acuity in childhood. Optom. Vis. Sci. 74, 741–750 (1997). [DOI] [PubMed] [Google Scholar]
- Zeevi Y. Y. & Mangoubi S. S. Vernier acuity with noisy lines: estimation of relative position uncertainty. Biol. Cybern. 50, 371–376 (1984). [DOI] [PubMed] [Google Scholar]
- Wickens T. D. Elementary signal detection theory. (Oxford University Press, USA, 2002). [Google Scholar]