Abstract
Objective
Postpartum major depression is a significant public health problem that strikes 15% of new mothers and confers adverse consequences for mothers, children, and families. The neural mechanisms involved in postpartum depression remain unknown, but brain processing of affective stimuli appears to be involved in other affective disorders. The authors examined activity in response to negative emotional faces in the dorsomedial prefrontal cortex and amygdala, key emotion regulatory neural regions of importance to both mothering and depression.
Method
Postpartum healthy mothers (N=16) and unmedicated depressed mothers (N=14) underwent functional magnetic resonance imaging blood-oxygen-level-dependent acquisition during a block-designed face versus shape matching task. A two-way analysis of variance was performed examining main effects of condition and group and group-by-condition interaction on activity in bilateral dorsomedial prefrontal cortical and amygdala regions of interest.
Results
Depressed mothers relative to healthy mothers had significantly reduced left dorsomedial prefrontal cortical face-related activity. In depressed mothers, there was also a significant negative correlation between left amygdala activity and postpartum depression severity and a significant positive correlation between right amygdala activity and absence of infant-related hostility. There was reliable top-down connectivity from the left dorsomedial prefrontal cortex to the left amygdala in healthy, but not depressed, mothers.
Conclusions
Significantly diminished dorsomedial prefrontal cortex activity and dorsomedial prefrontal cortical-amygdala effective connectivity in response to negative emotional faces may represent an important neural mechanism, or effect, of postpartum depression. Reduced amygdala activity in response to negative emotional faces is associated with greater postpartum depression severity and more impaired maternal attachment processes in postpartum depressed mothers.
The birth of a child is a greatly anticipated and desired life event, but it is paradoxically accompanied by maternal depression in 15% of new mothers (1). Postpartum depression is not only disabling and potentially life-threatening, but it also interferes with mother-infant relational processes, with consequent adverse effects on the socioemotional and cognitive development of offspring (2). Despite wider recognition of its tragic outcomes, stigma, lack of education about the disorder and its treatment, and poor discrimination of the disorder from normal maternal adjustment continue to be significant treatment barriers (3). For women who do seek treatment, outcomes are disappointing. Of depressed mothers who received 8 weeks of pharmacologic treatment (4) or 12 weeks of interpersonal psychotherapy (5), only 30%–50% successfully achieved remission, which is similar to remission rates for depression in the general population (6). Greater mechanistic understanding of postpartum depression is needed.
Hypothalamic-pituitary-adrenal axis dysregulation (7-9) and hypoestrogenemia appear to be important pathophysiological processes in postpartum depression (10). In contrast, there is little understanding of the neural mechanisms of emotion processing in the disorder. In nonpostpartum adult depression, neuroimaging studies have highlighted the role of dysfunction within key social cognition and emotion regulatory regions, including the dorsomedial prefrontal cortex, together with amygdala and striatal emotion processing regions, in response to self- and other-relevant emotional stimuli (11, 12). In normative motherhood, several investigators have reported medial prefrontal cortex and subcortical limbic activity in response to infant videos (13) and infant cries (14-16). Furthermore, healthy mothers displayed greater activity within the bilateral amygdala and medial prefrontal cortex in response to faces of their own child versus less familiar infants (17, 18). Such patterns suggest that maternal amygdala activity in response to salient emotional cues as well as engagement of social cognition regions involved in empathy and self-other relational processes (19) may comprise a neural circuitry that supports attunement to infant emotional states. Whether the brains of depressed mothers have the capacity to similarly engage these neural circuits when presented with noninfant-specific emotional stimuli has been brought into question by findings, in these mothers, of orbitofrontal cortex, amygdala, and striatal hypoactivity in response to emotionally valenced words (20). Thus, further examination of affective neural processing seems warranted.
In the present study, we used a negative emotional face matching paradigm (21) to examine prefrontal cortical and subcortical neural activity and connectivity in response to negative emotional stimuli in depressed mothers relative to healthy mothers. We considered two hypotheses. First, based upon reports of reduced amygdala activity in response to negatively valenced words in depressed mothers (20) and abnormally elevated amygdala activity in response to fearful and sad faces in nonpostpartum major depressive disorder (22, 23), we hypothesized that there would be altered amygdala activity in response to negative emotional faces in depressed mothers. Second, because postpartum depression occurs within a context of self-other relational processing with respect to the infant, we hypothesized that there would also be alterations in face-related dorsomedial prefrontal cortical activity in depressed mothers. We further explored dorsomedial prefrontal cortex-amygdala effective functional connectivity in response to negative emotional faces in postpartum depressed mothers relative to healthy mothers, based upon our previous findings of reduced prefrontal cortical-amygdala effective connectivity in other depressed cohorts (24).
Method
Participants
Subjects provided written informed consent as approved by the University of Pittsburgh Biomedical Institutional Review Board. Subjects delivered a healthy term infant in the preceding 12 weeks and were medication-free, multiparous, and breast- or bottle-feeding. Depression was defined by DSM-IV criteria for unipolar major depression (25) and a 25-item Hamilton Depression Rating Scale (HAM-D) score ≥15. Both prevalent (beginning antenatally) and incident (new-onset postpartum) cases of postpartum depression were included to maximize generalizability, since the disorder commonly begins antenatally (26). Women with bipolar illness were excluded. Healthy subjects had no personal or family history of an axis I affective disorder. Subjects were excluded if they had medical or neurological illnesses likely to affect cerebral physiology or anatomy, gross abnormalities of brain structure evident by magnetic resonance imaging, suicidal intent, substance abuse within 1 year, lifetime history of substance dependence (other than nicotine), eating disorders, use of hormonal contraception, or exposure to medications likely to alter cerebral physiology within 3 weeks.
Fourteen depressed and 16 healthy mothers were enrolled and imaged. Clinical variables collected on the scan day included HAM-D scores; Edinburgh Postnatal Depression Scale scores (a well-validated 10-item self-report measure of perinatal depression, anxiety, and function [27]); and Parent-to-Infant Attachment Questionnaire scores (a reliable and valid self-report of attachment quality, hostility, and pleasure in interaction during the first postpartum year [28]). Statistical tests on group differences in demographic, reproductive, psychiatric, and behavioral data were performed using Pearson chi-square test for categorical variables and Mann-Whitney U exact tests for continuous variables.
Experimental Stimuli
In order to fully engage neural regions implicated in emotion processing, we used a well-known emotional face matching task (21). The block-design paradigm consisted of four blocks of a perceptual face processing task interleaved with five blocks of a sensorimotor control task. Subjects were presented with a target stimulus and asked to select one of two images, presented on the lower half of the screen, which identically matched the target. Each face processing block consisted of fear and anger sub-blocks (three trials each), all derived from a standard set of facial affect pictures (29). During the sensorimotor control blocks, subjects viewed a trio of simple geometric shapes (six total trials). Each trial was presented for 4 seconds. Shape trials had a fixed interstimulus interval (2 seconds), and each shape block was displayed for 36 seconds. Face trials had a variable interstimulus interval (2–6 seconds, mean: 4 seconds), and thus face blocks were between 36 and 48 seconds each.
Functional Magnetic Resonance Imaging Data Acquisition
Scanning was performed on a Siemens 3 Tesla Trio scanner (Siemens Medical Solutions, Erlangen, Germany). High-resolution, T1-weighted anatomical images were acquired using an MPRAGE sequence (TR=1,630 msec; TE=2.48 msec; field of view=20.4 cm; α=8°; image matrix=256×256; voxel size=0.8×0.8×0.8 mm; 224 slices). Whole-brain functional images were acquired using a single-shot, gradient-recalled echoplanar pulse sequence (TR=2,000 msec; TE=30 msec; α=73°; field of view=20.4 cm; image matrix=64×64; voxel size=3.2×3.2×3.2 mm; 35 slices) sensitive to blood-oxygen-level-dependent (BO LD) contrast. Runs consisted of the acquisition of 195 successive brain volumes beginning with two discarded radio frequency excitations to allow for steady-state equilibrium.
Data Analysis
Data were preprocessed and analyzed using BrainVoyager QX1.9 (Brain Inno vation, Maastricht, the Netherlands). Preprocessing included slice time correction (using cubic spline interpolation), alignment of slices (using cubic spline interpolation to the first nondiscarded scan time), three-dimensional motion correction (using trilinear interpolation), spatial smoothing with a 4-mm Gaussian kernel, linear trend removal, and temporal highpass filtering (fast-Fourier transform based with a cutoff of three cycles/time courses). The functional data sets were coregistered to the Talairach-transformed (30) T1-weighted anatomical image series to create a four-dimensional data representation. Participant data were excluded for deviation in the estimated center of mass >3 mm in any direction.
A multiparticipant statistical analysis was performed by multiple linear regression of the time course of the BOLD response in each voxel of a priori regions of interest. The general linear model of the experiment was computed for each participant’s z-normalized volume time courses (30 total). Model predictors were defined by convolving an ideal boxcar response with a gammafunction model of the hemodynamic response (31). Boxcar values were equal to 1.0 during face blocks and 0.0 during shape blocks.
Region of Interest Analyses to Examine Main Effects of Experimental Condition, Group, and Group-by-Condition Interaction
We entered all data into a random-effects 2 (condition: faces, shapes)-×-2 (group: healthy, depressed) analysis of variance model. Analysis was focused on a priori regions of interest (amygdala and dorsomedial prefrontal cortex), by creating a single structural mask encompassing the bilateral Brodmann’s areas 10 and 32, and amygdala and parahippocampal gyrus regions of interest from the Analysis of Functional Neuroimages (National Institute of Mental Health, Bethesda, Md.). Activation maps were visualized on a Talairach-transformed template brain and displayed at a resolution of 1 mm3.
First, we computed the main effect of condition for the purpose of region selection for further correlational and connectivity analyses. Because of evidence of lactation (32) and oxytocin/prolactin effects of reducing anxiety and stress responses to fearful stimuli, potentially mediated by oxytocin-corticotropin-releasing hormone interactions in the central nucleus of the amygdala (33), we entered breast-feeding status as a categorical correlate and computed a point biserial correlation to determine whether this status was associated with face-related activity in a priori regions of interest.
Next, to examine abnormal activity in depressed mothers, we computed the main effect of group (healthy versus depressed mothers) and the group-by-condition interaction on facerelated activity in our regions of interest. For each statistical main effect and interaction map, p values were subjected to a significance threshold <0.005, uncorre cted. To control for multiple comparisons, we employed a cluster-size threshold adjustment, based on a Monte Carlo simulation approach extended to three-dimensional data sets, using the cluster threshold size estimator plug-in (34). This procedure takes input regarding the functional voxel size (3 mm3 native resolution), the total number of significant voxels within a map, and the estimated smoothness of a map and performs Monte Carlo simulations (1,000 iterations) to estimate the probability of clusters of a given size arising purely by chance. By applying a spatial cluster threshold of 753 mm3, or 26 contiguous voxels, the family-wise (corrected) false probability rate was set at p<0.05. Significant findings are therefore reported at p<0.05, using this correction for multiple comparisons.
Results
Subject Characteristics
With the exception of a higher breast-feeding rate among depressed mothers (p=0.052), demographic, reproductive, and medical variables (Table 1) were similar between groups. Subjects were 4 to 13 weeks (mean: 9 weeks [SD=2]) postpartum on the scan day. Subjects were hormonal contraception-free, with the exception of one woman who had a levonorgestrel intrauterine device inserted 6 days prior to the scan. Depressed mothers were unmedicated for at least 2 years at the time of the scan.
TABLE 1. Demographic and Clinical Characteristics of Depressed and Healthy Mothers.
| Healthy Mothers (N=16) |
Depressed Mothers (N=14) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Mean | SD | N | % | Mean | SD | N | % |
| Demographic | ||||||||
| Age (years) | 26.7 | 4.8 | 26.8 | 6.1 | ||||
| Education (years completed) | 15.2 | 3.1 | 15.1 | 2.4 | ||||
| Caucasian | 11 | 68.8 | 10 | 71.4 | ||||
| Medical/reproductive | ||||||||
| Right-handed | 15 | 93.8 | 12 | 85.7 | ||||
| Body mass index (kg/m2) | 27.9 | 3.7 | 29.3 | 5.8 | ||||
| Smoker | 3 | 18.8 | 3 | 21.4 | ||||
| Primiparous | 6 | 37.5 | 7 | 50.0 | ||||
| Breast-feeding | 7 | 43.8 | 11 | 78.6 | ||||
| Time since childbirth (weeks) | 9.4 | 2.0 | 8.2 | 2.2 | ||||
| Postpartum amenorrhea | 10 | 62.5 | 12 | 85.7 | ||||
| Vaginal delivery | 12 | 75.0 | 13 | 92.9 | ||||
| Psychiatric | ||||||||
| Antidepressant naive | 16 | 100.0 | 12 | 85.7 | ||||
| HAM-D (25-item) score | 2.8 | 2.5 | 21.2** | 6.2 | ||||
| HAM-A score | 0.6 | 0.8 | 14.8** | 7.3 | ||||
| Edinburgh Postnatal Scale for Depression score | 1.9 | 1.5 | 14.7** | 4.3 | ||||
| Quality of mother-infant attachment (Parent-to-Infant Attachment Questionnaire) |
43.6 | 1.8 | 35.3** | 6.9 | ||||
| Absence of maternal-infant hostility (Parent-to-Infant Attachment Questionnaire) |
22.0 | 2.2 | 15.3** | 3.6 | ||||
| Pleasure in maternal-infant interaction (Parent-to-Infant Attachment Questionnaire) |
22.3 | 1.7 | 18.3* | 5.5 | ||||
| Task behavioral performance | ||||||||
| Face matching accuracy (%) | 99.7 | 1.0 | 95.5 | 1.3 | ||||
| Face matching reaction time (msec) | 1,222.5 | 244.4 | 1,252.1 | 465.6 | ||||
| Shape matching accuracy (%) | 99.4 | 1.4 | 93.8 | 1.4 | ||||
| Shape matching reaction time (msec) | 1,131.0 | 219.4 | 1,246.0 | 411.4 | ||||
p<0.05
p<0.001.
Depressed women, compared with healthy women, had significantly more depression and anxiety and lower levels of attachment to their infants in three factors assessed via questionnaire (Table 1). Mean HAM-D scores on the scan day indicated mild to moderate depression in the depressed group (mean score: 21.2 [SD=6.2]). Twelve depressed subjects experienced major depressive episode onset during late pregnancy (N=2) or within the first postpartum month (N=10). Two depressed mothers experienced depression onset within the year prior to delivery. For 11 depressed mothers, the index episode represented a recurrence of major depressive illness, with prior episodes occurring during nonpostpartum and postpartum periods in most depressed subjects (N=10). Five depressed mothers had comorbid psychiatric disorders, including panic disorder (N=1), social phobia (N=3), generalized anxiety (N=1), agoraphobia (N=1), and past substance/alcohol use disorders (N=2).
Behavioral Results
There were no significant group differences in accuracy and reaction times during performance of the task (Table 1). Mean accuracy was >97%, and mean reaction time was <1,210 msec within the full cohort.
Main Effects of Experimental Condition, Group, and Group-by-Condition Interaction on Neural Activity in Regions of Interest
In bilateral amygdala and dorsomedial prefrontal cortical regions of interest, there was the following significant main effect of experimental condition (faces versus shapes) (F≥7.75, df=1, 28, p<0.05, corrected [also see Figure 1 in the data supplement accompanying the online version of this article]): activity in response to faces was significantly greater than to shapes across all participants in the bilateral amygdala, consistent with previous studies (21), and significantly greater in response to shapes than to faces in the left dorsomedial prefrontal cortex (t≥3.47, df=29, p<0.05, corrected). There were no neural regions in which breast-feeding status was significantly associated with face-related activity in all participants (point biserial correlation=0.57, df=28, p>0.05). Therefore, we did not include breast-feeding as a covariate in subsequent analyses.
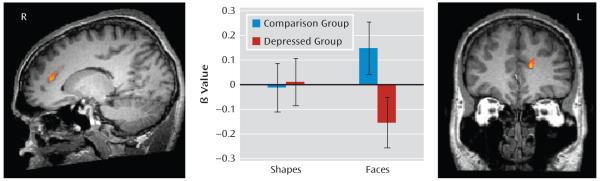
FIGURE 1. Group-by-Condition Interaction in the Dorsomedial Prefrontal Cortex Among Depressed and Healthy Mothersa.
a Error bars represent the standard deviation of the mean (F≥9.28, df=1, 28, p<0.05).
There was no main effect of group on neural activity in our regions of interest. There was a significant group-by-condition interaction (F≥9.28, df=1, 28, p<0.05, corrected) in the left dorsomedial prefrontal cortex. This interaction in the left dorsomedial prefrontal cortex was assessed further by extraction of beta values from this region and by independent t tests to compare groups, using a statistical threshold (p<0.0125 [0.05/4]) to control for the four possible pairwise post hoc tests (two groups-by-two conditions). The analysis revealed that healthy mothers showed significantly more activity than depressed mothers in response to face blocks (t≥4.51, df=28, p<0.005). Activity in response to shape blocks was minimal for both groups (Figure 1, Table 2) but was greater in depressed mothers relative to healthy mothers (t≥−4.51, df=28, p<0.005). Upon reduction of the voxel-wise threshold to p<0.05, the interaction effect within the dorsomedial prefrontal cortex remained left lateralized.
TABLE 2. Activated Regions of Interest in Healthy and Depressed Mothers.
| Right Amygdala |
Left Amygdala |
Dorsomedial Prefrontal Cortex |
|||||
|---|---|---|---|---|---|---|---|
| Variable | Peak Talairach Voxel (x,y,z) |
Voxels | Peak Talairach Voxel (x,y,z) |
Voxels | Peak Talairach Voxel (x,y,z) |
Voxels | Brodmann’s Area |
| Main effect condition | 18, −4, −11** | 806 | −20, −7, −11** | 611 | −9, 26, 30** | 9,758 | 10/32 |
| Group-by-condition interaction | −15, 31, 19** | 223 | 32 | ||||
| Depression severity correlation (Edin- burgh Postnatal Depression Scale)a |
−27, −10, −7* | 287 | |||||
| Absence of hostility (Parent-to-Infant Attachment Questionnaire) a |
21, −4, −11* | 63 | |||||
| Effective connectivity to the left amygdalab |
−6, 47, −2** | 97 | 32 | ||||
Data represent depressed group only.
Data represent healthy group only.
p<0.05
p<0.05 (corrected).
Exploratory Correlational Analyses
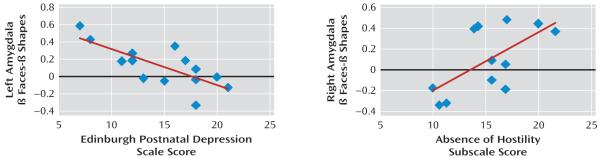
To examine the extent to which depression severity, mother-infant attachment, and time since childbirth were associated with face-related activity in depressed mothers, correlational analyses were computed between these clinical measures and activity in the dorsomedial prefrontal cortical region, where there was a significant group-by-condition interaction, and in bilateral amygdala regions of interest showing significantly greater activity in response to faces than to shapes, in all mothers, derived from our analysis of the main effect of condition (see the data supplement). There was no significant correlation between clinical variables and activity in the aforementioned dorsomedial prefrontal cortical region in either depressed or healthy mothers. For depressed mothers, Edinburgh Postnatal Depression Scale scores were negatively correlated with face-related left amygdala activity (r≥−0.81, df=12, p<0.05 [Figure 2, Table 2]). Subjects with greater depressive and anxiety symptoms had reduced left amygdala activity. There was a significant positive correlation between the absence of hostility subscale of the Parent-to-Infant Attachment Questionnaire and activity in the right amygdala (r≥0.58, df=10, p<0.05 [Figure 2, Table 2]). In the 12 depressed mothers who completed this questionnaire, less hostility toward the infant was associated with greater right amygdala activity. Neither right nor left face-related amygdala activity in depressed mothers was correlated with time since delivery (r=0.53, df=12, p>0.05).
FIGURE 2. Depression Severity and Attachment in Relation to Amygdala Activity in Depressed and Healthy Mothersa.
a Self-report of depression severity using the Edinburgh Postnatal Depression Scale (r≥−0.81, df=12, p<0.05) correlated negatively with face-related activity in the left amygdala, while attachment, using the absence of hostility subscale of the Parent-to-Infant Attachment Questionnaire, correlated positively with face-related activity (r≥0.58, df=10, p<0.05) in depressed mothers in the right amygdala.
Effective Connectivity Between the Amygdala and Dorsomedial Prefrontal Cortex
We used Granger causality mapping to determine the extent to which abnormally reduced left dorsomedial prefrontal cortical activity in response to faces may have impacted top-down (or preceding) effective connectivity between this region and the left amygdala region (seed region) that was activated in response to faces more than to shapes across all subjects (derived from our main effect of condition [also see the data supplement]) and in which face-related activity was negatively correlated with depression severity in depressed mothers. Granger causality theory states that a discrete time series X causes a discrete time series Y if the past values of X improve the prediction of the current value of Y, given that all other sources of influence have been taken into account (35). Thus, temporal information from the data is used to define direction of influence, at the whole-brain level, without establishing a model of assumed regional connectivity. Our Granger causality analysis was conducted at the group level to generate an individual t statistic image of the Granger causality map for both healthy and depressed mothers. For each Granger map, p values were subjected to a multiple comparison correction (false discovery rate [q] <0.05) (36) at the whole-brain level. This procedure deals with the problem of multiple comparisons by automatically identifying a threshold for statistical significance that ensures that, on average, the proportion of false positives among the activated voxels will be less than q.
In healthy mothers, there was significant preceding connectivity from the left dorsomedial prefrontal cortex to the left amygdala reference region, indicating dominant influence of this region on the left amygdala in response to faces (t≥1.26, df=29, q<0.05 [Figure 3, Table 2]). The left dorsomedial prefrontal cortex was the only area of interest of this whole brain analysis in which we observed significant connectivity to the left amygdala. In contrast, no connectivity was observed between the left amygdala and any neural region of interest in depressed mothers.
FIGURE 3. Effective Connectivity to the Left Amygdalaa.
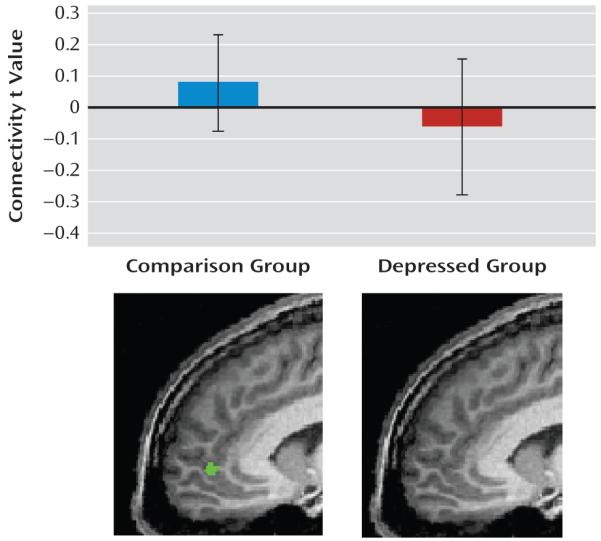
a Dorsomedial prefrontal cortex activity preceded left amygdala activity in healthy mothers, but no significant connectivity to the left amygdala in the depressed group was observed. Error bars represent the standard deviation of the mean.
Discussion
We measured neural activity during negative emotional face versus shape matching in unmedicated depressed and healthy mothers to explore mechanisms of postpartum depression. There was a main effect of task condition such that faces were associated with increased amygdala activity and shapes were associated with increased dorsomedial prefrontal cortex activity in all women.
Our first novel finding was that negative emotional faces activated the left dorsomedial prefrontal cortex over a large region in Brodmann’s area 32 significantly less in depressed mothers than in healthy mothers. While activity in this region did not correlate with depressive severity or maternal attachment, relationships between symptom severity and activity in this region may have been masked by a potential floor effect of postpartum depression on face-related dorsomedial prefrontal cortex activity. The dorsomedial prefrontal cortex is involved in voluntary and automatic control and reappraisal of emotional responses (37) and also in the social cognition network that allows an individual to recognize and consider the emotional experiences, values, and goals of others. Deficits in dorsomedial prefrontal cortex activity in response to negative faces in depressed mothers, therefore, might represent diminished awareness of and empathic responses to emotions of others, as has been reported in individuals with postpartum and nonpostpartum depression (37).
Our second novel finding was that preceding (top-down) connectivity between the left dorsomedial prefrontal cortex and left amygdala during negative emotional faces was present only in healthy, and not depressed, mothers. The presence of strong dorsomedial prefrontal cortex-amygdala effective connectivity in healthy mothers might represent a neural circuitry that supports social, empathic processes for regulation of amygdala activity in response to negative emotional stimuli. Engagement of this circuitry would be highly adaptive for generating optimal responses to environmental or infant challenges for new mothers. The absence of dorsomedial prefrontal cortex-amygdala connectivity during processing of negative emotional faces in depressed mothers, combined with reduced activity in the dorsomedial prefrontal cortex in response to these faces, suggests a disengagement of a critical prefrontal cortico-limbic circuitry for effective automatic emotional appraisal and voluntary regulation of emotional arousal in postpartum depression (38).
Our findings refuted our hypothesis, and contrasted with preliminary reports (20), that depressed mothers would show abnormal activity in the amygdala in response to negative emotional stimuli. While some studies reported greater (22, 23) and more sustained (39) amygdala activity in response to negative emotional stimuli in nonpostpartum depressed adults relative to healthy adults, this finding has not been universal (40, 41). The absence of amygdala hyperactivity in response to faces in depressed mothers relative to healthy mothers could also be explained by diminished hypothalamic-pituitary-adrenal axis drive following childbirth in women with postpartum “blues” and depression (7). Alternatively, the absence of elevated left amygdala activity in response to these cues in depressed mothers, which may have been predicted to result from the diminished top-down regulation of the left amygdala by the left dorsomedial prefrontal cortex, might have resulted from our finding that greater postpartum depression severity was in fact associated with reduced, not greater, left amygdala activity in response to negative emotional faces in depressed mothers. The impact of diminished top-down regulation of the left amygdala by the left dorsomedial prefrontal cortex may therefore have been lessened by this second finding of a negative relationship between postpartum depression severity and left amygdala activity in response to negative emotional faces in the more depressed mothers. It is also possible that our sample may have been too small to detect a significant between-group difference in amygdala activity, although we were able to detect a group-by-condition interaction in the dorsomedial prefrontal cortex.
Previous theories link approach and withdrawal-related emotion processing with left and right hemispheres, respectively (42). In our findings, left lateralization remained significant even after a reduction of the voxel-wise error rate to a liberal p value <0.05. It is therefore possible that our predominantly left-sided findings regarding dorsomedial prefrontal cortical activity, dorsomedial prefrontal cortical-amygdala connectivity, and relationships between symptom severity and amygdala activity in depressed mothers may reflect dysfunctional processing particular of approach-related emotional cues. While our study was not powered to formally test the laterality effect in our regions of interest, further studies could examine this effect.
This is the first study, to our knowledge, to examine negative face processing in postpartum depressed and healthy mothers. While a limitation was the relatively modest sample of participants, one strength of the study was the inclusion of unmedicated, largely antidepressant-naive, depressed mothers. Additionally, participant groups were well-matched for demographic, medical, and obstetric factors as well as behavioral performance. Future study of a larger sample will allow examination of the contributing roles of psychiatric comorbidity in postpartum depressed mothers, breast-feeding status, timing of depression onset, and parity to create an integrative model of risk for the neural circuitry deficits of interest.
Our finding that greater infant-related hostility was associated with reduced face-related right amygdala activity suggests that this might be a neural substrate for the reduced attunement and empathic responses reported among depressed mothers. It will be important to build upon our present findings with concurrent behavioral assessments of the mother-infant relationship to clarify neural mechanisms of mother-infant attachment.
In conclusion, diminished dorsomedial prefrontal cortical activity, diminished dorsomedial prefrontal cortical-amygdala effective connectivity, and a negative relationship of depression severity to amygdala activity may be important mechanisms or effects of postpartum depression. These preliminary findings, if replicated, may be useful biological targets that can be used to guide the development of more effective treatments for postpartum depression.
Acknowledgments
Dr. Wisner has received grant support from Nova-Gyne (donation of transdermal placebo patches for a National Institute of Mental Health-funded study of estradiol patch for postpartum depression treatment), the State of Pennsylvania, the Heinz Foundation, the Staunton Farm Foundation, the Fine Foundation, and the National Institute of Mental Health. Dr. Phillips receives grant support from the National Institute of Mental Health, the United States Army Research Office, and the Pennsylvania Department of Health. Drs. Moses-Kolko and Perlman and Mr. James and Ms. Saul report no financial relationships with commercial interests.
Supported by the National Institute of Mental Health grant R01 MH-079164 (Dr. Moses-Kolko, Dr. Wisner, Mr. James, Ms. Saul) and a NARSAD Young Investigator Award (Dr. Moses-Kolko); the University of Pittsburgh T32 grant MH-18951 (Dr. Perlman); and the National Institute of Mental Health grant MH-076971 (Dr. Phillips).
The authors thank all of the research participants and their families for their time dedicated to this study. The authors also thank the University of Pittsburgh Magnetic Resonance Research Center staff for performing the scan acquisition as well as Medela, Inc., for donation of a multi-use breast pump, and the University of Pittsburgh School of Medicine Clinical and Translational Science Institute for assistance with recruitment.
Footnotes
Previously presented as a poster at the Annual Meeting of the Society for Biological Psychiatry, Vancouver, British Columbia, May 14–16, 2009.
References
- 1.Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess. 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12:12–20. doi: 10.1016/s0883-9417(98)80004-6. [DOI] [PubMed] [Google Scholar]
- 3.Cindy-Lee D, Leinic C-L. Postpartum depression help-seeking barriers and maternal treatment preferences: a qualitative systematic review. Birth. 2006;33:323–331. doi: 10.1111/j.1523-536X.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- 4.Wisner KL, Hanusa BH, Perel JM, Peindl KS, Piontek CM, Sit DKY, Findling RL, Moses-Kolko EL. Postpartum depression: a randomized trial of sertraline vs nortriptyline. J Clin Psychopharmacol. 2006;26:353–360. doi: 10.1097/01.jcp.0000227706.56870.dd. [DOI] [PubMed] [Google Scholar]
- 5.O’Hara MW, Stuart S, Gorman LL, Wenzel A. Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry. 2000;57:1039–1045. doi: 10.1001/archpsyc.57.11.1039. [DOI] [PubMed] [Google Scholar]
- 6.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 7.Magiakou MA, Mastorakos G, Rabin D, Dubbert B, Gold PW, Chrousos GP. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab. 1996;81:1912–1917. doi: 10.1210/jcem.81.5.8626857. [DOI] [PubMed] [Google Scholar]
- 8.Yim IS, Glynn LM, Dunkel Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66:162–169. doi: 10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rich-Edwards JW, Mohllajee AP, Kleinman K, Hacker MR, Majzoub J, Wright RJ, Gillman MW. Elevated midpregnancy corticotropin-releasing hormone is associated with prenatal, but not postpartum, maternal depression. J Clin Endocrinol Metab. 2008;93:1946–1951. doi: 10.1210/jc.2007-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moses-Kolko EL, Berga SL, Kalro B, Sit DK, Wisner KL. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52:516–529. doi: 10.1097/GRF.0b013e3181b5a395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- 13.Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother’s response to infant’s attachment behaviors. Biol Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby-cry is affected by cesarean section delivery. J Child Psychol Psychiatry. 2008;49:1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hammer MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- 16.Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- 17.Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in response to pictures of their children and other children. Biol Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, Ammaniti M. Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cereb Cortex. 2009;19:1124–1133. doi: 10.1093/cercor/bhn153. [DOI] [PubMed] [Google Scholar]
- 19.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 20.Silverman ME, Loudon H, Safier M, Protopopescu X, Leiter G, Liu X, Goldstein M. Neural dysfunction in postpartum depression: an fMRI pilot study. CNS Spectr. 2007;12:853–862. doi: 10.1017/s1092852900015595. [DOI] [PubMed] [Google Scholar]
- 21.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 22.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 23.Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 24.Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition. New York State Psychiatric Institute, Biometrics Research Department; New York: 1998. [Google Scholar]
- 26.Stowe ZN, Hostetter AL, Newport DJ. The onset of postpartum depression: implications for clinical screening in obstetrical and primary care. Am J Obstet Gynecol. 2005;192:522–526. doi: 10.1016/j.ajog.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 27.Cox J, Holden J, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 28.Condon JT, Corkindale CJ. The assessment of parent-to-infant attachment: development of a self-report questionnaire instrument. J Reprod Infant Psychol. 1998;16:57–76. [Google Scholar]
- 29.Ekman P, Friesen WV. Unmasking the Face: A Guide to Recognizing Emotions From Facial Clues. Prentice Hall; Oxford, England: 1975. [Google Scholar]
- 30.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme Medical; New York: 1988. [Google Scholar]
- 31.Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:189–210. [Google Scholar]
- 32.Altemus M, Deuster PA, Galliven E, Carter CS, Gold PW. Suppression of hypothalamic-pituitary-adrenal axis responses to stress in lactating women. J Clin Endocrinol Metab. 1995;80:2954–2959. doi: 10.1210/jcem.80.10.7559880. [DOI] [PubMed] [Google Scholar]
- 33.Neumann I, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behavior in virgin, pregnant and lactating rats. Neuroscience. 2000;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- 34.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 35.Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage. 2005;25:230–242. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 37.Donges US, Kersting A, Dannlowski U, Lalee-Mentzel J, Arolt V, Suslow T. Reduced awareness of others’ emotions in unipolar depressed patients. J Nerv Ment Dis. 2005;193:331–337. doi: 10.1097/01.nmd.0000161683.02482.19. [DOI] [PubMed] [Google Scholar]
- 38.Phillips ML, Ladoucer CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 40.Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 42.Davidson RJ. What does the prefrontal cortex “do” in affect? Perspectives on frontal EEG asymmetry research. Biol Psychol. 2004;67:219–234. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]