Abstract
Deposition of the Aβ peptide in senile plaques and cerebral Aβ angiopathy can be stimulated in Aβ-precursor protein-transgenic mice by the intracerebral injection of dilute brain extracts containing aggregated Aβ seeds. Growing evidence implicates a prion-like mechanism of corruptive protein templating in this phenomenon, in which aggregated Aβ itself is the seed. Unlike prion disease, which can be induced de novo in animals that are unlikely to spontaneously develop the disease, previous experiments with Aβ seeding have employed animal models that, as they age, eventually will generate Aβ lesions in the absence of seeding. In the present study, we first established that a transgenic rat model expressing human Aβ-precursor protein (APP21 line) does not manifest endogenous deposits of Aβ within the course of its median lifespan (30 months). Next, we injected 3-month-old APP21 rats intrahippocampally with dilute Alzheimer brain extracts containing aggregated Aβ. After a 9-month incubation period, these rats had developed senile plaques and cerebral Aβ angiopathy in the injected hippocampus, whereas control rats remained free of such lesions. These findings underscore the co-dependence of agent and host in governing seeded protein aggregation, and show that cerebral Aβ-amyloidosis can be induced even in animals that are relatively refractory to the spontaneous origination of parenchymal and vascular deposits of Aβ.
Keywords: Alzheimer, amyloid, prion, proteopathy, senile plaques, transgenic rat
A pivotal occurrence in the development of Alzheimer’s disease (AD) is the self-assembly and accumulation of the β-amyloid (Aβ) peptide in the brain (Hardy & Selkoe 2002, Holtzman et al. 2011). In Aβ-precursor protein- (APP) transgenic mice, cerebral Aβ deposition can be stimulated by a single intracerebral injection of dilute brain extract containing aggregated Aβ (Kane et al. 2000, Meyer-Luehmann et al. 2006, Eisele et al. 2009, Eisele et al. 2010, Watts et al. 2011). Mechanistically, this induction, or seeding, of Aβ-deposition resembles the transmission of prion disease (Sigurdsson et al. 2002, Walker et al. 2006a, Soto et al. 2006, Walker et al. 2002, Walker et al. 2006b, Jucker & Walker in press, Langer et al. in press) in that a misfolded, aggregated form of Aβ appears to be the seeding agent (Meyer-Luehmann et al. 2006, Jucker & Walker in press). Aβ deposition also has been shown to be seeded in wild-type marmosets (Baker et al. 1994, Ridley et al. 2006), a New World monkey that, like all primates that have been studied to date, naturally generates human-sequence Aβ (Heuer et al. in press).
The characteristics of both the seeding agent and the host cortical milieu influence the pathologic signature of exogenous Aβ seeds (Meyer-Luehmann et al. 2006). Wild-type mice and rats, in which Aβ differs from that in humans by three amino acids (Otvos Jr et al. 1993), do not naturally generate senile (Aβ) plaques or cerebral Aβ-angiopathy (CAA), nor do they demonstrate seeded Aβ deposition (Kane et al. 2000, Meyer-Luehmann et al. 2006). The experimental animals successfully used for Aβ-seeding studies thus far – APP-transgenic mice and marmosets – do express human-sequence Aβ, and, with age, all of them eventually will develop Aβ-plaques and/or CAA in the absence of seeding. In contrast, prion disease can be transmitted to animals that are otherwise unlikely to manifest disease within their lifetimes. Thus, it is possible that prion disease and Aβ-amyloidosis differ in that prion disease can be induced in normally refractory hosts, whereas the seeded induction of Aβ aggregation simply involves the acceleration of a process that will eventually become manifest, in the absence of seeding, as the host animals age. In this study, we sought to determine whether Aβ deposition can be induced in a host that is relatively resistant to the endogenous generation of plaques and CAA. To achieve this, we chose an APP-transgenic rat model (APP21; (Agca et al. 2008)) that expresses human Aβ but does not generate endogenous Aβ lesions during the course of its median lifespan. We show that the intracerebral delivery of dilute cortical extracts from AD patients to 3-month old APP21 rats stimulates the formation of Aβ-plaques and CAA by 12 months of age.
Material and methods
Subjects
The APP21 transgenic rat line was produced on the inbred Fischer-344 strain (Agca et al. 2008). The human APP transgene includes the ‘Swedish’ (Swe) double mutation (K670N-M671L) along with the ‘Indiana’ (Ind) single AD mutation (V642F). The transgene is driven by the ubiquitin-C promoter, and the overall expression pattern of transgenic APP is similar to that of endogenous rat APP. Homozygous APP21 rats express ~2.9-fold more APP mRNA than do wild-type rats, and transgenic (human-sequence) Aβ is readily detectable in brain and in serum (Agca et al. 2008). To determine whether normal APP21 rats develop Aβ deposits with age, we analyzed homozygous APP21 rats (males and females) at 1, 3, 6, 12, 18, 24 and 30 months of age. 18-24 rats per group were studied in the groups ranging in age from 1-18 months, but due to age-associated attrition, only 10 rats were available for analysis at 24 months, and 8 rats at 30 months of age.
For the Aβ-seeding studies, eleven male, homozygous APP21-transgenic rats and 5 male, non-transgenic Fischer-344 control rats were studied (Table 1). Only male rats were used in the seeding studies to reduce variability caused by potential gender-related differences in Aβ deposition (Callahanet al. 2001). All rats were maintained under specific pathogen-free conditions, and the research protocols were approved by the institutional animal care and use committee.
Table 1.
Injectates and incubation times
| Incubation Time (months) | |||
|---|---|---|---|
| Injectate | 3 | 6 | 9 |
| AD | APP21 (n=3) | APP21 (n=2) | APP21 (n=4) Non-tg (n=5) |
|
Non-AD
Control |
APP21 (n=2) | ||
Preparation of donor brain tissue seeding extracts
Neocortical tissue samples were obtained at autopsy from clinically and histopathologically confirmed AD cases, as well as an aged, non-demented patient whose brain was free of AD lesions. The samples were immediately frozen at −80°C until use. The tissue extracts were prepared as previously described (Kane et al. 2000, Meyer-Luehmann et al. 2006). Briefly, the cortical blocks were homogenized at 10% or 20% (w/v) in PBS, vortexed, probe-sonicated 3 × 5 s with a Fisher Sonic Dismembrator 100 (setting 5) and centrifuged at 3000×G for 5 min. The clear supernatant was collected, divided into aliquots, and frozen. Total Aβ levels in the injectates were estimated by immunoassay to be ~0.5-2 ng/μl.
Stereotaxic injection of brain extracts
The rats were anesthetized with a mixture of ketamine (80mg/kg) and xylazine (8mg/kg) and maintained with 55mg/kg ketamine as needed. Seven APP21 rats and the five non-transgenic control rats were injected bilaterally with 5μl of clear, 10% AD extract into the dorsal hippocampus (interaural +5.86mm; lateral ±1.8mm; ventral 3.4mm (Paxinos & Watson 1998). Injections were made with a Hamilton syringe at the rate of 2.5μl/minute, and the syringe was left in place for an additional two minutes before slow withdrawal. In two additional transgenic rats, a 20% AD cortical extract (5μl) was similarly injected. As further controls, one rat received 5μl of 10% extract and one received 5μl of 20% extract from the brain of a 75 year-old nondemented control patient (histopathologically confirmed non-AD).
Tissue processing and analysis
Unseeded APP21 rats
The unseeded APP21 rats were transcardially perfused with PBS under deep sodium pentobarbital anesthesia (200mg/kg). One hemisphere for immunohistochemistry then was immersion-fixed in phosphate-buffered 4% paraformaldehyde for 4 hours, cryoprotected in 30% sucrose, frozen, and stored at −80°C until analysis. The other hemisphere was frozen (unfixed) with dry ice and stored at −80°C for analysis by immunoblot. In the 24- and 30-month old rats, only immunohistochemical analysis was performed on whole fixed brains due to age-related attrition of subjects at these ages.
Immunoblotting
Unfixed hemispheres from a subset of unseeded rats at 3, 9 and 18 months of age were analyzed by immunoblotting as previously described (Rosen et al. 2010), Briefly, the samples were homogenized in nine volumes of PBS, vortexed, probe-sonicated (10 × 0.5 sec), centrifuged at 5,000xg for 10 min at 4°C, and supernatants were stored at −80°C until use. Total protein in the samples was quantified with the bicinchoninic acid (BCA) assay. 76μg of total protein per sample were run on a 10-20% Tricine gel (Invitrogen) and blotted onto a nitrocellulose membrane. The membrane was boiled for 5 min in 1xPBS and probed with antibody 6E10 to Aβ (1:500). The secondary antibody was horseradish peroxidase-conjugated anti-mouse IgG (1:10,000). For signal detection, Super Signal West Pico electrochemiluminescence (Fisher Scientific) was used, after which the membrane was exposed to Kodak MR Biomax film (Kodak, New Haven, CT, USA).
Extract-injected rats
To optimize the sensitivity of detection, and to enable the localization and characterization of seeded Aβ deposits, whole brains from all cortical extract-injected rats were analyzed by immunohistochemistry. The rats were sacrificed under deep sodium pentobarbital anesthesia (200mg/kg) by transcardial perfusion with PBS (pH 7.4) followed by phosphate-buffered 4% paraformaldehyde. The brains were removed, post-fixed for 24-48 hours in phosphate-buffered 4% paraformaldehyde (4°C), cryoprotected in phosphate-buffered 30% sucrose, blocked, frozen on dry ice, and then stored at −80°C until processing.
Histochemistry
For histochemical analysis, brains were cut on a cryostat at 40μm thickness, and sections were stained with antibodies 6E10 (1:15,000; [Covance, Princeton, NJ]); 4G8 (1:10,000; mouse IgG2b monoclonal antibody [Covance] raised against residues 17-24 of Aβ); and rabbit polyclonal antibodies R361 and R398 (both at 1:15,000; [courtesy of Dr. Pankaj Mehta, Institute for Basic Research on Developmental Disabilities, Staten Island, NY] raised against synthetic Aβ32-40 and Aβ33-42, respectively)(see Rosen et al., 2008 for antibody details). β-amyloid load (percent area of the hippocampus occupied by Aβ-immunoreactive deposits) was determined on immunostained sections using point-counting methods (Mouton 2011). Additional sections were stained with thioflavin-S, and some were lightly counterstained with hematoxylin. Sections were photographed with a Spot Flex digital camera (Diagnostic Instruments, Sterling Heights, MI, USA) attached to a Leica DMLB microscope.
Statistical analysis
Values are expressed as means ± SEM. Because Aβ deposits were not found in normal, unseeded APP21 rats or in extract-injected non-transgenic rats (below), group differences in the induction of Aβ deposits were assessed using the nonparametric Fisher’s Exact Test (Graphpad Software). The significance threshold was set at p ≤ 0.05, two-tailed.
Results
Normal (unseeded) APP21 rats do not spontaneously generate cerebral Aβ deposits
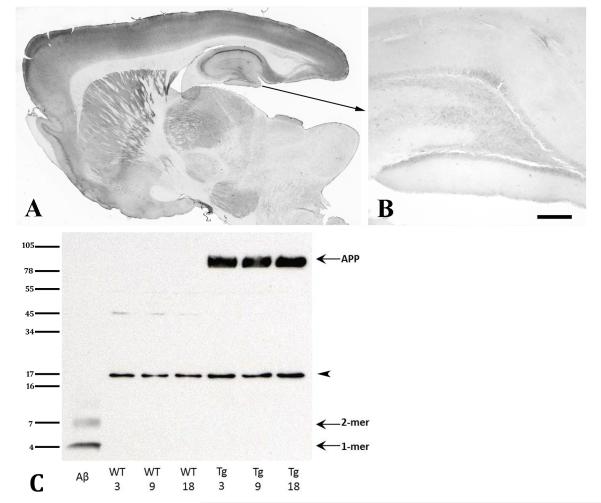
The median life-span of ad lib-fed Fischer-344 rats is slightly under 30 months (Ghirardi et al. 1995). To determine whether APP21 rats spontaneously generate Aβ plaques or CAA within this period, we analyzed unseeded, homozygous APP21 rats at 1, 3, 6, 12, 18, 24 and 30 months of age. Antibody 6E10 (which is highly selective for human-sequence Aβ) recognized normal-appearing intracellular human Aβ/APP in the transgenic rats at all ages, but none of the unseeded APP21 rats developed extracellular Aβ plaques or CAA at any age (Figure 1). Immunoblot analysis of fresh-frozen cortical samples from a subset of APP21 and wild-type rats at 3, 9 and 18 months of age detected strong bands corresponding to APP (~100 KDa) solely in APP21 rats (Figure 1C). Aβ was below the level of detection at these ages.
Fig. 1.
Analysis of senile plaques, CAA and APP in unseeded APP21 rats. A: Low magnification overview of a parasagittal section from an unseeded 30-month-old APP21 rat; B: Higher magnification of the hippocampal formation and dentate gyrus. Antibody 6E10 detected light immunoreactivity of transgenic (human-sequence) Aβ/APP in neuronal somata throughout the brain, but no extracellular deposition of Aβ was seen in any unseeded APP21 rat. Bar in panel B = 200μm. C: Western blot of cortical homogenates from APP21 and wild-type control rats at three different ages, immunostained with mouse monoclonal antibody 6E10. A preparation of aggregated, synthetic Aβ42 (10ng) is in the far left lane as a positive control. In the transgenic rats only, 6E10-immunoreactive bands corresponding to APP (~100kDa) were detected in similar quantities at all 3 ages. Aβ in all rats was below detection level. The bands at ~17kDa (arrowhead) are nonspecific cross-reactive material.
Aβ deposition can be exogenously seeded in APP21 rats
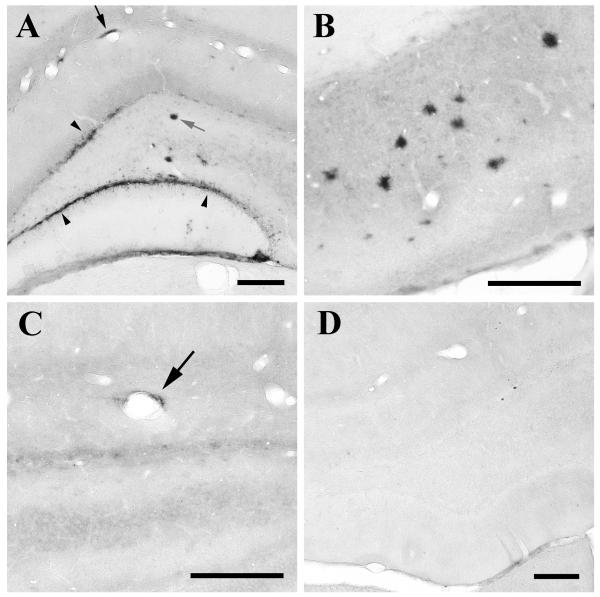
Nine months following the intrahippocampal infusion of AD cortical extracts into three month-old APP21 rats, all animals (n=4) showed seeded induction of Aβ deposition in the hippocampal formation (Figure 2A,B), whereas the AD extract-injected non-transgenic rats (n=5) were devoid of Aβ deposition (Figure 2D) (p=0.008, Fisher’s Exact Test). The seeded Aβ deposits were strongly immunoreactive with antibodies 6E10, 4G8, and R398, but they were negative or only weakly stained with antibody R361 (to Aβ40) and with thioflavin-S, indicating that the seeded deposits consisted primarily of diffuse aggregates of Aβ42. Three-month-old transgenic APP21 rats injected with cortical extract from a control (non-AD) case (n=2) were negative after a 9-month incubation period.
Figure 2.
Seeded deposition of Aβ in the hippocampus proper and dentate gyrus (A), and the subiculum (B) of a representative, 12-month old APP21 transgenic rat that had received an intracerebral infusion of dilute AD cortical extract at 3 months of age. Diffuse Aβ deposits occurred in the walls of blood vessels (black arrow), as sheet-like formations (arrowheads mark Aβ immunoreactivity in the granule cell layer), and as parenchymal, plaque-like deposits (gray arrow, middle). A mean of 2.3 ± 0.8% of the area of the dorsal hippocampus was occupied by Aβ deposits in the 9-month seeded rats. C: Light, perivascular Aβ deposition (arrow) in the hippocampal formation of an APP21 transgenic rat 3 months following infusion of cortical extract. Non-transgenic rats similarly injected (D) did not have immunoreactive Aβ in the hippocampus after 9 months. Antibodies 4G8 (A) and 6E10 (B-D); Bars = 200 μm.
Five additional APP21 rats (again 3 months old) were injected with AD brain extract and allowed to incubate for 3 months or 6 months. Two animals developed very light Aβ-immunoreactivity in the immediate vicinity of the injection site, one in the 6 month group and one in the 3 month group (Figure 2C). The other 3 rats assessed at these timepoints were negative.
Discussion
Previous studies with APP-transgenic mice have shown that Aβ deposition can be stimulated by the introduction of dilute brain extracts containing aggregated Aβ (Kane et al. 2000, Meyer-Luehmann et al. 2006, Eisele et al. 2009, Watts et al. 2011, Langer et al. in press). However, the host models used in these studies eventually will develop Aβ-plaques and CAA with age, so it is uncertain whether animals that are less likely to generate such lesions with age also are susceptible to seeded Aβ deposition. In the present study, we report that senile plaques and cerebral Aβ angiopathy can be induced in an APP-transgenic rat model that does not spontaneously form such deposits in the brain during the course of its median lifespan. While we cannot exclude the possibility that APP21 rats that survive into extreme old age (>30 months) would eventually manifest Aβ deposition, our findings indicate that protein aggregation can be exogenously precipitated in an animal model that is relatively resistant to the endogenous generation of Aβ lesions. This conclusion is strengthened by a recent report that Aβ deposition can be induced de novo in transgenic mice expressing wild-type human APP (Morales et al. in press).
Our findings reinforce the importance of host factors in governing the response of the brain to exogenous, Aβ-rich brain extracts. In APP-transgenic mice, the type of host has been shown to influence both the phenotype of the seeded lesions and the timecourse of lesion formation (Meyer-Luehmann et al. 2006). In general, transgenic mice that display a relatively aggressive emergence of endogenous plaques and/or CAA also respond more rapidly to the exogenous introduction of corruptive seeds (Meyer-Luehmann et al. 2006). In the APP21 transgenic rats that we investigated, substantial Aβ deposition was only apparent after 9 months of incubation, with little seeded deposition at 3 or 6 months post-injection. The importance of the host in modulating the timecourse of seeding is underscored by studies of marmosets (Baker et al. 1994, Maclean et al. 2000, Ridley et al. 2006). In the colony of marmosets investigated by this group, endogenous Aβ amyloidosis begins to materialize after 11 years of age (Ridley et al. 2006), and the seeded augmentation of Aβ load only is apparent after an incubation period of 5-6 years (Baker et al. 1994). Taken together, these studies in nonhuman primates and transgenic rodents indicate that Aβ deposition can be exogenously induced in animals that generate human-sequence Aβ, but that the lag time preceding the emergence of the lesions is proportional to the timecourse of endogenous Aβ deposition in the host.
Though they are incomplete models of human disease, transgenic mice expressing disease-related proteins have energized the experimental investigation of AD and other neurodegenerative disorders (Jucker 2010, Ashe & Zahs 2010, Wisniewski & Sigurdsson 2010, Harvey et al. 2011). In mice, the expression of transgenic, usually mutant, human APP typically results in the predictable development of senile plaques and/or CAA by a model-specific age (LeVine III & Walker 2006, Morrissette et al. 2009, Jucker 2010). The rate and degree of endogenous β-amyloid formation varies considerably among models, however, and is influenced by such factors as genetic background, gender, APP expression levels, the presence and type of pathogenic mutations, and the co-expression of other transgenes such as an AD-mutant form of presenilin-1, a protein that enhances the overproduction of Aβ peptides from full length APP (Hock et al. 2009, Jucker 2010). Transgenic rat models of AD pathology still are relatively uncommon, but they have certain advantages over mice owing to their larger size, unique genetics, and well-studied behavioral characteristics (Tesson et al. 2005). Compared to APP-transgenic mice, many APP-transgenic rats have not readily developed endogenous cerebral β-amyloidosis (Echeverria et al. 2004, Ruiz-Opazo et al. 2004, Folkesson et al. 2007, Clarke et al. 2007), although one APPSwe/Ind transgenic rat develops plaques beginning around 6 months of age (Leon et al. 2010), and, as in mice, the co-expression of presenilin-1 along with APP can stimulate the robust deposition of Aβ (Flood et al. 2007, Liu et al. 2008). The exogenous seeding paradigm might be employed to stimulate lesion formation in these resistant models, to synchronize the onset and progression of protein aggregation, and to evaluate the effects of seeding on neuronal integrity and behavior.
In summary, we show that Aβ deposition can be exogenously seeded in an APP-transgenic rat that is refractory to endogenous Aβ deposition during the course of its median lifespan. These findings in a new model and species support growing evidence that Aβ aggregation can be induced in the brain by a process of corruptive protein templating (Jucker & Walker in press), a molecular mechanism that may underlie the pathogenesis of numerous neurodegenerative diseases (Sigurdsson et al. 2002, Walker et al. 2002, Walker et al. 2006b, Soto et al. 2006, Jucker & Walker in press). The results also confirm that the expression of human-sequence Aβ by the host is necessary for seeding by Aβ-rich brain extracts, but also that other, as yet unidentified, host factors govern the lag phase preceding the appearance of senile plaques and CAA. At present, there is no evidence that AD per se is transmissible in the same manner as is prion disease. However, a more complete understanding of the determinants of protein seeding and accumulation in different hosts could inform therapeutic strategies to interrupt the proteopathic cascade in human neurodegenerative diseases.
Acknowledgments
This work was supported by NIH P51RR-000165, P50AG025688, University of Kentucky Faculty Support Grant 1012101660, the CART Foundation, the Competence Network on Degenerative Dementias (BMBF-01GI0705) and the BMBF in the frame of ERA-Net NEURON (MIPROTRAN). We gratefully acknowledge helpful discussions with Dr. Marla Gearing (Emory University).
Abbreviations used
- Aβ
amyloid-β (β-amyloid)
- AD
Alzheimer’s disease
- APP
Aβ-precursor protein
- BCA
bicinchoninic acid
- CAA
cerebral amyloid-β angiopathy
- IgG
immunoglobulin G
- kDa
kilodalton(s)
- Ind
‘Indiana’ AD mutation
- PBS
phosphate-buffered saline
- SEM
standard error of the mean
- Swe
‘Swedish’ AD double mutation
References
- Agca C, Fritz JJ, Walker LC, Levey AI, Chan AW, Lah JJ, Agca Y. Development of transgenic rats producing human beta-amyloid precursor protein as a model for Alzheimer’s disease: transgene and endogenous APP genes are regulated tissue-specifically. BMC Neurosci. 2008;9:28. doi: 10.1186/1471-2202-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe KH, Zahs KR. Probing the biology of Alzheimer’s disease in mice. Neuron. 2010;66:631–645. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Induction of beta (A4)-amyloid in primates by injection of Alzheimer’s disease brain homogenate. Comparison with transmission of spongiform encephalopathy. Molecular Neurobiology. 1994;8:25–39. doi: 10.1007/BF02778005. [DOI] [PubMed] [Google Scholar]
- Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC. Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am J Pathol. 2001;158:1173–1177. doi: 10.1016/s0002-9440(10)64064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J, Thornell A, Corbett D, Soininen H, Hiltunen M, Jolkkonen J. Overexpression of APP provides neuroprotection in the absence of functional benefit following middle cerebral artery occlusion in rats. Eur J Neurosci. 2007;26:1845–1852. doi: 10.1111/j.1460-9568.2007.05807.x. [DOI] [PubMed] [Google Scholar]
- Echeverria V, Ducatenzeiler A, Alhonen L, et al. Rat transgenic models with a phenotype of intracellular Abeta accumulation in hippocampus and cortex. J Alzheimers Dis. 2004;6:209–219. doi: 10.3233/jad-2004-6301. [DOI] [PubMed] [Google Scholar]
- Eisele YS, Bolmont T, Heikenwalder M, et al. Induction of cerebral beta-amyloidosis: intracerebral versus systemic Abeta inoculation. Proc Natl Acad Sci U S A. 2009;106:12926–12931. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Obermuller U, Heilbronner G, et al. Peripherally Applied A{beta}-Containing Inoculates Induce Cerebral {beta}-Amyloidosis. Science. 2010 doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood DG, Lin YG, Lang DM, Trusko SP, Hirsch JD, Savage MJ, Scott RW, Howland DS. A transgenic rat model of Alzheimer’s disease with extracellular Abeta deposition. Neurobiology of Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Folkesson R, Malkiewicz K, Kloskowska E, et al. A transgenic rat expressing human APP with the Swedish Alzheimer’s disease mutation. Biochemical and Biophysical Research Communications. 2007;358:777–782. doi: 10.1016/j.bbrc.2007.04.195. [DOI] [PubMed] [Google Scholar]
- Ghirardi O, Cozzolino R, Guaraldi D, Giuliani A. Within- and between-strain variability in longevity of inbred and outbred rats under the same environmental conditions. Exp Gerontol. 1995;30:485–494. doi: 10.1016/0531-5565(95)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harvey BK, Richie CT, Hoffer BJ, Airavaara M. Transgenic animal models of neurodegeneration based on human genetic studies. J Neural Transm. 2011;118:27–45. doi: 10.1007/s00702-010-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer E, Rosen RF, Cintron A, Walker LC. Nonhuman primate models of Alzheimer-like cerebral proteopathy. Current Pharmaceutical Design. doi: 10.2174/138161212799315885. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock BJ, Lattal KM, Kulnane LS, Abel T, Lamb BT. Pathology associated memory deficits in Swedish mutant genome-based amyloid precursor protein transgenic mice. Curr Aging Sci. 2009;2:205–213. doi: 10.2174/1874609810902030205. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr71. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat Med. 2010;16:1210–1214. doi: 10.1038/nm.2224. [DOI] [PubMed] [Google Scholar]
- Jucker M, Walker LC. Pathogenic Protein Seeding in Alzheimeŕs Disease and Other Neurodegenerative Disorders. Annals of Neurology. doi: 10.1002/ana.22615. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, Schwarz RD, Roher AE, Walker LC. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J Neurosci. 2000;20:3606–3611. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer F, Eisele YS, Fritschi SK, Staufenbiel M, Walker LC, Jucker M. Soluble Aβ seeds are potent inducers of cerebral β-amyloid deposition. J Neurosci. doi: 10.1523/JNEUROSCI.3088-11.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon WC, Canneva F, Partridge V, et al. A novel transgenic rat model with a full Alzheimer’s-like amyloid pathology displays pre-plaque intracellular amyloid-beta-associated cognitive impairment. J Alzheimers Dis. 2010;20:113–126. doi: 10.3233/JAD-2010-1349. [DOI] [PubMed] [Google Scholar]
- LeVine H, III, Walker LC. Models of Alzheimer’s disease. In: Conn PM, editor. Handbook of Models for Human Aging. Academic Press; Burlington: 2006. pp. 121–134. [Google Scholar]
- Liu L, Orozco IJ, Planel E, et al. A transgenic rat that develops Alzheimer’s disease-like amyloid pathology, deficits in synaptic plasticity and cognitive impairment. Neurobiol Dis. 2008;31:46–57. doi: 10.1016/j.nbd.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean CJ, Baker HF, Ridley RM, Mori H. Naturally occurring and experimentally induced beta-amyloid deposits in the brains of marmosets (Callithrix jacchus) J Neural Transm. 2000;107:799–814. doi: 10.1007/s007020070060. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- Morales R, Duran-Aniotz C, Castilla J, Estrada L, Soto C. De novo induction of amyloid-beta deposition in vivo. Mol Psychiatry. doi: 10.1038/mp.2011.120. in press. [DOI] [PubMed] [Google Scholar]
- Morrissette DA, Parachikova A, Green KN, LaFerla FM. Relevance of transgenic mouse models to human Alzheimer disease. The Journal of Biological Chemistry. 2009;284:6033–6037. doi: 10.1074/jbc.R800030200. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Unbiased Stereology: A Concise Guide. The Johns Hopkins University Press; Baltimore: 2011. [Google Scholar]
- Otvos L, Jr, Szendrei GI, Lee VM-Y, Mantsch HH. Human and rodent Alzheimer beta-amyloid peptides acquire distinct conformations in membrane-mimicking solvents. European Journal of Biochemistry. 1993;211:249–257. doi: 10.1111/j.1432-1033.1993.tb19893.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edition Academic Press; San Diego: 1998. [Google Scholar]
- Ridley RM, Baker HF, Windle CP, Cummings RM. Very long term studies of the seeding of beta-amyloidosis in primates. J Neural Transm. 2006;113:1243–1251. doi: 10.1007/s00702-005-0385-2. [DOI] [PubMed] [Google Scholar]
- Rosen RF, Tomidokoro Y, Ghiso JA, Walker LC. SDS-PAGE/immunoblot detection of Abeta multimers in human cortical tissue homogenates using antigen-epitope retrieval. J Vis Exp. 2010 doi: 10.3791/1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Opazo N, Kosik KS, Lopez LV, Bagamasbad P, Ponce LR, Herrera VL. Attenuated hippocampus-dependent learning and memory decline in transgenic TgAPPswe Fischer-344 rats. Mol Med. 2004;10:36–44. doi: 10.2119/2003-00044.herrera. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM, Wisniewski T, Frangione B. Infectivity of amyloid diseases. Trends Mol Med. 2002;8:411–413. doi: 10.1016/s1471-4914(02)02403-6. [DOI] [PubMed] [Google Scholar]
- Soto C, Estrada L, Castilla J. Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem Sci. 2006;31:150–155. doi: 10.1016/j.tibs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Tesson L, Cozzi J, Menoret S, Remy S, Usal C, Fraichard A, Anegon I. Transgenic modifications of the rat genome. Transgenic Res. 2005;14:531–546. doi: 10.1007/s11248-005-5077-z. [DOI] [PubMed] [Google Scholar]
- Walker L, Levine H, Jucker M. Koch’s postulates and infectious proteins. Acta Neuropathol (Berl) 2006a;112:1–4. doi: 10.1007/s00401-006-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Bian F, Callahan MJ, Lipinski WJ, Durham RA, LeVine H. Modeling Alzheimer’s disease and other proteopathies in vivo: is seeding the key? Amino Acids. 2002;23:87–93. doi: 10.1007/s00726-001-0113-7. [DOI] [PubMed] [Google Scholar]
- Walker LC, Levine H, 3rd, Mattson MP, Jucker M. Inducible proteopathies. Trends in Neurosciences. 2006b;29:438–443. doi: 10.1016/j.tins.2006.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Giles K, Grillo SK, Lemus A, DeArmond SJ, Prusiner SB. Bioluminescence imaging of Abeta deposition in bigenic mouse models of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2011;108:2528–2533. doi: 10.1073/pnas.1019034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Sigurdsson EM. Murine models of Alzheimer’s disease and their use in developing immunotherapies. Biochim Biophys Acta. 2010;1802:847–859. doi: 10.1016/j.bbadis.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]