Abstract
Bidirectional signaling between the urogenital sinus epithelium and mesenchyme is an essential element of prostate development that regulates ductal morphogenesis, growth, and differentiation. Comparable interactions between the epithelium and stroma in the adult prostate appear to regulate normal growth homeostasis. Alterations in the stromal–epithelial dialogue that recapitulate features of the mesenchymal–epithelial interactions of development may play a critical role in the development of benign prostatic hyperplasia and in the progression of prostate cancer. For this reason, the mesenchymal–epithelial interactions of development are of considerable interest. In this review, we provide an overview of the mesenchymal contribution to rodent prostate development with an emphasis on the stage just before ductal budding (embryonic day 16; E16) and describe the isolation, characterization and utility of a newly established E16 urogenital sinus mesenchymal cell line.
Keywords: UGSM-2, cell line, prostate, mesenchyme
Introduction
Viewed through a dissecting microscope, as when performing tissue separation for the purposes of a recombination experiment (Staack et al., 2003), the mesenchyme of the developing rodent prostate is all the “stuff” that is not epithelium (Fig. 1). What is this stuff? Where did it come from? And, what does it do?
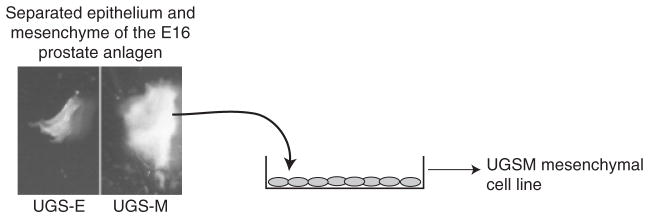
Fig. 1.
Isolation of UGSM-2 cells. The urogenital sinus of an E16 male INK4a −/− transgenic mouse embryo was subjected to collagenase digestion to separate epithelial (UGS-E) and mesenchymal (UGS-M) layers. UGS-M was placed into a culture dish and allowed to proliferate to confluence. Polyclonal UGSM cells were ring cloned to develop several stable clones, including the UGSM-2 cell line. Taken from Shaw et al. (2006).
At embryonic day 16 (E16), just before the onset of ductal budding, the fetal rodent prostate consists of a multilayered epithelial tube cloaked by an arrangement of mesenchymal cells. Urogenital epithelium (UGE) is derived from the embryonic endoderm that is separated from the hindgut when the cloaca is divided by the urogenital septum (Staack et al., 2003). Urogenital mesenchyme is derived from splanchnic mesoderm that is thought to originate from cells migrating through the primitive streak. Histologic examination of the E16 urogenital sinus (UGS) reveals a lumen surrounded by a multilayered epithelial lumen in turn surrounded by a collar of mesenchymal cells. Cells immediately adjacent to the epithelial layer are designated the periurethral mesenchyme and studies in the rat show that mesenchymal androgen receptor (AR) expression first appears in this layer (Hayward et al., 1996). Additional mesenchymal cells reinforce the periurethral layer and, together, comprise a substantial mesenchymal layer of varying thickness that runs the length of the prostatic urethra. A distinct mesenchymal condensation overlying the site of ventral prostate budding is termed the ventral mesenchymal pad (VMP) (Timms et al., 1995); similar, less prominent, condensations appear over the sites of dorso-lateral prostate budding. Although the VMP is not sexually dimorphic (Thomson et al., 2002), these areas of condensation correspond to regions of mesenchyme involved in extensive branching morphogenesis during postnatal prostate development. Tissue recombination experiments indicate that there is regional heterogeneity of the fetal mesenchyme’s ability to induce prostate development and confer ventral versus dorso-lateral lobe identity in the rodent (Timms et al., 1995). The basis for this regional specification is as yet unknown, although it has been suggested that Hox genes may play a role (Podlasek et al., 1999). The anterior prostate, also termed the coagulating gland, is unique with respect to its embryonic contributions. The buds of the anterior prostate arise from the UGS, like the buds of the ventral and dorso-lateral prostate, but postnatal branching morphogenesis of the anterior prostate occurs within the mesodermal sheath of the seminal vesicle (Podlasek et al., 1999).
The E16 mesenchyme contains myofibroblasts that coexpress vimentin and smooth muscle α-actin (SMA). Myofibroblasts are also found in the human prenatal prostate at weeks 23–25 (Bierhoff et al., 1997) and are thought to be precursors of the fibroblasts and smooth muscle cells that dominate the adult prostatic stroma. Detailed studies of stromal differentiation in the developing rat prostate showed that differentiation of mesenchymal cells to smooth muscle that surrounds the mature ducts occurs in the postnatal prostate in a proximal–distal fashion as ductal morphogenesis proceeds (Hayward et al., 1996). Myofibroblasts are present in the adult prostate at sites of benign prostatic hyperplasia (BPH) and in prostate carcinoma (Bierhoff et al., 1997; Ayala et al., 2003). Evaluation of the roles of these cells in the fetal prostate may explain any role they may have in adult prostate pathology.
Recent studies in a variety of organs and tissues have identified tissue-specific progenitor cells that exhibit enormous proliferative potential and the ability to differentiate into one or more terminally differentiated cell types. There are many questions regarding the origin of the adult prostate stroma since this aspect of prostate stromal biology has not been well studied. Is there a stromal stem/progenitor cell present in the adult prostate? If a stromal stem cell exists, is it separate from the epithelial stem cell, or is there a single stem cell that generates both the stromal and epithelial populations? Lin et al. (2007) cultured stromal cells derived from patients with BPH. These cells express several markers of mesenchymal stem cells (MSC) and can differentiate into smooth muscle, adipocyte, and osteogenic cell lineages. This suggests that at least part of the adult prostate stroma arises from MSC. It is important to identify the progenitor cell(s) for the adult stroma and determine the possible contribution of bone marrow cells to the adult stroma. Neoplasia or BPH may stimulate proliferation or migration of stromal progenitors or MSC. We are not aware of any published studies that have looked for the presence of stem or primitive progenitor cells in the UGS mesenchyme and/or examined their role in postnatal stromal proliferation and smooth muscle differentiation. The fetal mesenchyme may also contain the precursors of vascular and lymphatic components of the adult prostate; however, the ontogeny of vascular development in the prostate has not been studied and their precursors have not been clearly identified. Indeed, intensive efforts to characterize ductal budding and epithelial differentiation during prostate development stand in striking contrast to the relative lack of understanding of mesenchymal proliferation and differentiation during generation of the adult prostatic stroma. Tissue recombination of human prostate epithelial cells with rat UGM showed extensive smooth muscle sheet differentiation, which is characteristic of the human, but not the rodent, prostate. This reveals that epithelial cells control differentiation of prostate mesenchyme in a species-specific manner (Hayward et al., 1998). Paracrine interactions of epithelium and mesenchyme are clearly an area in need of further study.
Mesenchymal/stromal–epithelial interactions are a pivotal aspect of prostate development, differentiation, normal growth regulation, and neoplasia. The mesenchyme is the site of expression of many critical regulators of ductal budding and development including transforming growth factor-β, fibroblast growth factor (FGF-10, FGF-7), bone morphogenic protein (BMP-4, BMP-7), Noggin, secreted frizzled related protein 1, and several Hox genes (Marker et al., 2003; Tomlinson et al., 2004; Grishina et al., 2005; Joesting et al., 2005; Cook et al., 2007). Urogenital sinus mesenchyme (UGM) expresses ARs (Thompson et al., 1986) and participates in the metabolism of steroid hormones (Neubauer et al., 1985). The stroma contains aromatase (Ellem et al., 2004) and 5-alpha-reductase (Cowan et al., 1977), enzymes important for converting testosterone to estradiol or dihydrotestosterone, respectively. Estrogen and androgen are important for prostate growth, development, carcinogenesis and hyperplasia and our understanding of steroid effects in homeostasis may explain aberrant roles in the adult prostate (McPherson et al., 2007; Prins et al., 2007; Cunha et al., 2004). UGM is the target of regulation by signaling molecules secreted by the epithelium such as Sonic Hedgehog (Shh), Indian Hedgehog, and insulin-like growth factor-1. Several investigators have performed transcriptional profiling of rat or mouse prostate mesenchyme (Zhang et al., 2006; Vanpoucke et al., 2007). These studies identified new pathways controlling prostate development, but further understanding of the biology requires the use of mesenchymal cell lines. Such cell lines allow deletion or activation of specific signaling pathways in vitro and in vivo to study the biological effect of these perturbations. An ideal mesenchymal or stromal cell population would have the following properties: immortal, genetically stable, responds to known biochemical mediators, gene expression that is similar to freshly isolated tissue, allows in vivo and in vitro modeling and can be genetically modified.
Several immortalized prostate stromal cell lines exist: NbF-1, RSPC-2T, PS-1, WPMY-1, PS30, S2.13, and PM151T (Table 1). These cell lines have been evaluated with respect to androgen signaling and smooth muscle differentiation because these properties are important for prostate carcinoma studies and for description of the stromal cell type, respectively. Human prostate stromal cells were immortalized using large T antigen, HPV16 E6/E7 or telomerase catalytic subunit. Large T antigen and HPV16 E6/E7 use the p53 or pRb pathway to prevent senescence and can contribute to future genetic anomalies in the cell line because of reduced gene surveillance. This is an important factor to consider when selecting an immortalized cell line. NbF-1 cells were derived from the ventral prostate of four adult rats. Dihydrotestosterone, but not testosterone, stimulates DNA synthesis in these cells (Chang and Chung, 1989). RSPC-2T cells were derived from 10-day-old rats and were immortalized with v-myc. These cells express SMA, but not AR (Hoeben et al., 1995). PS-1 cells are derived from the adult rat ventral prostate. PS-1 cells are smooth muscle cells that express desmin and SMA. PS-1 cells express AR and androgen enhances cellular proliferation (Gerdes et al., 1996). WPMY-1 cells were acquired from a 54-year-old Caucasian male during cystoprostatectomy who had no evidence of neoplasia and immortalized with large T antigen. WPMY-1 cells express SMA, vimentin, and fibronectin, but not desmin and is therefore characterized as a myofibroblast cell line. WPMY-1 cells express AR and androgen increases proliferation (Webber et al., 1999). PS30 cells were derived by HPV16 E6/E7 immortalization of primary human prostate cells obtained from normal tissue at radical prostatectomy for localized prostate cancer. PS30 cells are myofibroblasts that express SMA, vimentin, but not desmin or heavy chain myosin. PS30 cells express AR, but androgen does not alter cellular proliferation (Price et al., 2000). S2.13 cells are clonal and derived from tissues from men aged 51–78 years via transurethral resection for BPH and immortalized with a temperature sensitive SV40 large T antigen. S2.13 cells are fibroblasts that express vimentin, but not SMA or AR (Daly-Burns et al., 2007). PM151T cells were derived from the prostatic normal transition zone of a 62-year-old man during radical prostatectomy performed for localized prostate cancer. Primary stromal cells were immortalized by overexpression of human telomerase catalytic subunit. Smooth muscle PM151T cells express SMA, calponin, heavy chain myosin, and AR (Kogan et al., 2006).
Table 1.
Adult prostate stromal cell lines
| Cell line | Phenotype | Species | How immortalized | Markers | AR | References |
|---|---|---|---|---|---|---|
| RSPC-2T | Smooth muscle/myofibroblast | Rat | v-myc | SMA | No | Hoeben et al. (1995) |
| PS-1 | Smooth muscle | Rat | Spontaneous | Desmin, SMA | Yes | Gerdes et al. (1996) |
| WPMY-1 | Myofibroblast | Human | Tag | SMA, VIM, FN | Yes | Webber et al. (1999) |
| PS30 | Myofibroblast | Human | HPV16 E6/E7 | SMA, VIM | Yes | Price et al. (2000) |
| S2.13 | Fibroblast | Human | ts-Tag | VIM | No | Daly-Burns et al. (2007) |
| PM151T | Smooth muscle | Human | hTERT | SMA, calponin, HCM | Yes | Kogan et al. (2006) |
AR, androgen receptor; SMA, smooth muscle actin; VIM, vimentin; FN, fibronectin; HCM, heavy chain myosin; Tag, T antigen; hTERT, human telomerase catalytic subunit.
These cells, derived from both rodents and humans, provide important models for study of stromal–epithelial interactions in the adult prostate. However, these are not suitable for study of mesenchymal roles in prostate development since these cells have differentiated and cannot be used to trace mesenchymal differentiation. These cell lines have not been tested for prostate inductive capacity or the ability to integrate normally into the developing prostate. These studies require a UGM line that is genetically stable, can be genetically manipulated, can participate in prostate development in vivo, is responsive to androgen, and models mesenchymal differentiation and signaling activities related to paracrine epithelial signals.
In this regard, three immortalized UGM cell lines are available: U4F, rUGM, and UGSM-2 (Table 2). U4F cells were derived from an E18 male rat embryo organ culture. Mesenchymal cells emanated from the UGS tissue onto the culture dish and immortalized spontaneously. U4F cells express AR, have vimentin intermediate filaments but do not express desmin (Rowley, 1992). Another cell line, rUGM, was also derived from an E18 male rat embryo. This cell line expresses AR and vimentin, but not desmin (Zhau et al., 1994). Neither the U4F nor the rUGM cell lines have been tested in the in vivo prostate recombination model. The UGM cell line, UGSM-2, also expresses vimentin and AR and does not express desmin. The UGSM-2 cell line has been further characterized in vivo.
Table 2.
Urogenital sinus mesenchyme cell lines
| Cell line | Phenotype | Age/species | How immortalized | Markers | AR | References |
|---|---|---|---|---|---|---|
| U4F | (Myo)fibroblast | E18 rat | Spontaneous | VIM | Yes | Rowley 1992 |
| rUGM | (Myo)fibroblast | E18 rat | Spontaneous | VIM | Yes | Zhau et al. (1994) |
| UGSM-2 | Myofibroblast | E16 mouse | INK4a −/− | SMA, VIM | Yes | Shaw et al. (2006) |
VIM, vimentin; SMA, smooth muscle actin.
The UGSM-2 cell line is a clonal cell line generated from an INK4a mouse E16 embryo, a transgenic knockout mouse that lacks the p16INK4a and p19ARF proteins that trigger senescence. UGSM-2 cells express AR, vimentin, and SMA but not smooth muscle markers desmin or heavy chain myosin (Shaw et al., 2006). UGSM-2 cells are myofibroblasts in culture. Their lack of mature smooth muscle markers is consistent with their origin from the prenatal UGM, because few mesenchymal cells express mature smooth muscle markers at E16 (Hayward et al., 1996). Not only do UGSM-2 cells express AR, but androgen treatment of UGSM-2 cells cultured in vitro changes the morphology of the cells and stimulates proliferation (A. Shaw et al., unpublished). The cells are stably tetraploid, contact inhibited, and non-tumorigenic in vivo. These cells participate in prostate development as shown by the ability of UGSM-2 cells grafted together with E16 intact UGS (i.e., both UGM and UGE) to proliferate and become part of the periductal stroma in the mature prostate grafts (Fig. 2). The capacity of UGSM-2 cells to differentiate into bona fide smooth muscle cells in vivo has not been fully characterized. A distinguishing feature of UGM tissue is its inductive capacity. Tissue recombination studies have shown that isolated mouse E16 UGM can induce prostate morphogenesis in epithelia derived from the UGS or adult bladder (Cunha et al., 2004). To screen for inductive capacity in UGSM-2 cells, we grafted UGSM-2 cells with isolated E16 UGS epithelium (separated from UGM) under the renal capsule of an adult male nude mouse. These grafts did not exhibit prostate formation (Shaw et al., 2006). However, since UGSM-2 cells can integrate into the developing prostate, UGSM-2 cells are a useful UGM cell line for studying prostate developmental pathways.
Fig. 2.
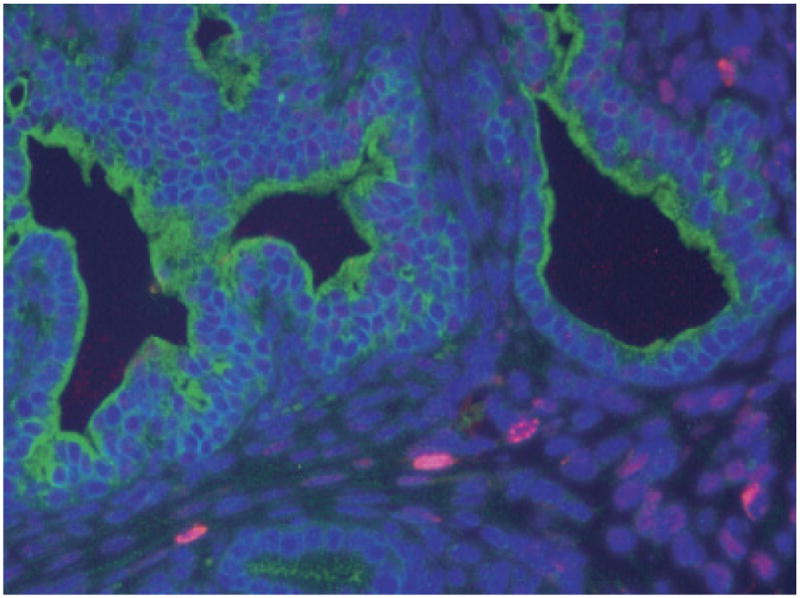
UGSM-2 cell fate in development of the urogenital sinus (UGS). UGSM-2 cells labeled with BrdU were seeded with dissected UGS tissue and allowed to develop under the renal capsule of male nude mice. Immunohistochemistry shows UGSM-2 cells (red) in the periductal regions of mature prostate. Epithelial cells are marked with pancytokeratin (green). Taken from Shaw et al. (2006).
We have used the UGSM-2 cell line to study Hedgehog (Hh) signaling. During prostate development, Hh peptide is secreted by the epithelium and acts upon the adjacent mesenchymal cells to activate the intracellular Hh signaling pathway. Our studies showed that the UGSM-2 cells are Hh responsive and that Shh peptide induces expression of the conserved Hh target genes Patched-1 and Gli1, replicating what is observed in the freshly isolated UGM (Shaw et al., 2006). Using UGSM-2 cells, we performed a microarray analysis to identify Hh target genes in the developing prostate (M. Yu et al., unpublished). The majority of putative target genes examined in detail exhibited Hh-regulated expression in the intact UGS and/or in primary UGS mesenchymal cells. We have also used UGSM-2 cells to characterize the role of Noggin in prostate development to examine the effect of BMP-4 on mesenchymal Noggin expression (Cook et al., 2007). These examples illustrate the broad potential of this cell line to identify potential targets of regulation by signaling pathways and examine the actions of signaling factors and transcriptional regulators important in prostate development. Further, the androgen responsiveness of these cells permits studies examining the influence of testosterone on these interactions.
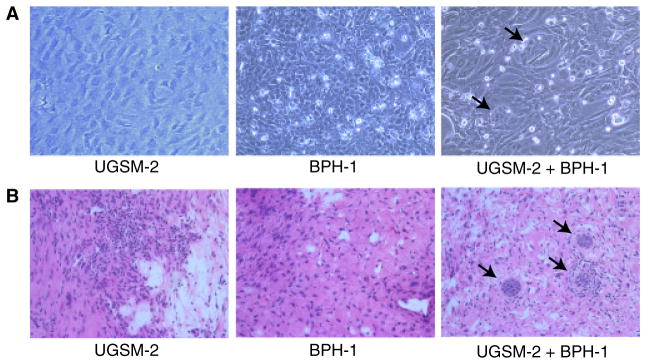
UGSM-2 cells grow as monolayers in cell culture and can be co-cultured with epithelial cells. We discovered that co-culture of UGSM-2 cells with BPH-1 prostate epithelial cells in two-dimensional culture resulted in congregation of human BPH-1 cells in acinar-like structures surrounded by UGSM-2 stromal cells (Fig. 3). A similar localization was seen when the cells were grafted together under the renal capsule (Fig. 3). These studies show that UGSM-2 cells interact with human BPH-1 epithelial cells by grouping cell types to resemble cell arrangements in solid epithelial cords seen in early prostate development. This provides a useful system to study mesenchymal–epithelial signaling pathways in vitro and in vivo.
Fig. 3.
Interaction of UGSM-2 and human BPH-1 epithelial cells. (A) UGSM-2 cells and/or BPH-1 cells grown on rat tail collagen. Co-cultures show small clusters of BPH-1 cells (arrows) surrounded by UGSM-2 cells (arrows). (B) UGSM-2 and/or BPH-1 cells renal capsule grafts after 4 weeks. Grafts of UGSM-2 cells alone yields only stromal tissue. BPH-1 cells grafted alone do not produce identifiable viable grafts. Co-grafting of UGSM-2 and BPH-1 cells results in small clusters of BPH-1 cells (arrows) surrounded by UGSM-2 cells.
We have stably labeled UGSM-2 cells with monomeric red fluorescent protein to enable visualization of the cells in vitro (Fig. 4) and in vivo. This facilitates monitoring of live cells in co-cultures and allows location of the cells in vivo. Co-culture of UGSM-2 cells with BPH-1 cells engineered to overexpress Shh results in activation of the Hh pathway and expression of Gli1 in UGSM-2 cells. Grafting of UGSM-2 cells engineered to overexpress the BMP antagonist Noggin with LNCaP tumor cells results in inhibition of BMP signaling in the xenograft or in culture (A. Shaw et al., unpublished). In both instances, the use of species-specific RT-PCR primers permits the selective quantitation of gene expression changes in the human BPH-1 epithelial cells and mouse-derived stroma. This allows selective monitoring of stromal–epithelial signaling pathways in which Shh elicits Hh signaling in UGSM-2 cells, but not in BPH-1 cells (Shaw et al., 2006).
Fig. 4.
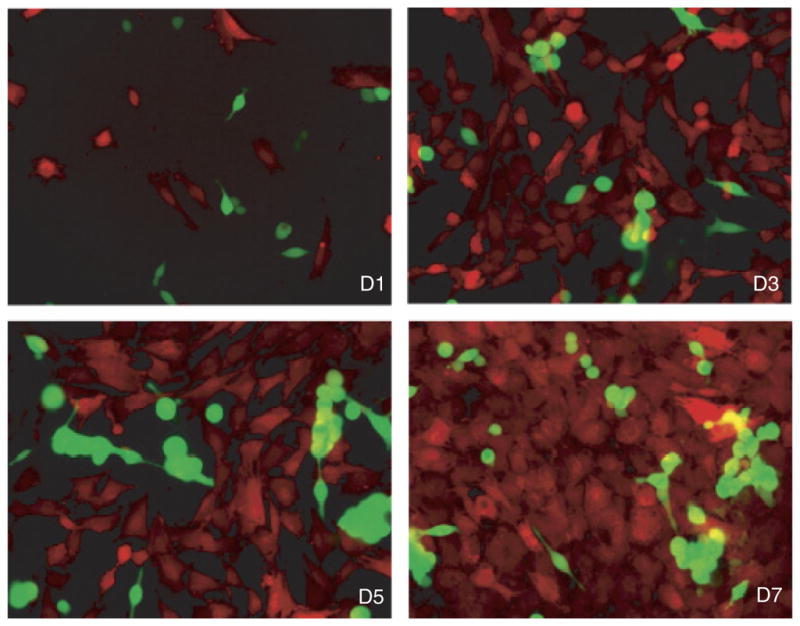
Labeling of cells enables tracking of cell proliferation, morphology, and motility in co-cultures. UGSM-2 cells are stably labeled with monomeric red fluorescent protein and LNCaP cells are labeled with green fluorescent protein. This shows proliferation of each cell type over 7 days in culture. Days 1, 3, 5, and 7 are shown. Taken from Shaw et al. (2006).
Since genetic manipulation of UGSM-2 cells may find use in a wide array of studies, we will point out several specific methodological considerations. As a consequence of being derived from the INK4a transgenic mutant created by insertion of a neomycin resistance gene, UGSM-2 cells are neomycin resistant. Plasmid vectors encoding hygromycin or zeocin resistance genes may be used for selection and maintenance of UGSM-2 cells in culture. However, UGSM-2 cells show a low efficiency of transfection using standard methods. We have found adenovirus vectors useful for transient expression studies and retroviral infection with vectors expressing green fluorescent protein selection to be most effective for generating stably transfected cells that can be sorted by fluorescence-activated cell sorting.
Conclusions
The UGSM-2 cell line possesses many features that make it useful to study the mesenchymal–epithelial interactions of prostate development: genetic stability, androgen responsiveness, and the ability to participate in prostate morphogenesis in vivo. Our studies of Hh signaling and Noggin regulation suggest that this cell line recapitulates the transcriptional and regulatory response of the UGS mesenchyme. However, no cell line can recapitulate in monolayer culture the microenvironment of mesenchymal cells in the fetal prostate and the regional variation in the identity, response and developmental fate of the UGS mesenchyme itself. For this reason, in vitro studies using these cell lines are best when complemented by in vivo studies to corroborate an in vitro observation and determine its biological significance.
Acknowledgments
We acknowledge support of National Institutes of Health grant T32 CA009614 Physician Scientist Training in Cancer Medicine (Dr. Attia), the Department of Defense Prostate Cancer Program Graduate Training Award W81XWH-06-1-0060 (Dr. Shaw), and the NIDDK Award DK056238-06.
Contributor Information
Aubie Shaw, McArdle Laboratory for Cancer Research, Madison, WI, USA, Department of Surgery, Division of Urology, School of Medicine and Public Health University of Wisconsin-Madison, K6/562 Clinical Science Center, 600 Highland Avenue, Madison WI 53792, USA, Tel: +608 265 8705, Fax: +608 265 8133.
Steven Attia, University of Wisconsin Paul P. Carbone, Comprehensive Cancer, Center Madison, WI, USA.
Wade Bushman, Email: bushman@surgery.wisc.edu, Department of Surgery, Division of Urology, School of Medicine and Public Health University of Wisconsin-Madison, K6/562 Clinical Science Center, 600 Highland Avenue, Madison WI 53792, USA, Tel: +608 265 8705, Fax: +608 265 8133.
References
- Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J, Rowley DR. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9:4792–4801. [PubMed] [Google Scholar]
- Bierhoff E, Walljasper U, Hofmann D, Vogel J, Wernert N, Pfeifer U. Morphological analogies of fetal prostate stroma and stromal nodules in BPH. Prostate. 1997;31:234–240. doi: 10.1002/(sici)1097-0045(19970601)31:4<234::aid-pros4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Chang SM, Chung LW. Interaction between prostatic fibroblast and epithelial cells in culture: role of androgen. Endocrinology. 1989;125:2719–2727. doi: 10.1210/endo-125-5-2719. [DOI] [PubMed] [Google Scholar]
- Cook C, Vezina CM, Allgeier SH, Shaw A, Yu M, Peterson RE, Bushman W. Noggin is required for normal lobe patterning and ductal budding in the mouse prostate. Dev Biol. 2007;312:217–230. doi: 10.1016/j.ydbio.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RA, Cowan SK, Grant JK, Elder HY. Biochemical investigations of separated epithelium and stroma from benign hyperplastic prostatic tissue. J Endocrinol. 1977;74:111–120. doi: 10.1677/joe.0.0740111. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Ricke W, Thomson A, Marker PC, Risbridger G, Hayward SW, Wang YZ, Donjacour AA, Kurita T. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol. 2004;92:221–236. doi: 10.1016/j.jsbmb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Daly-Burns B, Alam TN, Mackay A, Clark J, Shepherd CJ, Rizzo S, Tatoud R, O’Hare MJ, Masters JR, Hudson DL. A conditionally immortalized cell line model for the study of human prostatic epithelial cell differentiation. Diff Res Biol Divers. 2007;75:35–48. doi: 10.1111/j.1432-0436.2006.00113.x. [DOI] [PubMed] [Google Scholar]
- Ellem SJ, Schmitt JF, Pedersen JS, Frydenberg M, Risbridger GP. Local aromatase expression in human prostate is altered in malignancy. J Clin Endocrinol Metab. 2004;89:2434–2441. doi: 10.1210/jc.2003-030933. [DOI] [PubMed] [Google Scholar]
- Gerdes MJ, Dang TD, Lu B, Larsen M, McBride L, Rowley DR. Androgen-regulated proliferation and gene transcription in a prostate smooth muscle cell line (PS-1) Endocrinology. 1996;137:864–872. doi: 10.1210/endo.137.3.8603596. [DOI] [PubMed] [Google Scholar]
- Grishina IB, Kim SY, Ferrara C, Makarenkova HP, Walden PD. BMP7 inhibits branching morphogenesis in the prostate gland and interferes with Notch signaling. Dev Biol. 2005;288:334–347. doi: 10.1016/j.ydbio.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward SW, Baskin LS, Haughney PC, Foster BA, Cunha AR, Dahiya R, Prins GS, Cunha GR. Stromal development in the ventral prostate, anterior prostate and seminal vesicle of the rat. Acta Anat. 1996;155:94–103. doi: 10.1159/000147794. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Haughney PC, Rosen MA, Greulich KM, Weier HU, Dahiya R, Cunha GR. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Diff Res Biol Divers. 1998;63:131–140. doi: 10.1046/j.1432-0436.1998.6330131.x. [DOI] [PubMed] [Google Scholar]
- Hoeben E, Briers T, Vanderstichele H, De Smet W, Heyns W, Deboel L, Vanderhoydonck F, Verhoeven G. Characterization of newly established testicular peritubular and prostatic stromal cell lines: potential use in the study of mesenchymal–epithelial interactions. Endocrinology. 1995;136:2862–2873. doi: 10.1210/endo.136.7.7789311. [DOI] [PubMed] [Google Scholar]
- Joesting MS, Perrin S, Elenbaas B, Fawell SE, Rubin JS, Franco OE, Hayward SW, Cunha GR, Marker PC. Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res. 2005;65:10423–10430. doi: 10.1158/0008-5472.CAN-05-0824. [DOI] [PubMed] [Google Scholar]
- Kogan I, Goldfinger N, Milyavsky M, Cohen M, Shats I, Dobler G, Klocker H, Wasylyk B, Voller M, Aalders T, Schalken JA, Oren M, Rotter V. hTERT-immortalized prostate epithelial and stromal-derived cells: an authentic in vitro model for differentiation and carcinogenesis. Cancer Res. 2006;66:3531–3540. doi: 10.1158/0008-5472.CAN-05-2183. [DOI] [PubMed] [Google Scholar]
- Lin VK, Wang SY, Vazquez DV, Xu CC, Zhang S, Tang L. Prostatic stromal cells derived from benign prostatic hyperplasia specimens possess stem cell like property. Prostate. 2007;67:1265–1276. doi: 10.1002/pros.20599. [DOI] [PubMed] [Google Scholar]
- Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253:165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- McPherson SJ, Ellem SJ, Simpson ER, Patchev V, Fritzemeier KH, Risbridger GP. Essential role for estrogen receptor beta in stromal–epithelial regulation of prostatic hyperplasia. Endocrinology. 2007;148:566–574. doi: 10.1210/en.2006-0906. [DOI] [PubMed] [Google Scholar]
- Neubauer BL, Anderson NG, Cunha GR, Towell JF, Chung LW. Androgen metabolism in tissue recombinants composed of adult urinary bladder epithelium and urogenital sinus mesenchyme. J Steroid Biochem. 1985;23:95–101. doi: 10.1016/0022-4731(85)90266-3. [DOI] [PubMed] [Google Scholar]
- Podlasek CA, Clemens JQ, Bushman W. Hoxa-13 gene mutation results in abnormal seminal vesicle and prostate development. J Urol. 1999;161:1655–1661. [PubMed] [Google Scholar]
- Price DT, Rudner X, Michelotti GA, Schwinn DA. Immortalization of a human prostate stromal cell line using a recombinant retroviral approach. J Urol. 2000;164:2145–2150. [PubMed] [Google Scholar]
- Prins GS, Birch L, Tang WY, Ho SM. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod Toxicol. 2007;23:374–382. doi: 10.1016/j.reprotox.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley DR. Characterization of a fetal urogenital sinus mesenchymal cell line U4F: secretion of a negative growth regulatory activity. In Vitro Cell Dev Biol. 1992;28A:29–38. doi: 10.1007/BF02631077. [DOI] [PubMed] [Google Scholar]
- Shaw A, Papadopoulos J, Johnson C, Bushman W. Isolation and characterization of an immortalized mouse urogenital sinus mesenchyme cell line. Prostate. 2006;66:1347–1358. doi: 10.1002/pros.20357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. Mouse urogenital development: a practical approach. Diff Res Biol Divers. 2003;71:402–413. doi: 10.1046/j.1432-0436.2003.7107004.x. [DOI] [PubMed] [Google Scholar]
- Thompson TC, Cunha GR, Shannon JM, Chung LW. Androgen-induced biochemical responses in epithelium lacking androgen receptors: characterization of androgen receptors in the mesenchymal derivative of urogenital sinus. J Steroid Biochem. 1986;25:627–634. doi: 10.1016/0022-4731(86)90004-x. [DOI] [PubMed] [Google Scholar]
- Thomson AA, Timms BG, Barton L, Cunha GR, Grace OC. The role of smooth muscle in regulating prostatic induction. Development (Cambridge, UK) 2002;129:1905–1912. doi: 10.1242/dev.129.8.1905. [DOI] [PubMed] [Google Scholar]
- Timms BG, Lee CW, Aumuller G, Seitz J. Instructive induction of prostate growth and differentiation by a defined urogenital sinus mesenchyme. Microsc Res Tech. 1995;30:319–332. doi: 10.1002/jemt.1070300407. [DOI] [PubMed] [Google Scholar]
- Tomlinson DC, Freestone SH, Grace OC, Thomson AA. Differential effects of transforming growth factor-beta1 on cellular proliferation in the developing prostate. Endocrinology. 2004;145:4292–4300. doi: 10.1210/en.2004-0526. [DOI] [PubMed] [Google Scholar]
- Vanpoucke G, Orr B, Grace OC, Chan R, Ashley GR, Williams K, Franco OE, Hayward SW, Thomson AA. Transcriptional profiling of inductive mesenchyme to identify molecules involved in prostate development and disease. Genome Biol. 2007;8:R213. doi: 10.1186/gb-2007-8-10-r213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber MM, Trakul N, Thraves PS, Bello-DeOcampo D, Chu WW, Storto PD, Huard TK, Rhim JS, Williams DE. A human prostatic stromal myofibroblast cell line WPMY-1: a model for stromal–epithelial interactions in prostatic neoplasia. Carcinogenesis. 1999;20:1185–1192. doi: 10.1093/carcin/20.7.1185. [DOI] [PubMed] [Google Scholar]
- Zhang TJ, Hoffman BG, Ruiz de Algara T, Helgason CD. SAGE reveals expression of Wnt signalling pathway members during mouse prostate development. Gene Expr Patterns. 2006;6:310–324. doi: 10.1016/j.modgep.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Zhau HE, Hong SJ, Chung LW. A fetal rat urogenital sinus mesenchymal cell line (rUGM): accelerated growth and conferral of androgen-induced growth responsiveness upon a human bladder cancer epithelial cell line in vivo. Int J Cancer. 1994;56:706–714. doi: 10.1002/ijc.2910560516. [DOI] [PubMed] [Google Scholar]