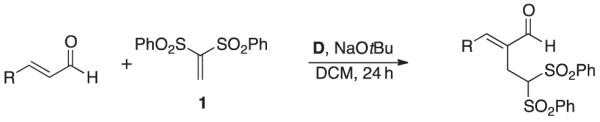
Abstract
N-heterocyclic carbenes (NHCs) promote the addition of 1,1-bis(phenylsulfonyl)ethylene to the α-position of α,β-unsaturated aldehydes. The proposed reaction pathway for this Rauhut-Currier-type reaction includes an unusual conjugate addition of an NHC to a bis-vinyl sulfone.
Introduction
Metal-free catalysis is an attractive field of methodology development because of its biomimetic and green nature. Intense efforts over the last decade to investigate organocatalysis have resulted in an eruption of selective and efficient strategies for the synthesis of organic molecules.[1] Specifically in the field of Lewis base catalysis,[2] N-heterocyclic carbenes (NHCs) facilitate a wide number of powerful bond-forming reactions.[3] Inspired by the biological conversion of pyruvate into acetyl Co-A by a thiamin cofactor, NHC catalysis utilizes the electron lone pair of nitrogen-containing heterocycles (i.e., thiazolium, imidazolium, and triazolium-derived carbenes) to promote a variety of reactions including acyl anion chemistry,[4] homoenolate[5] and enolate[6] equivalents, reductions,[3c] and oxidations.[7] Carbene catalysis is well known for the 1,2-addition of the carbene to a carbonyl group of a substrate, affording an acyl anion or homoenolate equivalent (Scheme 1). A less developed reactivity mode for these Lewis base catalysts is the conjugate addition of an α,β-unsaturated carbonyl-containing compound to generate a reactive enolate intermediate. Both Fu and Ye have independently reported NHC-catalyzed β-Umpolung-type and Morita-Baylis-Hillman (MBH) reactions[8] of α,β-unsaturated carbonyl substrates.[9] The Rauhut-Currier (RC) reaction[10] involves the nucleophile catalyzed coupling of a reactive intermediate (enolate) generated from an activated alkene to a secondary Michael acceptor, thereby forging a new C–C bond between the α-position of the alkene and the β-position of the secondary electrophile. To the best of our knowledge, there has been no report of a NHC-catalyzed RC reaction. This conjugate addition mode is an under-explored area for carbene catalysis and offers opportunities for reaction design and development beyond the established manifold resulting from 1,2-addition of the NHC to a carbonyl group (e.g., acyl anion and homoenolate equivalent reactions). In this work, we report that NHCs are compatible with an atypical NHC electrophile (non-carbonyl containing), resulting in the RC product of α,β-unsaturated aldehydes.
Scheme 1.
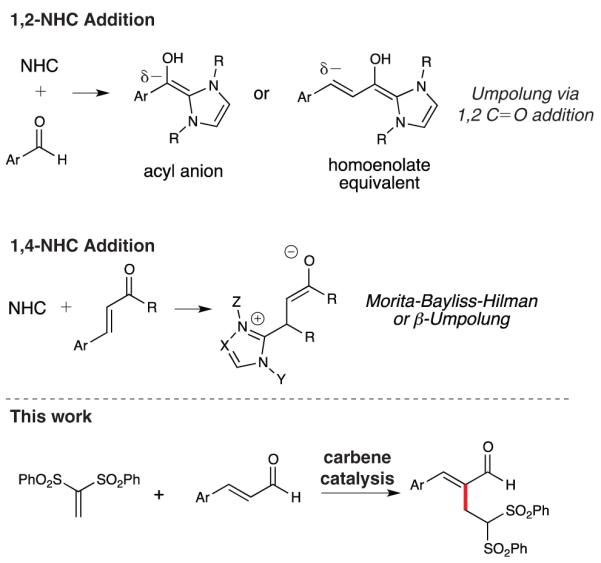
1,2 and 1,4-addition modes of N-heterocyclic carbene (NHC) catalysis.
Vinyl sulfones are versatile electrophiles in organic synthesis because of their high reactivity in organometallic and organocatalyis.[11] Our group has recently reported the use of vinyl sulfones as substrates in a carbene-catalyzed reaction with 1,3 dipolar electrophiles to form cycloaddition products with an unusual sulfonyl transposition.[12] In our initial investigations exploring homoenolate equivalent cycloadditions with vinyl sulfones, we discovered that NHCs promoted an unexpected process to deliver the apparent RC product (Scheme 1).
Results and Discussion
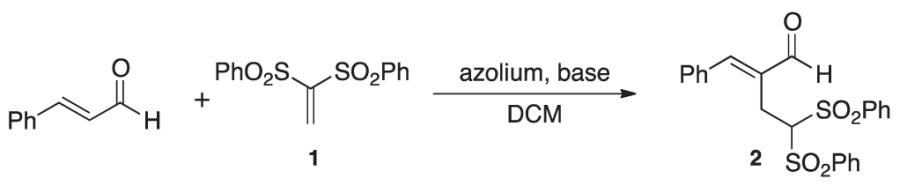
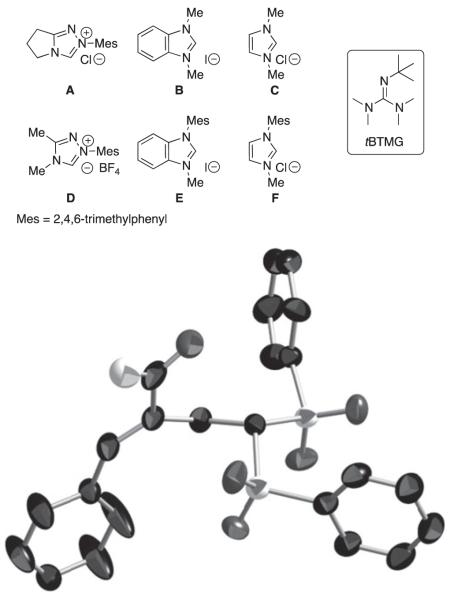
We initially explored the compatibility of NHC-homoenolate equivalents with vinyl sulfones to access cyclopentanones by a formal [3+2] annulation. However, the product isolated was confirmed as the intermolecular RC product (the X-ray crystallography data has been submitted to the Cambridge Crystallographic Data Centre) not the desired homoenolate equivalent addition product. This RC process represents a new mode of NHC reactivity and a different type of carbene catalysis compared with the benzoin/Umpolung modality. While intramolecular RC reactions have been reported by the groups of Krische,[10b] Roush,[10c,d] Miller,[10e] and others,[10h] we have been interested in Lewis base catalysis in general[13] and developing new intermolecular versions of this reaction for some time.[10g] After screening potential azolium salts as precatalysts, we found that triazolium D was the only catalyst that led to the formation of the RC products such as 2 (Table 1). The product structure was confirmed by single crystal X-ray diffraction. During reaction optimization, we focused on using non-nucleophilic bases to avoid possible complications of competitive base conjugate addition. Our best results were obtained with sodium tert-butoxide as the base and dichloromethane as the solvent. The choice of solvent and amount of base was important to avoid an unusual side product, (phenylperoxy)(1,3,3-tris(phenylsulfonyl)propyl)sulfane, which we observed in solvents such as THF and acetonitrile. This compound has been reported previously in studies of 1,1-bis(phenylsulfonyl)ethylene with enamine catalysis.[11d,i] The use of two equivalents of the enal partner was determined to be the ideal stoichiometry for this process. We found it odd that even though excess of the aldehyde was used, there was no evidence for the typical NHC-homoenolate chemistry present in the reaction mixture (the excess aldehyde was recovered during purification).
Table 1. Optimization table and ORTEP of 2 (displayed at 50% probability).
 | |||||
|---|---|---|---|---|---|
| Entry | Azolium | mol% | Base | Solventa | Yield 2 (%)b,c |
| 1 | A | 20 | tBTMG | CH2CL2 | 10 |
| 2 | B | 20 | tBTMG | CH2CL2 | 0 |
| 3 | C | 20 | tBTMG | CH2CL2 | 0 |
| 4 | D | 20 | tBTMG | CH2CL2 | 15 |
| 5 | E | 20 | tBTMG | CH2CL2 | 0 |
| 6 | F | 20 | tBTMG | CH2CL2 | 0 |
| 7 | D | 20 | NaOtBu | CH2CL2 | 39 |
| 8 | D | 20 | DBU | CH2CL2 | trace |
| 9 | D | 20 | Et3N | CH2CL2 | 0 |
| 10 | D | 20 | K2CO3 | CH2CL2 | 0 |
0.25 M.
isolated yields.
20 mol% azolium, 20 mol% base at 25°C for 25 h
With optimized reaction conditions that provided the intermolecular RC product between the vinyl sulfone and α,β-unsaturated aldehyde, we examined the scope of the reaction with respect to the enal component (Table 2). Having an electron withdrawing substituent on the aryl ring resulted in the products in good to moderate yields, potentially because of the increased electrophilicity of the β-position of the α,β-unsaturated aldehyde. Reactions with enals with non-electron withdrawing groups or groups that would block the β-position provided lower yields. The other product generated was trans-1,2-bis(phenylsulfonyl)ethylene and accounted for the mass balance. Unfortunately, the substrate scope of this process is currently limited and mixed sulfone/carbonyl group compounds are not suitable substrates.
Table 2. Enal substrate scope.
20 mol% D, 20 mol% base, 3Å MS, and 0.25M at 40°C.
isolated yield
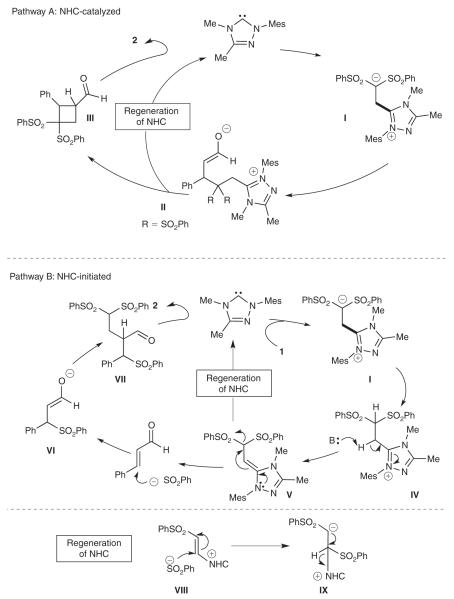
There are two main possible reaction pathways for the generation of the RC product (Scheme 2). In pathway A (NHC catalyzed), the NHC undergoes an initial conjugate addition to the vinyl sulfone, forming intermediate anion I that adds to cinnamaldehyde in a conjugate fashion. The resulting enolate II then undergoes a 4-exo-tet cyclization to produce a heavily substituted cyclobutane intermediate with concomitant regeneration of the carbene catalyst. Subsequent deprotonation of the α-position of the exocyclic aldehyde and elimination provides RC product 2. The alternative route involves a more complicated process (pathway B, NHC initiated). The NHC performs a conjugate addition to the vinyl sulfone to produce a sulfinate ion in situ. In previous reported studies, we have observed a smooth rearrangement of 1,1-bis vinyl sulfones to 1,2-bis sulfones by a sulfinate ejection/re-addition process.[12] The in situ sulfinate anion generated by NHC addition to the vinyl sulfone acts as the nucleophilic catalyst which then undergoes 1,4-addition to the enal, thereby forming enolate VI. At this point, anion VI would add to the vinyl sulfone and deprotonation results in the RC product 2. In support of pathway B, we have added sodium sulfinate (1 equiv.) to a solution of cinnamaldehyde and vinyl sulfone and observed the RC product with unidentified side products. However, the process did require a full equivalent of sulfinate (not 20 mol-% as per the normal reaction conditions) and does not rule out possible conjugate additions of the carbene to the vinyl sulfone (or enal) as depicted in pathway A.
Scheme 2.
Possible reaction mechanisms
Conclusion
We have demonstrated that a triazolium-derived NHC promotes the RC reaction between enals and vinyl sulfones. This process is noteworthy since the majority of NHC Lewis base reactivity comes from the addition to a carbonyl carbon, and not the β position of an α,β-unsaturated substrate. These results add to the growing list of and new reactivity modes for NHCs beyond the well established acyl anion and homoenolate equivalent strategies. Further development of this promising NHC reactivity pattern with other atypical NHC electrophilic partners is under investigation.
Experimental
All reactions were carried out under a nitrogen atmosphere in flame-dried glassware with magnetic stirring. Dichloromethane was purified by passage through a bed of activated alumina.[14] Reagents were purified before use unless otherwise stated following the guidelines of Perrin and Armarego.[15] Purification of reaction products was carried out by flash chromatography using EM Reagent silica gel 60 (230–400 mesh). Analytical thin-layer chromatography (TLC) was performed on EM Reagent 0.25 mm silica gel 60-F plates. Visualization of TLC plates was accomplished with UV light. Infrared spectra were recorded on a Perkin Elmer 1600 series FT-IR spectrometer. 1H NMR spectra were recorded on a Bruker A500 (500 MHz) spectrometer and are reported in ppm using solvent as an internal standard (CDCl3 at 7.26 ppm). Data are reported as (ap = apparent, s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad; coupling constant(s) in Hz; integration. Proton-decoupled 13C NMR spectra were recorded on a Bruker A500 (125 MHz) spectrometer and are reported in ppm using solvent as an internal standard (CDCl3 at 77.0 ppm). Mass spectrometric data for the products were acquired on an Agilent 6210 LC-Tof High Resolution Mass Spectrometer, using an Electro Spray Ionization source. Samples were introduced using 1 μL direct injection. The solvent used was 90 % MeOH/10 % water/0.1 % formic acid flowing at 0.5 mL min−1.
General Procedure for Formation of RC Product
To a reaction vial containing a magnetic stirring bar was added the corresponding 1,1-bis(phenylsulfonyl)ethylene (50 mg, 0.16 mmol), enal (2 equiv.), flame-dried 3Å molecular sieves, and dichloromethane (0.4 mL). A dichloromethane solution (0.2 mL) containing the azolium salt (0.03 mmol) and base (0.03 mmol) was then added, bringing the reaction solution to 0.25 M. The reaction was then heated to 408C for 24 h. The unpurified reaction was filtered through a pad of silica, washing with EtOAc and concentrated under vacuum. The material was purified by column chromatography (silica, 25 % EtOAc/hexanes), and after concentration of the appropriate fractions afforded the product.
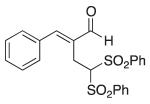
(E)-2-Benzylidene-4,4-bis(phenylsulfonyl)butanal (2)
Prepared according to the general procedure with dichloromethane, yielding 28 mg (39 %) as a white solid (25 % EtOAc/hexanes, RF = 0.18). νmax (film)/cm−1 3065, 2957, 2849, 1665, 1625, 1447, 1313, 1146, 1079. δH (500 MHz, CDCl3) 9.22 (s, 1H), 7.58–7.57 (m, 4H), 7.42 (m, 2H), 7.26–7.04 (m, 10H), 5.36 (t, J 6.9, 1H), 3.25 (d, J 6.9, 2H). δC (125 MHz, CDCl3) 195.0, 152.3, 137.6, 135.9, 134.6, 133.8, 130.2, 129.6, 129.19, 129.01, 78.9, 22.1. m/z HRMS (ESI) Calc. for C23H21O5S2+ [M + H]+ 441.0825. Found 441.0825.
A colourless tabular crystal of C23H20O5S2 having approximate dimensions of 0.52 × 0.29 × 0.12 mm3 was mounted using oil (Infineum V8512) on a glass fiber. All measurements were made on a Bruker APEX-II CCD Diffractometer with a CuK I S source at a temperature of 250(2) K with a theta range for data collection of 1.84° to 30.47°. Cell constants and an orientation matrix for data collection corresponded to a triclinic, space group P-1, with a 9.3104(5), b 10.4613(6), and c 11.7985(6)Å, α 89.825(3)°, β 70.317(2)° and γ 72.560(2)°. For Z 2 and FW = 440.51, the calculated density is 1.426 g cm3. The linear absorption coefficient, μ, for Mo Kα radiation is 0.293 mm−1. The maximum and minimum transmission factors were: 0.9662 and 0.8625, respectively. Of the 27 149 reflections collected, 6158 were unique (Rint 0.0668). The final cycle of full-matrix least-squares refinement on F2 was based on 6158 reflections and 271 variable parameters and converged with agreement factors of R1 0.0496 and wR2 0.1468 and goodness-of-fit on F2 = 0.990. Further information is contained in the CIF file, available from the Cambridge Crystallographic Data Centre, CCDC 833171.
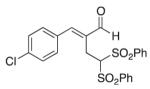
(E)-2-(4-Chlorobenzylidene)-4,4-bis(phenylsulfonyl) butanal (3)
Prepared according to the general procedure with dichloromethane, yielding 42 mg (55 %) as a white solid (25 % EtOAc/hexanes, RF = 0.18). νmax (film)/cm−1 3067, 2924, 2853, 1677, 1628, 1448, 1330, 1150, 1090, 1080. δH (500 MHz, CDCl3) 9.44 (s, 1H), 7.81–7.79 (m, 4H), 7.66 (ap t, 2H), 7.51–7.36 (m, 9H), 5.54 (t, J 6.9, 1H), 3.42 (d, J 6.9, 2H). δC (125 MHz, CDCl3) 194.8, 150.8, 137.6, 136.4, 134.7, 132.2, 130.8, 129.52, 129.46, 129.1, 78.8, 22.2. m/z HRMS (ESI) Calc. for C23H20ClO5S2+ [M + H]+ 475.0435. Found 475.0438.
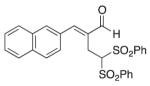
(E)-2-(Naphthalen-2-ylmethylene)-4, 4-bis(phenylsulfonyl)butanal (4)
Prepared according to the general procedure with dichloromethane, yielding 48 mg (61 %) as a pale yellow solid (25 % EtOAc/hexanes, RF = 0.17). νmax(film)/cm−1 3062, 2924, 2853, 1676, 1620, 1448, 1331, 1149, 1079. δH (500 MHz; CDCl3) 9.53 (s, 1H), 8.07 (s, 1H), 7.96 (dd, J 13.1, 5.9, 3H), 7.79 (d, J 8.4, 4H), 7.67–7.58 (m, 6H), 7.41–7.38 (m, 4H), 5.55 (t, J 6.8, 1H), 3.62 (d, J 6.8, 2H). δC (125 MHz; CDCl3) 194.9, 152.3, 137.5, 136.1, 134.5, 133.7, 133.1, 131.2, 130.0, 129.6, 128.94, 128.94, 127.92, 127.79, 127.1, 126.3, 79.1, 21.9 m/z HRMS (ESI) Calc. for C27H23O5S2+ [M + H]+: 491.0981. Found 491.0983.
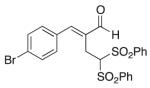
(E)-2-(4-Bromobenzylidene)-4,4-bis(phenylsulfonyl) butanal (5)
Prepared according to the general procedure with dichloromethane, yielding 47 mg (56 %) as a yellow solid (25 % EtOAc/hexanes, RF = 0.14). νmax (film)/cm−1 3067, 2923, 2852, 1677, 1628, 1448, 1330, 1149, 1079, 1009. δH (500 MHz, CDCl3) 9.43 (s, 1H), 7.80–7.78 (m, 4H), 7.68–7.60 (m, 5H), 7.49 (t, J 7.8, 4H), 7.38–7.34 (m, 2H), 5.52 (t, J 6.9, 1H), 3.41 (d, J 6.9, 2H). δC (125 MHz, CDCl3) 194.8, 150.8, 137.5, 136.5, 134.7, 132.7, 132.4, 131.0, 129.5, 129.1, 124.7, 78.9, 22.1. m/z HRMS (ESI) Calc. for C23H20BrO5S2+ [M + H]+: 518.9930. Found 518.9942.
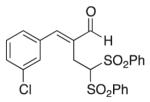
(E)-2-(3-Chlorobenzylidene)-4,4-bis(phenylsulfonyl) butanal (6)
Prepared according to the general procedure with dichloromethane, yielding 38 mg (49 %) as a yellow solid (25 % EtOAc/hexanes, RF = 0.17). νmax (film)/cm−1 3068, 2925, 2855, 1679, 1629, 1448, 1331, 1150, 1079. δH (500 MHz, CDCl3) 9.47 (s, 1H), 7.84–7.82 (m, 5H), 7.69–7.66 (m, 2H), 7.52–7.36 (m, 8H), 5.59 (t, J 7.0, 1H), 3.43 (d, J 6.9, 2H). δC (125 MHz, CDCl3) 194.7, 150.4, 137.5, 137.0, 135.5, 135.1, 134.7, 130.5, 130.1, 129.54, 129.36, 129.1, 127.4, 78.9, 22.3. m/z HRMS (ESI) Calc. for C23H20ClO5S2+ [M + H]+: 475.0435. Found 475.0440.
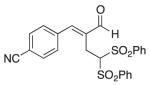
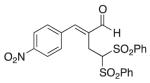
(E)-4-(2-Formyl-4,4-bis(phenylsulfonyl)but-1-en-1-yl) benzonitrile (7)
Prepared according to the general procedure with dichloromethane, yielding 49 mg (64 %) as a yellow solid (25 % EtOAc/hexanes, RF = 0.14). νmax (film)/cm−1 3067, 2929, 2229, 1681, 1448, 1331, 1313, 1148, 1079. δH (500 MHz, CDCl3) 9.48 (s, 1H), 7.75 (t, J 8.8, 6H), 7.66–7.60 (m, 4H), 7.49–7.41 (m, 5H), 5.53 (t, J 7.1, 1H), 3.37 (d, J 7.1, 2H). dC (125 MHz, CDCl3) 194.6, 149.4, 138.24, 138.20, 137.5, 134.8, 132.7, 129.9, 129.4, 129.1, 118.2, 113.4, 78.6, 22.5. m/z HRMS (ESI) Calc. for C24H20NO5S2+ [M + H]+: 466.0777. Found 466.0736.
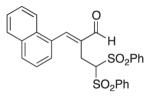
(E)-2-(Naphthalen-1-ylmethylene)-4,4-bis(phenylsulfonyl)butanal (8)
Prepared according to the general procedure with dichloromethane, yielding 46 mg (58 %) as a yellow solid (25 % EtOAc/hexanes, RF = 0.21). νmax (film)/cm−1 3063, 2925, 1679, 1627, 1448, 1331, 1151, 1079. δH (500 MHz, CDCl3) 9.75 (s, 1H), 8.11–7.99 (m, 5H), 7.71–7.48 (m, 8H), 7.33–7.29 (m, 5H), 5.56 (t, J 6.8, 1H), 3.42 (d, J 6.8, 2H). δC (125 MHz, CDCl3) 194.7, 150.5, 137.2, 134.5, 131.1, 130.7, 130.4, 129.6, 128.8, 127.4, 127.0, 126.9, 125.5, 124.2, 79.3, 22.3. m/z HRMS (ESI) Calc. for C27H23O5S2+ [M + H]+: 491.0981. Found 491.0982.
(E)-2-(4-Nitrobenzylidene)-4,4-bis(phenylsulfonyl) butanal (9)
Prepared according to the general procedure with dichloromethane, yielding 77 mg (98 %) as a pale orange solid (25 % EtOAc/hexanes, RF = 0.12). νmax (film)/cm−1 3070, 2926, 1682, 1572, 1448, 1341, 1313, 1150, 1079. δH (500 MHz, CDCl3) 9.56 (s, 1H), 8.29 (d, J 8.2, 2H), 7.91 (d, J 7.2, 2H), 7.79–7.48 (m, 11H), 5.57 (t, J 7.0, 1H), 3.20 (d, J 6.9, 2H). dC (125 MHz, CDCl3) 194.7, 149.5, 138.0, 134.6, 134.3, 129.2, 125.5, 78.5, 22.6 m/z HRMS (ESI) Calc. for C17H16NO5S+ [M H]+:486.0676. Found 486.0677.
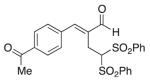
(E)-2-(4-Acetylbenzylidene)-4,4-bis(phenylsulfonyl) butanal (10)
Prepared according to the general procedure with dichloromethane, yielding 32 mg (41 %) as a pale yellow solid (25 % EtOAc/hexanes, RF = 0.23). νmax (film)/cm−1 3067, 2620, 2848, 1680, 1604, 1448, 1359, 1330, 1313, 1267, 1148, 1079. δH (500 MHz, CDCl3) 9.46 (s, 1H), 8.02 (d, J 8.3, 1H), 7.77–7.75 (m, 4H), 7.64–7.57 (m, 6H), 7.46–7.41 (m, 4H), 5.52 (t, J 7.0, 1H), 3.42 (d, J 7.0, 2H), 2.64 (s, 3H). δC (125 MHz; CDCl3) 197.3, 194.8, 150.5, 138.2, 137.6, 134.7, 129.62, 129.46, 129.09, 128.95, 78.7, 26.9, 22.4. m/z HRMS (ESI) Calc. for C25H23O6S2+ [M + H]+: 483.0931. Found 483.0924.
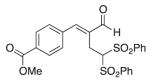
(E)-Methyl 4-(2-formyl-4,4-bis(phenylsulfonyl) but-1-en-1-yl)benzoate (11)
Prepared according to the general procedure with dichloromethane, yielding 40 mg (49 %) as a white solid (25 % EtOAc/hexanes, RF = 0.12). νmax (film)/cm−1 3065, 2953, 2847, 1721, 1678, 1448, 1437, 1331, 1313, 1282, 1183, 1149, 1114, 1079. δH (500 MHz, CDCl3) 9.48 (s, 1H), 8.12 (d, J 8.3, 1H), 7.80–7.78 (m, 4H), 7.66–7.63 (m, 3H), 7.55 (d, J 8.0, 2H), 7.49–7.44 (m, 5H), 5.54 (t, J 7.0, 1H), 3.98 (d, J 0.8, 2H), 3.43 (d, J 7.0, 3H). δC (125 MHz, CDCl3) 194.7, 166.3, 150.6, 138.1, 137.52, 137.43, 134.7, 131.2, 130.2, 129.50, 129.37, 129.1, 78.8, 52.5, 22.3. m/z HRMS (ESI) Calc. for C25H23O7S2+ [M + H]+: 499.0880. Found 499.0878.
Materials
Compound 1 was prepared as described previously and is also commercially available.[16]
Supplementary Material
Acknowledgements
Support for this work was provided by the NIH (GM73072-01), an NIGMS Research Supplement to Promote Diversity in Health-Related Research (R.L.A.), and an NIGMS Ruth L. Kirschstein NRSA fellowship (F31 GM96752). The authors thank S. Shafaie and C. L. Stern at Integrated Molecular Structure Education and Research Center (IMSERC) at Northwestern University for assistance with compound characterization, John Roberts (NU) for assistance with X-ray crystallography, and Daniel Cohen (NU) for providing α,β-unsaturated aldehyde substrates.
Footnotes
Accessory Publication
Spectroscopic and X-ray crystallography data are available as an accessory publication available on the Journal’s website. CCDC 833171 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
- [1].(a) Dalko P, Moisan L. Angew. Chem. Int. Ed. 2004;43:5138. doi: 10.1002/anie.200400650. doi:10.1002/ANIE.200400650. [DOI] [PubMed] [Google Scholar]; (b) List B. Chem. Commun. 2006;8:819. doi: 10.1039/b514296m. doi:10.1039/B514296M. [DOI] [PubMed] [Google Scholar]; (c) MacMillan DWC. Nature. 2008;455:304. doi: 10.1038/nature07367. doi:10.1038/ NATURE07367. [DOI] [PubMed] [Google Scholar]; (d) Doyle AG, Jacobsen EN. Chem. Rev. 2007;107:5713. doi: 10.1021/cr068373r. doi:10.1021/CR068373R. [DOI] [PubMed] [Google Scholar]; (e) Bertelsen S, Jorgensen KA. Chem. Soc. Rev. 2009;38:2178. doi: 10.1039/b903816g. doi:10.1039/B903816G. [DOI] [PubMed] [Google Scholar]; (f) Kampen D, Reisinger CM, List B. Top. Curr. Chem. 2009;291:395. doi: 10.1007/978-3-642-02815-1_1. doi:10.1007/128_2009_1. [DOI] [PubMed] [Google Scholar]
- [2].Denmark SE, Beutner GL. Angew. Chem. Int. Ed. 2008;47:1560. doi: 10.1002/anie.200604943. doi:10.1002/ANIE.200604943. [DOI] [PubMed] [Google Scholar]
- [3].(a) Enders D, Balensiefer T. Acc. Chem. Res. 2004;37:534. doi: 10.1021/ar030050j. doi:10.1021/AR030050J. [DOI] [PubMed] [Google Scholar]; (b) Nolan SP, editor. N-Heterocyclic Carbenes in Synthesis. Wiley-VCH; Weinheim: 2006. [Google Scholar]; (c) Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606. doi: 10.1021/cr068372z. doi:10.1021/CR068372Z. [DOI] [PubMed] [Google Scholar]; (d) Nair V, Vellalath S, Babu B. Chem. Soc. Rev. 2008;37:2691. doi: 10.1039/b719083m. doi:10.1039/B719083M. [DOI] [PubMed] [Google Scholar]; (e) Phillips EM, Chan A, Scheidt KA. Aldrichim. Acta. 2009;42:55. [PMC free article] [PubMed] [Google Scholar]
- [4].Ugai T, Tanaka S, Dokawa S. J. Pharm. Soc. Jpn. 1943;63:269. Breslow R. J. Am. Chem. Soc. 1958;80:3719. doi:10.1021/ JA01547A064. Hachisu Y, Bode JW, Suzuki K. J. Am. Chem. Soc. 2003;125:8432. doi: 10.1021/ja035308f. doi:10.1021/JA035308F. Chan A, Scheidt KA. J. Am. Chem. Soc. 2006;128:4558. doi: 10.1021/ja060833a. doi:10.1021/JA060833A. (e) For asymmetric variants, see: Enders D, Breuer K, Runsink J, Teles JH. Helv. Chim. Acta. 1996;79:1899. doi:10.1002/HLCA. 19960790712. Enders D, Breuer K, Teles JH. Helv. Chim. Acta. 1996;79:1217. doi:10.1002/HLCA.19960790427. Enders D, Kallfass U. Angew. Chem. Int. Ed. 2002;41:1743. doi: 10.1002/1521-3773(20020517)41:10<1743::aid-anie1743>3.0.co;2-q. doi:10.1002/1521-3773(20020517)41:10,1743::AID-ANIE1743.3.0.CO;2-Q. Kerr MS, de Alaniz JR, Rovis T. J. Am. Chem. Soc. 2002;124:10298. doi: 10.1021/ja027411v. doi:10.1021/JA027411V.
- [5].Burstein C, Glorius F. Angew. Chem. Int. Ed. 2004;43:6205. doi: 10.1002/anie.200461572. doi:10.1002/ANIE.200461572. Sohn SS, Rosen EL, Bode JW. J. Am. Chem. Soc. 2004;126:14370. doi: 10.1021/ja044714b. doi:10.1021/JA044714B. Chan A, Scheidt KA. Org. Lett. 2005;7:905. doi: 10.1021/ol050100f. doi:10.1021/ OL050100F. Nair V, Vellalath S, Poonoth M, Suresh E. J. Am. Chem. Soc. 2006;128:8736. doi: 10.1021/ja0625677. doi:10.1021/JA0625677. Chan A, Scheidt KA. J. Am. Chem. Soc. 2007;129:5334. doi: 10.1021/ja0709167. doi:10.1021/JA0709167. Nair V, Mathew SC, Biju AT, Suresh E. Angew. Chem. 2007;119:2116. doi: 10.1002/anie.200604025. doi:10.1002/ANGE.200604025. Chan A, Scheidt KA. J. Am. Chem. Soc. 2008;130:2740. doi: 10.1021/ja711130p. doi:10.1021/JA711130P. Phillips EM, Reynolds TE, Scheidt KA. J. Am. Chem. Soc. 2008;130:2416. doi: 10.1021/ja710521m. For NHC catalysis with doi:10.1021/JA710521M. (i) Lewis acids, see: Cardinal-David B, Raup DEA, Scheidt KA. J. Am. Chem. Soc. 2010;132:5345. doi: 10.1021/ja910666n. doi:10.1021/JA910666N. Raup DEA, Cardinal-David B, Holte D, Scheidt KA. Nat. Chem. 2010;2:766. doi: 10.1038/nchem.727. doi:10.1038/NCHEM.727. Cohen D, Cardinal-David B, Scheidt KA. Angew. Chem. Int. Ed. 2011;50:1678. doi: 10.1002/anie.201005908. doi:10.1002/ANIE.201005908. Cohen D, Cardinal-David B, Roberts JM, Sarjeant AA, Scheidt KA. Org. Lett. 2011;13:1068. doi: 10.1021/ol103112v. doi:10.1021/OL103112V.
- [6].(a) Chow K, Bode JW. J. Am. Chem. Soc. 2004;126:8126. doi: 10.1021/ja047407e. doi:10.1021/JA047407E. [DOI] [PubMed] [Google Scholar]; (b) Reynolds N, Rovis T. J. Am. Chem. Soc. 2005;127:16406. doi: 10.1021/ja055918a. doi:10.1021/JA055918A. [DOI] [PubMed] [Google Scholar]; (c) Phillips EM, Wadamoto M, Chan A, Scheidt KA. Angew. Chem. Int. Ed. 2007;46:3107. doi: 10.1002/anie.200605235. doi:10.1002/ANIE.200605235. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Song JJ, Tan Z, Reeves JT, Fandrick DR, Yee NK, Senanayake CH. Org. Lett. 2008;10:877. doi: 10.1021/ol703025b. doi:10.1021/OL703025B. [DOI] [PubMed] [Google Scholar]; (e) Huang X-L, Chen X-Y, Ye S. J. Org. Chem. 2009;74:7585. doi: 10.1021/jo901656q. doi:10.1021/JO901656Q. [DOI] [PubMed] [Google Scholar]; (f) Phillips EM, Wadamoto M, Roth HS, Scheidt KA. Org. Lett. 2009;11:105. doi: 10.1021/ol802448c. doi:10.1021/OL802448C. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Kawanaka Y, Phillips EM, Scheidt KA. J. Am. Chem. Soc. 2009;131:18028. doi: 10.1021/ja9094044. doi:10.1021/JA9094044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].(a) Castells J, Llitjos H, Morenomanas M. Tetrahedron Lett. 1977;18:205. doi:10.1016/S0040-4039(01)92589-4. [Google Scholar]; (b) Inoue H, Higashiura K. J. Chem. Soc., Chem. Commun. 1980;12:549. doi:10.1039/C39800000549. [Google Scholar]; (c) Miyashita A, Suzuki Y, Nagasaki I, Ishiguro C, Iwamoto K, Higashino T. Chem. Pharm. Bull. (Tokyo) 1997;45:1254. [Google Scholar]; (d) Maki BE, Chan A, Phillips EM, Scheidt KA. Org. Lett. 2007;9:371. doi: 10.1021/ol062940f. doi:10.1021/OL062940F. [DOI] [PubMed] [Google Scholar]; (e) Maki BE, Scheidt KA. Org. Lett. 2008;10:4331. doi: 10.1021/ol8018488. doi:10.1021/ OL8018488. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Maki BE, Chan A, Phillips EM, Scheidt KA. Tetrahedron. 2009;65:3102. doi: 10.1016/j.tet.2008.10.033. doi:10.1016/J.TET.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) De Sarkar S, Grimme S, Studer A. J. Am. Chem. Soc. 2010;132:1190. doi: 10.1021/ja910540j. doi:10.1021/JA910540J. [DOI] [PubMed] [Google Scholar]
- [8].(a) Morita K, Suzuki Z, Hirose H. Bull. Chem. Soc. Jpn. 1968;41:2815. doi:10.1246/BCSJ.41.2815. [Google Scholar]; (b) Baylis B, Hillman MED. German Patent 2155113. 1972;77:34174q. Chem. Abstr. 1972.; Drewes SE, Roos GHP. Tetrahedron. 1988;44:4653. doi:10.1016/S0040-4020(01)86168-8. Basavaiah D, Rao PD, Hyma RS. Tetrahedron. 1996;52:8001. doi:10.1016/0040-4020(96)00154-8. Langer P. Angew. Chem. Int. Ed. 2000;39:3049. doi: 10.1002/1521-3773(20000901)39:17<3049::aid-anie3049>3.0.co;2-5. doi:10.1002/ 1521-3773(20000901)39:17,3049::AID-ANIE3049.3.0.CO;2-5. Basavaiah D, Rao AJ, Satyanarayana T. Chem. Rev. 2003;103:811. doi: 10.1021/cr010043d. doi:10.1021/CR010043D.
- [9].(a) Fischer C, Smith SW, Powell DA, Fu GC. J. Am. Chem. Soc. 2006;128:1472. doi: 10.1021/ja058222q. doi:10.1021/JA058222Q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) He L, Jian T-Y, Ye S. J. Org. Chem. 2007;72:7466. doi: 10.1021/jo071247i. doi:10.1021/ JO071247I. [DOI] [PubMed] [Google Scholar]
- [10].(a) Rauhut MM, Currier H. US Patent 3 074 999. 1963; (b) Wang L-C, Luis AL, Agapiou K, Jang H-Y, Krische MJ. J. Am. Chem. Soc. 2002;124:2402. doi: 10.1021/ja0121686. doi:10.1021/JA0121686. [DOI] [PubMed] [Google Scholar]; (c) Frank SA, Mergott DJ, Roush WR. J. Am. Chem. Soc. 2002;124:2404. doi: 10.1021/ja017123j. doi:10.1021/JA017123J. [DOI] [PubMed] [Google Scholar]; (d) Thalji RK, Roush WR. J. Am. Chem. Soc. 2005;127:16778. doi: 10.1021/ja054085l. doi:10.1021/JA054085L. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Aroyan CE, Miller SJ. J. Am. Chem. Soc. 2007;129:256. doi: 10.1021/ja067139f. doi:10.1021/JA067139F. [DOI] [PubMed] [Google Scholar]; (f) Seidel F, Gladysz JA. Synlett. 2007;986 [Google Scholar]; (g) Reynolds TE, Binkley MS, Scheidt KA. Org. Lett. 2008;10:2449. doi: 10.1021/ol800745q. doi:10.1021/OL800745Q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Aroyan CE, Dermenci A, Miller SJ. Tetrahedron. 2009;65:4069. doi:10.1016/J.TET.2009.02.066. [Google Scholar]
- [11].(a) Fuchs PL, Braish TF. Chem. Rev. 1986;86:903. doi:10.1021/ CR00075A012. [Google Scholar]; (b) Simpkins N. Tetrahedron. 1990;46:6951. doi:10.1016/S0040-4020(01)87882-0. [Google Scholar]; (c) Grela K, Bieniek M. Tetrahedron Lett. 2001;42:6425. doi:10.1016/S0040-4039(01)01269-2. [Google Scholar]; (d) Mossé S, Alexakis A. Org. Lett. 2005;7:4361. doi: 10.1021/ol051488h. doi:10.1021/ OL051488H. [DOI] [PubMed] [Google Scholar]; (e) Mossé S, Laars M, Kriis K, Kanger T, Alexakis A. Org. Lett. 2006;8:2559. doi: 10.1021/ol0607490. doi:10.1021/OL0607490. [DOI] [PubMed] [Google Scholar]; (f) Zhu Q, Cheng L, Lu Y. Chem. Commun. 2008;47:6315. doi: 10.1039/b816307c. doi:10.1039/B816307C. [DOI] [PubMed] [Google Scholar]; (g) Zhu Q, Lu Y. Org. Lett. 2008;10:4803. doi: 10.1021/ol8019296. doi:10.1021/OL8019296. [DOI] [PubMed] [Google Scholar]; (h) Landa A, Maestro M, Masdeu C, Puente Á , Vera S, Oiarbide M, Palomo C. Chemistry. 2009;15:1562. doi: 10.1002/chem.200802441. doi:10.1002/ CHEM.200802441. [DOI] [PubMed] [Google Scholar]; (i) Sulzer-Mossé S, Alexakis A, Mareda J, Bollot G, Bernardinelli G, Filinchuk Y. Chemistry. 2009;15:3204. doi: 10.1002/chem.200801892. doi:10.1002/CHEM. 200801892. [DOI] [PubMed] [Google Scholar]; (j) Zhu Q, Lu Y. Org. Lett. 2009;11:1721. doi: 10.1021/ol9003349. doi:10.1021/OL9003349. [DOI] [PubMed] [Google Scholar]; (k) Moteki SA, Xu S, Arimitsu S, Maruoka K. J. Am. Chem. Soc. 2010;132:17074. doi: 10.1021/ja107897t. doi:10.1021/JA107897T. [DOI] [PubMed] [Google Scholar]; (l) Nielsen M, Jacobsen CB, Holub N, Paixão MW, Jørgensen KA. Angew. Chem. Int. Ed. 2010;49:2668. doi: 10.1002/anie.200906340. [DOI] [PubMed] [Google Scholar]; (m) Quintard A, Alexakis A, Mazet C. Angew. Chem. Int. Ed. 2011;50:1393. doi: 10.1002/anie.201007001. [DOI] [PubMed] [Google Scholar]; (n) Xiao J, Lu Y-P, Liu Y-L, Wong P-S, Loh T-P. Org. Lett. 2011;13:876. doi: 10.1021/ol102933q. doi:10.1021/OL102933Q. [DOI] [PubMed] [Google Scholar]
- [12].Atienza RL, Roth HS, Scheidt KA. Chem. Sci. 2011;2:1772–1776. doi: 10.1039/C1SC00194A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].For selected examples beyond our NHC catalysis program, see: Lettan RB, Scheidt K. Org. Lett. 2005;7:3227. doi: 10.1021/ol051030f. doi:10.1021/ OL051030F. Mattson AE, Zuhl AM, Reynolds TE, Scheidt KA. J. Am. Chem. Soc. 2006;128:4932. doi: 10.1021/ja056565i. doi:10.1021/JA056565I. Mattson AE, Scheidt KA. J. Am. Chem. Soc. 2007;129:4508. doi: 10.1021/ja068189n. doi:10.1021/JA068189N.
- [14].Pangborn AB, Giardello MA, Grubbs RH, Rosen RK, Timmers FJ. Organometal. 1996;15:1518. doi:10.1021/OM9503712. [Google Scholar]
- [15].Perrin DD, Armarego WL. Purification of Laboratory Chemicals. 3rd Edn Pergamon Press; Oxford: 1988. [Google Scholar]
- [16].Stetter H, Steinbeck K. Liebigs Ann. Chem. 1974;1315 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.