1. ABSTRACT
Calcium is a ubiquitous signaling molecule, indispensable for cellular metabolism of organisms from unicellular life forms to higher eukaryotes. The biological function of most eukaryotic cells is uniquely regulated by changes in cytosolic calcium, which is largely achieved by the universal phenomenon of store-operated calcium entry (SOCE). The canonical TRPs and Orai channels have been described as the molecular components of the store-operated calcium channels (SOCC). Importantly, the ER calcium-sensor STIM1 has been shown to initiate SOCE via gating of SOCC. Since the discovery of STIM1, as the critical regulator of SOCE, there has been a flurry of observations suggesting its obligatory role in regulating TRPC and Orai channel function. Considerable effort has been made to identify the molecular details as how STIM1 activates SOCC. In this context, findings as of yet has substantially enriched our understanding on, the modus operandi of SOCE, the distinct cellular locales that organize STIM1-SOCC complexes, and the physiological outcomes entailing STIM1-activated SOCE. In this review we discuss TRPC channels and provide an update on their functional regulation by STIM1.
Keywords: Calcium signaling, SOCE, TRPC channels, STIM1, Caveolin, Lipid raft, Gene Regulation, Proliferation
2. CALCIUM SIGNALING
Calcium (Ca2+) is one of the simplest and perhaps the most versatile cellular messengers. The idea that Ca2+ is intimately associated with cell physiology was realized over a century ago by Sidney Ringer. In 1883 he made the landmark observation that, it was a trace amount of Ca2+ which made isolated rat hearts placed in distilled water to beat continuously (1–2). Since this inadvertent discovery, there has been a prolific advance in the saga of Ca2+ signaling. In a cellular context the onset of Ca2+ signaling is marked primarily by an increase in the cytosolic Ca2+ ((Ca2+)cyt), which then engages in regulating a myriad of complex biological processes such as muscle contraction, neural transmission, hormonal, peptides, and fluid secretion, gene transcription, inflammation, cell proliferation and even cell death (3–4). In order to efficiently coordinate these physiologically diverse functions, cells strategically organize an array of PM Ca2+ channels, pumps, buffers, exchangers, and their regulators that spatiotemporally orchestrate (Ca2+)cyt levels in response to various signaling cues. It is thus apparent that, the deliberate positioning of purpose-specific signaling molecules at precise cellular compartments would be a judicious physiological act of a cell. Importantly, the specificity and accurate execution of almost all of these physiologically diverse processes depend on the controlled regulation of the multifarious Ca2+ signals that are initiated at precise cellular microdomains and are regulated by protein-protein interactions (5–6). Thus, compartmentalization of Ca2+ influx pathways to specific microdomains favors efficient communication between an assortment of receptors, channels, and their modulators. In addition, such microdomains can facilitate cellular signaling by providing an elaborately organized niche to cluster a unique set of molecular components that would otherwise be physically isolated (7–9).
Distinct signaling mechanisms are shown to be present in different cell types that trigger Ca2+ influx (6, 10). In most cells types, both release of Ca2+ from intercellular ER stores as well as Ca2+ influx across the PM is essential to maintain a critical control of various physiological functions (4, 10–12). The critical question, however, is how do complex biological systems translate different signaling cues to choreograph the generation and homeostasis of cellular Ca2+ signals in order to execute appropriate physiological responses in a controlled manner. To this end a multitude of defined molecular components of Ca2+ influx and release pathways have been identified and studied extensively. However, in this review we mainly focus on store-operated Ca2+ entry (SOCE), a unique cellular mechanism that maintains Ca2+ homeostasis in virtually every cell. As an important aspect of SOCE, we discuss TRPC channels and their regulation by STIM1 and other regulators. Furthermore, specific cellular microdomains involved in regulating TRPC function, STIM1 localization upon stimulation, the TRPC channel gating mechanism and the physiological outcomes of channel activation are also summarized.
3. MOLECULAR COMPONENTS OF SOCE
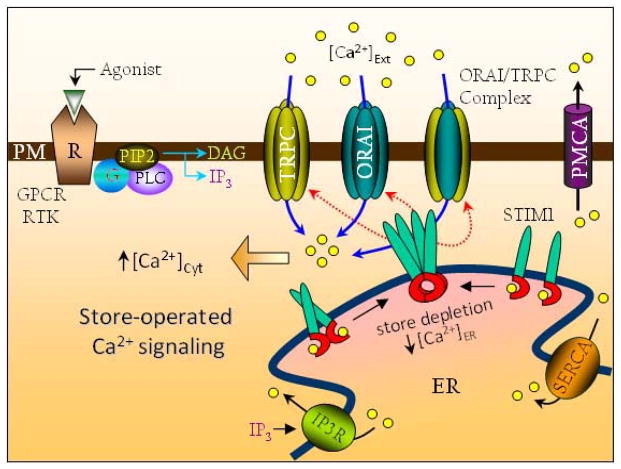
Most cellular processes require the release of Ca2+ from intracellular ER-stores followed by the influx of external Ca2+ to sustain many physiological responses. Ca2+ influx across the PM is not only essential for the refilling of internal ER-stores, but also contributes toward regulation and fine-tuning of several biological processes (5, 10, 13). This very process of activating PM Ca2+ channels by ER store-depletion is referred to as store-operated Ca2+ entry (SOCE) and the channels activated are broadly classified as store-operated Ca2+ entry channels (SOCC) (14–17). As illustrated in Figure 1, the first step in the initiation of SOCE is the binding of agonists (hormone or growth factors) to the PM receptors (e.g.-GPCRs/RTKs). Activation of the cell surface receptor engages a cascade of signaling events that culminates in the PLC- (Phospholipase C) mediated hydrolysis of the membrane PIP2 (phosphotidylinositol-4,5- bisphosphate) resulting in the generation of membrane bound diacylglycerol (DAG) and the diffusible messenger - inositol 1,4,5-trisphosphate (IP3). IP3 binds to IP3Rs that are localized in the ER and mediates the release of Ca2+ from intracellular ER stores, which results in activation of the PM-SOCC thereby elevating (Ca2+)cyt. This increase in (Ca2+)cyt is not only essential for regulating biological functions, but is also pumped back into the ER via the SERCA pump. The remaining Ca2+ is then pumped out that concertedly help in lowering the (Ca2+)cyt, and completing the SOCE cycle (14).
Figure 1.
STIM1 activated SOCE. The current molecular concept of SOCE is STIM1-mediated activation of PM-SOCC (TRPC/Orai channels). Agonist-induced receptor (GPCR/RTK) activation results in PLC-mediated hydrolysis of PIP2, generating the diffusible and membrane-bound cellular messengers – DAG (diacylglycerol) and IP3 respectively. IP3 binds to its receptor (IP3Rs) in the ER depleting the ER Ca2+ stores. This leads to STIM1 oligomerization by interaction of mono- or dimeric STIM1 molecules. The STIM1 oligomeric-clusters thus formed are subsequently recruited to ER-PM juxtaposed sites allowing STIM1 to physically activate the TRPC and Orai channels to bring about Ca2+ entry. This raises the (Ca2+)cyt which results in store-operated Ca2+ signaling and influences a variety of cellular functions. To complete the SOCE cycle, (Ca2+)cyt is sequestered back to the ER by the SERCA pump and/or extruded to the cells’ exterior by PMCA. The membrane-associated lipid messenger - DAG also has the ability to activate select TRPC channels independent of ER-stores and presumably STIM1 as well.
The concept of SOCE (also known as capacitative Ca2+ entry) was introduced about three decades back (18–19); however, the molecular identity of the ion channels and the regulators facilitating SOCE has just begun (20). Early genetic screens in Drosophila identified spontaneous gene mutations involved in phototransduction pathway, which eventually lead to the discovery of TRP channels (21). Mammalian homologs of the Drosophila trp genes have been suggested as candidate SOCC components. The TRPC (canonical TRP) family of ion channels constitutes a major subclass of the seven TRP subfamilies identified thus far. (22–27). TRPC1 was the first member of the TRPC family to be cloned and has been studied extensively (28–29). In addition to TRPC1, other TRPCs have also been shown to be regulated by store depletion per se. In addition, recent findings have also suggested that Orai proteins, which appear to be the molecular component of the elusive CRAC (Ca2+ release-activated Ca2+ entry) channels, to function as SOCC, however, this is predominantly seen in immune cells (10, 30–33). Moreover, TRPC channels have been shown to interact with Orai1, suggesting that both these channels can be the components of the illusive SOCE channel especially in non-immune cells.
One of the important aspects of SOCE that was poorly understood is the molecular mechanism (s) by which the status of the ER Ca2+ store is communicated to the PM channels, to initiate Ca2+ influx. Thus, identification of the working mechanism of SOCE has been a continuous scientific pursuit for over two decades. Multiple possibilities were put forward to provide a reasonable explanation to the question of how the status of ER stores is communicated to the PM Ca2+ channels (14–15, 34–36). For instance it has been suggested that, for SOCE activation the signal from the ER stores is transmitted to the PM Ca2+ channels by either a conformational-coupling mechanism, or via a diffusible messenger CIF (Ca2+ influx factor), which transits information about store-depletion, and activates the PM Ca2+ channels. In addition, store-depletion dependent recruitment of vesicle associated SOCC channel to the PM has also been proposed as a possible mechanism. However, none of these proposed models have either been refuted or have gotten universal acceptance.
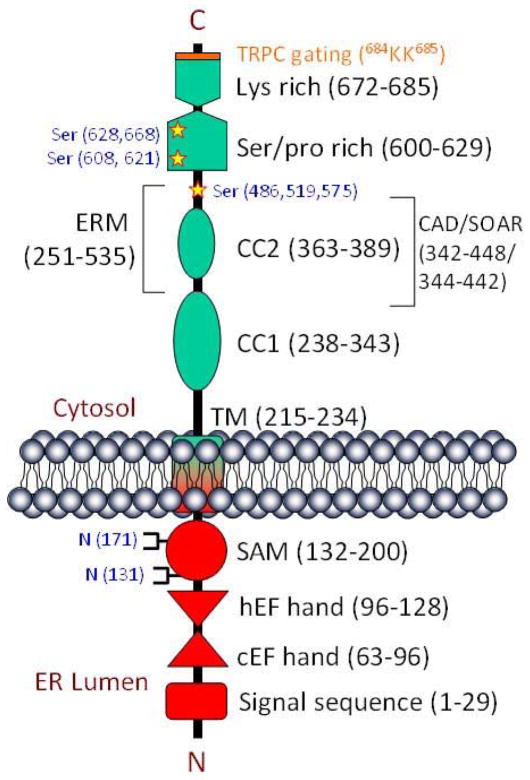
A recent seminal finding in the field of store-operated Ca2+ signaling has now solved the enigma of ER-PM communications during activation of SOCE. In mid-2005, two groups – one led by Tobias Meyer (Stanford University) and the other jointly led by Michael Cahalan (University of California at Irvine) and Kenneth Stauderman (Torrey Pines Therapeutics Inc., La Jolla, CA), independently identified the signaling molecule that was involved in linking ER store-depletion to the activation of PM-SOCC. Using large-scale RNA-interference screens on cellular signaling proteins, they identified STIM proteins (stromal interaction molecule 1 and 2) as the most important regulators of SOCE (37–38). Identification of STIM, as the missing link involved in transmitting the ER store-depletion message to the PM-SOCC, gave an answer for the most sought after query in store-operated Ca2+ signaling biology. STIM1 and STIM2 are both single-pass transmembrane proteins with N-terminal Ca2+ binding EF-hands (canonical and hidden) and SAM (sterile alpha motif) domain located either extracellular or in the ER lumen. On the other hand the C-terminus domain of STIM1 is localized in the cytoplasm and is shown to contain the ERM (ezrin-radixin-moesin) and coiled-coil protein interacting domains (39–40). STIM1 has been shown to be glycosylated and associate with the PM (41–43), however the precise role of PM-STIM1 in SOCE remains unclear (44). In addition, STIM1 is shown to be phosphorylated (41), predominantly at serine residues, and this appears to be important for SOCE regulation during cell division (45–46). An updated structure of STIM1 showing critical molecular domains identified thus far is shown in Figure 2. Similarly STIM2, which is about 45 percent identical with STIM1, was also identified as a second modulator; however recent reports indicate that STIM2 is primarily involved in maintaining basal Ca2+, rather than initiating SOCE (44).
Figure 2.
STIM1 domains. This model (not drawn to scale) depicts the critical domains of STIM1 with its N-terminal in the ER lumen and the C-terminal in the cytosol. The amino acids that span individual domain are shown within parentheses. The lysine (K) residues, critical for TRPC channel gating (shown in orange), lie within the poly-K region in the cytoplasmic domain on STIM1. The cytoplasmic domain of STIM1 also contains the overlapping Orai1 activating regions (CAD/SOAR). Shown in blue are the residues that are glycosylated (N-terminal) or phosphorylated on serine (C-terminal). Abbreviations - c/hEF hand (canonical/hidden), SAM (sterile alpha motif), TM (trans membrane), CC1/2 (coiled-coil), ERM (Ezrin-Radixin-Moesin), CAD (CRAC activation domain), SOAR (STIM1 Orai activating region)
Functionally, STIM1 in the ER responds to the changes in ER Ca2+ levels via reversible binding of Ca2+ with its EF-hand domain. Following depletion of ER Ca2+, STIM1 undergoes homotypic interactions and forms oligomeric clusters (puncta) at the ER-PM junctions. The STIM1 puncta at the peripheral ER can thus physically associate with PM-SOCC thereby activating SOCE (37, 47–52). Although the identification of the ER Ca2+ sensor, STIM1, has revolutionized our understanding of SOCE, the question on what is the importance of STIM1 puncta and how are they stabilized and recruited to sites of ER in apposition to the PM is still an unsolved mystery. Whether, the composition of the PM at these junctional sites influence the specificity of STIM1 recruitment is not known. Thus, information on the identity of the ER-PM junction sites, at sub-plasma membrane regions, where STIM1 clusters translocate and associate with the PM-SOCC to activate SOCE is warranted. Nevertheless, identification of STIM1 as a critical regulator of TRPC as well as the Orai channels has provided novel insight into their activation paradigms.
4. ACTIVATION MODALITIES OF NON-SELECTIVE TRPC CHANNELS
TRPC channels function as non-selective cation channels and conduct relatively large Na+, Ca2+, Ba2+ or Sr2+ inward currents (53). Although TRPC channels can be classified into two different groups depending on their mode of activation (store vs receptor mediated activation), under physiological condition all TRPC channels are activated by G-protein coupled mechanism. Several studies, using various cells, have shown that TRPC1, C4, and sometimes C5 can be activated by store depletion per se (54–55). While, the second group comprising of TRPC3, C6, and C7 can be activated by second messenger system. Also, it has been shown that the C3/C6/C7 subfamily can be gated by DAG (26, 54–55). Thus, this group forms a distinct subfamily of DAG-sensitive cation channels, coupling receptor/PLC-signaling pathways to cation entry. While there is no doubt that all members of the TRPC3/C6/C7 subfamily can principally be activated by DAG, it is still contentious issue as to whether DAG can be regarded as the physiological activator of the native channel. As deduced from pharmacological inhibition of DAG lipase and DAG kinase, it appears that endogenously generated DAG is sufficient for channel activation (17), however; this needs to be verified in other cell types. Most notably, receptor agonists and DAG did not display additive effects of C3 and C6 current amplitudes, suggesting that the same TRPC channels are activated by DAG and by PLC-linked receptors and that DAG may be the decisive second messenger generated by PLC. However, so far a direct interaction of DAG with these TRPC proteins has not been demonstrated.
Several mechanisms have been shown to activate/inactivate TRPC channels. N-terminus of IP3Rs is shown to interact with TRPC channels, which can activate TRPC1, TRPC3, and TRPC4 channels (56–57). In contrast, calmodulin is also shown to interact with TRPC channels and is involved in Ca2+-dependent feedback inhibition of TRPC channels by acting as a Ca2+ sensor (58–59). Interestingly, IP3Rs peptides compete with calmodulin for binding to TRPC channels (60), and addition of calmodulin prolongs the interval between Ca2+ release from intracellular stores and activation of Ca2+ influx (59–60). Additionally, since calmodulin binding affinity increases with elevated Ca2+, this mechanism may be important for certain adaptations and be influenced by cytosolic Ca2+ or release of Ca2+ from intercellular stores and might also be critical for allowing STIM1 to interact with TRPC channels. Phosphorylation has also been identified as a possible regulator of TRPC channels. TRPC1 is known to be phosphorylated by PKC and is important for Ca2+ entry in human endothelial cells (61). In contrast, TRPC3 activity is shown to be inhibited by both PKC and PKG (62). PKG inactivates TRPC3 by phosphorylating TRPC3 at Thr-11 and Ser-263, whereas, PKC inactivates TRPC3 by phosphorylation on Ser-712. On the other hand, Src kinases were found to be essential for the activation of TRPC3 (63); however no direct evidence with regard to phosphorylation of TRPC3 channel has been established. Similarly, Fyn (member of the Src family kinase) and CaMK II-dependent phosphorylation has been shown to regulate TRPC6 activity (64–65). Besides these mechanisms other proteins are also shown to be critical for the activation of TRPC channels. For example, Homer and caveolin1 (Cav1) has been shown to negatively regulate TRPC1 channels (66–67), whereas, STIM1 positively activate TRPC1 channels (68). Similarly, RyRs interaction with TRPC channels is shown to be essential for its activation (69). Although, it has been shown that Homer antibodies efficiently immunoprecipitate TRPC1-TRPC4 complexes, it was not equivalently able to precipitate using IP3Rs antibodies (70). These results suggest that when different subunits form a functional channel the mode of their regulation could be completely different. Moreover, since Homer and Cav1 could bind to different TRPC channels, intermolecular interactions of the TRPC channels could influence Homer and Cav1 binding, thereby modulating the channel activity differently. In addition, since these proteins bind at the C-terminus of TRPC channel, there could be competition between their interactions and several factors may decide as to which protein will interact with TRPC channels and regulate Ca2+ influx accordingly. Overall, these results suggest that diverse regulatory mechanism could be present, which can regulate these TRPC channels and depending on the need and the complexity of the cell, the cell may decide as to which regulatory protein TRPC channels will interact, in order to modulate Ca2+ influx. Thus, more research is needed to confirm these interactions within more dynamic conditions involving activation/inactivation of these TRPC channels and quantify the association of each protein with regard to channel activity.
TRPC proteins contain several assembly domains that could be important in regulating protein-protein interactions. N-terminal cytosolic domain of TRPC channels contains coiled-coil domains and several (3–4) ankyrin repeats, which are one of the most common protein–protein interaction motifs. Structural information on ankyrin domains includes a 33-amino-acid sequence representing a highly conserved helix–turn–helix structure that can be important for self multimerization, and also can play an essential role in protein-protein interactions. Importantly, deletion of the ankyrin domains in several TRPCs not only loses their ability to form tetramers, but also had issues with regard to membrane targeting and activation of TRPC channels. For example, deletion of TRPC3 ankyrin domains has been shown to decrease its PM localization (60, 71). Similarly, deletion of the first ankyrin domain in TRPC5, failed to form homo or heteromultimers thereby leading to non-functional channels (72). Although the amino acids involved in these interactions are not yet identified, point mutations within this region in TRPV6 have been shown to abolish channel assembly and resulted in non-functional channels, indicating that similar mechanisms can also exist in the canonical TRPs as well. Besides, the ankyrin domains, a coiled-coil region is also present at both the N and C-terminus of TRPC channels. Importantly, the N-terminal coiled-coil region is found to be essential for TRPC1 assembly and for interaction of Drosophila TRPL and TRPγ (73). Also, tetramerization of TRPC4 or TRPC6 channels requires both the coiled-coil motif and the ankyrin repeats (74).
In Drosophila, the TRP channels have been shown to be associated in a signalplex, which provides a unique platform to modulate TRP channel activity (26, 75). The key component of the Drosophila signalplex is INAD scaffold, which exhibits five PDZ domains and interacts with multiple proteins to orchestrate phototransduction. Similarly, mammalian TRPC channels not only interact among themselves to form homomeric and heteromeric channels, but have also been shown to interact with multiple proteins (55, 76). However, the scaffold protein similar to INAD has not yet been identified. Additionally, it is not clear if all these proteins interaction are constitutive or are dynamic. Thus, identification of interplay between these signalplex proteins can be essential for regulating the physiological function exhibited by these TRPC channels. Furthermore, alterations in their interactions can potentially inhibit or amplify channel function leading to pathological conditions. Recent research has indicated that TRPC channels are assembled into lipid raft domains, which are not only critical for the function of TRPC channels, but are also important in the assembly and retention of TRPC1 at the PM (55, 67, 76–80). Interestingly, mutations in the Drosophila INAD protein not only hamper TRP function, but also alter the localization of its interacting proteins (81). Thus, it can be anticipated that disruption of the mammalian TRPC signalplex will also lead to altered localization of TRPC channels and thus interfere in its ability to functionally interact with critical regulatory molecules.
Recent studies have demonstrated an obligatory role of STIM1 in activating TRPC channels and regulating SOCE (details provided in Table 1). In this context STIM1 activation of TRPC1 channel has been extensively studied. Physiologically, the interaction between STIM1 and TRPC1 is dynamic and reversible and is regulated by the status of ER Ca2+ stores in a way that, upon agonist-mediated store-depletion the STIM1-TRPC1 interaction is increased. However, following agonist clearance and subsequent refilling of the ER stores the STIM1-TRPC1 complex dissociate to a resting state (79). Although the precise control of this interaction cycle is not established, the kinetics underlying the homomultimeric clustering of STIM1 appears to be a prime driving force. STIM1 has been shown to associate with TRPC1 in a complex containing Orai1; however, independently their regulation by STIM1 is distinct, since functional interaction site on STIM1 is different (82–83). STIM1 binds to native TRPC channels as efficiently as it binds with ectopically expressed channels. With regard to STIM-TRPC associations, there seems to be a preference. STIM1 interacts with TRPC1, C2, C4 and C5 but doesn’t seem to interact with TRPC3, C6 and C7. It has been suggested that, although STIM1 doesn’t directly bind to certain TRPC channels it can still be able to influence their function indirectly since, native SOCCs can constitute heteromeric TRPC assemblies. This distinction in the activation mechanism of TRPC channels could explain as why TRPC1/C4/C5 are deemed as candidates for store depletion, whereas TRPC3/C6/C7 are mainly believed to be dependent on second messenger system. Similarly, TRPC1 has been shown to functionally interact with Orai1 channels and Orai1 channels have been shown to be regulators of TRPC channels (84–85). In addition, SOCE via the TRPC channels has been shown to be both Orai1 dependent and Orai1-independent (86–87), suggesting that positioning of specific channels along with precise interactions with other molecules at cellular compartments could co-ordinate Ca2+ entry. Overall, these results suggest that depending on the cell type and the need of Ca2+ signal, these interactions could have additive effect on the regulation of TRPC channels and that Ca2+ channels are more complex that requires multiple proteins for the generation of Ca2+ currents. In addition, compartmentalization of these key proteins could also play a significant role in these functional interactions and many of the TRPC interacting proteins have been shown to be partitioned in both lipid raft and non-lipid raft fractions (76).
Table 1.
TRPC-STIM1 association and physiological response
| Interaction | Cell line/tissue | Method | Physiology |
|---|---|---|---|
| TRPC1-STIM1 | HEK293 (40, 82, 87, 121, 123, 143) HSG (68, 79, 124, 144) HL-7702 (normal human liver cell line) (145) Human platelets (125, 146) Human Parathyroid (147) Human VSMCs (130, 132) Intestinal epithelial cells (133) Pulmonary artery cells (141–142) Human glomerular mesangial cells (148) Mouse pancreatic acinar cells (128) |
Co-IP, GST-pull down RNAi, TIRF, FRET Ca2+ measurements Confocal imaging Co-fractionation Co-expression |
SOCE activation Cell proliferation Cell migration Wound healing Transcriptional regulation (NFκB, CREB) Ternary complex with Orai1 |
| TRPC2-STIM1 | HEK293 (40, 123) | Co-IP, GST-pull down | n.d |
| TRPC3-STIM1 | HEK293 (40, 87, 123, 143, 149) | Co-IP (indirect) | Agonist-induced Ca2+ entry |
| TRPC4-STIM1 | HEK293 (40, 87, 123) Human glomerular mesangial cells (148) |
Co-IP, GST-pull down, RNAi, Ca2+ measurements | SOCE activation |
| TRPC5-STIM1 | HEK293 (87) | Co-IP | SOCE activation |
| TRPC6-STIM1 | HEK293 (40, 87, 143) | Co-IP (indirect) | Agonist-induced Ca2+ entry |
| TRPC7-STIM1 | HEK293 (150) | Co-IP (no interaction) | n.d |
The STIM1-TRPC interaction is shown to be mediated by the ERM domain present in the cytosolic region of STIM1 and deletion of the ERM domain has been shown to suppress SOCE. Interestingly, however, the binding and gating of TRPC channels by STIM1 seems to be dissociated. The poly-Lys (poly-K) region in STIM1 C-terminus is shown to be required for TRPC channel gating but is dispensable for STIM1-TRPC interactions. Deletion of this poly-Lys region impairs the ability of STIM1 to gate native TRPC1 channels and initiate SOCE. Further studies lead to the identification of the 684KK685 residues within the STIM1 poly-K region to be imperative for TRPC1 gating. This gating was shown to be facilitated by electrostatic associations between the STIM1684KK685 and TRPC1639DD640 which was proved by well thought out charge-swap experiments. These negatively charged aspartates is conserved in other TRPCs as well, thus it can be reasoned that the electrostatic association will be a common step in the STIM1-mediated gating (direct or indirect) of other TRPC channels. Indeed, with the exception to TRPC7, this commonality (presence of the conserved DD domain) exists in the STIM1-mediated activation of TRPC channels.
5. MEMBRANE RAFTS/CAVEOLAE AND CA2+ SIGNALING
The PM is spatially organized into multiple microdomains which constitute unique signaling nodes to efficiently relay external signals. The concept of lipid rafts was initially developed to convey the idea that these membrane microdomains form small, but discrete membrane platforms which function as signaling organizers (8, 88–91). A sub category of the lipid-rich membrane rafts are ‘caveolae’, which form omega-shaped membrane invaginations ranging from 50–200nm in diameter. This structural uniqueness of caveolae enables specific set of PM signaling complex to reach deeper into the cytosol thereby giving an added advantage for PM-organellar crosstalk. Thus, these invaginations can facilitate interactions between proteins that are cytosolic as well as located in separate organelles (e.g.-ER and mitochondria) (92), thereby mediating a communication between separate membrane compartments (e.g.-PM with ER), that would otherwise be several microns apart (7, 90–91, 93–94). Membrane rafts have been efficiently shown to be involved in organization of cell signaling machinery, including G protein-coupled receptor (GPCR) and receptor tyrosine kinase (RTK) pathways (95–99). Additionally, membrane rafts have now been suggested to play a significant role in many biological processes, including signal transduction pathways, apoptosis, viral infections, cell adhesion and migration, synaptic transmission, organization of the cytoskeleton, and plasma membrane protein sorting during both exocytosis and endocytosis (7, 94, 100). Importantly, since many of the above physiological process are also know to require Ca2+, it can be postulated that lipid rafts can potentially regulate these processes by modulating Ca2+ signaling.
Ca2+ signals are generated across wide spatial and temporal ranges that are efficiently coordinated through organization of specific Ca2+-channels, pumps, buffers, exchangers and protein scaffolds into common microdomains. Membrane rafts serve as such a microdomain wherein highly specific signaling events can be efficiently executed with precision. Involvement of lipid rafts/caveolae in Ca2+ regulation was identified over three decades back (101–102), but direct evidence was only shown recently (67, 80). The first evidence that identified the involvement of caveolae in Ca2+ homeostasis was observed in muscle cells where the SR was localized immediately underneath the plasma membrane and was in close proximity to caveolae (101). Soon after, other investigators identified that caveolae can effectively increase intracellular Ca2+, which may activate the contractile apparatus to produce a sustained vasoconstriction (103–104). Histochemical methods further confirmed that Ca2+ was found in the lumina of caveolae, suggesting the importance of caveolae in Ca2+ signaling (105). Further, X-ray spectral analysis confirmed that Ca2+-peak (corresponding to increases in (Ca2+)cyt) can be found within two different cellular compartments: in small invaginations of the sarcolemma, which is caveolae, and in the intrafibrillar sarcoplasmic reticulum (106). Additionally, PMCA pumps as well as IP3 -regulated Ca2+ channels were also shown to be localized in caveolae (107–109). Although these initial studies performed in mid-1990 provided clues that caveolae are important for Ca2+ signaling, not much research was performed to understand the mechanism or role of caveolae in Ca2+ influx per se. It was Anderson’s group that initially showed that agonist-stimulated Ca2+ signal originated in specific areas of the plasma membrane that were enriched in Caveolin1 (Cav1) (110–111). Interestingly, not only G-protein coupled receptors but also Gq/11, phospholipase C, IP3Rs, and SOCE channels, are now known to be present in lipid raft domains. The ability to concentrate most proteins of the SOCE cascade in a single microdomain suggests that proteins are grouped together to effectively coordinate Ca2+ signaling. Importantly, direct interaction between TRPC1 and Cav1 had been shown to have a direct role in regulating Ca2+ influx (23, 112).
Mammalian TRPC channels have been shown to associate with multiple proteins including STIM1, Gαq/11, PMCA, SERCA, IP3Rs, Homer, CaM, GPCRs, receptor tyrosine kinases, RyRs, PLCγ, PLCβ, NCX1, Na+/K+/ATPase, NHERF and Cav1, which are known to influence TRPC channel function (12, 24, 55, 113–116). Although a number of such TRPC interacting proteins have been identified, none of them have been shown to be a mammalian TRPC scaffold. Nonetheless, since most of TRPC interacting proteins are also known to be localized in lipid rafts, they together can presumably provide a unique platform/scaffold to coordinate Ca2+ entry. Lipid rafts are also dynamic entities, where small rafts merge into bigger platforms (117–118), which fits into the possibility of providing a preference for protein-protein interactions. Thus, lipid raft microdomains can in a dynamic, spatiotemporal fashion, facilitate direct physical, or functional, coupling between molecular components that are critical in the activation or inactivation of Ca2+ entry channels. In addition to its role of clustering related signaling molecules, Cav1 proteins can also regulate trafficking of various receptors/mediators to the PM (119). It is now evident that TRPC1 channels are assembled into lipid raft/caveolar microdomains, where they interact with Cav1. Importantly, all functional mammalian TRPC proteins have putative Cav1 binding domains at both their N and C terminus (67, 76, 78). The N-terminal domain of TRPC1 which interacts with Cav1 has been shown to be critical for its plasma membrane retention. However, the significance of the putative C-terminal binding domains remains to be explored. Studies performed with the expression of a mutant Cav1 (lacking its protein scaffolding and membrane anchoring domains) or cav1 gene knockout has been shown to disrupt the plasma membrane localization of TRPC1 leading to a significant decrease in Ca2+ influx upon store depletion (67, 80). Additionally, loss of membrane raft domains in non-excitable cells has been shown to decrease SOCE (67, 78, 120–122), indicating that these domains can explicitly regulate several Ca2+ channels, thereby differentially regulating Ca2+ entry. Thus, it can be reasonably proposed that the Cav1 containing membrane raft domains will be the organizers of the mammalian TRPC1 signalplex, analogous to the INAD scaffold of Drosophila TRP.
ER localized STIM1 undergoes clustering and translocation to the sub-plasma membrane regions of the cells where it displays a punctate localization (43, 47). The site of these puncta have been proposed to be the region of the cell where functional interaction between ER and PM occurs resulting in the activation of SOCE via plasma membrane channels. Indeed SOCE has been shown to occur at sites coincident with the STIM1 peripheral clusters (48–49, 51). STIM1-dependent clustering of the CRAC channel component, Orai1, in the plasma membrane requires STIM1 puncta and is coincident with the location of the puncta (48–49, 51). Similarly, the PM- SOCC component, TRPC1, is also co-localized with STIM1 clusters (123–124). These observations suggest that in order to mediate SOCE, STIM1 needs to be targeted to specific regions of the cell where the likelihood of its interaction with PM-SOCC will be high. Thus, the site of peripheral STIM1 clusters is critical for the regulation of SOCE. However, what determines the location of the STIM1 clusters in the ER-PM junctional regions and whether these represent specific sites in the cell is not yet known. Several studies including ours suggest that PM lipid raft domains determine the peripheral clustering of STIM1 and regulation of TRPC1-mediated SOCE (76, 79, 121, 125). Depletion of ER Ca2+ stores increases the association of STIM1 with lipid raft domains. Further, this association appears to be critical for the activation dependent translocation of STIM1-punctae to membrane raft domains at the ER-PM junctional region of the cells. In addition, disruption of the lipid raft domains severely attenuates stimulation-dependent association of TRPC1 and STIM1. Coincident with this, raft disrupted cells also displayed reduced SOCE. Importantly, STIM1D76A mutant was constitutively clustered in the cell periphery co-incident with membrane raft domains and the sub-plasma membrane localization of STIM1D76A was also dependent on lipid raft domains integrity (79). Furthermore, disruption of lipid rafts decreases STIM1D76A –mediated constitutive Ca2+ entry and its interaction with TRPC1 (67, 78, 80, 112, 126).
Ca2+ store depletion induces oligomerization of STIM1 which has been reported to occur prior to puncta formation in the cell periphery (37, 43, 47). The latter likely requires additional mechanisms for translocation and targeting of STIM1 oligomers to specific ER-plasma membrane junctional regions where STIM1 can interact with SOCE channels in the surface membrane (47, 49, 51). The coiled-coil domain in the C-terminus of STIM1 is reported to be crucial for its aggregation while the amino acids, 425–671, which contain a serine-proline-rich region, appear to be important for the correct targeting of the STIM1 cluster to the cell periphery after Ca2+ store-depletion. The polycationic region in the C-terminal tail of STIM1 also appears to help STIM1 targeting to PM region but is not essential for oligomerization after Ca2+ store depletion (40, 52, 127). Thus, aggregation of STIM1 that occurs in response to a decrease in ER- (Ca2+) and its translocation to the sub-plasma membrane region can be dissociated, although the latter is dependent on the former. These findings provide an important insight into the mechanism that is involved in the store-dependent regulation of TRPC1 channels by STIM1. Based on these findings, it can be suggested that caveolar microdomains in the PM can provide a unique platform for clustering and interaction of STIM1 and TRPC1 in the ER-plasma membrane junctions (see proposed model in Figure 3). Additionally, lipid rafts can anchor STIM1 and thus determine its localization in specific ER-PM junctions where it can functionally interact with plasma membrane channels and regulate SOCE. Furthermore, since caveolar lipid rafts have been shown to be relatively stable membrane domains they can serve as precise micro-compartments to facilitate the dynamic interactions between specific proteins in the ER and the surface membrane that are involved in regulation of SOCE.
Figure 3.
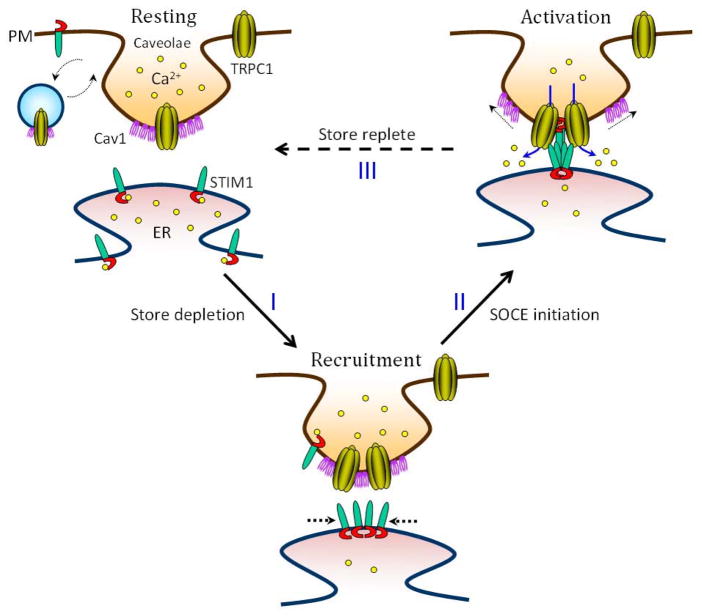
Steps in TRPC1 channel activation. This model shows the compartmentalization TRPC1-mediated Ca2+ influx at ER-caveolar/membrane raft juxtaposed microdomains. In ‘Resting’ state (ER-stores filled)- (a) STIM1, predominantly in the ER, is bound to Ca2+ and displays a diffused localization pattern, (b) PM-associated STIM1 is also shown, however, its contextual relevance is currently unknown, (c) PM-TRPC1 differentially associates with caveolin1 (cav1) enriched - caveolar and non-caveolar microdomains and (d) a steady-state PM trafficking of TRPC1 is achieved by vesicular activity. Between resting and activation, we propose an intermediate step of channel ‘Recruitment’, wherein, following ER Ca2+ store-depletion (step-I), (a) STIM1 unbinds Ca2+ and engages in oligomeric cluster (puncta) formation, (b) TRPC1 is recruited to caveolar raft domains wherein Cav1 scaffolds PM-TRPC1 thus retaining the channel at discrete ER apposed PM microdomains. The channel ‘Activation’ is marked by SOCE initiation (step II) where, (a) STIM1 interacts with TRPC1 and (b) activates the channel resulting in the increase of (Ca2+)cyt with the subsequent dissociation of Cav1. (Ca2+)cyt is then sequestered back to the ER to refill the ER-stores as indicated by store replete (step III), following which (a) STIM1 binds Ca2+ and dissociates from TRPC1 getting back to the resting state. As the filled status of ER controls the STIM1-puncta kinetics it also determines the dynamic and reversible STIM1-TRPC1 associations. The molecular events ensuing each step (step I through step III) outlined in this model may not necessarily reflect their exact physiological sequence and further investigation in this aspect is necessary to delineate the exact
Studies from our lab had identified a subtle molecular rearrangement in TRPC1-Cav1-STIM1 interaction that is indispensable for TRPC1-mediated Ca2+ entry. TRPC1 interaction with Cav1 precisely occurs at the membrane raft microdomains that regulate the PM association of TRPC1. Interestingly, Cav1 targets TRPC1 to same PM microdomains where the peripheral STIM1 clusters are organized in response to Ca2+ store depletion. Additionally, activation dependent raft recruitment of TRPC1 was severely impaired following Cav1 silencing. Consistent with the impaired caveolar association of TRPC1, SOCE was also significantly attenuated. In contrast, increased expression of Cav1 also inhibited SOCE, suggesting that Cav1 scaffolds TRPC1 in ER-PM domains but suppress its function. Importantly, it was further shown that Ca2+ store depletion increased the association of endogenous TRPC1 and STIM1, but decreased that of TRPC1 and Cav1. Additionally, only expression of STIM1 at higher levels, relative to Cav1 resulted in the dissociation of TRPC1-Cav1 complex and recovery of SOCE. These findings suggest that when STIM1 relocates into ER-PM junctional regions, in response to Ca2+ store-depletion, it associates with TRPC1 and mediates release of TRPC1 from Cav1 thus regulating the activation of TRPC1-SOCE. In addition, since Orai1 has been shown to interact with TRPC1 and STIM1, it could be speculative that Orai1 could also be present in these lipid rafts.
The positively charged 684KK685 in the C terminus of STIM1 has been recently shown to gate TRPC1 channels via electrostatic interaction with the negatively charged 639DD640 in the C terminus of TRPC1 (39). Importantly, reverse charged mutant of TRPC1, TRPC1 (DD to KK), which inhibits TRPC1-mediated Ca2+ entry also inhibited TRPC1-Cav1 dissociation (68). Interestingly, expression of a charge swapped mutant STIM1 (KK to EE), which would electrostatically complement the TRPC1 (DD to KK) mutant, resulted in the dissociation of TRPC1 from its scaffold – Cav1, following store depletion. As a consequence of TRPC1-Cav1 dissociation, a relative recovery in SOCE was also observed. These findings demonstrate that the same amino acid residues of STIM1 that are involved in gating of TRPC1 are also critical for mediating release of TRPC1 from Cav1, suggesting that molecular rearrangement involving TRPC1 (i.e. store-depletion induced association of TRPC1-STIM1 and dissociation of TRPC1-Cav1) is imperative for the activation of the channel by STIM1. Although the exact mechanism is not yet established, it can be hypothesized that STIM1 interaction with TRPC1 can induce a conformational change in TRPC1 that can result in its dissociation from Cav1 thereby allowing STIM1-mediated gating of TRPC1. Consistent with this, the Cav1 interacting domains have been found to overlap within the STIM1 gating domains and, further studies will be required to understand the structural details of this functional regulation. Overall, these findings suggest that Cav1 acts as a scaffold to retain inactive TRPC1 in the PM regions in juxtaposition to ER where STIM1 aggregates following store-depletion (see proposed model in Figure 3). STIM1-dependent activation of TRPC1-SOCE involves dissociation of TRPC1 from Cav1 and association with STIM1. Targeting of the channels and STIM1 to the same microdomains ensure the specificity and rate of interaction between these proteins that are essential for activation of SOCE. Importantly, interaction between STIM1 and TRPC1 has been shown to be mediated via the C-terminal domain. Furthermore, the C-terminal caveolin1 binding site overlaps with the STIM1 gating domains, indicating that a tight interplay between these two proteins will be critical for the regulation of TRPC1 channels.
6. PHYSIOLOGICAL SIGNIFICANCE OF STIM1 REGULATED TRPC FUNCTION
The role of TRPCs and STIM1 in regulating Ca2+ entry is well established; however the exact physiological role of these channels in regulating biological function is still not yet determined. Increase in (Ca2+)cyt within the cytosol is essential for cellular activities such as cell proliferation, gene expression, secretion, migration, and adhesion. Since, STIM1 and TRPC1 are key regulators of Ca2+ signaling, it can be anticipated that they will be critical for these biological functions (see Figure 4 for an illustration). Consistent with this, inhibition of STIM1, or deletion of TRPC1 is shown to reduce Ca2+ influx and frequency of Ca2+ oscillations in pancreatic acinar cells, which were essential for enzyme and fluid secretion (128). Importantly, TRPC1 knockout mice, also exhibited a significant decrease in SOCE along with severe loss of salivary gland fluid secretion (129). Although in this report, the role of STIM1 was not determined, TRPC1 has been shown to interact with STIM1 especially in salivary gland cells and is critical for its activation (68, 79, 124). Altogether, these results suggest that TRPC1-STIM1 association could regulate fluid and enzymatic secretion at least in the acinar cells of both salivary and pancreatic glands
Figure 4.
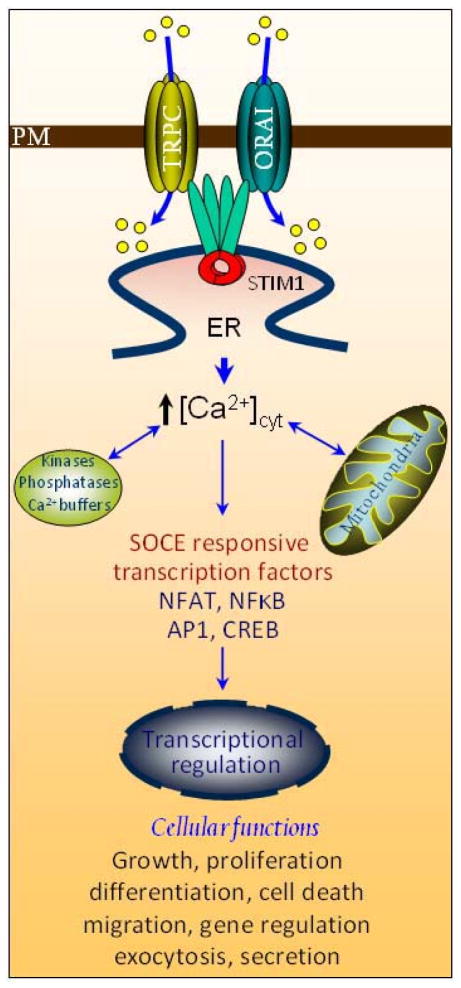
STIM1 activated SOCE and cell function. This model illustrates downstream signaling following STIM1-activated SOCE. Activation of PM-SOCC (TRPC/Orai channels) by STIM1 accounts for store-operated Ca2+ signaling following increase in (Ca2+)cyt. To impact on cell signaling, the SOCE-induced (Ca2+)cyt reciprocates with organelle such as mitochondria and signaling intermediates including kinases (such as PKC, CaMKs, ERK), phosphatases (such as calcineurin) and Ca2+ binding proteins like calmodulin. On a long term, SOCE influences a variety of cellular functions and brings about observable physiological changes by gene regulation. As STIM1 has the potential to influence many signaling pathways, it might be pivotal to studying pathological mysteries such as cancer and neurodegeneration.
In addition, to secretion, the role of TRPC1 and STIM1 in regulating cell migration and probably proliferation is more widely accepted. Vascular smooth muscle cells (VSMCs) retain the capacity for plasticity that enables switching to a non-contractile modulated phenotype that is important for blood vessel formation and vascular adaptation, which is as also dependent on Ca2+ entry. Interestingly, extracellular application of antibodies that specifically bound to STIM1 inhibited Ca2+ entry and cell migration, but not proliferation. STIM1 was shown to functionally, interact with TRPC1, and TRPC1 contributed to Ca2+ entry and cationic current. Importantly, TRPC1-containing channels were important for both cell proliferation as well as migration. These data suggest a complex situation in which STIM1 and TRPC1 are important for cell migration, and TRPC1 can have functions that are independent with at least PM STIM1 (130–131). Additionally, in another report it was shown that silencing of STIM1 suppressed phosphorylation of cAMP-responsive element binding protein (CREB) and cell growth (131–132). Consistent with this it has been shown that STIM1-induced Ca2+ influx viaTRPC1 regulates NFkB activation and cell proliferation (68). Importantly, STIM1 translocation to the plasma membrane promotes intestinal epithelial cell (IEC) migration after wounding by enhancing TRPC1-mediated Ca2+ signaling and provides new insight into the mechanism of intestinal epithelial restitution (133). Together these results suggest that STIM1, along with TRPC1 is an essential component of SOCE and are involved in cell proliferation (130–132). Similarly, endothelial progenitor cells (EPCs) also express TRPC1, STIM1 and Orai1 and inhibition of SOCE impaired proliferation of EPCs (134). Although in this study a direct interaction between TRPC1 and STIM1 was not observed, but since TRPC1 is essential in endothelial cell function, it can be hypothesized that Ca2+ entry via TRPC1 can increase cell proliferation. If this is true then, it can be regarded as a novel target to enhance the regenerative outcome of cell-based therapy.
NRK fibroblasts also express TRPC1, C5, C6, Orai1 and STIM1, and that the levels of their expression were dependent upon the growth stage of the cells (135). Similarly, Endothelin-1 (ET-1) treatment for 48h enhanced TRPC1 expression, SOCE, and transcription factor activation without upregulating STIM1. However, knockdown of STIM1 suppressed these effects, thereby preventing a hypertrophic response (136). These results suggest that STIM1 plays an essential role in the development of cardiomyocyte hypertrophy. Additionally, TRPC6-overexpressing Huh-7 cells proliferated 80 percent faster than did untransfected control cells and their SOCE amplitude was also significantly higher. In contrast, proliferation rate and SOCE amplitude was significantly decreased in TRPC6-knockdown cells, suggesting that TRPC6 or a channel that multimerise with TRPC6 is essential for cell proliferation of Huh-7 cells. Interestingly, SOCE was also reduced by STIM1 and Orai1 knockdowns, suggesting possible cooperation between these proteins in these cells. Consistent with this TRPC6 expression was decreased in isolated hepatocytes from healthy patients, but highly expressed in tumor samples, which strongly support a role for TRPC6 channels in liver oncogenesis (137).
Mast cell degranulation is dependent on Ca2+influx through “Ca2+ channels” that can also convey Sr2+. The release of histamine from rat peritoneal mast cells, whether supported by Ca2+ or Sr2+, was effectively blocked by low concentrations of La3+. Although STIM1 and Orai1 are being shown to be essential for the activation of mast cells, knockdown of TRPC5, substantially reduces influx of Ca2+ as well as degranulation in RBL-2H3 cells (138). Moreover, overexpression of Orai1 with STIM1 promotes constitutive influx of Ca2+ but not of Sr2+, whereas overexpression of TRPC5 with STIM1 promotes constitutive influx of both ions. These data suggest that Sr2+-permeable TRPC5 acts in conjunction with Orai1 and STIM1 to allow Sr2+ and other divalent ions to permeate and support degranulation in mast cells. Furthermore, ectopic expression of Fyn or TRPC1, in Fyn null mast cells restored Ca2+ responses and increased mast cell degranulation, suggesting that TRPC1 participates in Ca2+ influx required for mast cell degranulation. This demonstrates that in addition to a role described previously for Orai1 in promoting mast cell degranulation, nonselective cation channels also participate in promoting the exocytotic response (139). Consistent with this, STIM1 and TRPC1 have also been implicated in thrombin- and ADP-induced platelet aggregation (140), probably through the regulation of Ca2+ entry, which might become targets for the development of therapeutic strategies to treat platelet hyperactivity and thrombosis disorders). Additionally, in PASMCs Ca2+ entry was mediated by the TRPC1 channel through activation of STIM1, which may be an important model for future identification of SOCs in PASMCs and they may be useful targets for the development of new drugs to treat pulmonary hypertension (141). Although these examples indicate that TRPCs and STIM1 interactions are beneficial for cellular system, but could be harmful in conditions such as metabolic syndrome, where abnormally elevated adrenal TRPC expression may underlie increased plasma epinephrine and heart rate. The excess of plasma catecholamines and increased heart rate are risk factors for cardiovascular disease. Thus, TRPCs are also potential therapeutic targets in the fight against cardiovascular disease (142). Also, increased in cell proliferation due to increased activity of STIM1-TRPC1, could potentially lead to cancer and more research is needed to verify the possible role of TRPC1-STIM1 function in this regard.
7. CONCLUSION AND PERSPECTIVE
Ca2+ entry via the SOCE mechanism is essential for maintaining cellular functions. Intracellular Ca2+ stores communicate with the plasma membrane SOCE channels to orchestrate cellular Ca2+ homeostasis that regulate fundamental biological functions. Although STIM1 has been shown to be the regulators for both TRPCs and Orai channels and this process is essential for maintaining intracellular Ca2+ stores as well as influencing vital physiological processes, the exact biological function of many of these channels have not yet been fully explored. Furthermore, results using knockout mouse models, differ significantly from human cells and cells obtained from patient populations, where Orai1 was not found to be critical for T cell activation in humans but not in mice. This could be due to differential expression of certain Ca2+ channels (including TRPCs) and their regulators in a given cell type, which could not only dictate the composition of the SOCE channel, but can also be essential in their regulation. Additionally, STIM1 has been shown to form oligomers, but the significance of these oligomers is still not yet known. One possibility can be that STIM1 needs to form oligomers in order to influence ER restructuring to form ER-PM junctions. The second possibility may be that oligomeric STIM1 can initiate protein-protein interactions needed to functionally gate and regulate SOCE channels. In addition, Orai1 has been shown to interact with TRPC channels, but the significance of this interaction is not clear and more research is needed to decipher the role of individual SOCE channels. Also, nothing is known about the role of PM STIM1, one possibility can be that PM STIM1 can be present in lipid rafts and since STIM1 is known to multimerize, it can assist in establishing peripheral STIM1 puncta. Overall, although recent findings have helped in understanding the mechanisms of SOCE, future studies are still needed to completely evaluate the role of these functional interactions and finally use this information for therapeutic interventions.
Acknowledgments
We duly acknowledge the grant support from the National Institutes of Health (DE017102, 5P20RR017699).
References
- 1.Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci U S A. 2002;99(3):1115–22. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore B. In Memory of Sidney Ringer (1835–1910): Some account of the Fundamental Discoveries of the Great Pioneer of the Bio-Chemistry of Crystallo-colloids in Living Cells. Biochem J. 1911;5(6–7):i b3–xix. doi: 10.1042/bj005000i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40(5–6):405–12. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Ambudkar IS. Cellular domains that contribute to Ca2+ entry events. Sci STKE. 2004;2004(243):pe32. doi: 10.1126/stke.2432004pe32. [DOI] [PubMed] [Google Scholar]
- 5.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 6.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 7.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1(1):31–9. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 8.Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296(5574):1821–5. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 9.Harris TJ, Siu CH. Reciprocal raft-receptor interactions and the assembly of adhesion complexes. Bioessays. 2002;24(11):996–1003. doi: 10.1002/bies.10172. [DOI] [PubMed] [Google Scholar]
- 10.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85(2):757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 11.Putney JW., Jr Capacitative calcium entry: sensing the calcium stores. J Cell Biol. 2005;169(3):381–2. doi: 10.1083/jcb.200503161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beech DJ. TRPC1: store-operated channel and more. Pflugers Arch. 2005;451(1):53–60. doi: 10.1007/s00424-005-1441-3. [DOI] [PubMed] [Google Scholar]
- 13.Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11(10):611–24. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 14.Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2(6):387–96. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 15.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–47. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 16.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2002;4(11):E263–72. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- 18.Putney JW, Jr, Poggioli J, Weiss SJ. Receptor regulation of calcium release and calcium permeability in parotid gland cells. Philos Trans R Soc Lond B Biol Sci. 1981;296(1080):37–45. doi: 10.1098/rstb.1981.0169. [DOI] [PubMed] [Google Scholar]
- 19.Takemura H, Putney JW., Jr Capacitative calcium entry in parotid acinar cells. Biochem J. 1989;258(2):409–12. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev. 2009;231(1):10–22. doi: 10.1111/j.1600-065X.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- 21.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224(5216):285–7. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- 22.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. Faseb J. 2009;23(2):297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambudkar IS, Brazer SC, Liu X, Lockwich T, Singh B. Plasma membrane localization of TRPC channels: role of caveolar lipid rafts. Novartis Found Symp. 2004;258:63–70. discussion 70–4, 98–102, 263–6. [PubMed] [Google Scholar]
- 24.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay BC, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42(2):213–23. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Wang W, Singh BB, Lockwich T, Jadlowiec J, O’Connell B, Wellner R, Zhu MX, Ambudkar IS. Trp1, a candidate protein for the store-operated Ca (2+) influx mechanism in salivary gland cells. J Biol Chem. 2000;275(5):3403–11. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- 26.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82(2):429–72. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 27.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005(272):re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38(3–4):233–52. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci U S A. 1995;92(21):9652–6. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20(3):250–8. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7(9):690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 32.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 33.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443(7108):230–3. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 34.Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Sci STKE. 2001;2001(90):re1. doi: 10.1126/stke.2001.90.re1. [DOI] [PubMed] [Google Scholar]
- 35.Putney JW., Jr TRP, inositol 1,4,5-trisphosphate receptors, and capacitative calcium entry. Proc Natl Acad Sci U S A. 1999;96(26):14669–71. doi: 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putney JW, Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114(Pt 12):2223–9. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 37.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15(13):1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169(3):435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32(3):439–48. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42(2):205–11. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manji SS, Parker NJ, Williams RT, van Stekelenburg L, Pearson RB, Dziadek M, Smith PJ. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta. 2000;1481(1):147–55. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 42.Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, Smith PJ, Dziadek MA. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J. 2001;357(Pt 3):673–85. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437(7060):902–5. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131(7):1327–39. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smyth JT, Petranka JG, Boyles RR, DeHaven WI, Fukushima M, Johnson KL, Williams JG, Putney JW., Jr Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol. 2009;11(12):1465–72. doi: 10.1038/ncb1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu F, Sun L, Machaca K. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc Natl Acad Sci U S A. 2009;106(41):17401–6. doi: 10.1073/pnas.0904651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A. 2007;104(22):9301–6. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454(7203):538–42. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174(6):815–25. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Kahr H, Madl J, Gruber H, Groschner K, Romanin C. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283(12):8014–22. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 51.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174(6):803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun. 2006;350(4):969–76. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- 53.Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85(5):661–71. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- 54.Ambudkar IS, Bandyopadhyay BC, Liu X, Lockwich TP, Paria B, Ong HL. Functional organization of TRPC-Ca2+ channels and regulation of calcium microdomains. Cell Calcium. 2006;40(5–6):495–504. doi: 10.1016/j.ceca.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 55.Ambudkar IS, Ong HL. Organization and function of TRPC channelosomes. Pflugers Arch. 2007;455(2):187–200. doi: 10.1007/s00424-007-0252-0. [DOI] [PubMed] [Google Scholar]
- 56.Kiselyov K, Mignery GA, Zhu MX, Muallem S. The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Mol Cell. 1999;4(3):423–9. doi: 10.1016/s1097-2765(00)80344-5. [DOI] [PubMed] [Google Scholar]
- 57.Birnbaumer L, Boulay G, Brown D, Jiang M, Dietrich A, Mikoshiba K, Zhu X, Qin N. Mechanism of capacitative Ca2+ entry (CCE): interaction between IP3 receptor and TRP links the internal calcium storage compartment to plasma membrane CCE channels. Recent Prog Horm Res. 2000;55:127–61. discussion 161–2. [PubMed] [Google Scholar]
- 58.Singh BB, Liu X, Tang J, Zhu MX, Ambudkar IS. Calmodulin regulates Ca (2+)-dependent feedback inhibition of store-operated Ca (2+) influx by interaction with a site in the C terminus of TrpC1. Mol Cell. 2002;9(4):739–50. doi: 10.1016/s1097-2765(02)00506-3. [DOI] [PubMed] [Google Scholar]
- 59.Zhu MX. Multiple roles of calmodulin and other Ca (2+)-binding proteins in the functional regulation of TRP channels. Pflugers Arch. 2005;451(1):105–15. doi: 10.1007/s00424-005-1427-1. [DOI] [PubMed] [Google Scholar]
- 60.Wedel BJ, Vazquez G, McKay RR, St JBG, Putney JW., Jr A calmodulin/inositol 1,4,5-trisphosphate (IP3) receptor-binding region targets TRPC3 to the plasma membrane in a calmodulin/IP3 receptor-independent process. J Biol Chem. 2003;278(28):25758–65. doi: 10.1074/jbc.M303890200. [DOI] [PubMed] [Google Scholar]
- 61.Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation. 2006;13(8):693–708. doi: 10.1080/10739680600930347. [DOI] [PubMed] [Google Scholar]
- 62.Kwan HY, Huang Y, Yao X. Protein kinase C can inhibit TRPC3 channels indirectly via stimulating protein kinase G. J Cell Physiol. 2006;207(2):315–21. doi: 10.1002/jcp.20567. [DOI] [PubMed] [Google Scholar]
- 63.Vazquez G, Wedel BJ, Aziz O, Trebak M, Putney JW., Jr The mammalian TRPC cation channels. Biochim Biophys Acta. 2004;1742(1–3):21–36. doi: 10.1016/j.bbamcr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 64.Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mizutani A, Mikoshiba K. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem. 2004;279(18):18887–94. doi: 10.1074/jbc.M311274200. [DOI] [PubMed] [Google Scholar]
- 65.Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y, Inoue R. Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J Physiol. 2004;561(Pt 2):415–32. doi: 10.1113/jphysiol.2004.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114(6):777–89. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 67.Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem. 2003;278(29):27208–15. doi: 10.1074/jbc.M301118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pani B, Ong HL, Brazer SC, Liu X, Rauser K, Singh BB, Ambudkar IS. Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proc Natl Acad Sci U S A. 2009;106(47):20087–92. doi: 10.1073/pnas.0905002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kiselyov KI, Shin DM, Wang Y, Pessah IN, Allen PD, Muallem S. Gating of store-operated channels by conformational coupling to ryanodine receptors. Mol Cell. 2000;6(2):421–31. doi: 10.1016/s1097-2765(00)00041-1. [DOI] [PubMed] [Google Scholar]
- 70.Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, Worley PF, Muallem S. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. J Biol Chem. 2006;281(43):32540–9. doi: 10.1074/jbc.M602496200. [DOI] [PubMed] [Google Scholar]
- 71.Trebak M, St JBG, McKay RR, Birnbaumer L, Putney JW., Jr Signaling mechanism for receptor-activated canonical transient receptor potential 3 (TRPC3) channels. J Biol Chem. 2003;278(18):16244–52. doi: 10.1074/jbc.M300544200. [DOI] [PubMed] [Google Scholar]
- 72.Schindl R, Frischauf I, Kahr H, Fritsch R, Krenn M, Derndl A, Vales E, Muik M, Derler I, Groschner K, Romanin C. The first ankyrin-like repeat is the minimum indispensable key structure for functional assembly of homo- and heteromeric TRPC4/TRPC5 channels. Cell Calcium. 2008;43(3):260–9. doi: 10.1016/j.ceca.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 73.Xu XZ, Chien F, Butler A, Salkoff L, Montell C. TRPgamma, a drosophila TRP-related subunit, forms a regulated cation channel with TRPL. Neuron. 2000;26(3):647–57. doi: 10.1016/s0896-6273(00)81201-5. [DOI] [PubMed] [Google Scholar]
- 74.Lepage PK, Lussier MP, Barajas-Martinez H, Bousquet SM, Blanchard AP, Francoeur N, Dumaine R, Boulay G. Identification of two domains involved in the assembly of transient receptor potential canonical channels. J Biol Chem. 2006;281(41):30356–64. doi: 10.1074/jbc.M603930200. [DOI] [PubMed] [Google Scholar]
- 75.Montell C. Visual transduction in Drosophila. Annu Rev Cell Dev Biol. 1999;15:231–68. doi: 10.1146/annurev.cellbio.15.1.231. [DOI] [PubMed] [Google Scholar]
- 76.Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium. 2009;45(6):625–33. doi: 10.1016/j.ceca.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brownlow SL, Harper AG, Harper MT, Sage SO. A role for hTRPC1 and lipid raft domains in store-mediated calcium entry in human platelets. Cell Calcium. 2004;35(2):107–13. doi: 10.1016/j.ceca.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 78.Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem. 2000;275(16):11934–42. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- 79.Pani B, Ong HL, Liu X, Rauser K, Ambudkar IS, Singh BB. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE) J Biol Chem. 2008;283(25):17333–40. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murata T, Lin MI, Stan RV, Bauer PM, Yu J, Sessa WC. Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem. 2007;282(22):16631–43. doi: 10.1074/jbc.M607948200. [DOI] [PubMed] [Google Scholar]
- 81.Montell C. TRP channels in Drosophila photoreceptor cells. J Physiol. 2005;567(Pt 1):45–51. doi: 10.1113/jphysiol.2005.092551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim MS, Zeng W, Yuan JP, Shin DM, Worley PF, Muallem S. Native Store-operated Ca2+ Influx Requires the Channel Function of Orai1 and TRPC1. J Biol Chem. 2009;284(15):9733–41. doi: 10.1074/jbc.M808097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11(3):337–43. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng KT, Ong HL, Liu X, Ambudkar IS. Contribution of TRPC1 and Orai1 to Ca (2+) Entry Activated by Store Depletion. Adv Exp Med Biol. 2011;704:435–49. doi: 10.1007/978-94-007-0265-3_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salido GM, Jardin I, Rosado JA. The TRPC Ion Channels: Association with Orai1 and STIM1 Proteins and Participation in Capacitative and Non-capacitative Calcium Entry. Adv Exp Med Biol. 2011;704:413–33. doi: 10.1007/978-94-007-0265-3_23. [DOI] [PubMed] [Google Scholar]
- 86.Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S. An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett. 2010;584(10):2022–7. doi: 10.1016/j.febslet.2009.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee KP, Yuan JP, So I, Worley PF, Muallem S. STIM1-dependent and STIM1-independent function of TRPC channels tunes their store-operated mode. J Biol Chem. 2010;285(49):38666–73. doi: 10.1074/jbc.M110.155036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Connell KM, Martens JR, Tamkun MM. Localization of ion channels to lipid Raft domains within the cardiovascular system. Trends Cardiovasc Med. 2004;14(2):37–42. doi: 10.1016/j.tcm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–72. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 90.Pike LJ. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta. 2005;1746(3):260–73. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 91.Thomas CM, Smart EJ. Caveolae structure and function. J Cell Mol Med. 2008;12(3):796–809. doi: 10.1111/j.1582-4934.2008.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2008;44(1):1–5. doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 93.Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 94.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–91. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Manes S, Mira E, Gomez-Mouton C, Lacalle RA, Keller P, Labrador JP, Martinez AC. Membrane raft microdomains mediate front-rear polarity in migrating cells. Embo J. 1999;18(22):6211–20. doi: 10.1093/emboj/18.22.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sowa G, Pypaert M, Sessa WC. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci U S A. 2001;98(24):14072–7. doi: 10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patel HH, Murray F, Insel PA. G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handb Exp Pharmacol. 2008;(186):167–84. doi: 10.1007/978-3-540-72843-6_7. [DOI] [PubMed] [Google Scholar]
- 98.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273(10):5419–22. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 99.Jury EC, Flores-Borja F, Kabouridis PS. Lipid rafts in T cell signalling and disease. Semin Cell Dev Biol. 2007;18(5):608–15. doi: 10.1016/j.semcdb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–36. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 101.Gabella G. Caveolae intracellulares and sarcoplasmic reticulum in smooth muscle. J Cell Sci. 1971;8(3):601–9. doi: 10.1242/jcs.8.3.601. [DOI] [PubMed] [Google Scholar]
- 102.Popescu LM, Diculescu I, Zelck U, Ionescu N. Ultrastructural distribution of calcium in smooth muscle cells of guinea-pig taenia coli. A correlated electron microscopic and quantitative study. Cell Tissue Res. 1974;154(3):357–78. doi: 10.1007/BF00223732. [DOI] [PubMed] [Google Scholar]
- 103.Diculescu I. On the unity of cytomembrane system in the skeletal muscle. Morphol Embryol (Bucur) 1980;26(3):205–12. [PubMed] [Google Scholar]
- 104.Haack DW, Abel JH, Jr, Jaenke RS. Effects of hypoxia on the distribution of calcium in arterial smooth muscle cells of rats and swine. Cell Tissue Res. 1975;157(1):125–40. doi: 10.1007/BF00223235. [DOI] [PubMed] [Google Scholar]
- 105.Suzuki S, Sugi H. Evidence for extracellular localization of activator calcium in dog coronary artery smooth muscle as studied by the pyroantimonate method. Cell Tissue Res. 1989;257(2):237–46. doi: 10.1007/BF00261826. [DOI] [PubMed] [Google Scholar]
- 106.Meyer R, Stockem W, Schmitz M, Haas HG. Histochemical demonstration of an ATP-dependent Ca2+-pump in bullfrog myocardial cells. Z Naturforsch (C) 1982;37(5–6):489–501. doi: 10.1515/znc-1982-5-622. [DOI] [PubMed] [Google Scholar]
- 107.Anderson RG. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci U S A. 1993;90(23):10909–13. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993;120(5):1147–57. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fujimoto T, Nakade S, Miyawaki A, Mikoshiba K, Ogawa K. Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992;119(6):1507–13. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Isshiki M, Ando J, Korenaga R, Kogo H, Fujimoto T, Fujita T, Kamiya A. Endothelial Ca2+ waves preferentially originate at specific loci in caveolin-rich cell edges. Proc Natl Acad Sci U S A. 1998;95(9):5009–14. doi: 10.1073/pnas.95.9.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Isshiki M, Ying Y, Fujita T, Anderson RGW. A molecular sensor detects signal transduction from caveolae in living cells. The Journal of Biological Chemistry. 2002;277(45):23389–23398. doi: 10.1074/jbc.M205411200. [DOI] [PubMed] [Google Scholar]
- 112.Ambudkar IS. Ca2+ signaling microdomains:platforms for the assembly and regulation of TRPC channels. Trends Pharmacol Sci. 2006;27(1):25–32. doi: 10.1016/j.tips.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 113.Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium. 2003;33(5–6):433–40. doi: 10.1016/s0143-4160(03)00054-x. [DOI] [PubMed] [Google Scholar]
- 114.Kiselyov K, Shin DM, Kim JY, Yuan JP, Muallem S. TRPC channels: interacting proteins. Handb Exp Pharmacol. 2007;(179):559–74. doi: 10.1007/978-3-540-34891-7_33. [DOI] [PubMed] [Google Scholar]
- 115.Kiselyov K, Kim JY, Zeng W, Muallem S. Protein-protein interaction and functionTRPC channels. Pflugers Arch. 2005;451(1):116–24. doi: 10.1007/s00424-005-1442-2. [DOI] [PubMed] [Google Scholar]
- 116.Kiselyov K, Patterson RL. The integrative function of TRPC channels. Front Biosci. 2009;14:45–58. doi: 10.2741/3230. [DOI] [PubMed] [Google Scholar]
- 117.Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41(6):2075–88. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 118.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115(4):377–88. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 119.Luoma JI, Boulware MI, Mermelstein PG. Caveolin proteins and estrogen signaling in the brain. Mol Cell Endocrinol. 2008;290(1–2):8–13. doi: 10.1016/j.mce.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brownlow SL, Sage SO. Transient receptor potential protein subunit assembly and membrane distribution in human platelets. Thromb Haemost. 2005;94(4):839–45. doi: 10.1160/TH05-06-0391. [DOI] [PubMed] [Google Scholar]
- 121.Alicia S, Angelica Z, Carlos S, Alfonso S, Vaca L. STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: moving TRPC1 in and out of lipid rafts. Cell Calcium. 2008;44(5):479–91. doi: 10.1016/j.ceca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 122.Jardin I, Salido GM, Rosado JA. Role of lipid rafts in the interaction between hTRPC1, Orai1 and STIM1. Channels (Austin) 2008;2(6) doi: 10.4161/chan.2.6.7055. [DOI] [PubMed] [Google Scholar]
- 123.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I (crac) and TRPC1 channels. Nat Cell Biol. 2006;8(9):1003–10. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 124.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282(12):9105–16. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jardin I, Salido GM, Rosado JA. Role of lipid rafts in the interaction between hTRPC1, Orai1 and STIM1. Channels (Austin) 2008;2(6):401–3. doi: 10.4161/chan.2.6.7055. [DOI] [PubMed] [Google Scholar]
- 126.Kwiatek AM, Minshall RD, Cool DR, Skidgel RA, Malik AB, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol. 2006;70(4):1174–83. doi: 10.1124/mol.105.021741. [DOI] [PubMed] [Google Scholar]
- 127.Li Z, Lu J, Xu P, Xie X, Chen L, Xu T. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282(40):29448–56. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 128.Hee Hong J, Li Q, Seuk Kim M, Min Shin D, Feske S, Birnbaumer L, Tai Cheng K, Ambudkar IS, Muallem S. Polarized but Differential Localization and Recruitment of STIM1, Orai1 and TRPC Channels in Secretory Cells. Traffic. 2011;12(2):232–45. doi: 10.1111/j.1600-0854.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1 (−/−) mice. Proc Natl Acad Sci U S A. 2007;104(44):17542–7. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li J, Sukumar P, Milligan CJ, Kumar B, Ma ZY, Munsch CM, Jiang LH, Porter KE, Beech DJ. Interactions, functions, and independence of plasma membrane STIM1 and TRPC1 in vascular smooth muscle cells. Circ Res. 2008;103(8):e97–104. doi: 10.1161/CIRCRESAHA.108.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Takahashi Y, Watanabe H, Murakami M, Ohba T, Radovanovic M, Ono K, Iijima T, Ito H. Involvement of transient receptor potential canonical 1 (TRPC1) in angiotensin II-induced vascular smooth muscle cell hypertrophy. Atherosclerosis. 2007;195(2):287–96. doi: 10.1016/j.atherosclerosis.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 132.Takahashi Y, Watanabe H, Murakami M, Ono K, Munehisa Y, Koyama T, Nobori K, Iijima T, Ito H. Functional role of stromal interaction molecule 1 (STIM1) in vascular smooth muscle cells. Biochem Biophys Res Commun. 2007;361(4):934–40. doi: 10.1016/j.bbrc.2007.07.096. [DOI] [PubMed] [Google Scholar]
- 133.Rao JN, Rathor N, Zou T, Liu L, Xiao L, Yu TX, Cui YH, Wang JY. STIM1 translocation to the plasma membrane enhances intestinal epithelial restitution by inducing TRPC1-mediated Ca2+ signaling after wounding. Am J Physiol Cell Physiol. 2010;299(3):C579–88. doi: 10.1152/ajpcell.00066.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sanchez-Hernandez Y, Laforenza U, Bonetti E, Fontana J, Dragoni S, Russo M, Avelino-Cruz JE, Schinelli S, Testa D, Guerra G, Rosti V, Tanzi F, Moccia F. Store-operated Ca (2+) entry is expressed in human endothelial progenitor cells. Stem Cells Dev. 2010;19(12):1967–81. doi: 10.1089/scd.2010.0047. [DOI] [PubMed] [Google Scholar]
- 135.Dernison MM, Almirza WH, Kusters JM, van Meerwijk WP, Gielen CC, van Zoelen EJ, Theuvenet AP. Growth-dependent modulation of capacitative calcium entry in normal rat kidney fibroblasts. Cell Signal. 2010;22(7):1044–53. doi: 10.1016/j.cellsig.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 136.Ohba T, Watanabe H, Murakami M, Sato T, Ono K, Ito H. Essential role of STIM1 in the development of cardiomyocyte hypertrophy. Biochem Biophys Res Commun. 2009;389(1):172–6. doi: 10.1016/j.bbrc.2009.08.117. [DOI] [PubMed] [Google Scholar]
- 137.El Boustany C, Bidaux G, Enfissi A, Delcourt P, Prevarskaya N, Capiod T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology. 2008;47(6):2068–77. doi: 10.1002/hep.22263. [DOI] [PubMed] [Google Scholar]
- 138.Ma HT, Peng Z, Hiragun T, Iwaki S, Gilfillan AM, Beaven MA. Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J Immunol. 2008;180(4):2233–9. doi: 10.4049/jimmunol.180.4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Suzuki R, Liu X, Olivera A, Aguiniga L, Yamashita Y, Blank U, Ambudkar I, Rivera J. Loss of TRPC1-mediated Ca2+ influx contributes to impaired degranulation in Fyn-deficient mouse bone marrow-derived mast cells. J Leukoc Biol. 2010;88(5):863–75. doi: 10.1189/jlb.0510253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Galan C, Zbidi H, Bartegi A, Salido GM, Rosado JA. STIM1, Orai1 and hTRPC1 are important for thrombin- and ADP-induced aggregation in human platelets. Arch Biochem Biophys. 2009;490(2):137–44. doi: 10.1016/j.abb.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 141.Ng LC, McCormack MD, Airey JA, Singer CA, Keller PS, Shen XM, Hume JR. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J Physiol. 2009;587(Pt 11):2429–42. doi: 10.1113/jphysiol.2009.172254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ng LC, Airey JA, Hume JR. The Contribution of TRPC1 and STIM1 to Capacitative Ca (2+) Entry in Pulmonary Artery. Adv Exp Med Biol. 2010;661:123–35. doi: 10.1007/978-1-60761-500-2_8. [DOI] [PubMed] [Google Scholar]
- 143.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9(6):636–45. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu X, Ong HL, Pani B, Johnson K, Swaim WB, Singh B, Ambudkar I. Effect of cell swelling on ER/PM junctional interactions and channel assembly involved in SOCE. Cell Calcium. 2010;47(6):491–9. doi: 10.1016/j.ceca.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang ZY, Pan LJ, Zhang ZM. Functional interactions among STIM1, Orai1 and TRPC1 on the activation of SOCs in HL-7702 cells. Amino Acids. 2009 doi: 10.1007/s00726-009-0398-5. [DOI] [PubMed] [Google Scholar]
- 146.Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281(38):28254–64. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- 147.Lu M, Branstrom R, Berglund E, Hoog A, Bjorklund P, Westin G, Larsson C, Farnebo LO, Forsberg L. Expression and association of TRPC subtypes with Orai1 and STIM1 in human parathyroid. J Mol Endocrinol. 2010;44(5):285–94. doi: 10.1677/JME-09-0138. [DOI] [PubMed] [Google Scholar]
- 148.Sours-Brothers S, Ding M, Graham S, Ma R. Interaction between TRPC1/TRPC4 assembly and STIM1 contributes to store-operated Ca2+ entry in mesangial cells. Exp Biol Med (Maywood) 2009;234(6):673–82. doi: 10.3181/0809-RM-279. [DOI] [PubMed] [Google Scholar]
- 149.Woodard GE, Lopez JJ, Jardin I, Salido GM, Rosado JA. TRPC3 regulates agonist-stimulated Ca2+ mobilization by mediating the interaction between type I inositol 1,4,5-trisphosphate receptor, RACK1, and Orai1. J Biol Chem. 2010;285(11):8045–53. doi: 10.1074/jbc.M109.033605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yuan JP, Kim MS, Zeng W, Shin DM, Huang G, Worley PF, Muallem S. TRPC channels as STIM1-regulated SOCs. Channels (Austin) 2009;3(4):221–5. doi: 10.4161/chan.3.4.9198. [DOI] [PubMed] [Google Scholar]