Abstract
Aims
Previous studies have shown increased density of M2 receptors in hypertrophied rat bladders that possess an M2 contractile phenotype. The aim of the current study is to determine whether human bladders with an M2 contractile phenotype also have a greater density of bladder M2 receptors.
Materials and Methods
Human bladders were obtained from 24 different organ transplant donors. Darifenacin and methoctramine affinity was determined by the rightward shift of cumulative carbachol concentration contractile response curves for each bladder. Radioligand binding and immunoprecipitation was used to quantify M2 and M3 subtypes in isolated detrusor muscle and urothelium. In addition, pig bladder muscle and urothelial receptors were quantified for comparison.
Results
In the human urothelium total, M2 and M3 muscarinic receptor density is significantly negatively correlated with the affinity of darifenacin for inhibition of contraction of the detrusor muscle. In the detrusor muscle there is no correlation between receptor density and darifenacin affinity for inhibition of contraction. Muscarinic receptor density is greater in the muscle than in the urothelium in human bladders whereas in the pig bladder the density is greater in the urothelium than in the muscle.
Conclusions
The greater density of urothelial muscarinic receptors in human bladders with lower darifenacin affinity, indicative of a greater contribution of M2 receptors to the contractile response, points towards a possible role of the urothelium in controlling M2 mediated contractile phenotype. In comparison between human and pig bladders, the distribution of muscarinic receptor subtypes in the muscle and urothelium are quite different.
Keywords: bladder contraction, bladder mucosa, immunoprecipitation, urothelium
INTRODUCTION
Muscarinic receptors are responsible for the contraction of the detrusor muscle mediated by the neurotransmitter ACh. Five subtypes of muscarinic receptor have been identified by molecular techniques [Caulfield, 1993]. M2 and M3 receptor subtypes predominate in the detrusor muscle, with the population of the M2 subtype receptor in detrusor muscle greater than the M3 subtype. Previous immunoprecipitation data with subtype-selective antibodies and radioligand binding studies have reported a ratio of M2 to M3 receptors in the detrusor to be approximately three to one for human, guinea pig, and rabbit bladders [Wang et al., 1995] and selective radioligand binding data indicates a similar ratio for the pig bladder [Yamanishi et al., 2000]. Despite higher density of M2 receptors, contraction has been found to be primarily mediated by M3 receptors under normal conditions in all species studied. M3 receptor-activated cellular signaling pathways have been traditionally thought to induce contraction via activation of phospholipase C to generate inositol phosphates and diacyl-glycerol [Caulfield, 1993; Hegde, 2006]. However, PI-PLC inhibition has been reported to have no effect on cholinergic induced bladder contraction in either rat or human, whereas the activity of Rho kinase was found to be critical [Schneider et al., 2004a,b]. M2 receptors have been shown to play an indirect role in detrusor contraction by inhibiting bladder smooth muscle relaxation and enhancing detrusor contraction via Giinhibition of cAMP levels [Ehlert et al., 2005]. In pathologic states, the M2 receptor pathway has a greater role in mediating contraction in rats and humans [Braverman and Ruggieri, 2003; Pontari et al., 2004; Gevaert et al., 2006]. Thus, with pathophysiological stressors, the mechanisms that transduce the muscarinic contractile signal can become altered such that the M2 receptor subtype plays a more prominent role in the contractile response that is normally mediated by the M3 receptor subtype.
Bladder urothelium has been thought to merely act as a protective barrier for the underlying smooth muscle however it is now becoming recognized as an active participant in the contractile response [de Groat, 2004]. Recently, multiple subtypes of muscarinic receptors have been identified in human bladder urothelium. M1, M2, M3, and M5 have been detected, with a minor M1population, and an M2 to M3 and/or M5 ratio of 3:1in radioligand binding studies [Mansfield et al., 2005].The relationship of urothelial activity and its effect on muscle contraction has been investigated. Possible urothelial factors such as nitric oxide, guanylate cyclase, cyclo-oxygenase, P2Y receptors, cate-cholamines, GABA, and endothelium-derived hyperpolarizing factor (EDHF) do not mediate the inhibitory function of the pig urothelium [Hawthorn et al., 2000]. Although the mechanism remains unclear, there is a known inhibitory function of the urothelium on smooth muscle after urothelial activation in the human bladder [Chaiyaprasithi et al., 2003].
Treatment for overactive bladder (OAB) relies mainly on antimuscarinics which are thought to suppress detrusor contraction. With the recent release of several M3-selective muscarinic antagonists has come the realization that there are only slight differences in the clinical e/cacy between the M3-selective antagonists (darifenacin and solifenacin) and the more M2/M3 balanced antagonists (tolterodine and trospium). Clinically, distinctions between these new agents may appear to be in their side effects [Drutz et al., 1999; Appell et al., 2001; Chancellor et al., 2001; Diokno et al., 2002; Rovner and Wein, 2002; Siami et al., 2002; Sussman and Garely, 2002; Halaska et al., 2003; Haab et al., 2004; Chapple et al., 2005; MacDiarmid et al., 2005]. No studies have been reported on M2-selective anticholinergic medications in humans. The urothelial effect on bladder smooth muscle contraction could have substantial implications on future anti-muscarinic drug therapy. Therefore, we have quantified the density of the urothelial and detrusor muscle muscarinic receptor subtypes present within the human bladder, and have evaluated the relationship of these densities to the muscarinic receptor sub-type mediating in vitro bladder contractions in these same human bladder specimens.
METHODS
Materials
The following drugs or chemicals were obtained from the sources indicated:
[3H] quinuclidinyl benzilate (QNB, Perkin Elmer, Wellesley, MA), darifenacin was a gift from Pfizer Limited (Sandwich, Kent), carbachol, methoctramine and Sephadex G-50 (Sigma-Aldrich, St. Louis, MO), pansorbin (Calbiochem, La Jolla, CA). Frozen porcine bladders were obtained from Pel-Freeze Biologicals, Rogers, AZ. Whole human bladders were procured from brain dead organ transplant donors following informed consent of the next of kin by the National Disease Research Interchange (Philadelphia, PA). The procurement procedures were reviewed and approved by the Institutional Review Board of the University of Pennsylvania. Bladders were harvested within 30 min after cross clamping the aorta, immersed in Belzer’s Viaspan® University of Wisconsin Solution, and transported to the laboratory on wet ice by overnight courier.
Muscle Strips
Human bladder smooth muscle strips, approximately 2 × 8 mm (urothelium free), were dissected under fourfold magnification such that the direction of the smooth muscle bundles were primarily aligned along the length of the strip. Strips were stretched slowly to 1.0 g of isometric tension in tissue baths containing 10 ml of modified Tyrode’s solution (125 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 1.8 mM CaCl2, 0.5 mM MgCl2, 23.8 mM NaHCO3, and 5.6 mM glucose) and equilibrated with 95/5% O2/CO2 at 37 °C. The strips were tested for their ability to contract in response to electric field stimulation using bipolar platinum electrodes oriented 1cm apart along the length of the strip and a stimulus intensity of 8 volts, 30 Hz, 1msec duration. Strips that did not contract in response to this electric field stimulation were replaced (typically less than 10%).
Carbachol Concentration Response
Following equilibration to the bath solution for 30 min, bladder strips were incubated for 30 min in the presence or absence of the M3-selective muscarinic receptor antagonist darifenacin (0.03 μM, n = 3–8) or the M2-selective antagonist methoctramine (0.1 and 1.0 μM, n = 3–8). Each muscle strip was used as either an antagonist free control or exposed to darifenacin or methoctramine and thus each strip only underwent one cumulative carbachol concentration response curve. Concentration response curves were derived from the peak tension developed following cumulative addition of carbachol in one-half log increments (10 nM to 1 mM final bath concentration). An EC50 value for each strip was determined from a non-linear least squares sigmoidal curve fit of the data (Origin, OriginLab Corp., Northampton, MA). Concentration ratios were determined based on the average of the EC50 values of antagonist free strips (n = 3–8). Because higher concentrations of darifenacin appeared insurmountable and lower concentrations did not produce a significant shift of the concentration response curve, a single concentration of 30 nMdarifenacin was used. The estimated pKb for darifenacin was calculated using the formula pKb = −(log [darifenacin concentration] −log (concentration ratio−1)). For methoctramine, Schild plots were constructed, because the slope of all plots was not significantly different from 1, the slope was constrained to 1 and pKb are presented.
Immunoprecipitation
Detrusor muscle and urothelium were prepared for immunoprecipitation. The urothelium was carefully dissected from underlying muscle and scraped free of remaining smooth muscle cells.
Bladder tissue samples were homogenized in ice cold Tris EDTA buffer (TE) with protease inhibitory cocktail (PIC) at 100 mg tissue per ml. PIC contained 10 μg/ml of the following protease inhibitors: soybean and lima bean trypsin inhibitors, aprotinin, leupeptin, pepstatin, and α2-macroglobulin. Homogenization was performed using three 1 sec bursts with Brinkman homogenizer (model PT10/36). The homogenate was filtered through a four-layer gauze filter and 20 μl [3H] QNB (49 Curies/mM, approximately 4,000 cpm/μl) per ml assay homogenate was added. Incubation was performed at room temperature for 30 min with inversion every 5 min. Samples were then centrifuged at 20,000g for 10 min at 4 °C. Radiolabeled receptors were solubilized from this pellet by resuspension in TE buffer containing 1% digitonin and 0.2% cholic acid (1% TEDC) with PIC at 100 mg wet weight per ml. Samples were incubated for 50 min at 4 °C, with inversion every 5 min. Following incubation, samples were centrifuged at 30,000g for 45 min at 4 °C. The supernatant containing the solubilized receptors was incubated overnight after addition of the M2 antibody, the M3 antibody (described below), or vehicle in triplicate at 4 °C.
Samples underwent desalting over Sephadex G-50 mini-columns with 0.1% TEDC to determine total receptor density. For immunoprecipitation of M2 and M3 receptors and determining background, 200 μl of pansorbin was added and allowed to incubate at 4 °C for 50 min, with inversion every 5 min. Samples were centrifuged at 15,000g for 1 min at 4 °C. Surface wash was performed with the remaining pellet using 500 μl of 0.1% TEDC. Fifty microliters of 72.5 mM deoxycholate/750 mM NaOH was added to all tubes except totals. Incubation was performed for 30 min at room temperature. Resuspension was performed in 1 ml of TE. Fifty microliters of 1M HCl was added to all samples receiving deoxycholate/NaOH. Radioactive counts were determined by liquid scintillation spectrometry on a Beckman LS5000 TA liquid scintillation counter. The e/ciency of the scintillation counter was determined by counting a chemically identical non-radioactive sample spiked with a known amount of tritiated water in triplicate. The specific activity of the [3H] QNB is 49 Cu/mmole which equals 109 dpm/fmole or 54 cpm/fmole based on the e/ciency of the scintillation counter.
Protein Assay
Protein content was determined by a Coomassie blue dye binding protein assay (Sigma-Aldrich) using bovine serum albumin as a standard. Samples of homogenate tested were a 1:500 dilution in TE and samples of solubilized receptors at a 1:50 dilution in TE. Optical density was determined using a Beckman DU64 spectrophotometer.
Specificity of Antibodies
Rabbit polyclonal antibodies were used for the immunoprecipitation assays. The M2 antibody was raised to a fusion protein of glutathione-S-transferase and a sequence corresponding to the third intracellular loop of the M2 receptor. The M3 antibody was raised to a synthetic peptide corresponding to the C terminus of the M3 receptor coupled to thyroglobulin. The production and specificity of these antibodies as determined by immunoprecipitation of clonal cell lines expressing individual receptor subtypes has been previously described [Luthin et al., 1988; Yasuda et al., 1993; Wang et al., 1995].These studies have clearly demonstrated that these antibodies not only precipitate the appropriate receptor sub-type but also that neither antibody cross reacts with any of the other receptor subtypes. This immunoprecipitation assay makes use of dual, tandem specificity—the specificity of [3H]QNB binding to only muscarinic receptors and the specificity of the individual antibody binding to only the given muscarinic receptor subtype. If the antibody binds to other proteins that do not bind [3H]QNB then those proteins would not be detected in the assay. Likewise if [3H]QNB binds to other proteins that do not bind to the antibody then those proteins would also not be detected by the assay.
RESULTS
Bladder Specimens
All samples were procured by the National Disease Research Institute (NDRI) from organ transplant donors over a 3-year period. The majority had significant brain injury resulting in death (Table I). Darifenacin affinity was determined in 24 bladders, methoctramine affinity was determined in 9 of these specimens, and muscarinic receptor density was determined in 17 of these specimens. The specimens were separated into muscle and urothelium for quantification of receptor density. Donor age ranged from 4 to 70 years, with median age of 47. Of the 24 bladder specimens, 14 were from males and 10 were from females, 22 were Caucasian, 1 was Black and 1 was Hispanic. The donor age ranged from 4 to 70 years with a median age of 49 for the 17 bladders used for quantification of receptor density. Seven were from females and 10 were from males, all Caucasian. Additionally, quantification of receptor density was performed on three normal Yorkshire pig bladders using the same procedure.
TABLE I.
| Max | CARB | DAR | METH | Muscle receptors | Urothelial receptors | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Race | Sex | Wt. (kg) | History | (% KCl) | pEC50 | pKb | pKb | Total | M2 | M3 | Total | M2 | M3 |
| 70 | C | F | 108 | ICH | 133 | 7.07 | 9.3 | 175 | 112 | 27 | 14 7 | 98 | 9 | |
| 63 | C | F | 82 | SAH | 133 | 6.25 | 9.0 | 188 | 70 | 43 | 202 | 78 | 17 | |
| 59 | C | M | 101 | ICH | 183 | 6.49 | 8.6 | 950 | 574 | 269 | 131 | 81 | 13 | |
| 58 | C | M | 77 | SAH | 99 | 5.55 | 8.4 | 5.9 | 284 | 249 | 42 | 274 | 189 | 15 |
| 19 | C | M | 70 | MVA | 102 | 6.17 | 8.4 | |||||||
| 39 | C | M | 92 | CVA | 155 | 6.59 | 8.4 | 267 | 115 | 47 | 174 | 94 | 10 | |
| 39 | C | F | 76 | CB | 10 5 | 5.85 | 8.3 | 16 5 | 95 | 44 | 3 18 | 15 6 | 6 | |
| 4 | C | M | 14 | ANOXIA | 137 | 6.57 | 8.3 | 6.3 | 777 | 459 | 193 | 316 | 163 | 60 |
| 44 | C | F | 77 | MVA | 269 | 5.77 | 8.2 | 5.4 | ||||||
| 20 | C | M | 84 | HT | 83 | 6.94 | 8.2 | 6.1 | 531 | 313 | 157 | 277 | 146 | 44 |
| 25 | C | M | 89 | ANOXIA | 363 | 5.71 | 8.1 | 184 | 94 | 53 | 147 | 83 | 29 | |
| 61 | C | F | 72 | ICH | 195 | 5.99 | 8.0 | 5.7 | 290 | 190 | 72 | 229 | 218 | 27 |
| 58 | C | F | 99 | SAH | 124 | 6.05 | 8.0 | 5.6 | ||||||
| 44 | B | F | 118 | CB | 128 | 6.23 | 7.9 | |||||||
| 49 | C | M | 90 | HT | 155 | 6.21 | 7.9 | 207 | 113 | 31 | 400 | 179 | 30 | |
| 58 | C | M | 68 | CVA | 329 | 5.71 | 7.9 | |||||||
| 38 | C | F | 126 | CVA | 161 | 5.57 | 7.8 | 239 | 146 | 52 | 274 | 193 | 18 | |
| 46 | C | F | 55 | CVA | 122 | 6.13 | 7.8 | 6.0 | 277 | 182 | 75 | 171 | 103 | 17 |
| 59 | C | M | 98 | ICH | 133 | 6.60 | 7.7 | 637 | 371 | 126 | 495 | 350 | 74 | |
| 56 | H | F | 69 | CVA | 6.10 | 7.6 | ||||||||
| 34 | C | F | 60 | ICH | 114 | 6.66 | 7.6 | 286 | 146 | 76 | 146 | 88 | 13 | |
| 47 | C | M | 75 | ANOXIA | 107 | 5.28 | 7.5 | |||||||
| 69 | C | M | 64 | SDH | 117 | 5.13 | 7.4 | 6.5 | 363 | 185 | 64 | 225 | 151 | 25 |
| 70 | C | M | 73 | ICH | 148 | 6.19 | 7.2 | 5.6 | 391 | 275 | 82 | 344 | 199 | 37 |
Muscarinic receptor density is reported as fmoles/mg solubilized protein. CARB, carbachol; DAR, darifenacin; METH, methoctramine; ICH, intracerebral hemorrhage; SAH, sub-arachnoid hemorrhage; MVA, motor vehicle accident; CVA, cerebrovascular accident; CB, cerebral bleed; HT, head trauma; SDH, sub-dural hemorrhage.
Affinity of Anticholinergics for Inhibition of Carbachol Mediated Bladder Contraction
The affinity (pKb) of darifenacin for inhibition of carbachol stimulated contraction ranges from a high of 9.3 to a low of 7.2 (Table I). The affinity of methoctramine ranged from 5.4 to 6.5. The slope of the Schild plots for methoctramine are not statistically different from unity (mean = 1.13 ± 0.24). There is no correlation between darifenacin affinity and methoctramine affinity.
Contractile Parameters
There is no correlation between darifenacin affinity and the carbachol maximal contraction. However, there is a significant correlation (r = 0.43, P < 0.05) between darifenacin affinity (pKb) and the pEC50 for carbachol (Fig. 1) such that the lower the darifenacin affinity, the lower the carbachol potency. No other donor parameters including age, time on life support, sex, or time from harvest to performing the experiments correlate with the affinity of darifenacin (data not shown).
Fig. 1.
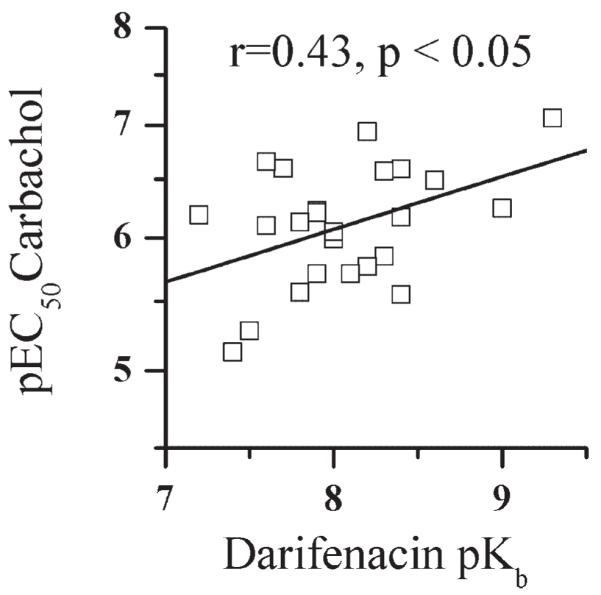
Correlation of carbachol pEC50 with darifenacin affinity. For carbachol affinity pEC50 indicates -Log EC50 expressed as the molar concentration of carbachol that produced 50% of the maximal response to 120 mM KCl.
Human Bladder Muscle Receptors
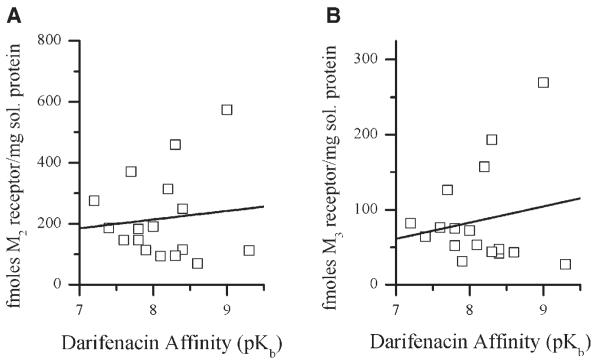
The detrusor samples were evaluated by immunoprecipitation to quantify receptor density. All assays were performed in triplicate. There is no correlation between darifenacin affinity and either M2 or M3 receptor density in the muscle (Table I, Fig. 2). The ratio of M2 to M3 receptors in the muscle specimens is approximately 3:1.
Fig. 2.
Correlation of human detrusor M2 and M3 receptor density with darifenacin affinity. Immunoprecipitation was performed as described in the Methods section. Each symbol represents receptor density in fmoles per mg solubilized protein for M2 (A) and M3 (B) determined in triplicate from each individual bladder detrusor muscle specimen from an individual organ donor.
Human Bladder Urothelial Receptors
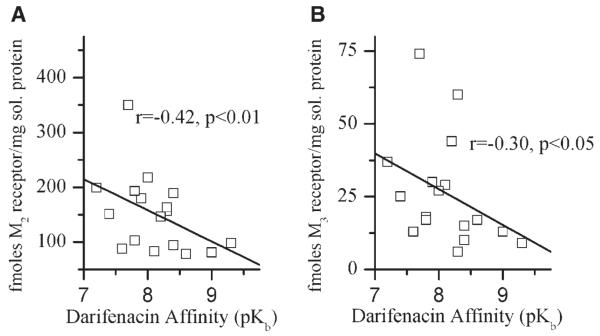
In the urothelium, M2 and M3 muscarinic receptor density has a significant negative correlation (M2 r = −0.42, P < 0.01; M3 r = −0.30, P < 0.05) with darifenacin affinity (Table I, Fig. 3). Thus, the greater the darifenacin affinity, the lower the density of urothelial M2 and M3 receptors. The ratio of M2 to M3 receptors in the urothelium specimens is approximately 8:1.
Fig. 3.
Correlation of urothelial M2 and M3 receptor density with darifenacin affinity determined using the underlying detrusor. Immunoprecipitation was performed as described in the Methods section. Each symbol represents receptor density in fmoles per mg solubilized protein for M2 (A) and M3 (B) determined in triplicate from each individual bladder urothelial specimen from an individual organ donor.
Pig Bladder Detrusor and Urothelial Receptors
ThreeYorkshire pig bladders underwent immunoprecipitation. Total muscarinic receptor density in the detrusor muscle specimens and urothelial specimens is 83 ±15 and 153 ± 10 fmol/mg solubilized protein, respectively. M2 receptor density in the pig detrusor and urothelium is 52 ± 9 and 91 ± 5 fmol/mg solubilized protein. The M3 receptor density in the detrusor and urothelium is 5 ± 0.3 and 2 ± 0.5 fmol/mg solubilized protein, respectively.
DISCUSSION
Although the muscarinic receptor subtype mediating contraction of the normal human bladder is thought to be the M3 subtype, this is based on the following reports in a limited total number of human bladder specimens [Waldeck et al.,1997; Yono et al., 1999; D’Agostino et al., 2000; Chess-Williams et al., 2001; Fetscher et al., 2002; Miyamae et al., 2003; Pontari et al., 2004; Schneider et al., 2004a].These various studieshave demonstrated that M3-selective antagonists such as 4-DAMP and darifenacin inhibit carbachol mediated contractions with high affinity and M2-selective antagonists such as methoctramine inhibit contraction with low affinity, concluding that the M3 receptor mediates normal human detrusor contraction. In all of these reports other than our own, mean affinity values are reported for the antagonists across all of the different bladder specimens, all of which were obtained at cystectomy for bladder cancer. This averaging of affinity values across different patient specimens eliminates direct observation of the individual variation between the different subjects. Reporting average affinities across different human specimens also implicitly assumes that all of the specimens included in the average are the same.We have previously demonstrated that the M2 receptor is involved in mediating bladder contraction in patients with neurogenic bladder dysfunction from spinal cord injury and myelodysplasia as well as in selected bladders obtained from organ donors and patients with neurogenic bladder. We found that the affinity of M3-selective muscarinic antagonists (p-F-HHSiD and darifenacin) in some of the bladder specimens from organ transplant donors fell well below the range for M3 receptors reported in the literature (six of six specimens for p-F-HHSiD and three of five specimens for darifenacin) [Pontari et al., 2004]. The distribution of darifenacin affinity presented here with a larger number of organ donor specimens (n = 24) is similar to our previously published results (n = 5) although the darifenacin affinities in this larger sample size have a greater range.
The affinity of darifenacin for inhibition of bladder contractions in individual specimens from organ donors was determined in order to evaluate the receptor subtypes mediating detrusor contraction. The darifenacin affinity from donor tissue varied between subjects, consistent with M2, M3, and both M2 and M3 receptors involved in mediation of contraction (Table I). A number of experimental conditions in the rat are associated with an increased contribution of the M2 receptor subtype to the contractile response. These include sarcoplasmic reticulum calcium depletion with thapsigargin [Braverman et al., 2002], bladder denervation [Braverman et al., 1999; Braverman and Ruggieri, 2003; Ruggieri and Braverman, 2006], spinal cord injury [Braverman et al., 1999], bladder decentralization [Braverman and Ruggieri, 2003], and bladder outlet obstruction [Braverman and Ruggieri, 2003; Ruggieri and Braverman, 2006]. It is unclear if previous bladder hypertrophy, neurogenic lesions, or bladder over activity symptoms were present in the medical history of bladder donors based on the limited information available from the organ procurement agency. There are multiple possible explanations for the variation in darifenacin affinity observed in these specimens in addition to changes due to bladder dysfunction, including anoxia or ischemia from harvesting, drug treatments, sustained bladder inactivity during periods of life support and autonomic drug administration during life support. It is also possible that this variation observed in organ transplant donors reflects the natural variation of normal human bladder contractile response. Possible explanations for the discrepancy between our findings and previous findings that only M3 receptors mediate contraction of normal human bladder [Waldeck et al., 1997; Yono et al., 1999; D’Agostino et al., 2000; Chess-Williams et al., 2001; Fetscher et al., 2002; Miyamae et al., 2003; Schneider et al., 2004a] include not only differences in the source of bladder tissue (cancer cystectomy vs. organ transplant donors) and averaging the data across all of the different subjects but also other methodologic differences. All of the previous studies with the exception of our own have used each strip as its own control, repeating agonist concentration response curves up to five times in succession with increasing concentrations of the antagonists whereas in our studies each strip was exposed to only a single cumulative agonist concentration response curve either in the presence or absence of the antagonist in separate groups of strips.
For all of the nine specimens in which methoctramine affinity was determined the affinity was low, significantly lower than the range of 7.8–8.3 reported for the affinity of methoctramine at M2 receptors, and closest to the range of 6.3–6.9 reported for M3 receptors [Caulfield and Birdsall, 1998]. Low methoctramine affinity was found even in the bladders which had the lowest darifenacin affinity (Table I). This is similar to our previous findings in bladders from organ transplant donors [Pontari et al., 2004] in which apparent contradictory data was produced in certain specimens that demonstrated a low affinity of the M3-selective antagonists indicating an M2 contribution to the contractile response together with a low methoctramine affinity indicating an M3 mediated response. We interpret this as being consistent with both M2 and M3 receptor subtypes contributing to the contractile response. In the presence of low methoctramine concentrations the M3 receptor mediates the contraction which is not inhibited until the methoctramine concentration is high enough to inhibit the M3 receptors. The same would hold for the M3-selective antagonist darifenacin in which the M2 receptors mediate the contraction at low darifenacin concentrations. Thus in a tissue in which both M2 and M3 receptors are capable of mediating contraction the affinity of both M2- and M3-selective antagonists would beexpected to be low and within the range of the least sensitive receptor subtype.
We performed immunoprecipitation on Yorkshire pig bladders to reproduce previous data of urothelial and detrusor muscarinic receptor populations. Our determination of receptor density closely matches data from receptor binding protocols [Hawthorn et al., 2000; Mansfield et al., 2005], with a greater density in the urothelium than in the smooth muscle for pig bladders. The immunoprecipitated M2 and M3 receptors accounted for 69% of the total urothelial and 61% of the total muscle receptors. This is different that the human bladder in which the muscarinic receptor density is greater in the muscle than the urothelium and the immunoprecipitated M2 and M3 receptors accounted for 71% of the total urothelial and 81% of the total muscle receptors.While immunoprecipitation studies in intact tissues routinely report that some fraction of the total receptors are resistant to immunoprecipitation [Luthin et al., 1988; Cawley et al., 1993; Emala et al., 1995; Ruggieri et al., 1995; Wang et al., 1995; Pontari et al., 1998] this does not rule out the possible presence of subtypes other than M2 and M3 in these tissues.
Studies have shown urothelial expression of receptors for ATP, capsaicin, temperature, acetylcholine, norepinephrine, and mechanosensitivity [Birder, 2005], as well as release of chemical mediators such as NO, ATP, acetylcholine, substance P, and prostaglandins [de Groat, 2004]. Mansfield et al. [2005] demonstrated in binding studies using four subtype specific antagonists the presence of a majority of M2 receptors in the human urothelium, with minor populations of darifenacin-preferring sites representing either M3 and/or M5 receptors, with no difference in total receptor density between the detrusor and urothelium. We performed immunoprecipitation assays using M2 and M3 specific antibodies to calculate the density of muscarinic receptors within the detrusor and urothelium of human bladders and found that total receptor density for human bladder is higher compared to this previous report,with a higher ratio of muscle to mucosa muscarinic receptors. Unlike the pig model, in the human bladder, there are more muscarinic receptors in the detrusor compared to the urothelium. A similar 3:1 ratio of M2/M3 receptors for human detrusor was found compared to prior reported data [Mansfield et al., 2005], although we report a higher ratio in the urothelium of 8:1.
There was a statistically significant relationship between the affinity of darifenacin and the population of urothelial receptors. The lower the darifenacin affinity (M2 receptor contributing to contraction) the greater the density of M2 and M3 muscarinic receptors. No such relationship is seen in the detrusor muscle. This alludes to a role of bladder urothelium in causing the change in phenotype of detrusor smooth muscle from the M3 contractile phenotype to either mixed M2 and M3 phenotype or the M2 contractile phenotype, due to the increased density of muscarinic receptors in the urothelium. Inhibition of smooth muscle contraction is induced by functional urothelium, suggesting the presence of a diffusible inhibitory factor [Hawthorn et al., 2000]. This diffusible inhibitory factor has yet to be identified, however, studies in pig and human but not rat bladder strips have suggested that carbachol stimulation of muscarinic urothelial receptors leads to the release of this diffusible inhibitory factor which can regulate detrusor contractility [Hawthorn et al., 2000; Chess-Williams, 2002; Chaiyaprasithi et al., 2003]. Similarly, it is possible that a urothelial derived diffusable factor determines the phenotype of the detrusor contraction. Because the contractility data described here was obtained following removal of the urothelium, this alteration in contractile phenotype must either be irreversible or slow to revert.
CONCLUSIONS
Our data from human bladder detrusor and urothelium demonstrates that sub-populations of muscarinic receptors within the urothelium are different between bladders with normal and pathologic contraction.This adds to evidence that the urothelium may be capable of exerting control over the expression of the contractile signal transduction mechanism of the underlying smooth muscle. While no physiologic connection has been confirmed, we speculate that the increased urothelial receptor density in bladders with an M2 mediated contractile component could have implications on future anti-muscarinic drug therapy. Further study is needed to clarify the role of the urothelium in the change in contractile phenotype of the detrusor smooth muscle from M3 to M2 mediated contractions.
ACKNOWLEDGMENTS
This work was supported by NIH grant, PHS, grant no. RO1DK43333 (to M.R.R.); Industry grants by Pfizer (to A.S.B.); Unrestricted grants by Yamanuchi (to M.R.R.), Astra-Zeneca (to M.R.R.); Paid consultant: Novartis (to M.R.R.), Pfizer (to M.R.R.).
Grant sponsor: PHS (NIH); Grant number: RO1DK43333; Grant sponsor: Pfizer; Grant sponsor: Yamanuchi; Grant sponsor: Astra-Zeneca; Grant sponsor: Novartis.
Footnotes
See Acknowledgments for conflict of interest statement.
REFERENCES
- Appell RA, Sand P, Dmochowski R, et al. Prospective randomized controlled trial of extended-release oxybutynin chloride and tolterodine tartrate in the treatment of overactive bladder: Results of the OBJECT Study. Mayo Clinic Proc. 2001;76:358–63. see comment. [PubMed] [Google Scholar]
- Birder LA. More than just a barrier: Urothelium as a drug target for urinary bladder pain [Review] [78 refs] Am J Physiol Renal Physiol. 2005;289:F489–95. doi: 10.1152/ajprenal.00467.2004. [DOI] [PubMed] [Google Scholar]
- Braverman AS, Ruggieri MR., Sr. Hypertrophy changes the muscarinic receptor subtype mediating bladder contraction from M3 toward M2. Am J Physiol Regul Integr Comp Physiol. 2003;285:R701–08. doi: 10.1152/ajpregu.00009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman A, Legos J, Young W, et al. M2 receptors in genito-urinary smooth muscle pathology. Life Sci. 1999;64:429–36. doi: 10.1016/s0024-3205(98)00582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman AS, Tallarida RJ, Ruggieri MR., Sr. Interaction between muscarinic receptor subtype signal transduction pathways mediating bladder contraction. Am J Physiol Regul Integr Comp Physiol. 2002;283:R663–68. doi: 10.1152/ajpregu.00116.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors characterization, coupling and function. Pharmacol Ther. 1993;58:319–79. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJ. International union of pharmacology. XVII. Classification of muscarinic acetylcholine receptors [Review] [146 refs] Pharmacol Rev. 1998;50:279–90. [PubMed] [Google Scholar]
- Cawley TA, Jr., Shickley TJ, Ruggieri MR, et al. Effect of chronic neuro-leptic treatment on central and peripheral muscarinic receptors. J Pharmacol ExpTher. 1993;267:134–39. [PMC free article] [PubMed] [Google Scholar]
- Chaiyaprasithi B, Mang CF, Kilbinger H, et al. Inhibition of human detrusor contraction by a urothelium derived factor. J Urol. 2003;170:1897–900. doi: 10.1097/01.ju.0000091870.51841.ae. [DOI] [PubMed] [Google Scholar]
- Chancellor MB, Appell RA, Sathyan G, et al. A comparison of the effects on saliva output of oxybutynin chloride and tolterodine tartrate. ClinTher. 2001;23:753–60. doi: 10.1016/s0149-2918(01)80024-2. see comment. [DOI] [PubMed] [Google Scholar]
- Chapple CR, Martinez-Garcia R, Selvaggi L, et al. for the Ssg. 2005. A comparison of the e/cacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: Results of the STAR trial. Eur Urol. 48:464–70. doi: 10.1016/j.eururo.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Chess-Williams R. Muscarinic receptors of the urinary bladder: Detrusor, urothelial and prejunctional. Auton Autacoid Pharmacol. 2002;22:133–45. doi: 10.1046/j.1474-8673.2002.00258.x. [DOI] [PubMed] [Google Scholar]
- Chess-Williams R, Chapple CR, Yamanishi T, et al. The minor population of M3-receptors mediate contraction of human detrusor muscle in vitro. J Auton Pharmacol. 2001;21:243–48. doi: 10.1046/j.1365-2680.2001.00231.x. [DOI] [PubMed] [Google Scholar]
- D’Agostino G, Bolognesi ML, Lucchelli A, et al. Prejunctional muscarinic inhibitory control of acetylcholine release in the human isolated detrusor: Involvement of the M4 receptor subtype. BrJ Pharmacol. 2000;129:493–500. doi: 10.1038/sj.bjp.0703080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC. The urothelium in overactive bladder: Passive bystander or active participant? Urology. 2004;64:7–11. doi: 10.1016/j.urology.2004.08.063. [DOI] [PubMed] [Google Scholar]
- Diokno A, Sand P, Labasky R, et al. Long-term safety of extended-release oxybutynin chloride in a community-dwelling population of participants with overactive bladder: A one-year study. Int Urol Nephrol. 2002;34:43–9. doi: 10.1023/a:1021372426421. [DOI] [PubMed] [Google Scholar]
- Drutz HP, Appell RA, Gleason D, et al. Clinical e/cacy and safety of tolterodine compared to oxybutynin and placebo in patients with overactive bladder. Int Urogynecol J. 1999;10:283–89. doi: 10.1007/s001929970003. [DOI] [PubMed] [Google Scholar]
- Ehlert FJ, Gri/n MT, Abe DM, et al. The M2 muscarinic receptor mediates contraction through indirect mechanisms in mouse urinary bladder. J Pharmacol ExpTher. 2005;313:368–78. doi: 10.1124/jpet.104.077909. [DOI] [PubMed] [Google Scholar]
- Emala CW, Aryana A, Levine MA, et al. Basenji-greyhound dog: Increased m2 muscarinic receptor expression in trachealis muscle. Am J Physiol. 1995;268:L935–40. doi: 10.1152/ajplung.1995.268.6.L935. [DOI] [PubMed] [Google Scholar]
- Fetscher C, Fleichman M, Schmidt M, et al. M(3) muscarinic receptors mediate contraction of human urinary bladder. Br J Pharmacol. 2002;136:641–43. doi: 10.1038/sj.bjp.0704781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevaert T, Ost D, De Ridder D. Comparison study of autonomous activity in bladders from normal and paraplegic rats. Neurourol Urodyn. 2006;24:368–78. doi: 10.1002/nau.20206. [DOI] [PubMed] [Google Scholar]
- Haab F, Stewart L, Dwyer P. Darifenacin, an M3 selective receptor antagonist, is an effective and well-tolerated once-daily treatment for overactive bladder [Review] [36 refs] Eur Urol. 2004;45:420–29. doi: 10.1016/j.eururo.2004.01.008. discussion 429. [DOI] [PubMed] [Google Scholar]
- Halaska M, Ralph G, Wiedemann A, et al. Controlled, double-blind, multicentre clinical trial to investigate long-term tolerability and e/cacy of trospium chloride in patients with detrusor instability. World J Urol. 2003;20:392–99. doi: 10.1007/s00345-003-0321-8. [DOI] [PubMed] [Google Scholar]
- Hawthorn MH, Chapple CR, Cock M, et al. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol. 2000;129:416–19. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde SS. Muscarinic receptors in the bladder: From basic research to therapeutics. Br J Pharmacol. 2006;147:S80–7. doi: 10.1038/sj.bjp.0706560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthin GR, Harkness J, Artymyshyn RP, et al. Antibodies to a synthetic peptide can be used to distinguish between muscarinic acetylcholine receptor binding sites in brain and heart. Mol Pharmacol. 1988;34:327–33. [PubMed] [Google Scholar]
- MacDiarmid SA, Anderson RU, Armstrong RB, et al. E/cacy and safety of extended release oxybutynin for the treatment of urge incontinence: An analysis of data from 3 flexible dosing studies. J Urol. 2005;174:1301–05. doi: 10.1097/01.ju.0000173076.93737.d5. discussion 1305. [DOI] [PubMed] [Google Scholar]
- Mansfield KJ, Liu L, Mitchelson FJ, et al. Muscarinic receptor subtypes in human bladder detrusor and mucosa, studied by radioligand binding and quantitative competitive RT-PCR: Changes in ageing. Br J Pharmacol. 2005;144:1089–99. doi: 10.1038/sj.bjp.0706147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamae K, Yoshida M, Murakami S, et al. Pharmacological effects of darifenacin on human isolated urinary bladder. Pharmacology. 2003;69:205–11. doi: 10.1159/000073665. [DOI] [PubMed] [Google Scholar]
- Pontari MA, Luthin GR, Braverman AS, et al. Characterization of muscarinic cholinergic receptor subtypes in rat prostate. J Recept Signal Transduct Res. 1998;18:151–66. doi: 10.3109/10799899809047742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontari MA, Braverman AS, Ruggieri MR., Sr. The M2 muscarinic receptor mediates in vitro bladder contractions from patients with neurogenic bladder dysfunction. Am J Physiol Regul Integr Comp Physiol. 2004;286:R874–80. doi: 10.1152/ajpregu.00391.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovner ES, Wein AJ. Once-daily, extended-release formulations of anti-muscarinic agents in the treatment of overactive bladder: A review [Review] [59 refs] Eur Urol. 2002;41:6–14. doi: 10.1016/s0302-2838(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Ruggieri MR, Sr., Braverman AS. Regulation of bladder muscarinic receptor subtypes by experimental pathologies. Auton Autacoid Pharmacol. 2006;26:311–26. doi: 10.1111/j.1474-8673.2006.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri MR, Colton MD, Wang P, et al. Human prostate muscarinic receptor subtypes. J Pharmacol ExpTher. 1995;274:976–82. [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Fetscher C, Krege S, et al. Signal transduction underlying carbachol-induced contraction of human urinary bladder. J Pharmacol ExpTher. 2004a;309:1148–53. doi: 10.1124/jpet.103.063735. [DOI] [PubMed] [Google Scholar]
- Schneider T, Hein P, Michel MC. Signal transduction underlying carbachol-induced contraction of rat urinary bladder. I. Phospholipases and Ca2+ sources. J Pharmacol ExpTher. 2004b;308:47–53. doi: 10.1124/jpet.103.058248. [DOI] [PubMed] [Google Scholar]
- Siami P, Seidman LS, Lama D. A multicenter, prospective, open-label study of tolterodine extended-release 4 mg for overactive bladder: The speed of onset of therapeutic assessment trial (STAT) Clin Ther. 2002;24:616–28. doi: 10.1016/s0149-2918(02)85137-2. [DOI] [PubMed] [Google Scholar]
- Sussman D, Garely A. Treatment of overactive bladder with once-daily extended-release tolterodine or oxybutynin: The antimuscarinic clinical effectiveness trial (ACET) [see comment] Curr Med Res Opin. 2002;18:177–84. doi: 10.1185/030079902125000570. [DOI] [PubMed] [Google Scholar]
- Waldeck K, Larsson B, Andersson KE. Comparison of oxybutynin and its active metabolite, N-desethyl-oxybutynin, in the human detrusor and parotid gland. J Urol. 1997;157:1093–97. [PubMed] [Google Scholar]
- Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol ExpTher. 1995;273:959–66. [PMC free article] [PubMed] [Google Scholar]
- Yamanishi T, Chapple CR, Yasuda K, et al. The role of M(2)-muscarinic receptors in mediating contraction of the pig urinary bladder in vitro. Br J Pharmacol. 2000;131:1482–88. doi: 10.1038/sj.bjp.0703719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda RP, Ciesla W, Flores LR, et al. Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: Distribution of m4 and m5 receptors in rat brain. Mol Pharmacol. 1993;43:149–57. [PubMed] [Google Scholar]
- Yono M, Yoshida M, Wada Y, et al. Pharmacological effects of tolterodine on human isolated urinary bladder. Eur J Pharmacol. 1999;368:223–30. doi: 10.1016/s0014-2999(99)00036-9. [DOI] [PubMed] [Google Scholar]