Abstract
A variety of substituted benzisoxazolines have been synthesized by the [3 + 2] cycloaddition of nitrones and arynes. The reaction scope is broad, the reaction conditions are mild, and the process tolerates a variety of functional groups.
Introduction
Benzisoxazoline is a critical sub-unit in pharmaceuticals with important biological and pharmacological activities, including antimicrobial activity against Salmonella paratyphi, Proteus vulgaris, Xanthomonas spp., Fusarium solanii, and Botrytis cinerea.1 Benzisoxazolines can also be important synthetic intermediates for the preparation of more complex molecules.2 Much effort has been devoted to the synthesis of this heterocyclic ring system.3
Since a convenient approach to aryne generation by the fluoride induced 1,2-elimination of o-(trimethylsilyl)aryl triflates was first reported,4 highly electrophilic arynes have been employed extensively in recent years for the construction of many heteroaromatic structures.5 Our group has recently reported that simple nucleophilic reactions,6 annulation reactions7 and a variety of 1,3-dipolar cycloadditions8 provide useful new synthetic routes to a variety of heterocycles, generally affording excellent yields under mild reaction conditions. In a continuation of our work on the reactions of arynes with dipoles, we have investigated the cycloaddition reactions of nitrones and arynes. During this project, related work was communicated by other groups.9 However, that work generally involved less convenient reaction conditions and aryne precursors, such as n-BuLi-promoted halogen exchange of o-haloaryl triflates under low temperatures,9b and PhI(OAc)2/TfOH-promoted generation of benzynes from benzobisoxadisiloles.9c More importantly, the authors failed to examine the scope and effect of various functional groups on their processes. Herein, we wish to report our more detailed and extensive results on the 1,3-dipolar cycloaddition reaction of nitrones and arynes generated from o-silylaryl triflates to afford a wide variety of substituted benzisoxazolines.10
Results and Discussion
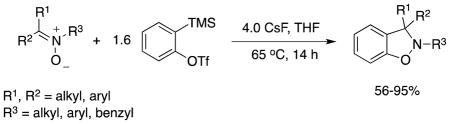
We optimized the reaction conditions for the reaction of the unsubstituted benzyne precursor o-(trimethylsilyl)phenyl triflate (1a) and N-benzylidenebenzylamine N-oxide (2a) (Table 1). Employing acetonitrile as the solvent and 3.0 equiv of CsF as the fluoride source provides the desired benzisoxazoline 3a in a 60% yield (entry 1). Adding more CsF or more benzyne precursor failed to improve the yield significantly (entries 2– 3). Another fluoride ion source, TBAF,11 was also examined (entries 4 and 5). By utilizing 2.0 equiv of 1a and 4.0 equiv of TBAF in THF (entry 5), a high yield of the corresponding benzisoxazoline (92%) was obtained. These reaction conditions, however, turned out not to be suitable for other nitrones, probably due to the relative rapid rate of benzyne generation. In order to better control the rate of benzyne generation, differing amounts of CsF in THF were examined, since CsF has limited solubility in THF, thus reducing the rate of benzyne generation (entries 6–9). Using 2.0 equiv of 1a and 5.0 equiv of CsF at 65 °C in THF, a 93% yield of the desired cycloadduct was produced (entry 7). Using reduced amounts of the benzyne precursor (1.6 equiv) and CsF (4.0 equiv) still afforded a high 91% yield (entry 8).
Table 1.
Optimization studies of the reaction between o-(trimethylsilyl)phenyl triflate (1a) and N-benzylidenebenzylamine N-oxide (2a) a
 | ||||||
|---|---|---|---|---|---|---|
| entry | benzyne (equiv) | CsF (equiv) | solvent | temp. (°C) | time (h) | % yield of 3ab |
| 1 | 1.2 | 3.0 | MeCN | rt | 24 | 60 |
| 2 | 1.2 | 4.0 | MeCN | rt | 24 | 65 |
| 3 | 2.0 | 3.0 | MeCN | rt | 24 | 56 |
| 4 | 1.2 | 2.0 TBAFc | THF | rt | 24 | 45 |
| 5 | 2.0 | 4.0 TBAFc | THF | 45 | 24 | 92 |
| 6 | 1.2 | 3.0 | THF | 65 | 24 | 68 |
| 7 | 2.0 | 5.0 | THF | 65 | 14 | 93 |
| 8 | 1.6 | 4.0 | THF | 65 | 14 | 91 |
| 9 | 1.6 | 3.0 | THF | 65 | 24 | 83 |
All reactions were conducted on a 0.25 mmol scale in 5 mL of solvent for 24 h.
Yields of products isolated by column chromatography.
1M TBAF in THF solution.
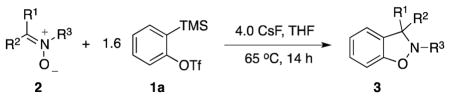
Using the optimal conditions shown in Table 1, entry 8, the scope and limitations of this methodology have been examined. Various substituted nitrones have been examined in this reaction and the results are summarized in Table 2.
Table 2.
Cycloadditions of Benzyne with Nitrones a
 | ||
|---|---|---|
| entry | product | yield (%)b |
| 1 |
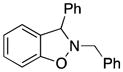 3a |
91 |
| 2 |
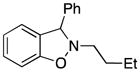 3b |
88 |
| 3 |
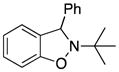 3c |
95 |
| 4 |
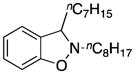 3d |
90 |
| 5 |
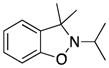 3e |
79 |
| 6 |
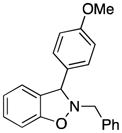 3f |
90 |
| 7 |
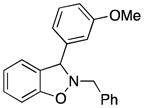 3g |
79 |
| 8 |
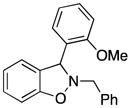 3h |
65 |
| 9 |
 3i |
69 |
| 10 |
 3j |
92 |
| 11 |
 3k |
90 |
| 12 |
 3l |
93 |
| 13 |
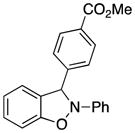 3m |
90 |
| 14 |
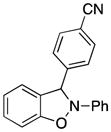 3n |
88 |
| 15 |
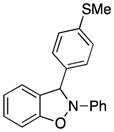 3o |
77 |
| 16 |
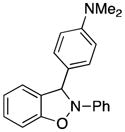 3p |
74 |
| 17 |
 3q |
57 |
| 18 |
 3r |
70 |
| 19 |
 3s |
56 |
| 20 |
 3t |
64 |
| 21 |
 3u |
65 |
| 22 |
 3v |
60 |
| 23 |
 3w |
64 |
Unless otherwise stated, all reactions have been carried out on a 0.25 mmol scale, with 1.6 equiv of benzyne precursor 1a and 4.0 equiv of CsF in 5 mL of THF at 65 °C for 14 h.
Yields of products isolated by column chromatography.
Excellent yields have been obtained from both N-alkyl (entries 2–5) and N-benzyl- substituted nitrones (entries 1, and 6–9). The results suggest that the steric effect of substituents on the carbon of the nitrone double bond are significant. Lower yields are observed for the substrates with more steric hindrance on the carbon of the C-N double bond. For example, 2e, which has two methyls on the carbon of the C-N double bond (entry 5), and 2h, which has an ortho-methoxy-substituted phenyl on that carbon (entries 8) both give lower yields. The process has also been applied to N-aryl-substituted nitrone 2j (entry 10), which affords a high 92% yield of benzisoxazoline 3j.
Nitrones with a variety of functionally-substituted phenyl groups on the nitrone carbon have been examined under our optimal conditions (entries 11–19). Many functional groups are well tolerated, including ester, cyano, amino, halogen and other groups. Nitrones with electron-donating groups tend to result in lower yields (entries 15 and 16) than nitrones with electron-withdrawing groups (entries 11–14), presumably due to the reduced electrophilicity of the carbon-nitrogen double bond due to the presence of such groups (see the later mechanistic discussion). Nitrone 2q also afforded a relatively low yield of 57%, mainly due to the steric hindrance produced by the ortho-methoxy group (entry 17). Due to the presence of the additional carbon-carbon double bond in 2s, the reaction was not as clean as that of other substrates, but still produced a good yield (entry 19).12 Several cyclic nitrones have also been examined and moderate yields have been obtained (entries 21–23).
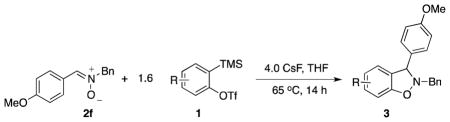
After a variety of substituted nitrones were examined, the behavior of various aryne precursors was studied in this reaction (Table 3).
Table 3.
Investigation of Different Arynes in the Coupling Reaction with Nitrone 2f a
 | |||
|---|---|---|---|
| entry | aryne | product | yield (%)b |
| 1 |
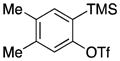 1b |
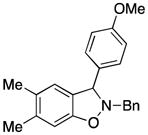 3fb |
72 |
| 2 |
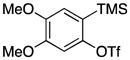 1c |
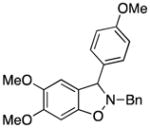 3fc |
70 |
| 3 |
 1d |
 3fd |
41 |
| 4 |
 1e |
 3fe |
75 |
Unless otherwise stated, all reactions have been carried out on a 0.25 mmol scale, with 1.6 equiv of benzyne precursor 1a and 4.0 equiv of CsF in 5 mL of THF at 65 °C for 14 h.
Yields of products isolated by column chromatography.
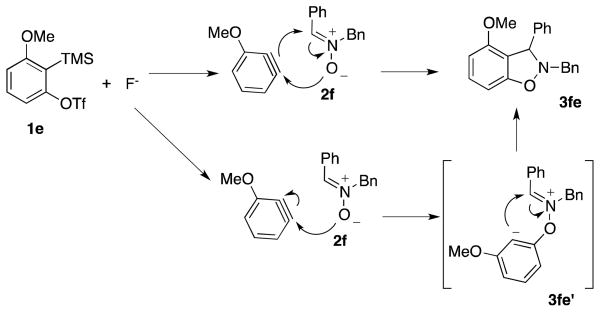
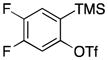
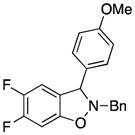
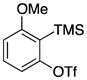
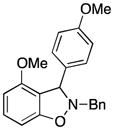
Symmetrical dimethyl- (1b), dimethoxy- (1c) and difluoro-substituted (1d) benzyne precursors have been employed under our optimal reaction conditions with nitrone 2f. All of these substrates generated comparable, but lower yields than benzyne itself. Similar results have been observed before in other reaction systems.6 The unsymmetrical methoxy-substituted benzyne precursor 1e provided benzisoxazoline 3fe as a single regioisomer in a 75% yield. Two possible mechanisms for this process are proposed in Scheme 1 based on the experimental results and previous experience.8
Scheme 1.
Possible Mechanisms
Following the formation of benzyne induced by fluoride from benzyne precursor 1e, a [3 + 2] cycloaddition can occur. Nucleophilic attack of the oxygen anion of 2f on benzyne, quickly followed by cyclization of 3fe′, can be envisioned as a mechanistic alternative. The significant steric and electronic effects of the substituents on the carbon-nitrogen double bond of the nitrone on the reaction results are consistent with both of these proposed mechanisms. The steric hindrance arising from the substituent on the carbon of the C-N double bond (see the results of entries 5, 8 and 17 in Table 2) are expected to disfavor the cycloaddition of 2f to 3fe or the cycloaddition of 3fe′ to 3fe. Furthermore, the presence of electron-withdrawing substituents on the phenyl group present on the carbon of the C-N double bond (see the results of entries 12–16 in Table 2) would be expected to increase the electrophilicity of the C-N double bond in 3fe′ producing higher yields of the products.
Conclusions
In conclusion, we have developed a facile and general method for the synthesis of substituted benzisoxazolines by the 1,3-dipolar cycloaddition of arynes and nitrones under mild reaction conditions, which provides excellent yields. A variety of functional groups are well tolerated in this process, allowing further synthesis of more complicated molecules.
Experimental Section
General
The 1H and 13C NMR spectra were recorded at 300 and 75.5 MHz or 400 and 100 MHz respectively. All melting points are uncorrected. High resolution mass spectra were recorded using EI at 70 eV. All reagents were used directly as obtained commercially unless otherwise noted. All reactions were carried out in oven-dried glassware and monitored by thin layer chromatography (SiO2, hexanes or hexanes/EtOAc). THF was distilled over Na. The silylaryl triflate 1a, CsF, TBAF solution (1M in THF) and acetonitrile were purchased from Sigma-Aldrich Co. 4,5-Dimethyl-substituted silylaryl triflate 1b, 4,5-dimethoxy-substituted silylaryl triflate 1c and 4,5-difluoro-substituted silylaryl triflate 1d were prepared according to a literature procedure.13
Non-commercially available compounds
Non-commercially available starting materials were prepared according to literature procedures.14
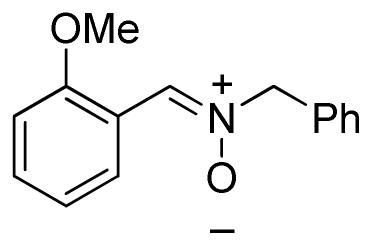
(2-Methoxyphenylmethylene)benzylamine-N-oxide (2h)
light yellow solid, mp 78–79 °C; 1H NMR (300 MHz, CDCl3) δ 9.28 (dd, J = 7.8, 1.5 Hz, 1H), 7.96 (s, 1H), 7.51-7.26 (m, 7H), 6.99 (t, J = 8.1 Hz, 1H), 6.85 (d, J = 7.5 Hz, 1H), 5.05 (s, 2H), 3.83 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 157.0, 133.9, 131.7, 129.1, 128.9, 128.8, 128.8, 120.8, 119.7, 109.8, 71.7, 55.6; HRMS (EI) calcd for C15H15NO2: 241.1103, found 241.1101.
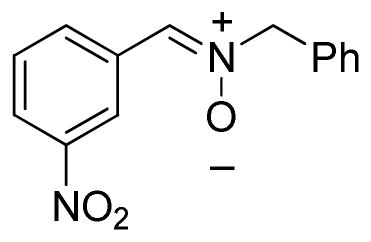
(3-Nitrophenylmethylene)benzylamine-N-oxide (2i)
yellow solid, mp 142–144 °C; 1H NMR (300 MHz, CDCl3) δ 9.04 (s, 1H), 8.57 (d, J = 7.8 Hz, 1H), 8.20 (d, J = 8.1 Hz, 1H), 7.57-7.41 (m, 7H), 5.11 (s, 2H); 13C NMR (75 MHz, CDCl3) δ 148.2, 133.8, 132.8, 132.1, 132.0, 129.6, 129.5, 129.5, 129.2, 124.7, 123.1, 71.9; HRMS (EI) calcd for C14H12N2O3: 256.0848, found 256.0843.
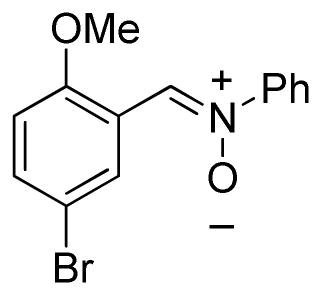
(5-Bromo-2-methoxyphenylmethylene)phenylamine-N-oxide (2q)
yellow solid, mp 135–137 °C; 1H NMR (300 MHz, CDCl3) δ 9.66 (d, J = 2.7 Hz, 1H), 8.33 (s, 1H), 7.78-7.74 (m, 2H), 7.51-7.46 (m, 4H), 6.79 (d, J = 9.0 Hz, 1H), 3.86 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 156.5, 149.6, 134.6, 131.1, 130.1, 129.3, 128.2, 121.9, 121.7, 113.6, 111.7, 56.1; HRMS (EI) calcd for C14H12NO2Br: 305.0051, found 305.0049.
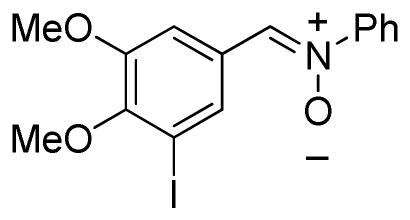
(4,5-Dimethoxy-3-iodophenylmethylene)phenylamine-N-oxide (2r)
yellow solid, mp 162–164 °C; 1H NMR (300 MHz, CDCl3) δ 8.60 (d, J = 1.5 Hz, 1H), 7.94 (d, J = 1.8 Hz, 1H), 7.82 (s, 1H), 7.75-7.72 (m, 2H), 7.45 (m, 3H), 3.92 (s, 3H), 3.89 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 152.2, 150.8, 148.9, 132.8, 132.3, 130.2, 129.3, 128.8, 121.7, 112.5, 92.0, 60.7, 56.1; HRMS (EI) calcd for C15H14NO3I: 383.0018, found 383.0014.
Representative procedure for the cycloaddition of arynes and nitrones
An oven dried 6-dram vial equipped with a stir bar was charged with 0.25 mmol of the nitrone, 0.40 mmol (1.6 equiv) of the aryne precursor and 1.00 mmol (152 mg, 4.0 equiv) of CsF, followed by 5 ml of dry THF. The vial was sealed and placed in an oil bath at 65 °C for about 14 h. The resultant mixture was cooled and concentrated under vacuum to yield the crude product, which was purified by flash chromatography on silica gel with ethyl acetate/hexanes or dichloromethane/hexane as the eluent.
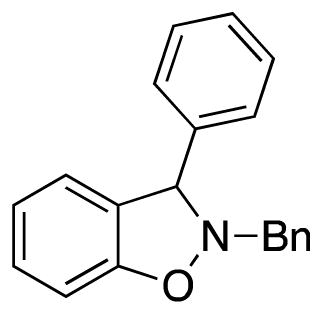
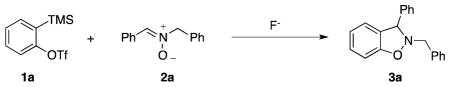
2-Benzyl-3-phenyl-2,3-dihydrobenzo[d]isoxazole (3a)
white solid (65.5 mg, 91%), mp 94–95 °C; 1H NMR (400 MHz, CDCl3) δ 7.43 (s, 2H), 7.39-7.28 (m, 8H), 7.23 (t, J = 7.7 Hz, 1H), 7.04 (d, J = 7.5 Hz, 1H), 6.93 (t, J = 7.4 Hz, 1H), 6.88 (d, J = 8.0 Hz, 1H), 5.37 (s, 1H), 4.41 (d, J = 13.3 Hz, 1H), 4.18 (d, J = 13.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 156.3, 140.7, 136.6, 129.5, 129.1, 129.0, 128.8, 128.7, 128.2, 128.0, 127.9, 124.5, 121.7, 117.6, 108.4, 72.9, 63.0; HRMS (EI) calcd for C20H17NO: 287.1310, found 287.1304.
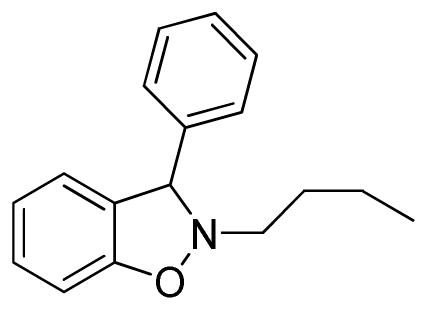
2-Butyl-3-phenyl-2,3-dihydrobenzo[d]isoxazole (3b)
colorless oil (55.7 mg, 88%); 1H NMR (300 MHz, CDCl3) δ 7.45-7.33 (m, 5H), 7.22 (t, J = 6.0 Hz, 1H), 7.00 (d, J = 6.0 Hz, 1H), 6.93-6.87 (m, 2H), 3.27 (m, 1H), 3.01 (m, 1H), 1.78-1.71 (m, 2H), 1.51-1.38 (m, 2H), 0.95 (t, J = 6.0 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 156.1, 140.8, 129.5, 129.0, 128.1, 128.0, 124.1, 121.3, 108.0, 74.5, 59.6, 30.0, 20.6, 14.1; HRMS (EI) calcd for C17H19NO: 253.1467, found 253.1463.
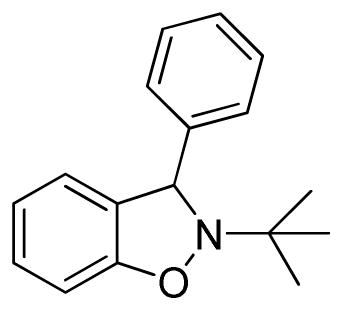
2-tert-Butyl-3-phenyl-2,3-dihydrobenzo[d]isoxazole (3c)
white solid (60.2 mg, 95%), mp 94–96 °C; 1H NMR (300 MHz, CDCl3) δ 7.43-7.26 (m, 5H), 7.14 (d, J = 9.0 Hz, 1H), 6.90 (d, J = 6.0 Hz, 1H), 6.83-6.79 (m, 2H), 5.61 (s, 1H), 1.20 (s, 9H); 13C NMR (75 MHz, CDCl3) δ 156.4, 144.1, 130.0, 128.8, 127.6, 127.5, 123.8, 120.9, 106.9, 67.2, 61.2, 29.9, 25.7; HRMS (EI) calcd for C17H19NO: 253.1467, found 253.1468.
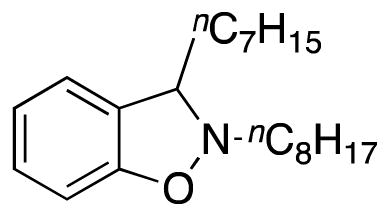
2-Octyl-3-heptyl-2,3-dihydrobenzo[d]isoxazole (3d)
colorless oil (74.8 mg, 90%); 1H NMR (300 MHz, CDCl3) δ 7.21-7.09 (m, 2H), 6.91 (t, J = 7.3 Hz, 1H), 6.80 (d, J = 8.0 Hz, 1H), 4.09 (dd, J = 7.7, 5.3 Hz, 1H), 3.02 (dd, J = 13.2, 6.5 Hz, 1H), 2.79-2.58 (m, 1H), 1.70-1.66 (m, 4H), 1.49-1.17 (m, 20H), 0.88 (t, J = 6.4 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 156.2, 129.3, 128.6, 123.9, 121.1, 108.4, 70.0, 60.4, 36.8, 32.1, 29.8, 29.7, 29.5, 29.5, 27.8, 27.6, 26.0, 22.9, 14.3; HRMS (EI) calcd for C22H37NO: 331.2875, found 331.2878.
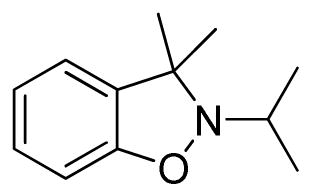
2-Isopropyl-3,3-dimethyl-2,3-dihydrobenzo[d]isoxazole (3e)
light brown oil (37.6 mg, 79%); 1H NMR (300 MHz, CDCl3) δ 7.21-7.15 (m, 2H), 6.92 (t, J = 6.0 Hz, 1H), 6.81 (d, J = 9.0 Hz, 1H), 4.05 (t, J = 6.0 Hz, 1H), 3.03 (m, 1H), 2.68 (m, 1H), 1.71 (m, 4H), 1.01 (td, J = 9.0, 6.0 Hz, 6H); 13C NMR (75 MHz, CDCl3) δ 156.3, 129.0, 128.7, 123.9, 121.1, 108.3, 71.2, 62.3, 29.5, 21.0, 12.0, 10.3; HRMS (EI) calcd for C12H17NO: 191.1310, found 191.1308.
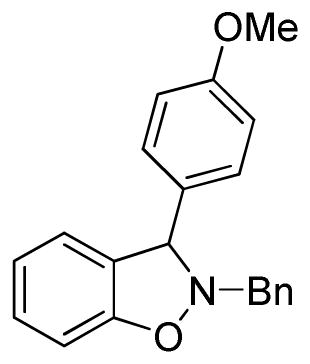
2-Benzyl-3-(4-methoxyphenyl)-2,3-dihydrobenzo[d]isoxazole (3f)
white solid (71.2 mg, 90%), mp 121–123 °C; 1H NMR (300 MHz, CDCl3) δ 7.46-7.31 (m, 5H), 7.26-7.20 (m, 3H), 7.02 (d, J = 6.0 Hz, 1H), 6.95-6.86 (m, 4H), 5.34 (s, 1H), 4.38 (d, J = 12.0 Hz, 1H), 4.18 (d, J = 12.0 Hz, 1H), 3.81 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 159.6, 156.4, 136.7, 132.6, 129.5, 129.2, 129.2, 129.0, 128.6, 127.8, 124.3, 121.6, 114.2, 108.3, 72.5, 62.6, 55.5; HRMS (EI) calcd for C21H19NO2: 317.1416, found 317.1420.
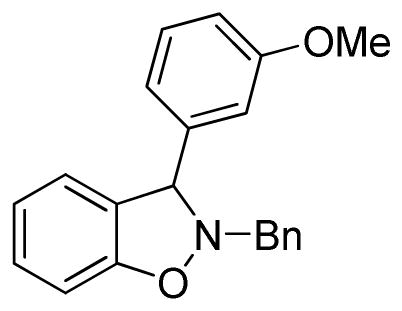
2-Benzyl-3-(3-methoxyphenyl)-2,3-dihydrobenzo[d]isoxazole (3g)
white solid (63.0 mg, 79%), mp 69–72 °C; 1H NMR (300 MHz, CDCl3) δ 7.46 (d, J = 6.0 Hz, 2H), 7.41-7.32 (m, 3H), 7.83 (m, 2H), 7.29-7.21 (m, 2H), 7.07 (d, J = 6.0 Hz, 1H), 6.96-6.83 (m, 5H), 5.36 (s, 3H), 4.42 (d, J = 12.0 Hz, 1H), 4.18 (d, J = 12.0 Hz, 1H), 3.77 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 160.0, 156.3, 142.2, 136.5, 129.8, 129.5, 129.1, 128.8, 128.6, 127.9, 124.4, 121.6, 120.2, 113.8, 113.3, 108.3, 72.7, 62.9, 55.4; HRMS (EI) calcd for C21H19NO2: 317.1416, found 317.1422.
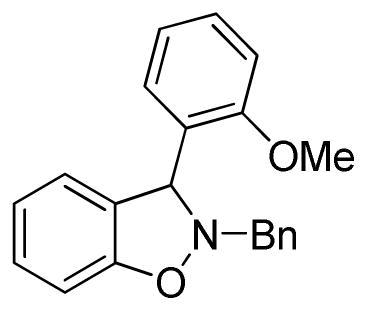
2-Benzyl-3-(2-methoxyphenyl)-2,3-dihydrobenzo[d]isoxazole (3h)
white solid (51.8 mg, 65%), mp 71–73 °C; 1H NMR (300 MHz, CDCl3) δ 7.46 (d, J = 6.0 Hz, 2H), 7.38-7.19 (m, 8H), 6.95-6.86 (m, 4H), 5.89 (s, 1H), 4.37 (d, J = 15.0 Hz, 1H), 4.18 (d, J = 15.0 Hz, 1H), 3.84 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 156.7, 156.4, 137.1, 129.6, 129.4, 128.9, 128.9, 128.5, 127.6, 124.8, 121.6, 121.1, 110.4, 108.5, 66.4, 63.5, 55.5; HRMS (EI) calcd for C21H19NO2: 317.1416, found 317.1419.
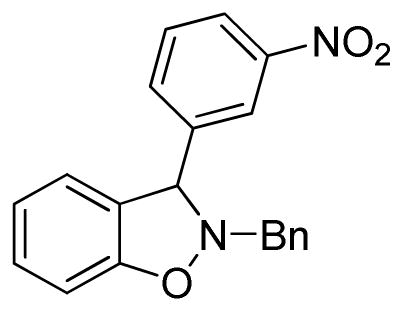
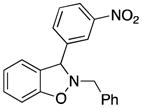
2-Benzyl-3-(3-nitrophenyl)-2,3-dihydrobenzo[d]isoxazole (3i)
white solid (57.1 mg, 69%), mp 118–120 °C; 1H NMR (300 MHz, CDCl3) δ 8.11 (dd, J = 6.0, 3.0 Hz, 2H), 7.62 (d, J = 6.0 Hz, 2H), 7.49-7.25 (m, 8H), 7.09 (d, J = 9.0 Hz, 1H), 6.97 (d, J = 9.0 Hz, 1H), 6.91 (d, J = 9.0 Hz, 1H), 5.44 (s, 1H), 4.50 (d, J = 12.0 Hz, 1H), 4.13 (d, J = 12.0 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 156.2, 148.1, 143.3, 135.7, 133.7, 129.8, 129.8, 129.6, 128.8, 128.2, 127.3, 124.4, 123.0, 122.6, 122.1, 108.8, 71.5, 63.2; HRMS (EI) calcd for C20H16N2O3: 332.1161, found 332.1166.
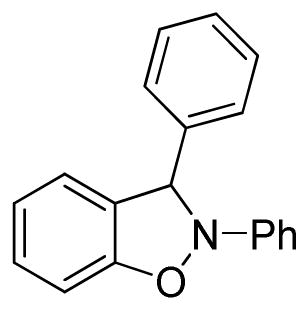
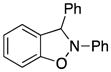
2,3-Diphenyl-2,3-dihydrobenzo[d]isoxazole (3j)
light brown oil (62.7 mg, 92%); 1H NMR (300 MHz, CDCl3) δ 7.19-7.15 (m, 2H), 7.09-6.85 (m, 7H), 6.74 (d, J = 9.0 Hz, 2H), 6.62 (t, J = 6.0 Hz, 1H), 6.53-6.49 (m, 1H), 6.43 (dd, J = 6.0, 3.0 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 150.6, 138.6, 136.5, 131.5, 129.9, 129.5, 129.1, 127.7, 127.2, 122.8, 121.1, 118.3, 110.2, 108.9, 99.3; HRMS (EI) calcd for C19H15NO: 273.1154, found 273.1152.
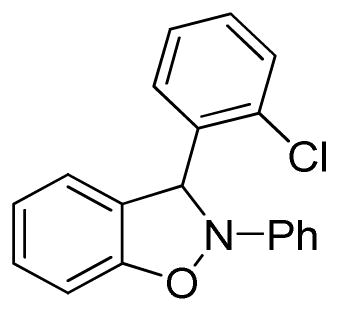
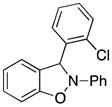
3-(2-Chlorophenyl)-2-phenyl-2,3-dihydrobenzo[d]isoxazole (3k)
white solid (69.2 mg, 90%), mp 74–76 °C; 1H NMR (300 MHz, CDCl3) δ 7.54 (d, J = 6.0 Hz, 1H), 7.44 (d, J = 9.0 Hz, 1H), 7.29-7.15 (m, 5H), 7.08 (dt, J = 9.0, 3.0 Hz, 2H), 6.99 (t, J = 9.0 Hz, 1H), 6.92-6.87 (m, 1H), 6.82-6.79 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 150.3, 142.3, 135.3, 134.4, 133.5, 131.0, 130.3, 129.6, 128.8, 127.6, 122.7, 121.4, 121.3, 117.5, 110.5, 109.2, 95.6; HRMS (EI) calcd for C19H14NOCl: 307.0764, found 307.0769.
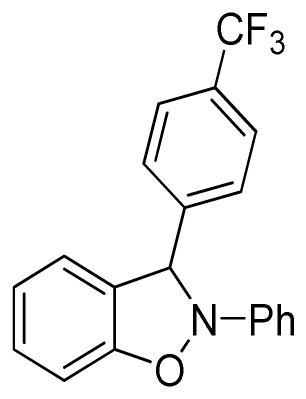
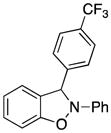
2-Phenyl-3-(4-trifluoromethylphenyl)-2,3-dihydrobenzo[d]isoxazole (3l)
white solid (79.4 mg, 93%), mp 86–88 °C; 1H NMR (300 MHz, CDCl3) δ 7.66 (t, J = 9.6 Hz, 4H), 7.32-7.24 (m, 2H), 7.13-7.01 (m, 4H), 6.94-6.79 (m, 4H); 13C NMR (75 MHz, CDCl3) δ 150.4, 142.6, 142.4, 134.6, 129.8, 127.7, 126.2, 126.2, 126.1, 126.1, 123.3, 121.6, 118.4, 110.8, 109.1, 98.4; HRMS (EI) calcd for C20H14NOF3: 341.1027, found 341.1032.
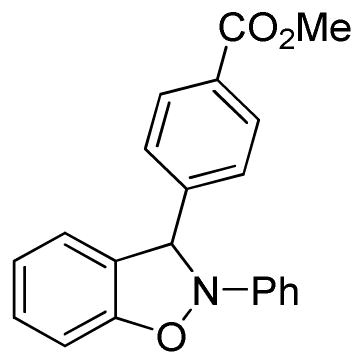
3-(4-Carbomethoxyphenyl)-2-phenyl-2,3-dihydrobenzo[d]isoxazole (3m)
white solid (74.2 mg, 90%), mp 118–120 °C; 1H NMR (300 MHz, CDCl3) δ 8.06 (d, J = 8.4 Hz, 2H), 7.62 (d, J = 8.1 Hz, 2H), 7.27 (t, J = 7.5 Hz, 2H), 7.10 (m, 3H), 7.02 (t, J = 7.5 Hz, 1H), 6.92-6.79 (m, 4H), 3.90 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 166.8, 150.5, 143.1, 142.5, 134.7, 131.5, 130.4, 129.7, 127.3, 123.2, 121.5, 121.4, 118.5, 110.5, 109.0, 98.6, 52.4; HRMS (EI) calcd for C21H17NO3: 331.1208, found 331.1215.
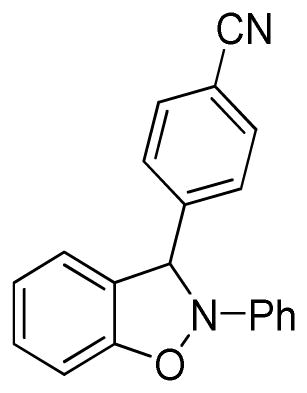
3-(4-Cyanophenyl)-2-phenyl-2,3-dihydrobenzo[d]isoxazole (3n)
white solid (65.7 mg, 88%), mp 105–108 °C; 1H NMR (300 MHz, CDCl3) δ 7.72-7.65 (m, 4H), 7.30 (t, J = 7.5 Hz, 2H), 7.12-7.03 (m, 4H), 6.94-6.79 (m, 4H); 13C NMR (75 MHz, CDCl3) δ 150.3, 143.5, 133.0, 129.8, 128.0, 123.5, 121.7, 121.7, 118.6, 118.5, 113.7, 111.0, 109.2, 98.2; HRMS (EI) calcd for C20H14N2O: 298.1106, found 298.1106.
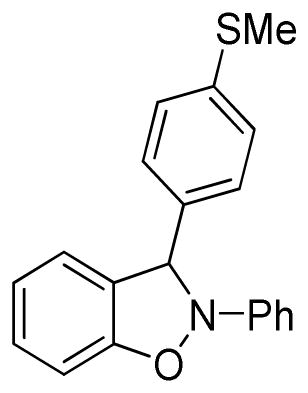
3-(4-Methylthiophenyl)-2-phenyl-2,3-dihydrobenzo[d]isoxazole (3o)
white solid (61.8 mg, 77%), mp 82–84 °C; 1H NMR (300 MHz, CDCl3) δ 7.45 (d, J = 9.0 Hz, 2H), 7.29-7.23 (m, 4H), 7.12-7.08 (m, 3H), 7.00 (t, J = 9.0 Hz, 1H), 6.90-6.83 (m, 1H), 6.80-6.76 (m, 3H), 2.46 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 150.5, 142.5, 140.8, 135.2, 130.2, 129.6, 127.7, 126.7, 122.8, 121.2, 121.1, 118.4, 110.1, 108.9, 98.9, 15.7; HRMS (EI) calcd for C20H17NOS: 319.1031, found 319.1034.
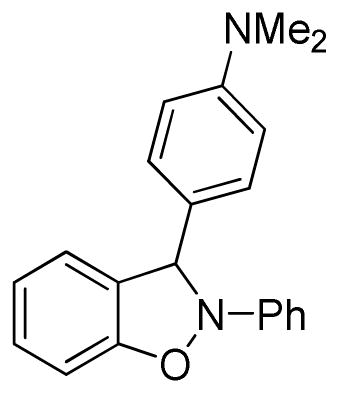
3-[4-(Dimethylamino)phenyl]-2-phenyl-2,3-dihydrobenzo[d]isoxazole (3p)
light brown oil (58.3 mg, 74%); 1H NMR (300 MHz, CDCl3) δ 7.40 (d, J = 8.7 Hz, 2H), 7.29-7.20 (m, 3H), 7.16-7.08 (m, 3H), 6.98 (t, J = 7.2 Hz, 1H), 6.86 (td, J = 6.0, 3.0 Hz, 1H), 6.79-6.77 (m, 2H), 6.70 (d, J = 9.0 Hz, 2H), 2.96 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 151.7, 150.7, 142.5, 129.6, 129.4, 128.4, 125.8, 122.5, 120.9, 120.7, 118.4, 112.4, 109.6, 108.7, 99.6, 40.6; HRMS (EI) calcd for C21H20ON2: 316.1576, found 316.1583.
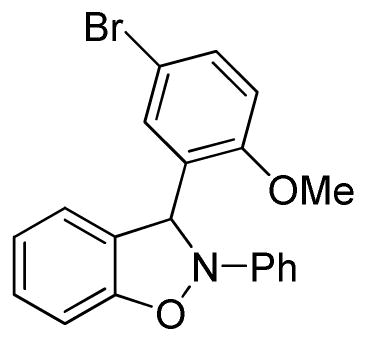
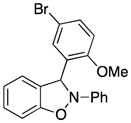
3-(5-Bromo-2-methoxyphenyl)-2-phenyl-2,3-dihydrobenzo[d]isoxazole (3q)
white solid (54.6 mg, 57%), mp 73–75 °C; 1H NMR (300 MHz, CDCl3) δ 7.55 (d, J = 3.0 Hz, 1H), 7.43 (dd, J = 6.0, 3.0 Hz, 1H), 7.27 (t, J = 6.0 Hz, 2H), 7.19-7.16 (m, 2H), 7.11-7.08 (dd, J = 9.0, 3.0 Hz, 2H), 7.00 (t, J = 9.0 Hz, 1H), 6.90 (td, J = 6.0, 3.0 Hz, 1H), 6.85 (s, 1H), 6.80 (dd, J = 9.0, 3.0 Hz, 2H), 3.89 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 156.7, 150.5, 142.8, 134.3, 133.7, 130.8, 129.6, 128.4, 122.6, 121.4, 121.2, 117.4, 113.5, 113.2, 111.0, 109.1, 93.4, 56.3; HRMS (EI) calcd for C20H16NO2Br: 381.0364, found 381.0368.
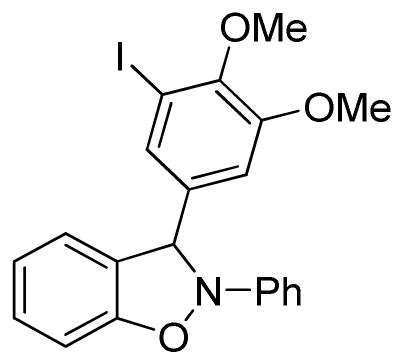
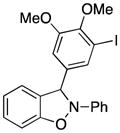
3-(4,5-Dimethoxy-3-iodophenyl)-2-phenyl-2,3-dihydrobenzo[d]isoxazole (3r)
light brown oil (80.3 mg, 70%); 1H NMR (300 MHz, CDCl3) δ 7.55 (d, J = 3.0 Hz, 1H), 7.33 (d, J = 6.0 Hz, 2H), 7.16-7.05 (m, 6H), 6.93 (td, J = 9.0, 6.0 Hz, 1H), 6.83 (t, J = 6.0 Hz, 2H), 6.70 (s, 1H), 3.85 (s, 3H), 3.83 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 153.2, 150.3, 150.2, 142.6, 136.3, 134.8, 129.7, 129.5, 123.3, 121.4, 118.6, 111.4, 110.6, 109.0, 98.4, 92.9, 60.6, 56.2; HRMS (EI) calcd for C21H18NO3I: 459.0331, found 459.0320.
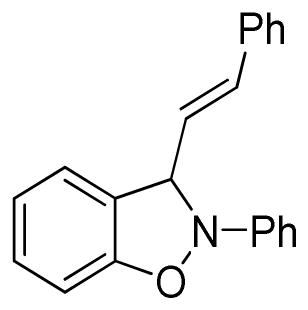
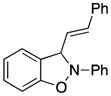
2-Phenyl-3-(E-2-phenylethenyl)-2,3-dihydrobenzo[d]isoxazole (3s)
light brown oil (42.1 mg, 56%); 1H NMR (300 MHz, CDCl3); δ 7.48-7.43 (m, 3H), 7.38-7.28 (m, 6H), 7.09-7.05 (m, 2H), 6.92-6.72 (m, 4H), 6.48-6.38 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 150.9, 142.8, 135.3, 131.5, 129.6, 129.3, 128.8, 128.7, 127.3, 125.4, 122.9, 121.2, 121.1, 118.5, 110.8, 108.9, 99.1; HRMS (EI) calcd for C21H17ON: 299.1310, found 299.1305.
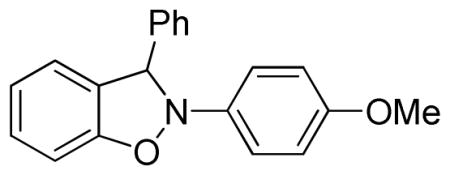
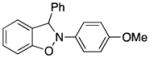
2-(4-Methoxyphenyl)-3-phenyl-2,3-dihydrobenzo[d]isoxazole (3t)
white solid (48.6 mg, 64%), mp 118–120 °C; 1H NMR (300 MHz, CDCl3) δ 7.53 (dd, J = 6.0, 3.0 Hz, 2H), 7.36 (t, J = 3.0 Hz, 1H), 7.03 (d, J = 9.0 Hz, 2H), 6.82-6.74 (m, 6H), 6.64 (s, 1H), 3.71 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 156.4, 150.4, 138.6, 136.9, 135.6, 129.9, 128.9, 127.6, 122.6, 121.3, 120.3, 114.8, 108.9, 108.4, 100.5, 55.6; HRMS (EI) calcd for C20H17NO2: 303.1259, found 303.1255.
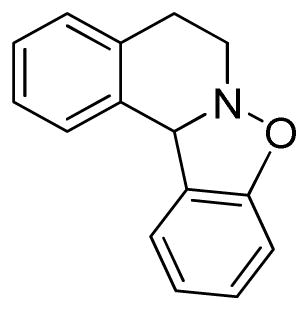
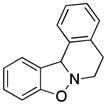
5,12-Dihydro-6H-[1,2]benzisoxazolo[3,2]isoquinoline (3u)
white solid (36.4 mg, 65%), mp 69–72 °C; 1H NMR (300 MHz, CDCl3) δ 7.37 (d, J = 9.0 Hz, 1H), 7.29 (t, J = 9.0 Hz, 1H), 7.22-7.09 (m, 4H), 6.90-6.83 (m, 2H), 5.80 (s, 1H), 3.36-3.30 (m, 2H), 3.00-2.77 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 156.7, 134.1, 133.5, 129.1, 128.8, 128.6, 127.2, 127.0, 126.9, 123.6, 121.6, 108.7, 65.7, 49.9, 26.9; HRMS (EI) calcd for C15H13NO: 223.0997, found 223.1003.
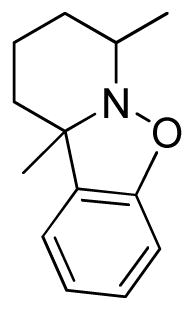
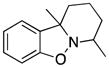
7,10a-Dimethylpyrido-8,9,10-trihydro[1,2]benzisoxazole (3v)
colorless oil (30.5 mg, 60%); 1H NMR (300 MHz, CDCl3) δ 7.13 (t, J = 6.0 Hz, 1H), 7.03 (d, J = 9.0 Hz, 1H), 6.87 (t, J = 6.0 Hz, 1H), 6.78 (d, J = 9.0 Hz, 1H), 3.22 (m, 1H), 1.63-1.51 (m, 9H), 1.41 (d, J = 9.0 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 155.5, 136.9, 128.0, 121.0, 120.8, 108.0, 65.6, 54.0, 33.3, 26.3, 22.8, 20.3, 19.3; HRMS (EI) calcd for C13H17NO: 203.1310, found 203.1306.
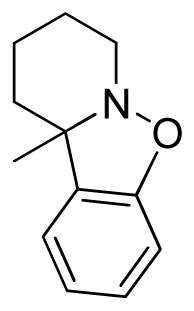
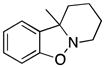
10a-Methyl-7,8,9,10-tetrahydropyrido[1,2]benzisoxazole (3w)
colorless oil (30.4 mg, 64%); 1H NMR (300 MHz, CDCl3) δ 7.19 (d, J = 9.0 Hz, 1H), 7.07 (d, J = 6.0 Hz, 1H), 6.97 (t, J = 6.0 Hz, 1H), 6.89 (d, J = 9.0 Hz, 1H), 3.39 (m, 1H), 2.64 (td, J = 9.0, 6.0 Hz, 1H), 2.29 (m, 1H), 1.86 (m, 1H), 1.65-1.51 (m, 3H), 1.35 (s, 3H), 1.35-1.23 (m, 3H); 13C NMR (75 MHz, CDCl3) δ 157.2, 133.4, 128.2, 121.8, 121.7, 110.0, 67.0, 52.7, 32.8, 30.0, 23.5, 20.5; HRMS (EI) calcd for C12H15NO: 189.1154, found 189.1149.
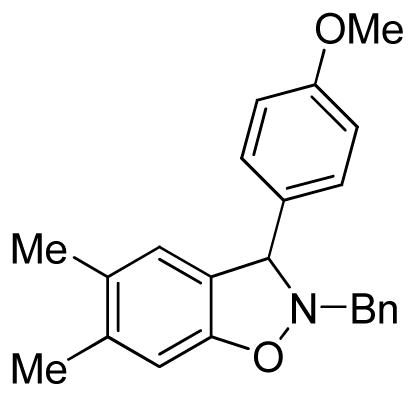
2-Benzyl-3-(4-methoxyphenyl)-2,3-dihydro-5,6-dimethylbenzo[d]isoxazole (3fb)
yellow solid (62.0 mg, 72%), mp 121–123 °C; 1H NMR (300 MHz, CDCl3) δ 7.43-7.26 (m, 5H), 7.18 (d, J = 8.4 Hz, 2H), 6.85 (d, J = 9.0 Hz, 2H), 6.78 (s, 1H), 6.68 (s, 1H), 5.25 (s, 1H), 4.35 (d, J = 13.0 Hz, 1H), 4.12 (d, J = 13.0 Hz, 1H), 3.79 (s, 3H), 2.25 (s, 3H), 2.17 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 159.5, 154.8, 137.5, 136.8, 133.2, 129.7, 129.6, 129.5, 129.1, 128.6, 127.7, 126.5, 125.1, 114.1, 109.3, 72.3, 62.8, 55.5, 20.5, 19.6; HRMS (EI) calcd for C23H23NO2: 345.1729, found 345.1721.
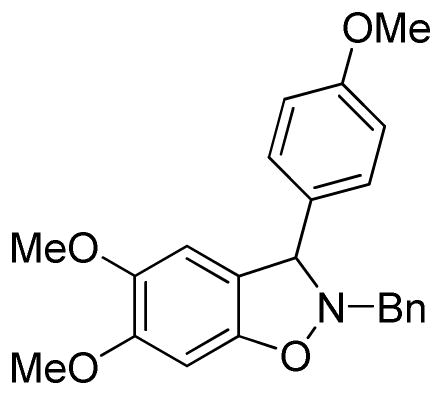
2-Benzyl-5,6-dimethoxy-3-(4-methoxyphenyl)-2,3-dihydrobenzo[d]isoxazole (3fc)
light brown oil (66.2 mg, 70%); 1H NMR (300 MHz, CDCl3) δ 7.42-7.27 (m, 5H), 7.16 (d, J = 9.0 Hz, 2H), 6.86 (d, J = 9.0 Hz, 2H), 6.53 (d, J = 9.0 Hz, 2H), 5.27 (s, 1H), 4.35 (d, J = 12.0 Hz, 1H), 4.14 (d, J = 15.0 Hz, 1H), 3.86 (s, 3H), 3.80 (s, 3H), 3.77 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 159.6, 150.8, 150.3, 144.5, 136.8, 132.8, 129.5, 129.4, 128.6, 127.8, 118.7, 114.2, 107.9, 93.6, 73.0, 62.8, 57.0, 56.4, 55.5; HRMS (EI) calcd for C23H23NO4: 377.1627, found 377.1620.
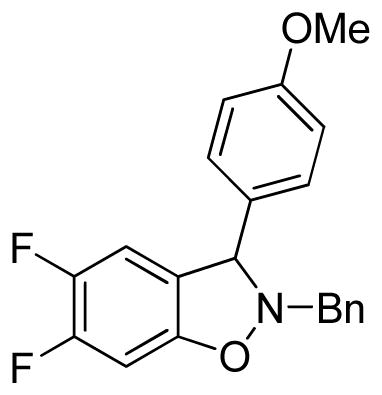
2-Benzyl-5,6-difluoro-3-(4-methoxyphenyl)-2,3-dihydrobenzo[d]isoxazole (3fd)
light brown oil (36.3 mg, 41%); 1H NMR (300 MHz, CDCl3) δ 7.40-7.31 (m, 6H), 7.20 (d, J = 9.0 Hz, 2H), 6.89 (d, J = 9.0 Hz, 2H), 6.77 (t, J = 9.0 Hz, 1H), 6.66 (dd, J = 9.0, 6.0 Hz, 1H), 5.27 (s, 1H), 4.34 (d, J = 12.0 Hz, 1H), 4.16 (d, J = 12.0 Hz, 1H), 3.82 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 159.9, 136.2, 129.4, 129.3, 128.7, 128.0, 114.4, 112.7, 112.4, 98.3, 98.0, 72.5, 62.5, 55.5; HRMS (EI) calcd for C21H17NO2F2: 353.1227, found 353.1230.
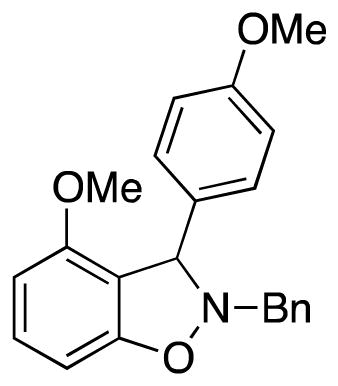
2-Benzyl-4-methoxy-3-(4-methoxyphenyl)-2,3-dihydrobenzo[d]isoxazole (3fe)
colorless oil (64.8 mg, 75%); 1H NMR (600 MHz, CDCl3) δ 7.44 (d, J = 7.0 Hz, 2H), 7.36 (t, J = 7.3 Hz, 2H), 7.32 (t, J = 7.2 Hz, 1H), 7.22 (t, J = 8.1 Hz, 1H), 7.12 (d, J = 8.7 Hz, 2H), 6.80 (d, J = 8.7 Hz, 2H), 6.57 (d, J = 8.0 Hz, 1H), 6.51 (d, J = 8.2 Hz, 1H), 5.37 (s, 1H), 4.39 (d, J = 12.8 Hz, 1H), 4.05 (d, J = 12.9 Hz, 1H), 3.77 (s, 3H), 3.75 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 158.9, 157.9, 156.6, 136.3, 132.9, 130.4, 129.5, 128.5, 128.4, 127.7, 115.1, 113.7, 104.1, 101.8, 69.3, 63.0, 55.5, 55.2; HRMS (EI) calcd for C22H21NO3: 347.1521, found 347.1521. Note: in the 1D-NOE experiment a correlation of 5.37 (s, 1H) to 3.75 (s, 3H), but not to 6.57 (d, J = 8.0 Hz, 1H) or 6.51 (d, J = 8.2 Hz, 1H), is observed. This would not be the case if the compound 3fe were the other regioisomer.
Supplementary Material
Acknowledgments
We thank the National Institute of General Medical Sciences (GM070620 and GM079593) and the National Institutes of Health Kansas University Center of Excellence in Chemical Methodology and Library Development (P50 GM069663) for their generous financial support. Thanks are also extended to Dr. Feng Shi, Dr. Shilpa Worlikar and Dr. Donald Rogness for preparation of the benzyne precursors.
Footnotes
1H and 13C NMR data for compounds. This material is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Raut AW, Doshi AG, Raghuwanshi RB. Orient J Chem. 1998;14:363. and references therein. [Google Scholar]
- 2.(a) Dai M, Wang Z, Danishefsky SJ. Tetrahedron Lett. 2008;49:6613. doi: 10.1016/j.tetlet.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jaeger V, Buss V, Schwab W. Tetrahedron Lett. 1978;34:3133. [Google Scholar]
- 3.(a) Sangeeta S, Rathi R, Doshi AG. Orient J Chem. 2006;22:169. [Google Scholar]; (b) Barluenga J, Aznar F, Palomero MA. Chem Eur J. 2001;7:5318. doi: 10.1002/1521-3765(20011217)7:24<5318::aid-chem5318>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]; (c) Kitamura T, Todaka M, Shin-machi I, Fujiwara Y. Heterocycl Commun. 1998;4:205. [Google Scholar]; (d) Lokhande PD, Ghiya BJ. J Ind Chem Soc. 1991;68:412. [Google Scholar]; (e) Matsumoto T, Sohma T, Hatazaki S, Suzaki K. Synlett. 1993:843. [Google Scholar]
- 4.Himeshina Y, Sonoda T, Kobayashi H. Chem Lett. 1983:1211. [Google Scholar]
- 5.For the most recent comprehensive review on arynes, see: Chen Y, Larock RC. In: Modern Arylation Methods. Ackerman J, editor. Wiley/VCH; New York: 2009. pp. 401–473.
- 6.Liu Z, Larock RC. J Org Chem. 2006;71:3198. doi: 10.1021/jo0602221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Zhao J, Larock RC. J Org Chem. 2007;72:583. doi: 10.1021/jo0620718. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rogness D, Larock RC. Tetrahedron Lett. 2009;50:4003. doi: 10.1016/j.tetlet.2009.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rogness D, Larock RC. J Org Chem. 2010;75:2289. doi: 10.1021/jo1000687. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Dubrovskiy AV, Larock RC. Org Lett. 2010;12:3117. doi: 10.1021/ol101017z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Shi F, Larock RC. Tetrahedron Lett. 2009;50:4067. doi: 10.1016/j.tetlet.2009.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu Z, Shi F, Martinez PDG, Raminelli C, Larock RC. J Org Chem. 2008;73:219. doi: 10.1021/jo702062n. [DOI] [PubMed] [Google Scholar]; (c) Shi F, Waldo J, Chen Y, Larock RC. Org Lett. 2008;10:2409. doi: 10.1021/ol800675u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Dubrovskiy AV, Larock RC. Org Lett. 2010;12:1180. doi: 10.1021/ol902921s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Wu Q, Li B, Lin W, Shi C, Chen Y, Chen Y. Hecheng Huaxue. 2007;15:292. [Google Scholar]; (b) Dai M, Wang Z, Danishefsky SJ. Tetrahedron Lett. 2008;49:6613. doi: 10.1016/j.tetlet.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wu K, Chen Y, Lin Y, Cao W, Zhang M, Chen J, Lee AWM. Tetrahedron. 2010;66:578. [Google Scholar]
- 10.For examples of the cycloaddition of nitrones and arynes generated by other methods, such as halogen-lithium exchange of an ortho-haloaryl triflate, see: Matsumoto T, Sohma T, Hatazaki S, Suzuki K. Synlett. 1993;11:843.Pellissier H, Santelli M. Tetrahedron. 2003;59:701.
- 11.1M TBAF in THF solution.
- 12.Dai M, Wang Z, Danishefsky SJ. Tetrahedron Lett. 2008;49:6613. doi: 10.1016/j.tetlet.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Peña D, Pérez D, Guitián E, Castedo L. J Am Chem Soc. 1999;121:5827. [Google Scholar]; (b) Peña D, Pérez D, Guitián E, Castedo L. J Org Chem. 2000;65:6944. doi: 10.1021/jo000535a. [DOI] [PubMed] [Google Scholar]; (c) Yoshida H, Sugiura S, Kunai A. Org Lett. 2002;4:2767. doi: 10.1021/ol0262845. [DOI] [PubMed] [Google Scholar]; (d) Liu Z, Zhang X, Larock RC. J Am Chem Soc. 2005;127:15716. doi: 10.1021/ja055781o. [DOI] [PubMed] [Google Scholar]; (e) Peña D, Escudero S, Pérez D, Guitián E, Castedo L. Angew Chem, Int Ed. 1998;37:2659. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2659::AID-ANIE2659>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (f) Yoshida H, Ikadai J, Shudo M, Ohshita J, Kunai A. J Am Chem Soc. 2003;125:6638. doi: 10.1021/ja034571d. [DOI] [PubMed] [Google Scholar]
- 14.(a) Cardona F, Bonanni M, Soldaini G, Goti A. Chem Sus Chem. 2008;1:327. doi: 10.1002/cssc.200700156. [DOI] [PubMed] [Google Scholar]; (b) Soldaini G, Cardona F, Goti A. Org Lett. 2007;9:473. doi: 10.1021/ol062862w. [DOI] [PubMed] [Google Scholar]; (c) Lo MM-C, Fu GC. J Am Chem Soc. 2002;124:4572. doi: 10.1021/ja025833z. [DOI] [PubMed] [Google Scholar]; (d) Murray RW, Iyanar K. J Org Chem. 1996;61:8099. doi: 10.1021/jo961252e. [DOI] [PubMed] [Google Scholar]; (e) Goti A, Nannelli L. Tetrahedron Lett. 1996;37:6025. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.