Abstract
Chronic infiltration of lymphocytes into the salivary and lacrimal glands of Sjögren’s Syndrome patients leads to destruction of acinar cells and loss of exocrine function. Protein kinase C-delta (PKCδ) is known to play a critical role in B cell maintenance. Mice in which the PKCδ gene has been disrupted have a loss of B cell tolerance, multiple organ lymphocytic infiltration, and altered apoptosis. To determine if PKCδ contributes to the pathogenesis of Sjögren’s Syndrome, we quantified changes in indicators of Sjögren’s Syndrome in PKCδ−/− mice as a function of age. Salivary gland histology, function, the presence of autoantibodies, and cytokine expression were examined. Materials and Methods: Submandibular glands were examined for the presence of lymphocytic infiltrates, and the type of infiltrating lymphocyte and cytokine deposition was evaluated by immunohistochemistry. Serum samples were tested by autoantibody screening, which was graded by its staining pattern and intensity. Salivary gland function was determined by saliva collection at various ages. Results: PKCδ−/− mice have reduced salivary gland function, B220+ B cell infiltration, anti-nuclear antibody production, and elevated IFN-γ in the salivary glands as compared to PKCδ+/+ littermates. Conclusions: PKCδ−/− mice have exocrine gland tissue damage indicative of a Sjögren’s Syndrome-like phenotype.
Keywords: PKCδ, autoimmunity, Sjögren’s syndrome
Introduction
Sjögren’s Syndrome (SS) is a chronic, autoimmune disorder marked by lymphocytic infiltration of exocrine glands, particularly the salivary and lacrimal glands (Fox & Kang, 1992). Destruction of acinar cells and the loss of exocrine function lead to the development of dry eyes (keratoconjunctivitis sicca) and dry mouth (xerostomia) (Kroneld et al., 1997, Humphreys-Beher et al., 1999). SS affects 0.5% of the population, however women are affected at a rate eight times that of men (Bowman et al., 2004). The disease can occur as a primary disease, or secondary to other autoimmune disorders such as scleroderma, rheumatoid arthritis, or systemic lupus erythematosus (Bowman et al., 2004). The pathogenesis of SS is poorly understood, although most studies suggest that immune-mediated damage to the exocrine glands underlie the functional deficiencies seen. Animal models have been developed to study the pathogenesis of the disease, however many fail to produce the persistent lesions and functional loss seen in human patients (Jonsson et al., 2007).
T cell-mediated autoimmune responses have been observed to be central to the pathogenesis of SS, and in many spontaneous mouse models of SS CD4+ T cells predominate in the salivary gland infiltrates (Soyfoo et al., 2007). However recent studies have suggested that functionally impaired B cells and alterations in apoptosis may also play an important role in the pathogenesis of SS (Youinou et al., 2007). Evidence of a dominant role of B cells in the genesis of SS includes the loss of immune tolerance, systemic antibodies to self antigens, and accumulation of memory-type B cells in the inflamed parotid glands of human patients (Stott et al., 1998). SS patients may also have increased circulation of B cell activating factor (BAFF) (7). Interestingly, transgenic mice that over-express BAFF have an excess of mature B cells and a propensity to develop certain autoimmune diseases, including a SS-like syndrome that results in increased B cell infiltration into the salivary glands, along with salivary hypofunction (Ware, 2000, Groom et al., 2002). Destruction of circulating B cells in human patients with the anti-CD20 antibody, Rituximab, leads to improvement of primary SS (Devauchelle-Pensec et al., 2007), supporting a crucial role for B cells in the pathogenesis of SS-like autoimmune disease (Khare et al., 2000).
Protein kinase C-delta (PKCδ), is a ubiquitously expressed member of the novel subfamily of PKC isoforms (Nishizuka, 1992) that is known to be critical for apoptosis (Reyland, 2009). Mice deficient for PKCδ (δKO) have defects in apoptosis, particularly in response to genotoxic agents (Humphries, 2006, Allen-Petersen, 2010). Notably, δKO mice develop systemic autoimmune disease associated with hyperproliferation of B220+ B cells, lymphocytic infiltrates in peripheral tissue, the presence of auto-reactive antibodies, and immune-complex-type glomerulonephritis, suggesting that PKCδ is important for the establishment of B-cell tolerance (Miyamoto et al., 2002). Adoptive transfer experiments suggest that the hyperproliferation phenotype observed in δKO mice is B-cell autonomous. To further delineate specific aspects of autoimmune disease in the δKO mice, we have focused on salivary gland pathology and function. Here we report that δKO mice display exocrine gland tissue injury and salivary gland dysfunction indicative of a SS-like autoimmune disease. This suggests that PKCδ is important for maintaining salivary gland homeostasis and perhaps for protecting salivary and other exocrine glands from immune-injury.
Materials and Methods
Animals
δKO mice on the C57Bl6 background were a generous gift from Dr. K. Nakayama of Kyushu University, Fukuoka, Japan (Miyamoto et al., 2002). Wild type C57BL/6 littermates were used as controls for all studies. Mice were bred and maintained under specific pathogen-free conditions in the Center for Comparative Medicine at University of Colorado Anschutz Medical Campus which is an AAALAC-approved facility. The protocols used in this study were approved by the University of Colorado IACUC. Except as noted, only female mice, with ages ranging from 6 weeks to 12 months, were used for these studies.
Salivary flow rates
To stimulate salivation mice were given an intraperitoneal injection of carbachol (Sigma, St Louis, MO) dissolved in saline (0.25 mg/kg of body weight) two min prior to measuring salivary flow rate. Saliva was collected for 5 minutes using a micropipette. Salivary flow rates are expressed as μl/min.
Immunohistochemistry
Mice were anesthetized with Advertin, exsanguinated, and the submandibular glands (SMG) were removed and fixed in 10% formalin prior to embedding in paraffin. Hematoxylin and eosin (H&E) stained sections were used for standard histological examination. For immunohistochemical staining, 4μM sections were deparaffinized by heating at 60°C for one hour and re-hydrated. Following antigen retrieval, endogenous peroxidases were quenched with 3% H2O2 and sections were blocked using Vectastain Elite ABC Kit (Vector Laboratories PK-6100). Sections were incubated with anti-Ki-67 (1:50, Dako M7249), anti-CD45R (1:100 BD Pharmigen 550286), or anti-CD3 (1:100 Nova Castra NCL CD3-12), or anti-IL-4 (1:100 Abcam, Cambridge, MA), or anti-IFN-γ (1:50 R&D Systems, Minneapolis, MN) for one hour at room temperature, followed by incubation with secondary antibody (Vector Laboratories PK-6100) for 30 min at room temperature. Signal was detected with diaminobenzadine (DAB) substrate (Sigma D6190) and counterstained with Gill’s Hematoxylin (1:3; Vector Laboratories H-3401) or methyl green (Vector Laboratories H-3402).
Foci counting and scoring
Submandibular glands (SMG) were removed, fixed in 10% formalin and paraffin embedded. Five 4μM sections were cut per animal, at least 50 μM apart, and stained with H&E. Female mice six-eight months old (WT, n=7; δKO, n=13) or 12 months old (WT, n=7; δKO, n=6), and 12 month old male mice (WT, n=4; δKO, n=6) were used in the study. The slides were blinded and evaluated by a pathologist for total number of foci of lymphocytic infiltrates, and were graphed as number of foci/section. The same sections were subsequently scored a second time to access the degree of infiltration and tissue damage and a score was assigned as previously described (White & Casarett, 1974). A focal score of 1 indicates that 1-5 foci of mononuclear cells were seen (more than 50 cells per focus) per section; 2 indicates that more than 5 foci of mononuclear cells were seen, but without significant parenchymal destruction; 3 indicates that multiple confluent foci were seen, with moderate degeneration of parenchymal tissue; 4 indicates extensive infiltration of the gland with mononuclear cells, and extensive parenchymal destruction.
Detection of Anti-Nuclear Autoantibody (ANA)
Indirect immunofluorescence was performed using HEP-2 cell substrate slides according to the manufacture’s directions (INOVA, San Diego, CA). Wild-type and δKO sera were diluted 1:100, and goat anti-mouse IgG-FITC antibodies were diluted 1:400. Slides were mounted using Vectashield Mounting Medium with 4′,6- diamidino-2-phenylindole (DAPI) (VECTOR Laboratories, Burlingame, CA). Fluorescence images were taken with Zeiss Axiovert 200M microscope and a Zeiss AxioCam MRm camera using the 20x or 40x 0.75 NA objectives for evaluation. Color images were evaluated using Adobe Photoshop version 7.
Results
The submandibular glands of δKO mice contain lymphocytic foci typical of Sjogren’s Syndrome
Lymphocytic infiltrates are most often detected in the SMGs in the animal models and present as focal, periductal accumulations of mononuclear cells, with or without destruction of the parenchymal tissue (Greenspan et al., 1974). Examination of sections of stained SMGs from female δKO and WT littermates showed that as the mice aged there was an increase in the number and size of lymphocytic foci, however foci number and size was significantly increased in δKO mice compared to WT mice of the same age (Figure 1A, 1B and 1C). Foci were not observed in either the WT or the δKO mice at six weeks or four months of age (data not shown). Beginning at six months of age, small foci were observed in both WT and δKO females and the number of foci increased with age (Figure 1B). Female δKO mice had a significantly increased total number of foci compared to WT mice at both six-eight months (p <0.05) and 12 months (p<0.05) of age (Figure 1B ands 1C). In addition, the foci present in the δKO mice were significantly larger in size (p<0.05) (Figure 1A). δKO male mice at 12 months of age also displayed more lymphocytic foci than control male mice of the same age (p= need to find this out) were slightly less foci, and the foci present were smaller in size, when compared to their female δKO counterparts (Figure 1B).
Figure 1. Lymphocytic infiltration in the submandibular glands of δKO mice with age.
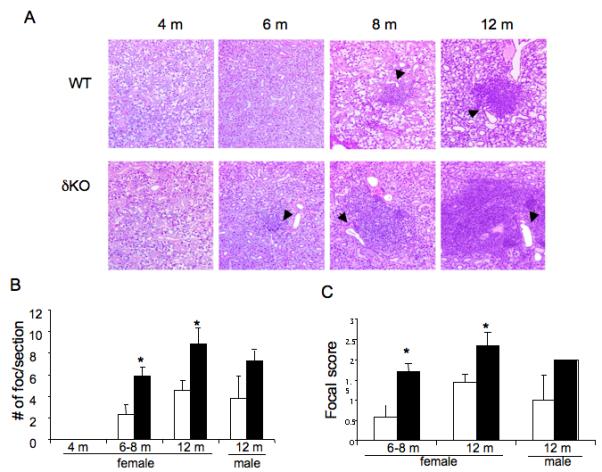
Panel A: Pathology of submandibular glands from WT and δKO mice. Four uM section of the submandibular glands from WT (top panel) and δKO (bottom panel) female mice at 4 months, 8 months, and 12 months old were stained with H + E. Representative images at 200X are shown. Arrows indicate the location of interlobular ducts. Panel B: Quantification of lymphocytic infiltrates in the SMG gland. Graph shows a comparison of the foci number in the submandibular glands of female mice at 4 months, 6-8 months and 12 months of age, and male mice at 12 months of age; WT = white bars and δKO = black bars. The total number of foci in five 4 μM sections per animal (50 μM apart) was counted blindly and the data is presented as average number of foci/ section; *= p<.05 compared to WT using a 2-tailed t-test. Panel C: Graph shows scoring of lymphocytic foci in the submandibular glands of WT and δKO female mice at 6-8 month (WT, n=7; δKO, n=13) and 12 month of age (WT, n=7; δKO, n=6), and in 12 month old male mice (WT, n=4; δKO, n=6); WT (white bars) and δKO (black bars). Scoring was done blindly by a pathologist using the White-Cassaret method (15). The average score of the five sections was used to determine the total focal score for the animal; *=p<0.05 compared to WT using a 2-tailed t-test.
The focal score reflects the degree of infiltration and tissue damage present in the gland (White & Casarett, 1974). As seen in Figure 1C, δKO female mice had an increased focal score at 6-8 months and 12 months, compared to WT mice of the same age. In 12 month old female δKO mice, which had the highest focal score, there were vast areas of the gland replaced by the infiltrate, with some mice having a focal score of three based on multiple confluent foci with moderate parenchymal destruction. The foci appeared to form around or adjacent to the large interlobular ducts, starting at six months of age (Figure 1A, arrows). This is consistent with what is observed in human labial salivary gland biopsies (Greenspan et al., 1974), and some animal models of SS ((Jonsson et al., 1987, Takada et al., 2004). The acini and ducts were extensively replaced by these infiltrates in the more severe foci, however, fibrosis and destruction of acini and ducts in the areas adjacent to the foci was not observed. Since there was no damage of acini in areas adjacent to the foci, the majority of the secretory tissue in the SMGs were intact. Histological examination of glands from 12 month old mice indicated that male δKO mice tended to have much smaller foci than observed in females δKO animals (Figure 1A); all 12 month male δKO mice had a focal score of two, while female mice typically displayed higher grade lesions (Figure 1C).
B-cells predominate in the lymphocytic infiltrate in submandibular glands
To determine the subset of immune cells present in the infiltrates, immunohistochemistry was performed using anti-B220 to detect B cells and anti-CD3 to detect T cells (Figure 2A). Lymphocytic infiltrates in the SMGs of δKO mice showed intense staining for B220, suggesting that B cells represent the major infiltrating lymphocytes. No change in staining intensity was observed between the small, early foci and the larger foci that appeared in older mice. The increase in peripheral B cells in the SMGs in δKO mice is consistent with increased expansion of B cells observed in these mice (Miyamoto et al., 2002, Mecklenbrauker et al., 2002). Immunohistochemical staining with anti-CD3 antibody showed weak staining at all time points observed, suggesting a smaller population of T cells was present in the infiltrates (Figure 2A). No difference in the extent of anti-CD3 staining was observed between WT and δKO mice, regardless of the age of the mice examined (Figure 2A).
Figure 2. B220+ B cells are the predominant lymphocyte present in the foci.
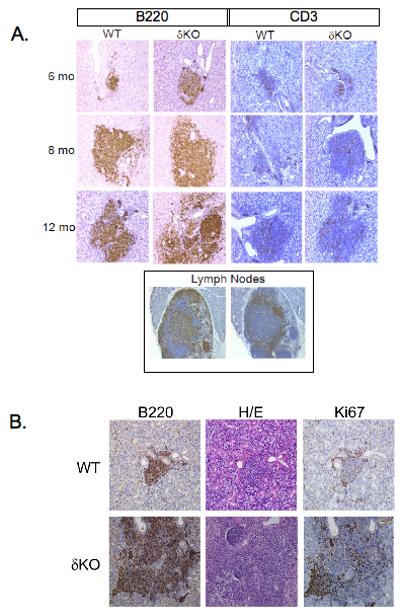
Panel A: Four uM sections of the submandibular glands from WT and δKO female mice at 6 months, 8 months, and 12 months old were stained with either anti-B220 (left panel) to stain B lymphocytes, or anti-CD-3 (right panel) to stain T lymphocytes. Sections were counterstained with hematoxylin. Pictures were taken at 200X magnification. Lymph nodes (bottom panel) were used as positive controls for anti-B220 and anti-CD-3 antibodies. Panel B. Four μM serial sections were taken from WT (top panel) or δKO (bottom panel) female mice at 12 months of age and stained for anti-B220 (left panel), H+E (middle panel), and anti-Ki-67 (right panel) Pictures were taken at 200x magnification.
Some human patients with autoimmune and/or other inflammatory diseases, including patients with SS, have infiltrates in non-lymphoid tissue that closely resemble organized lymphoid tissue with germinal centers (GCs) (Stott et al., 1998, Salomonsson et al., 2003). These GC-like structures have dense populations of proliferating B cells, along with T cells and plasma cells. To determine whether the B220+ B cells present in the lymphocytic infiltrates in the SMG were proliferating, we stained serial sections with anti-B220 and Ki-67, a marker for proliferating cells. Ki-67 staining was observed in foci from both WT and δKO SMGs, and the Ki-67 staining overlapped the B220 staining (Figure 2B). The intensity of Ki-67 staining correlated with the size of the foci; in small foci there were considerably fewer cells that stained positive for Ki-67 (Figure 2B, top panel) than were observed in very large foci (Figure 2B, bottom panel). Interestingly, focus size and not the loss of PKCδ correlated with the number of proliferating cells in these lesions as large foci that were present in the glands of WT mice displayed strong Ki-67 staining (data not shown). The presence of proliferating B220+ B cells in the foci, and large amount of proliferating cells in these larger, more confluent foci, suggested that GC-like structures may be present in the SMGs of mice that have extensive lymphocytic infiltrates.
Salivary gland hypofunction in δKO mice
To determine if the δKO mice display salivary hypofunction consistent with a SS-like disorder, saliva was collected after stimulation of salivation with carbachol. WT and δKO female mice at six and 12 weeks of age exhibited similar salivary flow rates (Figure 3). At four months of age, however, the flow rate observed in δKO mice was reduced by 35% compared to WT mice and was significantly reduced at all later time points examined (Figure 3). A trend toward decreased salivary flow rates was also observed in 10 and 12 month old WT mice, compared to the flow rates observed in WT mice at four to eight months of age. This may reflect the increased number of lymphocytic infiltrates observed in the SMGs of older WT mice, however this decrease in salivary flow rate was not statistically significant. It is interesting to note that salivary gland hypofunction was detectable at four months of age in the δKO mice, which proceeds the time at which lymphocytic infiltrates first appear in these mice (six months of age; Figure 1A). This differs from other SS animal models and human SS patients in which the appearance of lymphocytic infiltrates occurs prior to, or concurrent with, salivary hypofunction (Jonsson et al., 2007). It should be noted that even though lymphocytic infiltrates were observed in WT control mice at later time points (Figure 1A) only the δKO mice displayed statistically significant salivary hypofunction. The differences in salivary flow rates in WT and δKO mice may be explained by the extent of lymphocytic infiltrates, which are more severe in δKO mice, or perhaps differences in immunoglobulin subsets and/or the cytokines expressed in δKO mice versus WT mice. The secretory dysfunction observed in these mice does not appear to correlate with extensive loss of secretory tissue, since the majority of the secretory tissue remains intact. Also the secretory dysfunction was observed as early as four months, prior to the presence of lymphocytic infiltrates.
Figure 3. Salivary flow rates decrease with age in δKO females.
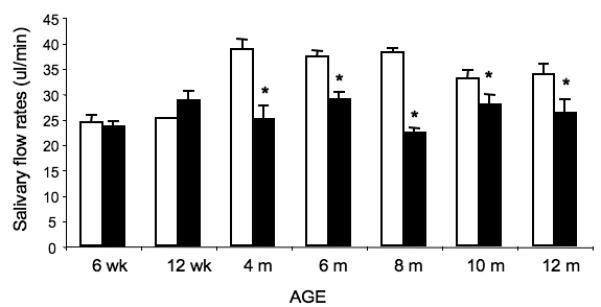
Saliva was collected for 5 min following injection of 0.25 mg/kg of carbachol. Total saliva volume was measured using a pipette and salivary flow rates are depicted as μl of saliva/min of collection; WT = white bars, δKO = black bars; *= p< 0.5 compared to wild type, using a 2-tailed t-test.
Anti-nuclear autoantibodies (ANA) are present in the serum of δKO mice
Since autoantibodies that are specific for nuclear proteins, DNA, or RNA molecules are commonly detected in rheumatic autoimmune diseases, serum samples from WT and δKO mice were screened for the present of ANA by staining of HEp-2 cells and evaluated for signal intensity and the pattern of staining by three independent evaluators. Figure 4 illustrates different patterns of staining that are indicative of ANA present in SS. None of the samples of mouse serum showed the diffuse staining pattern that is commonly observed in patients with either rheumatoid arthritis or systemic lupus erythematosus. It is important to mention the presence of ANA in serum is not a disease specific marker since the serum of normal individuals can also be positive for ANA, however, the absence of ANA in serum from patients with one of these autoimmune diseases, including SS, is very rare. As indicated in Table 1, 100% of serum samples from δKO mice were positive for ANA beginning at an early age, while of 12.5% of the serum samples from WT mice were positive for ANA at 6 weeks, although more WT mice became positive as they aged. At younger ages, both mouse strains showed a coarse speckled staining pattern. With aging, the staining pattern tended to become more diversified in both strains (Table 1).
Figure 4. Examples of ANA speckled nuclear staining patterns detected in δKO sera.
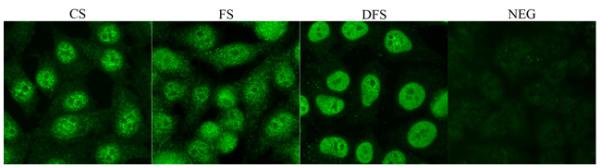
HEp-2 slides were incubated with WT and δKO sera diluted 1:100 followed by anti-mouse IgG conjugated to FITC (green). Examples of nuclear coarse speckled (CS), fine speckled (FS), dense fine speckled (DFS) and negative (NEG) staining patterns as detected in δKO sera are shown. A rating of the intensity of staining (1 through 3) was also assigned based on signal intensity by 3 different evaluators (Table 1).
Table 1.
CS, coarse speckled; FS, fine speckled; DFS, dense fine speckled; NEG, negative; WT, wild-type mice; KO, PKCδ−/− mice. Numbers in parenthesis indicate mouse number for screening. Intensity graded from 1-3 with 1 being weakest and 3 being strongest in expression.
| 6 WEEKS | 4 MONTHS | 8 MONTHS | 12 MONTHS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| WT (8) | KO (2) | WT (4) | KO (4) | WT (0) | KO (5) | WT (9) | KO (6) | ||
| PATTERN | INTENSITY | #MICE | #MICE | #MICE | #MICE | #MICE | #MICE | #MICE | #MICE |
| CS | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 |
| 2 | 0 | 1 | 0 | 3 | 0 | 0 | 1 | 2 | |
| 3 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | |
| FS | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| DFS | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 0 | |
| NEG | NA | 7 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| % positive | 12.5% | 100% | 50% | 100% | NA | 100% | 100% | 100% | |
Elevated pro-inflammatory IFN- γ expression in the salivary glands of δKO mice
Previous studies have demonstrated the up regulation of Th1 driven cytokine mRNAs such as IFN-γ in the salivary glands from SS patients, while in contrast expression levels of IL-4 mRNA are either absent, or slightly upregulated (Fox et al., 1994, Ohyama et al., 1996). Therefore we examined the protein levels of IFN-γ and IL-4 in salivary glands from eight month old WT and δKO mice by immunohistochemistry (Figure 5). Interestingly, IL-4 expression was slightly up regulated in the SMG from δKO mice compared to WT mice while IFN-γ expression was strongly up regulated in the SMG from δKO mice compared to WT mice, This pattern is similar to that observed in SS, suggesting that these cytokines may contribute to the progression of disease in the δKO mouse model.
Figure 5. Upregulated IFN-γ expression in salivary glands of PKCδ−/− mice.
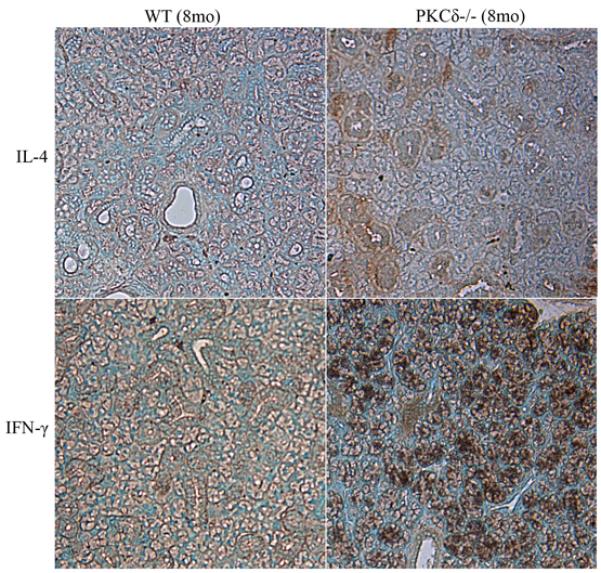
IL-4 and IFN-γ expression in salivary gland sections from 8 month old WT and δKO mice was examined by immunohistochemistry. IL-4 expression was slightly up regulated in δKO mice, while IFN-γ expression was greatly up regulated in δKO mice (brown).
Discussion
Recent studies have suggested a crucial role of B cells and other lymphoid cells (Youinou et al., 2007) in various mouse models of SS, as well as in humans. Mice deficient for δKO develop systemic autoimmune disease, however salivary gland injury and function has not been studied in this model (Miyamoto et al., 2002). Here we report that δKO mice have several important and clinical features of human SS (Soyfoo et al., 2007).
Histopathological examination of the SMGs of δKO female mice revealed the presence of focal and interlobular infiltrates beginning at six months of age, which increased in both number and intensity with age (Figure 1). Many of the larger foci had replaced extensive areas of acini and ducts with lymphocytes, however no damage was observed in adjacent acini and ducts. The presence of infiltrates in the SMGs of the WT mice is consistent with previous reports, in which infiltrates were observed starting at six months of age due to age-related breakdown of tolerance (Hayashi et al., 1989). However, δKO mice displayed an increased number and intensity of infiltrates at all time points measured compared to WT mice (Figure 1). δKO male mice at 12 months of age had fewer and less intense infiltrates than the same structures observed in female mice, although the differences observed did not reach statistical significance. Although not addressed here, it is possible that loss of PKCδ may accelerate the breakdown in tolerance associated with aging in the C57Bl/6 mice.
Immunohistochemical analysis of the infiltrates revealed that the predominant lymphocyte present in the foci were B220+ B cells, with a smaller amount of CD3+ T cells interspersed throughout the foci (Figure 2B). The increase in peripheral B cells in the SMGs of δKO mice is consistent with increased expansion of B cells reported in these mice (Mecklenbrauker et al., 2002, Miyamoto et al., 2002). The predominance of B cells in the lymphocytic infiltrates observed in the δKO mice differ from that observed in human SS patients, in which CD4+ and CD8+ T cells are predominant in the infiltrates, at least during the early stage of the disease (1). However, the B cell hyperactivity observed in δKO mice is consistent with that which has been observed in many human SS patients (Hansen et al., 2002, Hansen et al., 2005, Fox & Kang, 1992).
δKO mice display many of the characteristics of BAFF transgenic mice, such as lymphocytic infiltrates consisting largely of B220+ cells, salivary hypofunction, and increased production of immunoglobulins and autoantibodies (Groom et al., 2002). BAFF has been shown to promote B cell survival by inhibiting nuclear accumulation of PKCδ; nuclear accumulation of PKCδ is essential to its ability to induce apoptosis (Mecklenbrauker et al., 2004) (DeVries et al., 2002). δKO B cells do not die upon BAFF-withdrawal, however reconstitution with a constitutively nuclear form of PKCδ restores BAFF sensitivity; suggesting that PKCδ is the critical downstream target of BAFF signaling (Mecklenbrauker et al., 2004). Consistent with this scenario, enhanced proliferation and survival of B cells and the present of germinal centers in unstimulated lymphoid tissue has been reported in δKO mice (Miyamoto et al., 2002). Structures resembling germinal centers are likewise seen in the salivary glands of patients with SS (Stott et al., 1998). The presence of Ki-67 staining B220+ cells also suggests the presence of germinal cell–like structures in the salivary glands of δKO mice (Figure 2).
A critical aspect of SS, which is surprisingly missing from many models, is loss of secretory function (Soyfoo et al., 2007). Analysis of salivary flow rates of the δKO mice revealed a reduction in salivary flow rates which began at 4 months and preceded inflammation of SMGs (Figure 3). Although WT mice exhibited infiltration of lymphocytes into their SMGs, they did not display salivary hypofunction to the extent observed in the δKO mice. Interestingly, although there are reports that C57BL/6 mice sometimes develop autoimmunity as they age (25), this autoimmune reaction is clearly distinct from the autoimmunity we observe in δKO mice as salivary gland function was not affected in the C57Bl/6 mice (Figure 3) and IFN-γ was not significantly upregulated in the salivary glands (Fig 5). These characteristics are known to distinguish SS-like disease from other inflammatory conditions.
Previous reports have also shown that δKO mice have hypergammaglobulinemia, characterized particularly by the increased expression of IgG1 and IgA at 6 months of age and older (Miyamoto et al., 2002). In addition, auto-antibodies such as anti-chromatin and ANA have been described in the sera of δKO mice as they age, although there are conflicting reports with regard to the presence of ANA as well as elevated serum levels of IL-6 (Mecklenbrauker et al., 2002, Miyamoto et al., 2002). Unlike previous studies, we show that sera from δKO mice contains ANA starting at six weeks of age, and we describe distinct nuclear staining patterns of ANA, which are commonly seen in autoimmune SS. All sera from δKO mice was positive regardless of age; in contrast, only 1/7 control mice produced ANA at six weeks, 50% of the serum samples from WT mice were positive for ANA at 8 months, and all control serum samples were positive by 12 months of age (Table 1). This clearly indicates that auto-reactive B cells in δKO mice may be due to failure of tolerance induction to nuclear antigens. In contrast, WT C57Bl6 mice tend to lose tolerance to self-antigens over time, which is indicative of age-induced breakdown of tolerance. The overall staining patterns tend to diversify as both groups of mice get older, potentially implying diversification of B cell repertoire engaging epitope spreading as the inflammation progresses.
Taken together, an inflammatory phenotype, indicated by lymphocytic infiltration, ANA production, and pro-inflammatory cytokine expression in the salivary glands, along with secretory dysfunction, which are clinical hallmarks of SS, were observed in the δKO mouse model. In most animal models of SS, inflammation precedes secretory dysfunction, even if the level of inflammation does not always correlate with the degree of glandular hypofunction (Jonsson et al., 2007). In human patients’ histological manifestations can likewise be variable from a complete absence of infiltrates to monoclonal B cell expansion followed by development of B cell lymphoma. Our model resembles human SS based on focal infiltration, loss of secretion, and autoantibody production and maybe more reflective of human patients with germinal centers in the salivary glands, which is only 17% of the patient population (Hansen et al., 2002, Kroneld et al., 1997, Youinou et al., 2007). Finally, our model showing secretory dysfunction prior to immune cell infiltration supports the roles of soluble factors such as autoantibodies, cytokines, and/or nitric oxide in functional suppression of fluid secretion and/or altering structural integrity of the salivary glands in SS (Cha et al., 2006) (Baker et al., 2008, Caulfield et al., 2009). Taken together, our data support the conclusion that δKO mice have exocrine gland tissue damage indicative of a SS-like phenotype.
Acknowledgments
This work was supported by Public Health Service grants DE015648 (MER), DE019644 (SHC) and DE016354 (SMA).
References
- Baker OJ, Camden JM, Redman RS, Jones JE, Seye CI, Erb L, Weisman GA. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am J Physiol Cell Physiol. 2008;295:C1191–201. doi: 10.1152/ajpcell.00144.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SJ, Ibrahim GH, Holmes G, Hamburger J, Ainsworth JR. Estimating the prevalence among Caucasian women of primary Sjogren’s syndrome in two general practices in Birmingham, UK. Scand J Rheumatol. 2004;33:39–43. doi: 10.1080/03009740310004676. [DOI] [PubMed] [Google Scholar]
- Caulfield VL, Balmer C, Dawson LJ, Smith PM. A role for nitric oxide-mediated glandular hypofunction in a non-apoptotic model for Sjogren’s syndrome. Rheumatology (Oxford) 2009;48:727–33. doi: 10.1093/rheumatology/kep100. [DOI] [PubMed] [Google Scholar]
- Cha S, Singson E, Cornelius J, Yagna JP, Knot HJ, Peck AB. Muscarinic acetylcholine type-3 receptor desensitization due to chronic exposure to Sjogren’s syndrome-associated autoantibodies. J Rheumatol. 2006;33:296–306. [PubMed] [Google Scholar]
- Devauchelle-Pensec V, Pennec Y, Morvan J, Pers JO, Daridon C, Jousse-Joulin S, Roudaut A, Jamin C, Renaudineau Y, Roue IQ, Cochener B, Youinou P, Saraux A. Improvement of Sjogren’s syndrome after two infusions of rituximab (anti-CD20) Arthritis Rheum. 2007;57:310–7. doi: 10.1002/art.22536. [DOI] [PubMed] [Google Scholar]
- DeVries TA, Neville MC, Reyland ME. Nuclear import of PKCdelta is required for apoptosis: identification of a novel nuclear import sequence. EMBO J. 2002;21:6050–60. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RI, Kang HI. Pathogenesis of Sjogren’s syndrome. Rheum Dis Clin North Am. 1992;18:517–38. [PubMed] [Google Scholar]
- Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren’s syndrome. J Immunol. 1994;152:5532–9. [PubMed] [Google Scholar]
- Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjogren’s syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37:217–29. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway J, Mauri D, Cavill D, Gordon TP, Mackay CR, Mackay F. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A, Odendahl M, Reiter K, Jacobi AM, Feist E, Scholze J, Burmester GR, Lipsky PE, Dorner T. Diminished peripheral blood memory B cells and accumulation of memory B cells in the salivary glands of patients with Sjogren’s syndrome. Arthritis Rheum. 2002;46:2160–71. doi: 10.1002/art.10445. [DOI] [PubMed] [Google Scholar]
- Hansen A, Reiter K, Ziprian T, Jacobi A, Hoffmann A, Gosemann M, Scholze J, Lipsky PE, Dorner T. Dysregulation of chemokine receptor expression and function by B cells of patients with primary Sjogren’s syndrome. Arthritis Rheum. 2005;52:2109–19. doi: 10.1002/art.21129. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Utsuyama M, Kurashima C, Hirokawa K. Spontaneous development of organ-specific autoimmune lesions in aged C57BL/6 mice. Clin Exp Immunol. 1989;78:120–6. [PMC free article] [PubMed] [Google Scholar]
- Humphreys-Beher MG, Brayer J, Yamachika S, Peck AB, Jonsson R. An alternative perspective to the immune response in autoimmune exocrinopathy: induction of functional quiescence rather than destructive autoaggression. Scand J Immunol. 1999;49:7–10. doi: 10.1046/j.1365-3083.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- Jonsson MV, Delaleu N, Jonsson R. Animal models of Sjogren’s syndrome. Clin Rev Allergy Immunol. 2007;32:215–24. doi: 10.1007/s12016-007-8012-7. [DOI] [PubMed] [Google Scholar]
- Jonsson R, Tarkowski A, Backman K, Holmdahl R, Klareskog L. Sialadenitis in the MRL-l mouse: morphological and immunohistochemical characterization of resident and infiltrating cells. Immunology. 1987;60:611–6. [PMC free article] [PubMed] [Google Scholar]
- Khare SD, Sarosi I, Xia XZ, McCabe S, Miner K, Solovyev I, Hawkins N, Kelley M, Chang D, Van G, Ross L, Delaney J, Wang L, Lacey D, Boyle WJ, Hsu H. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:3370–5. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroneld U, Halse AK, Jonsson R, Bremell T, Tarkowski A, Carlsten H. Differential immunological aberrations in patients with primary and secondary Sjogren’s syndrome. Scand J Immunol. 1997;45:698–705. doi: 10.1046/j.1365-3083.1997.d01-449.x. [DOI] [PubMed] [Google Scholar]
- Mecklenbrauker I, Kalled SL, Leitges M, Mackay F, Tarakhovsky A. Regulation of B-cell survival by BAFF-dependent PKCdelta-mediated nuclear signalling. Nature. 2004;431:456–61. doi: 10.1038/nature02955. [DOI] [PubMed] [Google Scholar]
- Mecklenbrauker I, Saijo K, Zheng NY, Leitges M, Tarakhovsky A. Protein kinase Cdelta controls self-antigen-induced B-cell tolerance. Nature. 2002;416:860–5. doi: 10.1038/416860a. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002;416:865–9. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–14. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Ohyama Y, Nakamura S, Matsuzaki G, Shinohara M, Hiroki A, Fujimura T, Yamada A, Itoh K, Nomoto K. Cytokine messenger RNA expression in the labial salivary glands of patients with Sjogren’s syndrome. Arthritis Rheum. 1996;39:1376–84. doi: 10.1002/art.1780390816. [DOI] [PubMed] [Google Scholar]
- Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front Biosci. 2009;14:2386–99. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmstrom P, Wahren-Herlenius M, Jonsson R. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjogren’s syndrome. Arthritis Rheum. 2003;48:3187–201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- Soyfoo MS, Steinfeld S, Delporte C. Usefulness of mouse models to study the pathogenesis of Sjogren’s syndrome. Oral Dis. 2007;13:366–75. doi: 10.1111/j.1601-0825.2007.01376.x. [DOI] [PubMed] [Google Scholar]
- Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjogren’s syndrome. J Clin Invest. 1998;102:938–46. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K, Takiguchi M, Konno A, Inaba M. Spontaneous development of multiple glandular and extraglandular lesions in aged IQI/Jic mice: a model for primary Sjogren’s syndrome. Rheumatology (Oxford) 2004;43:858–62. doi: 10.1093/rheumatology/keh209. [DOI] [PubMed] [Google Scholar]
- Ware CF. APRIL and BAFF connect autoimmunity and cancer. J Exp Med. 2000;192:F35–8. doi: 10.1084/jem.192.11.f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SC, Casarett GW. Induction of experimental autoallergic sialadenitis. J Immunol. 1974;112:178–85. [PubMed] [Google Scholar]
- Youinou P, Devauchelle V, Hutin P, Le Berre R, Saraux A, Pers JO. A conspicuous role for B cells In Sjogren’s syndrome. Clin Rev Allergy Immunol. 2007;32:231–7. doi: 10.1007/s12016-007-8000-y. [DOI] [PubMed] [Google Scholar]


