Abstract
INTRODUCTION
Radial scars are benign breast lesions; their appearance on mammography may, however, mimic carcinoma. Needle core biopsy is performed for pre-operative diagnosis and, currently in Wales, all lesions with benign biopsy results are surgically excised. We have reviewed all cases of needle core biopsy-diagnosed radial scars from the Welsh breast screening programme, Breast Test Wales (BTW), and investigated the outcome of radial scars based on histology from surgical excision in order to evaluate the appropriateness of the current management of these lesions in Wales.
PATIENTS AND METHODS
All needle core biopsy diagnosed radial scars were identified from the BTW screening database from the start of screening in 1989 until the end of 2007.
RESULTS
A total of 118 patients were diagnosed with radial scars on needle core biopsy; two patients had bilateral radial scars. Median patient age was 54 years (range, 49-68 years). Ninety-five lesions (79%) were thought to be pure radial scars on needle core biopsy; however, only 81 pure radial scars were identified on excision biopsy histology. Carcinoma was present in seven patients and ductal carcinoma in situ in nine patients at excision biopsy. In two patients, the cancers occurred in lesions reported as pure radial scars on needle core biopsy. Twenty-two lesions showed atypical ductal or lobular hyperplasia (ADH/ALH) or both on excision biopsy; 14 of these lesions were classed as pure radial scars by needle core biopsy.
CONCLUSIONS
All core biopsy diagnosed radial scars, presenting as screen detected abnormalities, should be excised due to their association with premalignant and malignant conditions.
Keywords: Radial scars, Breast cancer, Breast screening, Core biopsy
Radial scars are benign breast lesions with unknown aetiology. Most radial scars are microscopic; however, larger lesions may be detected on mammography, where they may mimic breast cancer. Between 1989 and 2007, radial scars were identified in diagnostic or therapeutic surgical excision specimens in 0.06% of women screened by the Welsh screening service (Breast Test Wales; BTW) which is comparable with the incidence of radial scars reported in the literature of 0.03-0.07%. 1
Though usually asymptomatic, radial lesions with palpable masses have been described.2 Although pure radial scars are benign, they can be associated with atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH) as well as in situ or invasive carcinoma. Diagnosis of ADH on core biopsy is an indication for surgical biopsy.3 There is limited evidence to suggest that even pure radial scars on core biopsy is an indication for surgical biopsy.4
Currently in Wales, women with mammographic abnormalities suggestive of radial scars are recalled for needle core biopsy of the lesion (although needle core biopsy was not performed at BTW until the mid-1990s). Core biopsy is carried out using a spring loaded biopsy gun with a size 14 or 16 French needle or, more recently, with a vacuum-assisted mammotome (VAM) under ultrasound or stereotactic guidance. All patients with radial scars diagnosed by Breast Test Wales are advised to have the lesion excised with a localisation biopsy.
The aim of this study was to assess the policy for radial scar excision by investigating the prevalence of ADH and carcinoma found by needle core biopsy and subsequent surgical excision biopsy in these lesions.
Patients and Methods
The study was carried out at BTW using prospectively collected data from the screening centres in south east (Cardiff), north (Llandudno) and west (Swansea) Wales. Data from the screening programme database were used to identify all cases where radial scar was included in the diagnosis at surgical diagnostic and or therapeutic biopsy from the start of BTW in 1989 until the end of 2007. In the study period, 1,285,716 women were screened by appointment. Of these, there were 764 (0.05%) radial scars lesions identified at surgery (diagnostic or therapeutic). Overall, 118 patients with a total of 120 lesions were identified as having been reported as radial scars on needle core biopsy specimens (2 patients were diagnosed with bilateral radial scars). The remaining patients were excluded as they had either fine needle aspiration cytology performed or had a localisation biopsy without a needle core biopsy. These 118 women form the study population on which a retrospective review of patient's notes and films was conducted.
For all cases, the mammograms, ultrasound scans and clinical assessment were reviewed. Core biopsy histology and excision biopsy reports were retrospectively analysed for the presence of atypical hyperplasia and in situ or invasive carcinoma and a comparison between the two sets of results was made.
Results
The median age at first screening appointment was 54 years (range, 49-68 years). Sixty-two radial scars occurred in the left breast and 58 in the right (two women had bilateral radial scars). Some 58% of needle core biopsy identified radial scars were diagnosed at the prevalent screen.
Of the 118 patients, 65 (54%) were considered radiologically to be probably malignant (R4). Forty-three (36%) had features suggestive of a radial scar (R2 or R3; Fig. 1). Of the 28 clinically palpable lesions, 16 were described as a nodule or thickening and a further nine described as a lump, two as a ‘lumpy area’ and one had an associated skin dimple. A single core biopsy was performed in 108 cases, 55 under ultrasound guidance, 50 with stereotactic guidance and three stereotactic mammotome biopsies.
Figure 1.
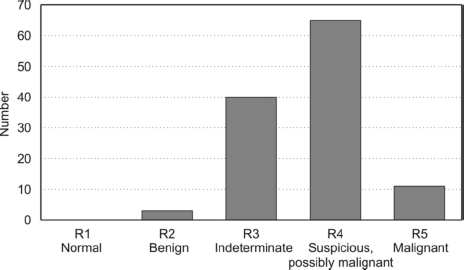
Overall imaging score of the needle core biopsy confirmed radial scars.
Twelve patients underwent repeat biopsy to achieve better sampling of the lesion. Ten initially had ultrasound-guided needle core biopsy, four went onto have a second ultrasound-guided needle core biopsy, five had a stereotactically guided needle core biopsy and one a VAM. The remaining two patients both had two stereotactic needle core biopsies.
The median number of cores taken was 3 (range, 1-10). Twenty-one lesions were described as having had ‘multiple’ cores and four lesions had ‘a few’ cores in the pathology reports. Of the 120 lesions, 95 were diagnosed as pure radial scars based on needle core biopsy histology and 81 on final excision biopsy histology. These 95 cases were scored as benign (B2; 27), equivocal (B3; 66) or suspicious (B4; 2). The remaining 25 lesions were reported as showing atypia (14), DCIS (9), LCIS (1) or invasive cancer (1) associated with the radial scar. These 25 cases were scored B2 (3), B3 (9), B4 (11) and B5 (2) as summarised in Figure 2.
Figure 2.
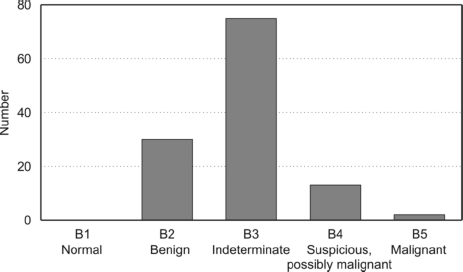
Histological grade of R5 lesions based on needle core biopsy specimens.
All 120 lesions were excised, 96 by localisation biopsy using a guide wire, seven lesions were easily palpable and did not require localisation and the method of biopsy was not documented in 17 cases.
Six excision biopsies showed grade 1 invasive carcinoma (5%) and eight contained DCIS (7%) of which one also had associated lobular carcinoma in situ (LCIS; Table 1). One case of invasive tubular carcinoma had been reported at time of excision as a benign radial scar, but a small grade 1 tubular breast cancer was identified when the histology was reviewed 18 months later. Of the six invasive cancers identified on excision biopsy, four were thought to be pure radial scars on needle core biopsy (67%). In addition there were 22 lesions (18%) with associated atypia - ADH (13), ALH (6) and both ADH and ALH (3) - and, finally, three cases (2.5%) with LCIS. Only 81 lesions were deemed to be pure radial scars on final histological assessment, giving an overall false negative rate of 12% for needle core biopsy. There was a single false positive, with a core needle biopsy showing invasive cancer that was not confirmed on excision biopsy.
Table 1.
Lesion diagnosis on needle core biopsy compared with final excision biopsy histology
| Core needle biopsy histology | Excision biopsy histology | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pure radial scar | ADH | ALH | ADH/ALH | DCIS | DCIS/LCIS | LCIS | Invasive cancer | ||
| Pure radial scar | 95 | 70 | 10 | 4 | 2 | 3 | 0 | 2 | 4 |
| ADH | 12 | 8 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| ADH/ALH | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| ALH | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| LCIS | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| DCIS | 9 | 3 | 2 | 0 | 0 | 3 | 0 | 0 | 1 |
| Invasive cancer | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Total | 120 | 81 | 13 | 6 | 3 | 7 | 1 | 3 | 6 |
ADH, atypical ductal hyperplasia; ALH; atypical lobular hyperplasia; LCIS, lobular carcinoma in situ; DCIS, ductal carcinoma in situ.
The underestimation rate for carcinoma in the pure radial scar diagnosis group was 4% (4/95), for DCIS was 3% (3/95) and 2% (2/95) for LCIS. Of the total number of carcinomas identified by excision biopsy, 67% (4/6) had been reported as pure radial scars on core biopsy (Table 1). In addition, a further 16 lesions initially classed as pure radial scars had associated ADH, ALH or both on excision (Fig. 3). The overall underestimation rate for all abnormalities in pure radial scars lesions was 26% (25/95).
Figure 3.
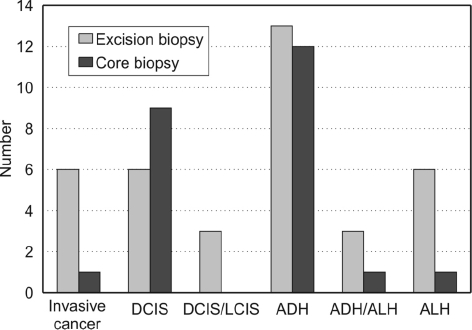
Comparison of R5 lesion histology on initial needle core biopsy and final excision biopsy.
Discussion
The management of core biopsy confirmed radial scars remains a contentious issue. It has been claimed that all radial scars should be excised because of their association with premalignant and malignant lesions.5 However, it has also been suggested that pure radial scars diagnosed by needle core biopsy do not require excision and can be managed safely by mammographic surveillance.6
There are mammographic features suggestive of radial scars (for example, the Tabar and Dean criteria; Table 2).2 However, only 68% of radial scars present as a typical mammographic lesion and less than 50% display all the Tabar and Dean criteria.7 In this study, only 43 cases (36%) were diagnosed as radial scars on mammography. To add to the diagnostic difficulties posed by radial scars, they cannot be distinguished from spiculate cancers on mammography. Frouge et al.8 assessed 40 cases (20 radial scars and 20 carcinomas) exhibiting at least three of the Tabar and Dean criteria. The authors were unable to differentiate between the mammographic appearances of the radial scars and the cancers.
Table 2.
Tabar and Dean criteria for the appearance of a radial scar on mammography
| • Central radiolucency ('black star’ appearance) |
| • Radiating, long, thin spicules |
| • Varying appearance in different projections |
| • Radiolucent linear structures parallel to the spicules |
| • Absence of a palpable lesion or skin change |
Most (54%) of the lesions in this study were given R4 (suspicious possibly malignant) and overall 64% were classed as R4 or R5. However, only 12% (9/77) of lesions given R4 or R5 contained DCIS or invasive cancer on final histology. Because of the difficulties assessing these lesions on radiological imaging, the final diagnosis of a radial scar should still be made on histology.9
The incidence of premalignant or malignant histology in radial scars in this study is comparable with rates reported in the literature. Fasih et al.10 have reported rates from the Newcastle breast screening programme of 67% for pure radial scars, 17% for ADH and 16% for in situ or invasive carcinoma, compared with 68%, 18% and 14% (including LCIS), respectively, in this study. Lower, but comparable, results have been shown in a retrospective analysis of 175 patients with screen-detected radial scars,11 showing concurrent carcinoma in 3% and carcinoma in situ in 5% (5% and 7%, respectively, in this study).
It has been suggested that radial scars may act as an independent risk factor for breast cancer, and that women with radial scars have a risk of breast cancer double that of those without radial scars.12 However, this risk may be due to co-existent pro-liferative disease as Sanders et al.13 found that the co-existence of a radial scar adds little risk of cancer. ADH is a recognised risk factor for breast cancer, with a relative risk of breast cancer in ADH of 9.8 compared with 2.6 for non-proliferative ductal hyperplasia.14
Core biopsy is a sampling tool and so malignant foci within the radial scar may be missed, particularly if the malignant area is found at the periphery of the radial scar. The role of core biopsy is, therefore, to identify pre-invasive and invasive lesions in order to enable optimal surgical planning. Areas of malignant change within radial scars are typically small and can be missed by core biopsies, there are interobserver variations in the diagnosis of ADH and problems with patient compliance with mam-mographic follow-up all make conservative management of radial scars unsafe.15 The false-negative rate in needle core biopsies from benign radial scars that contain malignancy on excision biopsy varies from 3.9%5 to 40%.16 As a result of these problems, excision biopsy should be carried out on all needle core biopsy diagnosed radial scars.
Previous studies advocating conservative management of radial scar have generally included small numbers. Philpotts et al.17 included nine radial scars in a retrospective review of lesions sampled by stereotactic needle core biopsy, of which eight were excised. Excision biopsy showed four cases with associated ADH but no malignancies were found. The sensitivity of core biopsy to detect malignancy may increase when greater numbers of cores are taken. Brenner et al.4 found no malignancies are missed if 12 or more cores are obtained (none of the lesions in our study however had more than 12 cores). Conversely, Kirwan et al.18 did not find a statistically significant improvement in the accuracy of core biopsies if more than one core was taken. The use of a vacuum-assisted mammotome increases the diagnostic yield14 and this technique may have a future diagnostic and therapeutic role in the excision of radial scars,19 although the piecemeal excision of the radial scars may still miss an abnormal focus peripherally.
Conclusions
In this study, 33% of ‘benign’ radial scars diagnosed on needle core biopsy had associated atypia and carcinoma on excision biopsy. All screen-detected radial scars diagnosed on needle core biopsy should be surgically excised.
References
- 1.Kennedy M, Masterson AV, Kerin M, Flanagan F. Pathology and clinical relevance of radial scars: a review. J Clin Pathol. 2003;56:721–4. doi: 10.1136/jcp.56.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel A, Steel Y, McKenzie J, Letcher M, Della Rovere GQ, Morgan MW. Radial scars: a review of 30 cases. Eur J Surg Oncol. 1997;23:202–5. doi: 10.1016/s0748-7983(97)92244-7. [DOI] [PubMed] [Google Scholar]
- 3.Liberman L, Cohen MA, Dershaw DD, Abramson AF, Hann LE, Rosen PP. Atypical ductal hyperplasia diagnosed at stereotactic core biopsy of breast lesion: an indication for surgical biopsy. AJR Am J Roentgenol. 1995;164:1111–3. doi: 10.2214/ajr.164.5.7717215. [DOI] [PubMed] [Google Scholar]
- 4.Brenner RJ, Jackman RJ, Parker SH, Evans P, Deutch B, et al. Percutaneous core needle biopsy of radial scars of the breast: When is excision necessary? AJR Am J Roentgenol. 2002;179:1179–84. doi: 10.2214/ajr.179.5.1791179. [DOI] [PubMed] [Google Scholar]
- 5.Douglas-Jones AG, Denson JL, Cox AC, Harries IB, Stevens G. Radial scar lesions of the breast diagnosed by needle core biopsy: analysis of cases containing occult malignancy. J Clin Pathol. 2007;60:295–8. doi: 10.1136/jcp.2006.037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cawson JN, Malara F, Kavannagh A, Hill P, Balasubramanium G, Henderson M. Fourteen-gauge needle core biopsy of mammographically evident radial scars: is excision necessary? Cancer. 2003;97:345–51. doi: 10.1002/cncr.11070. [DOI] [PubMed] [Google Scholar]
- 7.Boute V, Goyat I, Denoux Y, Lacroix J, Marie B, Michels JJ. Are the criteria of Tabar and Dean still relevant to radial scars? Eur J Radiol. 2006;60:243–9. doi: 10.1016/j.ejrad.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Frouge C, Tristant H, Guinebretiere JM, Meunier M, Contesso G, et al. Mammographic lesions suggestive of radial scars: microscopic findings in 40 cases. Radiology. 1995;195:623–5. doi: 10.1148/radiology.195.3.7753984. [DOI] [PubMed] [Google Scholar]
- 9.Ciatto S, Morrone D, Catarzi S, Rosselli Del Turco M, Bianchi S, et al. Radial scars of the breast: review of 38 consecutive mammographic diagnoses. Radiology. 1993;187:757–60. doi: 10.1148/radiology.187.3.8388568. [DOI] [PubMed] [Google Scholar]
- 10.Fasih T, Jain M, Shrimankar J, Staunton M, Hubbard J, Griffith CD. All radial scars/complex sclerosing lesions seen on breast screening mammograms should be excised. Eur J Surg Oncol. 2005;31:1125–8. doi: 10.1016/j.ejso.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Patterson JA, Scott M, Anderson N, Kirk SJ. Radial scar, complex sclerosing lesion and risk of breast cancer. Analysis of 175 cases in Northern Ireland. Eur J Surg Oncol. 2004;30:1065–8. doi: 10.1016/j.ejso.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ. Radial scars in benign breast biopsy specimens and the risk of breast cancer. N Engl J Med. 1999;340:430–6. doi: 10.1056/NEJM199902113400604. [DOI] [PubMed] [Google Scholar]
- 13.Sanders ME, Page DL, Simpson JF, Schuyler PA, Dale Plummer W, Dupont WD. Interdependence of radial scar and proliferative disease with respect to invasive breast carcinoma risk in patients with benign breast biopsies. Cancer. 2006;106:1453–61. doi: 10.1002/cncr.21730. [DOI] [PubMed] [Google Scholar]
- 14.Balu-Maestro C, Ettore F, Chapellier C, Peyrottes I, Leblanc-Talent P. When should caution be used with regards to histopathologic findings of image guided breast micro- and macro-biopsies? J Radiol. 2006;87:265–73. doi: 10.1016/s0221-0363(06)74000-8. [DOI] [PubMed] [Google Scholar]
- 15.Farshid G, Rush G. Assessment of 142 stellate lesions with imaging feature suggestive of radial scar discovered during population-based screening for breast cancer. Am J Surg Pathol. 2004;28:1626–31. doi: 10.1097/00000478-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Jackman RJ, Marzoni FA, Jr, Nowels KW. Percutaneous removal of benign mammographic lesions: comparison of automated large-core and directional vacuum-assisted stereotactic biopsy techniques. AJR Am J Roentgenol. 1998;171:1325–30. doi: 10.2214/ajr.171.5.9798873. [DOI] [PubMed] [Google Scholar]
- 17.Philpotts LE, Shaheen NA, Jain KS, Carter D, Lee CH. Uncommon high-risk lesions of the breast diagnosed at stereotactic core-needle biopsy: clinical importance. Radiology. 2000;216:831–7. doi: 10.1148/radiology.216.3.r00se31831. [DOI] [PubMed] [Google Scholar]
- 18.Kirwan SE, Denton ERE, Nash RM, Humphreys SS, Michell MG. Multiple 14G stereotactic core biopsies in the diagnosis of mammographically detected stellate lesions of the breast. Clin Radiol. 2000;55:763–6. doi: 10.1053/crad.2000.0513. [DOI] [PubMed] [Google Scholar]
- 19.Royal College of Radiologists Breast Group. The current state of vacuum assisted biopsy in the UK-; Conclusions from the second mammotome user group conference; Nottingham, UK. 2006. < http://ww.rcrbreastgroup.com/ASM/Edin06/files/PosterPDFs/P3.pdf> [accessed 27 March 2009] [Google Scholar]


