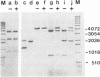
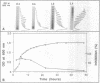
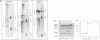Abstract
The availability of DNA structural probes that can be applied to living cells is essential for the analysis of biological functions of unusual DNA structures adopted in vivo. We have developed a chemical probe assay to detect and quantitate left-handed Z-DNA structures in recombinant plasmids in growing E. coli cells. Potassium permanganate selectively reacts with B-Z or Z-Z junction regions in supercoiled plasmids harbored in the cells. Restriction enzyme recognition sites located at these junctions are not cleaved by the corresponding endonuclease after modification with KMnO4. This inhibition of cleavage allows the determination of the relative amounts of B- and Z-forms of the cloned inserts inside the cell. We have successfully applied this method to monitor the extent of Z-DNA formation in E. coli as a function of the growth phase and mutated topoisomerase or gyrase activities. The assay can in principle be used for any unusual DNA structure that contains a restriction recognition site inside or near the structural alteration. It can be a useful tool to analyze in vivo correlations between DNA structure and gene regulatory events.
Full text
PDF

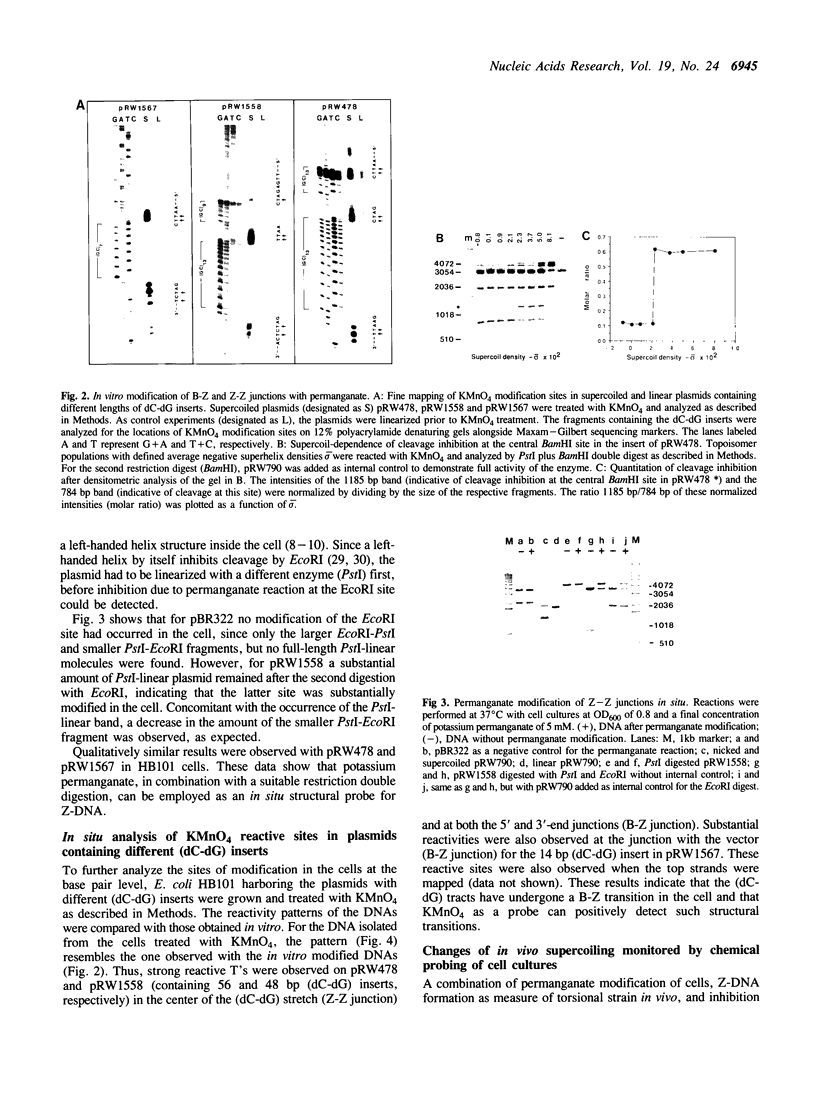
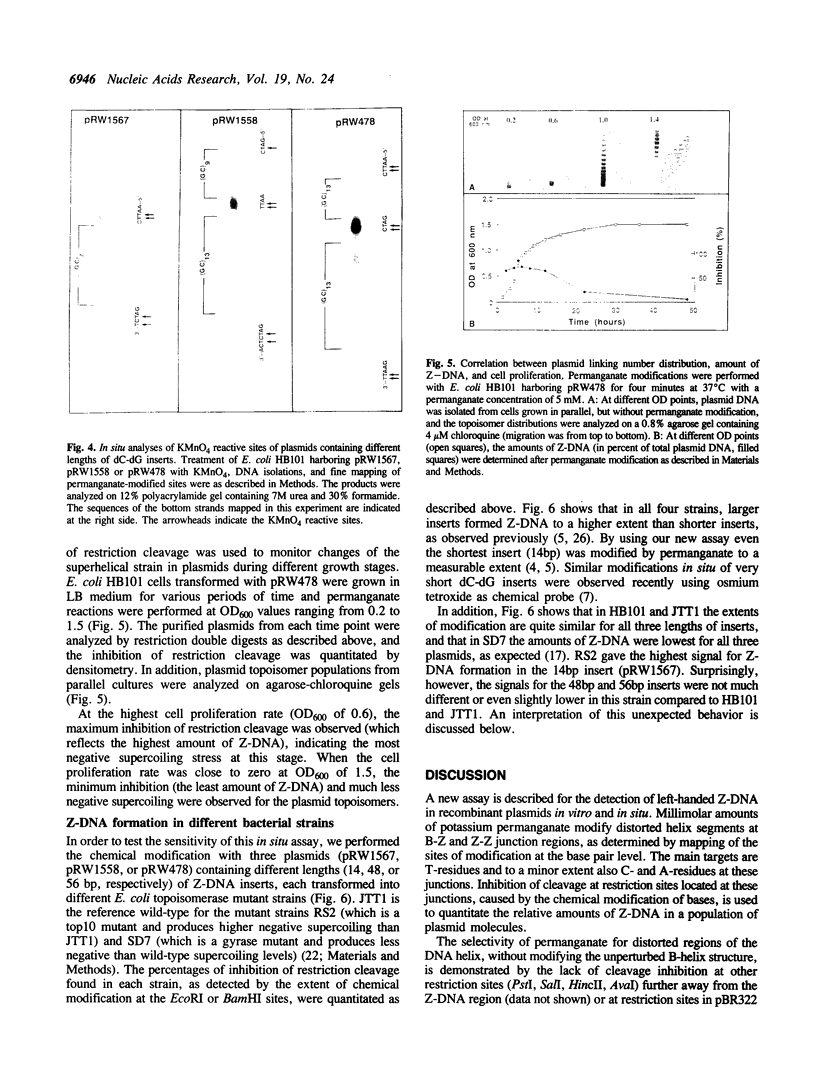
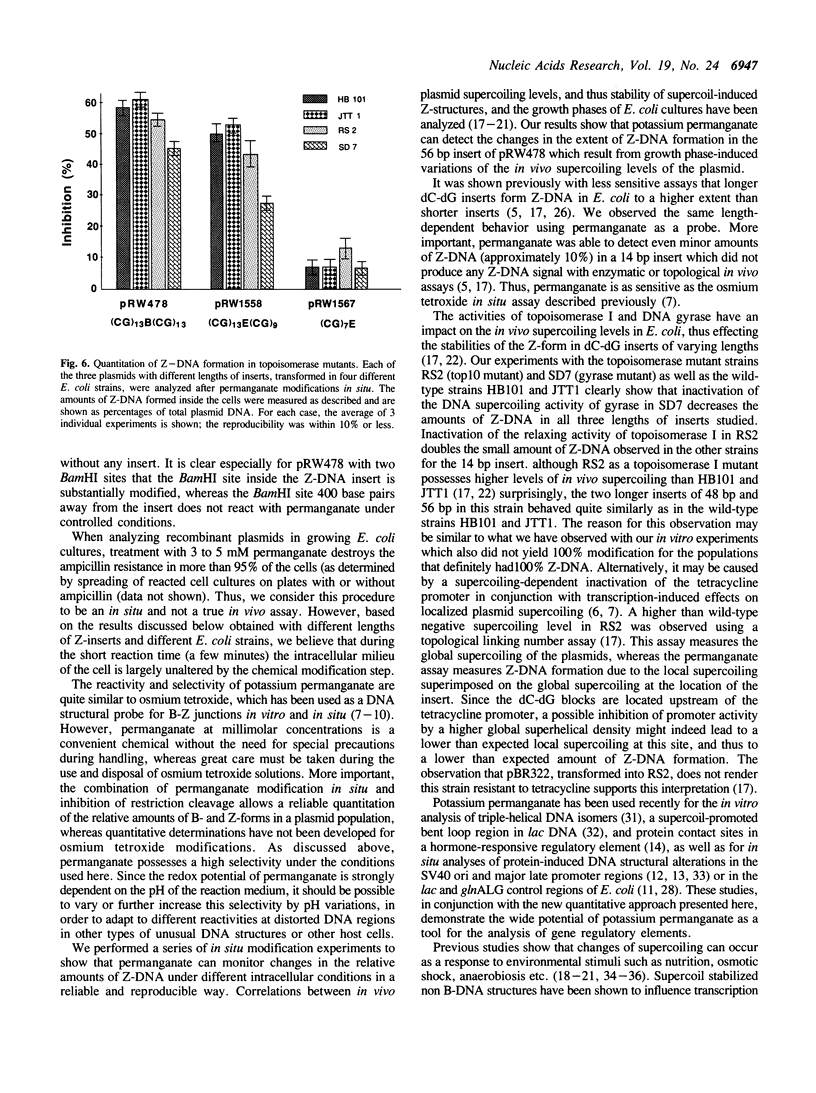

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke V. L., Gralla J. D. Changes in the linking number of supercoiled DNA accompany growth transitions in Escherichia coli. J Bacteriol. 1987 Oct;169(10):4499–4506. doi: 10.1128/jb.169.10.4499-4506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barat-Gueride M., Dufresne C., Rickwood D. Effect of DNA conformation on the transcription of mitochondrial DNA. Eur J Biochem. 1989 Aug 1;183(2):297–302. doi: 10.1111/j.1432-1033.1989.tb14928.x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Hurwitz J. Localized melting and structural changes in the SV40 origin of replication induced by T-antigen. EMBO J. 1988 Oct;7(10):3149–3158. doi: 10.1002/j.1460-2075.1988.tb03182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Zhang L., Sasse-Dwight S., Gralla J. D. DNA supercoiling promotes formation of a bent repression loop in lac DNA. J Mol Biol. 1987 Jul 5;196(1):101–111. doi: 10.1016/0022-2836(87)90513-4. [DOI] [PubMed] [Google Scholar]
- Boublíková P., Palecek E. Osmium tetroxide, N,N,N',N'-tetramethylethylendiamine. A new probe of DNA structure in the cell. FEBS Lett. 1990 Apr 24;263(2):281–284. doi: 10.1016/0014-5793(90)81393-3. [DOI] [PubMed] [Google Scholar]
- Boublíková P., Palecek E. Probing of B-Z junctions in recombinant plasmids in vitro and in the cell with different osmium tetroxide complexes. Gen Physiol Biophys. 1989 Oct;8(5):475–490. [PubMed] [Google Scholar]
- Camilloni G., Di Martino E., Di Mauro E., Caserta M. Regulation of the function of eukaryotic DNA topoisomerase I: topological conditions for inactivity. Proc Natl Acad Sci U S A. 1989 May;86(9):3080–3084. doi: 10.1073/pnas.86.9.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K. A., Sternglanz R., Reynolds A. E., Wright A. Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell. 1982 Nov;31(1):43–51. doi: 10.1016/0092-8674(82)90403-2. [DOI] [PubMed] [Google Scholar]
- Dorman C. J., Ni Bhriain N., Higgins C. F. DNA supercoiling and environmental regulation of virulence gene expression in Shigella flexneri. Nature. 1990 Apr 19;344(6268):789–792. doi: 10.1038/344789a0. [DOI] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. The in-vivo occurrence of Z DNA. J Biomol Struct Dyn. 1983 Dec;1(3):593–609. doi: 10.1080/07391102.1983.10507467. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Ukita T. The selective degradation of pyrimidines in nucleic acids by permanganate oxidation. Biochem Biophys Res Commun. 1967 Nov 30;29(4):556–561. doi: 10.1016/0006-291x(67)90521-9. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S. Transcription regulation in vitro by an E. coli promoter containing a DNA cruciform in the '-35' region. Nucleic Acids Res. 1989 Jul 25;17(14):5537–5545. doi: 10.1093/nar/17.14.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski A., Blaho J. A., Larson J. E., Shimizu M., Wells R. D. Tetracycline promoter mutations decrease non-B DNA structural transitions, negative linking differences and deletions in recombinant plasmids in Escherichia coli. J Mol Biol. 1989 Jun 5;207(3):513–526. doi: 10.1016/0022-2836(89)90461-0. [DOI] [PubMed] [Google Scholar]
- Jaworski A., Higgins N. P., Wells R. D., Zacharias W. Topoisomerase mutants and physiological conditions control supercoiling and Z-DNA formation in vivo. J Biol Chem. 1991 Feb 5;266(4):2576–2581. [PubMed] [Google Scholar]
- Jaworski A., Hsieh W. T., Blaho J. A., Larson J. E., Wells R. D. Left-handed DNA in vivo. Science. 1987 Nov 6;238(4828):773–777. doi: 10.1126/science.3313728. [DOI] [PubMed] [Google Scholar]
- Johnston B. H., Quigley G. J., Ellison M. J., Rich A. The Z-Z junction: the boundary between two out-of-phase Z-DNA regions. Biochemistry. 1991 May 28;30(21):5257–5263. doi: 10.1021/bi00235a020. [DOI] [PubMed] [Google Scholar]
- Johnston B. H., Rich A. Chemical probes of DNA conformation: detection of Z-DNA at nucleotide resolution. Cell. 1985 Oct;42(3):713–724. doi: 10.1016/0092-8674(85)90268-5. [DOI] [PubMed] [Google Scholar]
- Kowalski D., Eddy M. J. The DNA unwinding element: a novel, cis-acting component that facilitates opening of the Escherichia coli replication origin. EMBO J. 1989 Dec 20;8(13):4335–4344. doi: 10.1002/j.1460-2075.1989.tb08620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J Bacteriol. 1979 Nov;140(2):424–435. doi: 10.1128/jb.140.2.424-435.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx J. L. Z-DNA: still searching for a function. Science. 1985 Nov 15;230(4727):794–796. doi: 10.1126/science.2997919. [DOI] [PubMed] [Google Scholar]
- McCarthy J. G., Williams L. D., Rich A. Chemical reactivity of potassium permanganate and diethyl pyrocarbonate with B DNA: specific reactivity with short A-tracts. Biochemistry. 1990 Jun 26;29(25):6071–6081. doi: 10.1021/bi00477a027. [DOI] [PubMed] [Google Scholar]
- McLean M. J., Blaho J. A., Kilpatrick M. W., Wells R. D. Consecutive A X T pairs can adopt a left-handed DNA structure. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5884–5888. doi: 10.1073/pnas.83.16.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M. J., Lee J. W., Wells R. D. Characteristics of Z-DNA helices formed by imperfect (purine-pyrimidine) sequences in plasmids. J Biol Chem. 1988 May 25;263(15):7378–7385. [PubMed] [Google Scholar]
- Mitsui Y., Langridge R., Shortle B. E., Cantor C. R., Grant R. C., Kodama M., Wells R. D. Physical and enzymatic studies on poly d(I-C)-poly d(I-C), an unusual double-helical DNA. Nature. 1970 Dec 19;228(5277):1166–1169. doi: 10.1038/2281166a0. [DOI] [PubMed] [Google Scholar]
- Ni Bhriain N., Dorman C. J., Higgins C. F. An overlap between osmotic and anaerobic stress responses: a potential role for DNA supercoiling in the coordinate regulation of gene expression. Mol Microbiol. 1989 Jul;3(7):933–942. doi: 10.1111/j.1365-2958.1989.tb00243.x. [DOI] [PubMed] [Google Scholar]
- Palecek E., Rasovská E., Boublíková P. Probing of DNA polymorphic structure in the cell with osmium tetroxide. Biochem Biophys Res Commun. 1988 Jan 29;150(2):731–738. doi: 10.1016/0006-291x(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Rahmouni A. R., Wells R. D. Stabilization of Z DNA in vivo by localized supercoiling. Science. 1989 Oct 20;246(4928):358–363. doi: 10.1126/science.2678475. [DOI] [PubMed] [Google Scholar]
- Richardson S. M., Higgins C. F., Lilley D. M. DNA supercoiling and the leu-500 promoter mutation of Salmonella typhimurium. EMBO J. 1988 Jun;7(6):1863–1869. doi: 10.1002/j.1460-2075.1988.tb03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. KMnO4 as a probe for lac promoter DNA melting and mechanism in vivo. J Biol Chem. 1989 May 15;264(14):8074–8081. [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. Probing the Escherichia coli glnALG upstream activation mechanism in vivo. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8934–8938. doi: 10.1073/pnas.85.23.8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton C. K., Wells R. D. The facile generation of covalently closed, circular DNAs with defined negative superhelical densities. Anal Biochem. 1982 May 15;122(2):253–257. doi: 10.1016/0003-2697(82)90277-9. [DOI] [PubMed] [Google Scholar]
- Truss M., Chalepakis G., Beato M. Contacts between steroid hormone receptors and thymines in DNA: an interference method. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7180–7184. doi: 10.1073/pnas.87.18.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueshima R., Fujita N., Ishihama A. DNA supercoiling and temperature shift affect the promoter activity of the Escherichia coli rpoH gene encoding the heat-shock sigma subunit of RNA polymerase. Mol Gen Genet. 1989 Jan;215(2):185–189. doi: 10.1007/BF00339716. [DOI] [PubMed] [Google Scholar]
- Umek R. M., Kowalski D. The ease of DNA unwinding as a determinant of initiation at yeast replication origins. Cell. 1988 Feb 26;52(4):559–567. doi: 10.1016/0092-8674(88)90469-2. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Rich A. In Z-DNA the sequence G-C-G-C is neither methylated by Hha I methyltransferase nor cleaved by Hha I restriction endonuclease. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3268–3272. doi: 10.1073/pnas.81.11.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig B., Dorbic T., Rich A. The level of Z-DNA in metabolically active, permeabilized mammalian cell nuclei is regulated by torsional strain. J Cell Biol. 1989 Mar;108(3):755–764. doi: 10.1083/jcb.108.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias W., Jaworski A., Larson J. E., Wells R. D. The B- to Z-DNA equilibrium in vivo is perturbed by biological processes. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7069–7073. doi: 10.1073/pnas.85.19.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias W., Jaworski A., Wells R. D. Cytosine methylation enhances Z-DNA formation in vivo. J Bacteriol. 1990 Jun;172(6):3278–3283. doi: 10.1128/jb.172.6.3278-3283.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias W., Larson J. E., Kilpatrick M. W., Wells R. D. HhaI methylase and restriction endonuclease as probes for B to Z DNA conformational changes in d(GCGC) sequences. Nucleic Acids Res. 1984 Oct 25;12(20):7677–7692. doi: 10.1093/nar/12.20.7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Gralla J. D. In situ nucleoprotein structure at the SV40 major late promoter: melted and wrapped DNA flank the start site. Genes Dev. 1989 Nov;3(11):1814–1822. doi: 10.1101/gad.3.11.1814. [DOI] [PubMed] [Google Scholar]
- Zhang L., Gralla J. D. In situ nucleoprotein structure involving origin-proximal SV40 DNA control elements. Nucleic Acids Res. 1990 Apr 11;18(7):1797–1803. doi: 10.1093/nar/18.7.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]