Abstract
Limited analyses of cerebrospinal fluid from patients with central nervous system infections have shown that the oligoclonal IgG is antibody directed against the agent that causes disease. Using a new method involving binding of IgG to beads coated with lysates prepared from candidate infectious antigens, we showed that the oligoclonal IgG in cerebro-spinal fluid of a patient with chronic varicella zoster virus vasculopathy is directed against the causative virus. This approach holds promise in identifying and purifying the relevant oligoclonal IgGs in inflammatory central nervous system diseases of unknown cause.
Clinical pathology laboratories define oligoclonal immunoglobulin (Ig) as discrete bands seen on isoelectric focusing gels of cerebrospinal fluid (CSF), typically near the cathode, that are not present in serum of the same patient. These oligoclonal bands (OGBs) are found most often in chronic infectious diseases of the central nervous system (CNS). In diseases in which the specificities of the OGBs have been determined, the bands have been shown to be antibody directed against the infectious agent that caused disease (reviewed in Gilden and colleagues1). For example, most OGBs in subacute sclerosing panencephalitis (SSPE), a form of chronic measles encephalitis, are directed against measles virus (MV).2,3 Similarly, OGBs in cryptococcal meningitis and neurosyphilis are antibody directed against Cryptococcus neoformans4 and Treponema pallidum,5 respectively. OGBs are also found in other inflammatory CNS diseases of unknown cause, such as multiple sclerosis, sarcoidosis, and Behçet’s disease. Identification of the specificity of those oligoclonal Igs might be key in determining the cause of these disorders.
In chronic infectious CNS diseases, additional bands of IgG have been found in both the CSF and serum of the same patient and have been shown to be antibody directed against the same antigen.3,6 Thus, the OGBs seen exclusively in the CSF are part of a larger immune response in both the CSF and periphery that is directed against the causative agent. Herein, we developed a technique to absorb OGBs directed against specific antigens and determined the specificity of OGBs present in the CSF of a patient with VZV vasculopa-thy.
Materials and Methods
Cerebrospinal Fluid Samples
A CSF sample obtained at the onset of VZV vasculopathy 5 months after bilateral sacral distribution zoster contained a high titer of anti-VZV-specific IgG.7 The CSF was frozen at -20°C. At the time of this study, enzyme-linked immunosorbent assay (ELISA) reconfirmed the presence of anti-VZV antibody in CSF, the quality of VZV antigen in the VZV-infected cell lysate, and the presence of antibody to measles virus in the CSF of an SSPE patient (a gift from Dr B. Vandvik, Oslo, Norway) (Table). CSF protein concentra-tions were determined by bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL).
Table. Properties of Cerebrospinal Fluids from VZV Vasculopathy and SSPE Patients.
| CSF (ELISA concentration) |
Total Protein (mg/dl) |
IgG (%) | ELISA (A405) |
|||
|---|---|---|---|---|---|---|
| VZV-MeWo | Uninfected | MV-Vero | Titer | |||
| VZV vasculopathy (30 μg/ml) |
153 | 13 | 0.293 | −0.019 | nd | 392 |
| SSPE (30 μg/ml) | 280 | 15 | nd | 0.082 | 0.953 | nd |
VZV = varicella zoster virus; SSPE = subacute sclerosing panencephalitis; CSF = cerebrospinal fluid; ELISA = enzyme-linked immunosorbent assay; MV = measles virus; nd = not done.
Enzyme-Linked Immunosorbent Assay
To assess initial virus specificity by ELISA, we tested the CSFs on VZV-infected, MV-infected, or uninfected cell ly-sates as described.8 In brief, CSFs were diluted to 30μg/ml in phosphate-buffered saline (PBS), and 50μl was incubated for 1 hour at 37°C in microtiter wells coated with a 1 to 30 dilution of VZV-infected MeWo cells, MV-infected Vero cells, or uninfected MeWo or Vero cells. After washing 10 times with PBS for 5 minutes each time, the wells were incubated with a 1 to 200 dilution of alkaline phosphataseconjugated goat anti-human IgG (Vector Laboratories, Burlingame, CA) for 1 hour at 37°C and washed 10 times with PBS for 5 minutes each time. Bound antibody was measured after 30 minutes’ incubation with 50μl of p-nitrophenyl phosphate substrate (Sigma, St. Louis, MO) monitored at 405nm.
Covalent Coupling of Lysates to Sepharose Beads
At the height of cytopathic effect, subconfluent cultures of VZV-infected MeWo cells and MV (Chicago strain)-infected Vero cells, as well as control uninfected MeWo and Vero cells, were rinsed with PBS, scraped from the flask with a cell scraper into PBS, and Dounce-homogenized, followed by brief centrifugation at 600rpm to remove large particulate matter from the lysates. Protein concentrations of the lysates were determined by bicinchoninic acid (BCA) protein assay (Pierce). Lysates were frozen as aliquots at -70°C until use. The same batches of infected cell lysates were used for conjugation to beads in all experiments. Cyanogen bromide-activated Sepharose 4b beads (Sigma) were reconstituted and 300μl was mixed with an excess of 3 to 6mg of lysate in siliconized vials overnight at 4°C on an orbital rotator at 30rpm. Vials were centrifuged at 10,000rpm for 1 minute, and the supernatants were removed for protein assay and calculation of lysate coupling efficiency (usually 1.5-3mg of protein was coupled to 300μl of Sepharose beads). Lysatebead conjugates were incubated with 1M ethanolamine (pH 8.0) for 4 hours at 4°C to block unreacted binding sites on the beads and washed alternately with basic and acidic wash buffers (four times each) according to the manufacturer’s instructions to remove any unbound reagents. Lysate-bead conjugates were used immediately or stored briefly in PBS with 1M NaCl at 4°C.
Binding of Cerebrospinal Fluid to Lysate-Coupled Beads
VZV vasculopathy and SSPE CSFs (20-60μl) were diluted in PBS to equivalent amounts of IgG (3-4μg), mixed with 75μl of lysate-coupled beads of infected or uninfected lysates overnight at 4°C in tubes preblocked with 1% bovine serum albumin in PBS for 2 hours, and rinsed five times with PBS. After centrifugation at 10,000rpm for 1 minute, unbound supernatant was removed for isoelectric focusing (IEF) analysis, and beads were washed three times (5 minutes each) with PBS. Protein bound to the beads was eluted by incubation with 300mM glycine, pH 3.0, for 5 minutes. After centrifugation at 10,000rpm for 1 minute, the eluate supernatant was removed and immediately neutralized to pH 8.0 with Tris buffer. The eluate was concentrated and buffer exchanged with double-distilled water by centrifugation through Centricon concentrators with a MW cutoff of 10,000 (Millipore, Bedford, MA). Approximately one third of the eluate (15μl) was electrophoresed on IEF gels (pH range, 3.0-10.0), in parallel with the original CSF and with 1/10 of the unbound CSF sample that did not bind to the beads. Gels were silver-stained according to the manufacturer’s instructions (Cerebrospinal Fluid Test Kit FR-8030, method 1, Perkin-Elmer Life Sciences, Norton, OH) to visualize protein. Each gel was silver-stained to maximize protein detection.
Results
CSF from a 71-year-old man with VZV vasculopathy was analyzed for specificity of the oligoclonal Ig. The CSF was acellular, but total protein and IgG levels were elevated and had been shown to contain a high titer of anti-VZV IgG antibody (see Table). Quantitative analysis of the VZV CSF by ELISA9 showed that the anti-VZV IgG titer was 392, the highest CSF dilution at which reactivity to VZV antigen was more than twice that of control CSF. IEF analysis of serum from the VZV vasculopathy patient also showed a pattern similar to CSF, with at least four additional bands in CSF that were not visible in serum (data not shown). CSF from an SPPE patient with oligoclonal IgG directed against MV was also analyzed as a control. Each CSF was diluted to 30μg/ml protein and examined by ELISA to confirm the specificity of the IgG as well as the presence of viral antigen in the infected cell lysates (see Table). CSF from the patient with VZV vasculopathy reacted specifically with VZV-infected MeWo cells; the SSPE CSF reacted specifically with MV-infected Vero cells. Reactivity of SSPE CSF for MV-infected cells was approximately three times greater than the reactivity of VZV vasculopathy CSF for VZV-infected cells; this may indicate either agreater abundance or affinity of MV-specific IgG in SSPE CSF.
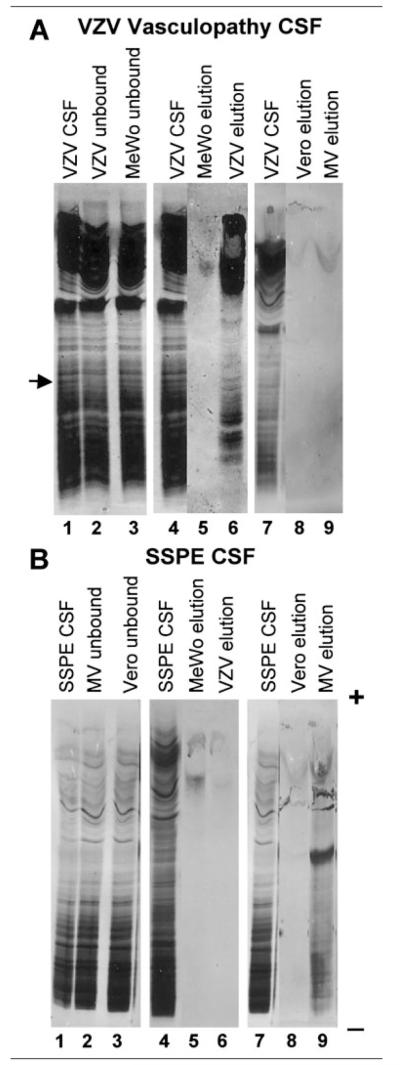
Both CSFs were incubated with Sepharose 4b beads coated with cell lysate from infected or uninfected cells to absorb the antigen-specific Ig. Beads were freshly prepared in every experiment using the same preparations of VZV-infected or MV-infected cell lysates. CSF protein that bound to the lysates was eluted, immediately neutralized, concentrated, and visualized by electrophoresis on IEF gels and silver-staining. Only beads coated with VZV-infected MeWo cells bound VZV CSF protein (Fig, A, lane 6). Most eluted bands were present in the cathodal region of the gel, where oligoclonal IgG is characteristically visualized. In addition, a broad band of protein also was eluted from the VZV beads in the anodal (acidic) region (see Fig, A, lane 6), which might represent either IgG or IgM antibody that bound to the VZV lysate. Neither uninfected nor MV-infected cells bound protein from the VZV CSF (see Fig, A, lanes 5, 8, and 9). Similarly, SSPE CSF bound only to beads coated with MV-infected cells (see Fig, B, lane 9). The low pH elutions with glycine buffer effectively removed all CSF protein bound to lysates. Additional eluates obtained with 4M urea or 1% sodium dodecyl sulfate or elution for longer times did not release additional protein detectable on IEF gels (data not shown).
Fig.
Absorption of cerebrospinal fluid (CSF) Ig to virusinfected lysates coupled to beads. (A) Resolution by isoelectric focusing (IEF) and silver-staining of proteins in CSF from patient with varicella zoster virus (VZV) vasculopathy (lane 1, CSF) and in CSF that does not bind to lysates of VZV-infected (lane 2, VZV unbound) or uninfected (lane 3, MeWo unbound) cells. Arrow denotes a band from the VZV vasculopathy CSF that disappeared after absorption with VZV lysate (lane 2) but not after absorption with uninfected lysate (lane 3). CSF from patient with VZV vasculopathy (lane 4, CSF) was also compared with CSF protein bound and eluted from lysates of uninfected (lane 5, MeWo elution) and VZV-infected (lane 6, VZV elution) cells. The same CSF (lane 7, CSF) was again compared with CSF protein bound and eluted from lysates of uninfected (lane 8, Vero elution) and MV-infected (lane 9, MV elution) cells. Multiple bands of IgG in the CSF of the patient with VZV vasculopathy were eluted only from the VZV lysate (lane 6, VZV elution), but not from any other lysates (lanes 5, 8, and 9). (B) Resolution by IEF and silver-staining of protein in CSF from patient with SSPE (lane 1, SSPE CSF), and in CSF that does not bind to lysates of MV-infected (lane 2, MV unbound) or uninfected (lane 3, Vero unbound) cells. CSF from SSPE patient (lane 4, SSPE CSF) was also compared with CSF protein bound and eluted from lysates of uninfected (lane 5, MeWo elution) and VZV-infected (lane 6, VZV elution) cells. The same CSF (lane 7, SSPE CSF) was again compared with CSF protein bound and eluted from lysates of uninfected (lane 8, Vero elution) and MV-infected (lane 9, MV elution) cells. Multiple bands of IgG in the CSF of the patient with SSPE were eluted only from the MV lysate (lane 9, MV elution), but not from any other lysates (lanes 5, 6, and 8). pH scale indicated on gel, + anode, − cathode.
The CSF protein that bound to its cognate VZV-infected lysate represented only a small proportion of the total CSF protein applied to the beads. From approximately 4μg of total CSF protein applied to the beads, it can be estimated from the silver-staining that 10 to 100ng of protein was specifically eluted from the infected lysate-coated beads. Most CSF protein remained in the unbound fractions of CSF separated from the VZV-infected cell lysates (see Fig, A). However, at least one band disappeared from the control CSF after absorption with the VZV lysate, but not after absorption with the MV lysate (see arrow in Fig, A). Additional CSF bands bound specifically to and were eluted from the VZV-infected lysate (see Fig, A, lane 6); these IgGs represent many of the cathodal bands seen after IEF of the original CSF that were incompletely removed from unbound CSF (see Fig, A, lane 2).
Discussion
Using a new method in which oligoclonal IgG is bound to beads coated with lysates prepared from candidate infectious antigens, we show that OGBs in the CSF of a patient with chronic VZV vasculopathy (not a primary viral encephalitis) are directed against the causative virus. Thus, even in a vasculopathy where the persisting antigen is primarily in vessels rather than brain, the intrathecally derived IgG is still specific for the agent that causes disease, as previously shown in cases of chronic meningitis and encephalitis.3-5,10 Although the CSF of the patient with VZV vasculopathy contained three to four bands that did not appear in the serum,7 many more bands in the CSF eluted specifically from beads coated with VZV lysate. Such high levels of intrathecally synthesized, VZV-specific antibody was shown in a previous study of this patient in which the serum to CSF ratio of VZV IgG was profoundly reduced compared with albumin or total IgG.7
Our study further shows that when the patient developed vasculopathy, the oligoclonal antibody in the CSF was directed against VZV. Virtually all of the CSF protein that bound to and was eluted from beads was VZV-specific because it bound only to VZV-infected lysates and not to beads coated with MV lysates or other controls. Furthermore, most of the eluted bands of protein were focused in the basic (cathodal) regions of the IEF gels where IgG typically migrates. Some VZV-specific eluted bands also migrated to the anodal region, possibly representing virus-specific IgG or even IgM, which typically focuses at more acidic pH ranges.11,12 The study was controlled by validating the MV-specificity of OGBs in the CSF of a patient with SSPE.
The specificity of OGBs in chronic CNS infection has been analyzed previously using two methodologies. In the first, IgG was absorbed from CSF by incubation with antigen and the Ig-antigen complex was sedimented by centrifugation (immunocomplex sedimentation). The relevant OGBs were determined by their subsequent absence in the supernatants after IEF or by eluting the IgG from the IgG-antigen complex and analysis by IEF. Using this technique, Vandvik and colleagues3 removed OGBs from SSPE CSF and showed that they were directed against MV. This technique also established the specificity of OGBs for the causative agent in mumps and cryptococcal meningitis,4,10 and in progressive rubella panencephalitis.13 A second method assessed the ability of OGBs from IEF gels to absorb to membranes precoated with antigens (immunoelectrofixation). This method was used to demonstrate that the oligoclonal IgG was directed against T. pallidum in neurosyphilis,5 against herpes simplex virus (HSV)-specific glycoproteins in HSV encephalitis and against HTLV-1-specific proteins in HTLV-1 myelopathy.6,14,15 Our technique has several advantages over these methodologies. For example, we were able to utilize less than 100μl of CSF with microgram amounts of antibody and visualize less than 1μg of eluted protein by silver-staining, as compared with the milligram quantities of purified IgG required for immunocomplex sedimentation. Moreover, our method avoids the loss of reactive IgG from the CSF as it binds to antigen-coated membranes in immunoelectrofixation. By eluting the bound protein from beads coated with antigen under the same conditions used to purify active IgG from protein A affinity columns, we expect the purified IgG in our studies to be functional and available as soluble antibody for additional studies. On the other hand, the OGBs that specifically bound to and were eluted from beads coated with virusinfected lysates in our study were still visible in the unbound fractions on IEF gels, indicating that they had not been completely removed from CSF. We calculated that 3 to 4μg of CSF IgG was applied to the beads, and that approximately 10 to 100ng of CSF IgG bound to its specific antigen (see Fig, A, lane 6; and B, lane 9). The use of larger amounts of antigencoated beads (or even purified antigen), or repeated binding and elution, should enable complete absorption of all OGBs directed against specific antigen. In SSPE, almost all the oligoclonal IgG was removed by immunocomplex sedimentation after repeated absorptions with MV.2
We used silver-staining in the development of this method to visualize all the protein that bound to the lysate-coated beads. The IEF lanes that compared eluates from beads coated with infected and uninfectedlysates demonstrate the striking specificity of the pro-tein that was bound and eluted. In future applications, immunodetection of the eluted IgG with a standard secondary antibody to visualize only IgG that binds to the beads would simplify the analysis of IEF profiles while matching the exquisite sensitivity of silver-staining.16 Finally, the immunodetection method may also identify irrelevant antibody in the unbound fractions that is not directed against the candidate antigen present on beads. Such IgG has been found in SSPE and shown not to be MV specific.17
Overall, our technique served to demonstrate that OGBs in the CSF of VZV vasculopathy are directed against the virus (VZV) that caused the disease. This technique also holds promise in identifying or confirming the specificity of the OGBs in inflammatory CNS diseases in which the relevant antigen is unknown.
Acknowledgments
This work was supported by grants from the Public Health Service, NIH (NS41549, M.P.B.; NS32623, D.H.G., M.P.B., G.P.O.; AG06127, D.H.G.) and a NIH Training Grant in Neurovirology-Molecular Biology (NS07321, B.N.H.).
We thank Dr B. Vandvik for generously providing SSPE CSF, and the assistance of the University of Colorado Hospital Clinical Laboratory. We also thank M. Hoffman for editorial review and C. Allen for preparing the manuscript.
References
- 1.Gilden DH, Devlin ME, Burgoon MP, Owens GP. The search for virus in multiple sclerosis brain. Mult Scler. 1996;2:179–183. doi: 10.1177/135245859600200403. [DOI] [PubMed] [Google Scholar]
- 2.Norrby E, Vandvik B. Relationship between measles virusspecific antibody activities and oligoclonal IgG in the central nervous system of patients with subacute sclerosing panencephalitis and multiple sclerosis. Med Microbiol Immunol (Berl) 1975;162:63–72. doi: 10.1007/BF02123578. [DOI] [PubMed] [Google Scholar]
- 3.Vandvik B, Norrby E, Nordal HJ, Degre M. Oligoclonal measles virus-specific IgG antibodies isolated from cerebrospinal fluids, brain extracts, and sera from patients with subacute sclerosing panencephalitis and multiple sclerosis. Scand J Immunol. 1976;5:979–992. doi: 10.1111/j.1365-3083.1976.tb03050.x. [DOI] [PubMed] [Google Scholar]
- 4.Porter KG, Sinnamon DG, Gillies RR. Cryptococcus neoformans-specific oligoclonal immunoglobulins in cerebrospinal fluid in cryptococcal meningitis. Lancet. 1977;1:126. doi: 10.1016/s0140-6736(77)92473-4. [DOI] [PubMed] [Google Scholar]
- 5.Vartdal F, Vandvik B, Michaelsen TE, et al. Neurosyphilis: intrathecal synthesis of oligoclonal antibodies to Treponema pallidum. Ann Neurol. 1982;11:35–40. doi: 10.1002/ana.410110107. [DOI] [PubMed] [Google Scholar]
- 6.Link H, Cruz M, Gessain A, et al. Chronic progressive myelopathy associated with HTLV-I: oligoclonal IgG and anti-HTLV-I IgG antibodies in cerebrospinal fluid and serum. Neurology. 1989;39:1566–1572. doi: 10.1212/wnl.39.12.1566. [DOI] [PubMed] [Google Scholar]
- 7.Gilden DH, Lipton HL, Wolf JS, et al. Two patients with unusual forms of varicella-zoster virus vasculopathy. N Engl J Med. 2002;347:1500–1503. doi: 10.1056/NEJMoa020841. [DOI] [PubMed] [Google Scholar]
- 8.Burgoon MP, Williamson RA, Owens GP, et al. Cloning the antibody response in humans with inflammatory CNS disease: isolation of measles-specific antibodies from phage display li-braries of a subacute sclerosing panencephalitis brain. J Neuroimmunol. 1999;94:204–211. doi: 10.1016/s0165-5728(98)00243-4. [DOI] [PubMed] [Google Scholar]
- 9.Forghani B. Varicella-zoster virus antibody. Methods in enzymatic analysis. In: Bergmeyer H, editor. Antigens and antibodies 1. 3rd ed Vol. 10. Verlag Chemie; Weinheim, Germany: 1986. pp. 267–284. [Google Scholar]
- 10.Vandvik B, Norrby E, Johnson J, Sensvold K. Mumps meningitis: prolonged pleocytosis and occurrence of mumps virus-specific oligoclonal IgG in the cerebrospinal fluid. Eur Neurol. 1978;17:13–22. doi: 10.1159/000114916. [DOI] [PubMed] [Google Scholar]
- 11.Villar LM, Gonzalez-Porque P, Masjuan J, et al. A sensitive and reproducible method for the detection of oligoclonal IgM bands. J Immunol Methods. 2001;258:151–155. doi: 10.1016/s0022-1759(01)00492-6. [DOI] [PubMed] [Google Scholar]
- 12.Sharief MK, Thompson EJ. Distribution of cerebrospinal fluid oligoclonal IgM bands in neurological diseases: a comparison between agarose electrophoresis and isoelectric focusing. J Neurol Sci. 1992;109:83–87. doi: 10.1016/0022-510x(92)90098-6. [DOI] [PubMed] [Google Scholar]
- 13.Coyle PK, Wolinsky JS. Characterization of immune complexes in progressive rubella panencephalitis. Ann Neurol. 1981;9:557–562. doi: 10.1002/ana.410090608. [DOI] [PubMed] [Google Scholar]
- 14.Grimaldi LM, Roos RP, Manservigi R, et al. An isolelectric focusing study in herpes simplex virus encephalitis. Ann Neurol. 1988;24:227–232. doi: 10.1002/ana.410240209. [DOI] [PubMed] [Google Scholar]
- 15.Grimaldi LM, Roos RP, Devare SG, et al. HTLV-I-associated myelopathy: oligoclonal immunoglobulin G bands contain anti-HTLV-I p24 antibody. Ann Neurol. 1988;24:727–731. doi: 10.1002/ana.410240606. [DOI] [PubMed] [Google Scholar]
- 16.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1988. [Google Scholar]
- 17.Mehta PD, Patrick BA, Thormar H, Wisniewski HM. Oligoclonal IgG bands with and without measles antibody activity in sera of patients with subacute sclerosing panencephalitis (SSPE) J Immunol. 1982;129:1983–1985. [PubMed] [Google Scholar]