Abstract
New methods are needed for the nondestructive measurement of tooth demineralization and remineralization to monitor the progression of incipient caries lesions (tooth decay) for effective nonsurgical intervention and to evaluate the performance of anti-caries treatments such as chemical treatments or laser irradiation. Studies have shown that optical coherence tomography (OCT) has great potential to fulfill this role since it can be used to measure the depth and severity of early lesions with an axial resolution exceeding 10-μm, it is easy to apply in vivo and it can be used to image the convoluted topography of tooth occlusal surfaces. In this paper we attempt to determine the earliest stage at which we can detect significant differences in lesion severity. Automated methods of analysis were used to measure the depth and severity of demineralized bovine enamel produced using a simulated caries model that emulates demineralization in the mouth. Significant differences in the depth and integrated reflectivity from the lesions were detected after only a few hours of demineralization. These results demonstrate that cross polarization OCT is ideally suited for the nondestructive assessment of early demineralization.
Keywords: polarization, optical coherence tomography, tooth demineralization, dental caries
1. INTRODUCTION
New tools are needed to non-destructively assess carious lesion depth and severity, efficacy of chemical intervention, and testing of anti-caries agents. The National Institute of Dental and Craniofacial research has requested the validation of new technologies for the measurement of tooth surface demineralization or remineralization to serve as a likely surrogate end point in dental clinical trials (1). Several studies have demonstrated that polarization sensitive optical coherence tomography (PS-OCT) can be used to nondestructively measure the severity of subsurface demineralization in enamel and dentin and is therefore well suited for this role (2–10).
Polarization sensitivity is particularly valuable for imaging caries lesions due to the enhanced contrast of caries lesions caused by depolarization of the incident light by the lesion and the confounding influence of the strong surface reflectance of the tooth surface is reduced in the orthogonal polarization. Baumgartner et al. (11–13) presented the first polarization resolved images of dental caries. PS-OCT images are typically processed in the form of phase and intensity images (14) (15), such images best show variations in the birefringence of the tissues. Caries lesions rapidly depolarize or scramble the polarization of incident polarized light and the image of the orthogonal polarization to that of the incident polarization can provide improved contrast of caries lesions. We developed an approach to quantifying the severity of caries lesion by integrating the reflectivity of the orthogonal axis (⊥) or cross polarization image (3). There are two mechanisms in which intensity can arise in the cross polarization image. The native birefringence of the tooth enamel can rotate the phase angle of the incident light beam between the two orthogonal axes (similar to a waveplate) as the light propagates through the enamel without changing the degree of polarization. The other mechanism is depolarization or polarization scrambling from scattering in which the degree of polarization is reduced. It is this later mechanism that is exploited to measure the severity of demineralization. Demineralization of the enamel due to dental decay causes an increase in the scattering coefficient by a 1–2 orders of magnitude, thus demineralized enamel induces a very large increase in the reflectivity along with depolarization. This in turn causes a large rise in the cross polarization image.
This approach also has the added advantage of reducing the intensity of the strong reflection from the tooth surface that can prevent resolution of the lesion area near the tooth surface, which is important for measurement of the lesion surface zone that can potentially provide information about the lesion activity. A conventional OCT system cannot differentiate the strong reflectance from the tooth surface from increased reflectivity from the lesion itself(16, 17). This facilitates direct integration of the lesion reflectivity to quantify the lesion severity, regardless of the tooth topography and the difficult task of having to deconvolve the strong surface reflection from the lesion surface can be circumvented. Longitudinal studies have demonstrated that PS-OCT can be used for monitoring erosion, demineralization and remineralization (2–10). The progression of artificially produced caries lesions in the pit and fissure systems of extracted molars can also be monitored non-destructively and the integrated reflectivity in the cross polarization image correlates well with the growth of the lesion (4, 18). Since the most important information about the lesion is near the surface, a polarization sensitive OCT system is invaluable for imaging dental caries particularly early lesions.
Last year we demonstrated that automated algorithms can be applied successfully to calculate the depth of demineralization and the overall or integrated reflectivity from the zone of demineralization (19). This approach has significant advantages because PS-OCT can be used to rapidly acquire 2D and 3D tomographic images of areas of early demineralization on tooth surfaces. In order to rapidly process the images and effectively quantify the lesion severity algorithms are needed to automatically extract lesion depth and severity information. Moreover, the high dynamic range of the reflectivity and the lack of a sharp demarcation between the sound and demineralized enamel at the lesion margins makes it challenging to define the lesion depth and we have found that edge finding algorithms are suitable for determining the lesion depth. Once the lesion depth is accurately calculated the lesion severity is calculated by integrating the reflectivity over that depth. In previous studies we integrated over a fixed lesion depth that was chosen to be greater than any of the simulated lesions in the study. This later approach should be more accurate since the reflectivity of sound enamel is not zero.
In this paper we employed a simulated lesion model with varying degrees of demineralization representing increasing lesion severity. Our objective was to determine the earliest or shallowest lesions for which significant differences can be determined using PS-OCT.
2. MATERIALS AND METHODS
2.1 Sample Preparation
Enamel blocks, approximately 8 to 12-mm in length with a width of ~ 3-mm and a thickness of 2-mm of bovine enamel were prepared from extracted bovine tooth incisors acquired from a slaughterhouse. Each enamel sample was partitioned into six regions or windows (two sound and 4 lesion areas) by etching small incisions 1.4-mm apart across each of the enamel blocks using a laser (see Fig. 1). Incisions were etched using a transverse excited atmospheric pressure (TEA) CO2 laser operating at 9.3-μm, Impact 2500, GSI Lumonics (Rugby, UK). The incision area also has an increased resistance to acid dissolution that serves to more effectively isolate each group (20). A thin layer of acid resistant varnish in the form of red nail polish, Revlon (New York, NY) was applied to protect the sound enamel control area on each end of the block before exposure to the demineralization solutions. The samples were immersed in demineralization solutions for different time periods to produce lesions of varying severity. The surface softened lesion model, produces subsurface demineralization without erosion of the surface (21). The mineral loss profiles are fairly uniform in these lesions and they emulate an active lesion. Surface softened lesions were produced on thirty enamel blocks. There were six windows on each sample and the blocks were exposed to a demineralization solution at pH 4.8 composed of a 40-mL aliquot of 2.0 mmol/L calcium, 2.0 mmol/L phosphate, and 0.075 mol/L acetate for either four 1-hr periods (1–4-hrs), four 4-hour periods (4–16-hrs), or four 8-hour periods (8–32-hours) with ten blocks used for each series. The groups overlapped each other by one period so that they could be compared to ensure consistency.
Fig. 1.
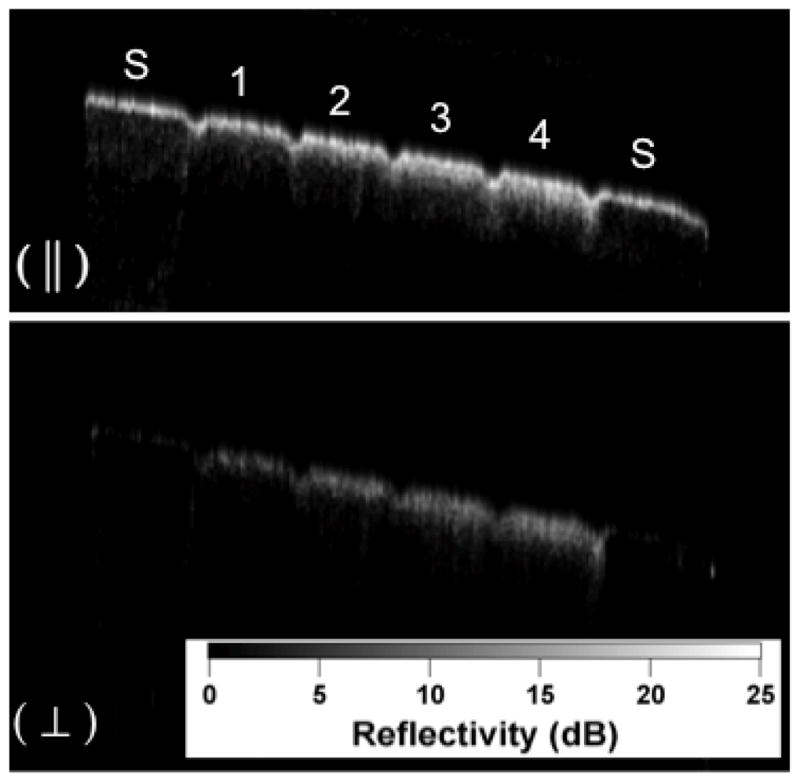
PS-OCT b-scan images of one of the samples with 8-hour period of demineralization created using the surface softened lesion model. The sound areas on each end of the samples are marked (S) and the four areas of increasing demineralization separated by the laser incisions are labeled 1–4. Only the orthogonal polarization (⊥) or cross polarization image was used for analysis in these studies.
Each sample was then placed into the demineralization solution and incubated at 37°C. After each period of demineralization, one region of each sample was covered with a thin layer of the same acid resistant varnish to prevent further demineralization. After the fourth period, the samples were removed from the demineralization solution and the acid resistant varnish was removed using acetone. Each sample was then stored in 0.1% thymol solution to prevent fungal and bacterial growth.
2.2 PS-OCT System
An all fiber-based Optical Coherence Domain Reflectometry (OCDR) system with polarization maintaining (PM) optical fiber, high speed piezoelectric fiber-stretchers and two balanced InGaAs receivers that was designed and fabricated by Optiphase, Inc., Van Nuys, CA. This two-channel system was integrated with a broadband superluminescent diode (SLD) Denselight (Jessup, MD) and a high-speed XY-scanning system (ESP 300 controller & 850G-HS stages, National Instruments, Austin, TX) for in vitro optical tomography. This system is based on a polarization-sensitive Michelson white light interferometer. The high power (15-mW) polarized SLD source operated at a center wavelength of 1317 nm with a spectral bandwidth FWHM of 84 nm was aligned to deliver 15-mW into the slow axis of the PM fiber of the source arm of the interferometer. This light was split into the reference and sample arms of the Michelson interferometer by a 50/50 pm-fiber coupler. The sample arm was coupled to an AR coated fiber-collimator to produce a 6-mm in diameter, collimated beam. That beam was focused onto the sample surface using a 20-mm focal length AR coated plano-convex lens. This configuration provided axial and lateral resolution of approximately 20 μm with a signal to noise ratio of greater than 40–50 dB. Both orthogonal polarization states of the light scattered from the tissue are coupled into the slow and fast axes of the pm- fiber of the sample arm. A quarter wave plate set at 22.5° to horizontal in the reference arm rotated the polarization of the light by 45° upon reflection. After being reflected from the reference mirror and the sample, the reference beams were recombined by the pm fiber-coupler. A polarizing cube splits the recombined beam into its horizontal and vertical polarization components or “slow” and “fast” axis components, which were then coupled by single mode fiber optics into two detectors. The light from the reference arm was polarized at 45° and therefore split evenly between the two detectors. Readings of the electronically demodulated signal from each receiver channel represent the intensity for each orthogonal polarization of the backscattered light. Neutral density filters are added to the reference arm to reduce the intensity noise for shot limited detection. The all-fiber OCDR system is described in (22). The PS-OCT system is completely controlled using Labview™ software (National Instruments, Austin, TX). Acquired scans are compiled into b-scan files. Image processing was carried out using Igor Pro™, data analysis software (Wavemetrics Inc, Lake Oswego, Oregon).
PS-OCT scans acquired from PM fiber based PS-OCT systems typically contain artifacts (additional peaks) due to cross-talk and the limited extinction ratio of the fiber that may confound analysis. Automated removal of such artifacts can be carried out successfully with a few extra data alteration steps after data collection. A reference a-scan was acquired from a mirror prior to scanning the samples. The reference a-scan contains several weak artifact signals along with the primary reflection. A smaller 400-point a-scan array was extracted from the 2000-pt reference a-scan containing the principal artifacts. The reference array was normalized to the intensity of the point of interest and subtracted to selectively remove the artifacts.
2.3 Calculation of Integrated Reflectivity and Lesion Depth
The integrated reflectivity, ΔR in units of (dB × μm) was calculated for each of the five windows (one sound, four demineralized) for every sample. Line profiles were taken from cross polarization OCT images in each of the five regions, and the reflectivity was integrated from the enamel surface to various depths, yielding the integrated reflectivity, ΔR, of the regions in units of decibels per micron. Previous studies have shown that ΔR can be correlated with the integrated mineral loss (volume % mineral × microns) called ΔZ (6,31).
An initial background subtraction was carried out for each OCT scan and a 2 × 2 convolution filter was applied to remove speckle noise. In the edge-detection approach, the enamel edge and the lower lesion boundary were determined by applying an edge locator. Two passes were required for each a-scan to locate each respective boundary with each pass starting from opposite ends of the a-scan and identifying the first pixel that exceeds the threshold of e−2 of the maximum value. The minimum threshold values for edge detection were previously experimentally determined by comparison of lesion depths measured using polarized light microcopy with measurements using OCT in order to avoid overestimation of lesion depth due to weak signals caused by birefringence in sound enamel (19). Distance (micron) per pixel conversion factor was obtained experimentally by system calibration. The two cutoff points for the lesion surface and endpoint represent the calculated lesion depth and the integration between these two positions represents the integrated reflectivity. A 1-mm square area was chosen for analysis in the center of each of the 1.4-mm by 3-mm areas demarcating each group on each sample. Therefore, 400 a-scans were analyzed for each group.
Typically there are large variation in the depth and integrated mineral loss from sample to sample for these types of demineralization experiments resulting in large standard deviations for each group. Sample groups were compared using Repeated Measures Analysis of Variance (ANOVA) with a Tukey–Kramer post hoc multiple comparison test. Having all the study groups (5) for each series on each sample allowed us to decrease intersample variability. InStat™ from GraphPad software (San Diego, CA) was used for statistical calculations.
3. RESULTS
Figure 1 shows PS-OCT images from one of the samples containing lesions produced in 8-hr intervals. Linearly polarized light was incident on the sample and the reflected light was measured in the same polarization (||) to the incident light and also in the orthogonal polarization (⊥) to the incident light. It is much harder to see differences in the lesion severity in the original (||) polarization than in the orthogonal polarization or cross polarization image due to the intense reflectivity at the surface. With the exception of the first group representing only 1-hour intervals of demineralization, differences in the depth of the lesions in each group are visible in the individual scans. PS-OCT was able to detect significant differences between most of the groups in each demineralization series with the exception of the 1-hour demineralization period. PS-OCT was able to detect significant differences in the integrated reflectivity from the lesion area after only two hours of demineralization and significant differences in lesion depth between the sound and demineralized groups after only 4 hours of demineralization. Analysis of the cross polarization OCT scans suggest that lesions as small as 10–20-μm were detected. Such small lesions are much smaller than the lesions previously measured with OCT.
4. DISCUSSION
These measurements demonstrate that a PS-OCT system operating with an axial resolution of 9-μm in air (SLD band width 84-nm) and 6-μm in enamel at 1317-nm is capable of detecting extremely shallow lesions. The use of algorithms to automatically calculate the depth and integrated reflectivity from the lesion area were essential since the edge detection approach enabled reliable calculation of the lesion depth and the automatic processing allowed analysis of large areas on each sample area. Being able to analyze the entire lesion area provides a larger effective sample size and also reduces variability caused by variations in the lesion severity over each sample window. In previous PS-OCT studies, the severity of lesion areas was accessed by integration of single a-scans (2, 3, 6, 7, 14, 23, 24). Better discrimination of lesion areas is possible if the entire lesion cross-section area or volume is integrated as opposed to a single a-scan since such lesions are seldom uniform. Since this approach involves large volumes of data it is only practical with the development of algorithms for automated processing. This becomes even more important for the efficient implementation of fast Fourier domain (FD) OCT systems that are capable of the acquisition of entire lesion volumes, e.g., 2 × 2 × 3 mm3 volumes, at video rates.
It is important to point out that we have yet to analyze these samples using transverse microradiography and polarized light microscopy that will provide a gold standard for comparison. This analysis requires complete destruction of the samples and we have withheld this analysis since the samples are also being analyzed using other imaging methods.
A simulated lesion model was utilized which produces subsurface lesions while maintaining an intact surface without erosion. The “surface softened” model simulates a highly active incipient lesion without a highly mineralized outer layer. Other lesion models such as the “pH cycling” model simulate a more slowly developing lesion by replicating periods of demineralization followed by remineralization (25). This is what occurs naturally in the mouth and this model produces lesions with a highly mineralized outer layer since the outer pores of the lesion produced by the demineralization periods are filled preferentially by mineral during the remineralization cycle. The pH cycling model produces less dramatic lesion gradients that are more difficult to detect and future studies will evaluate our approach with these more challenging models and on natural lesions.
Acknowledgments
This work was supported by NIH/NIDCR Grant R01-DE17869.
References
- 1.NIH. NIH Consensus Statement. 2001. Diagnosis and Management of Dental Caries throughout Life; pp. 1–24. [PubMed] [Google Scholar]
- 2.Chong SL, Darling CL, Fried D. Nondestructive measurement of the inhibition of demineralization on smooth surfaces using polarization-sensitive optical coherence tomography. Lasers Surg Med. 2007;39(5):422–427. doi: 10.1002/lsm.20506. [DOI] [PubMed] [Google Scholar]
- 3.Fried D, Xie J, Shafi S, Featherstone JDB, Breunig T, Lee CQ. Early detection of dental caries and lesion progression with polarization sensitive optical coherence tomography. J Biomed Optics. 2002;7(4):618–627. doi: 10.1117/1.1509752. [DOI] [PubMed] [Google Scholar]
- 4.Jones RS, Darling CL, Featherstone JDB, Fried D. Imaging artificial caries on occlusal surfaces with polarization sensitive optical coherence tomography. Caries Res. 2006;40(2):81–89. doi: 10.1159/000091052. [DOI] [PubMed] [Google Scholar]
- 5.Ngaotheppitak P, Darling CL, Fried D. Polarization Optical Coherence Tomography for the Measuring the Severity of Caries Lesions. Lasers Surg Med. 2005;37(1):78–88. doi: 10.1002/lsm.20169. [DOI] [PubMed] [Google Scholar]
- 6.Jones RS, Darling CL, Featherstone JDB, Fried D. Remineralization of in vitro Dental Caries Assessed with Polarization Sensitive Optical Coherence Tomography. J Biomed Opt. 2006;11(1):014016, 1–9. doi: 10.1117/1.2161192. [DOI] [PubMed] [Google Scholar]
- 7.Jones RS, Fried D. Remineralization of Enamel Caries Can Decrease Optical Reflectivity. J Dent Res. 2006;85(9):804–808. doi: 10.1177/154405910608500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C, Darling C, Fried D. Polarization Sensitive Optical Coherence Tomographic Imaging of Artificial Demineralization on Exposed Surfaces of Tooth Roots. Dent Mat. 2009;25(6):721–728. doi: 10.1016/j.dental.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manesh SK, Darling CL, Fried D. Nondestructive assessment of dentin demineralization using polarization sensitive optical coherence tomography. J Biomed Mater Res B. 2009;90(2):802–812. doi: 10.1002/jbm.b.31349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manesh SK, Darling CL, Fried D. Polarization sensitive optical coherence tomography for the nondestructive assessment of the remineralization of dentin. J Biomed Optics. 2009;14(044002):1–6. doi: 10.1117/1.3158995. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner A, Hitzenberger CK, Dicht S, Sattmann H, Moritz A, Sperr W, Fercher AF. Optical coherence tomography for dental structures. Lasers in Dentistry IV. 1998;3248:130–136. [Google Scholar]
- 12.Dicht S, Baumgartner A, Hitzenberger CK, Sattmann H, Robi B, Moritz A, Sperr W, Fercher AF. Polarization-sensitive optical optical coherence tomography of dental structures. Lasers in Dentistry V. 1999;3593:169–176. doi: 10.1159/000016571. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner A, Dicht S, Hitzenberger CK, Sattmann H, Robi B, Moritz A, Sperr W, Fercher AF. Polarization-sensitive optical optical coherence tomography of dental structures. Caries Res. 2000;34:59–69. doi: 10.1159/000016571. [DOI] [PubMed] [Google Scholar]
- 14.Everett MJ, Colston BW, Sathyam US, Silva LBD, Fried D, Featherstone JDB. Non-invasive diagnosis of early caries with polarization sensitive optical coherence tomography (PS-OCT) Lasers in Dentistry V. 1999;3593:177–183. [Google Scholar]
- 15.Wang XJ, Zhang JY, Milner TE, Boer JFd, Zhang Y, Pashley DH, Nelson JS. Characterization of Dentin and Enamel by use of Optical Coherence Tomography. Appl Opt. 1999;38(10):586–590. doi: 10.1364/ao.38.002092. [DOI] [PubMed] [Google Scholar]
- 16.Amaechi BT, Podoleanu A, Higham SM, Jackson DA. Correlation of quantitative light-induced fluorescence and optical coherence tomography applied for detection and quantification of early dental caries. J Biomed Opt. 2003;8(4):642–647. doi: 10.1117/1.1606685. [DOI] [PubMed] [Google Scholar]
- 17.Amaechi BT, Podoleanu AG, Komarov G, Higham SM, Jackson DA. Quantification of root caries using optical coherence tomography and microradiography: a correlational study. Oral Health Prev Dent. 2004;2(4):377–382. [PubMed] [Google Scholar]
- 18.Ngaotheppitak P, Darling CL, Fried D, Bush J, Bell S. PS-OCT of Occlusal and Interproximal Caries Lesions viewed from Occlusal Surfaces. Lasers in Dentistry XII. 2006;61370L:1–9. [Google Scholar]
- 19.Le MH, Darling CL, Fried D. Methods for calculating the severity of demineralization on tooth surfaces. Lasers in Dentistry VX. 2009;7162:1–7. doi: 10.1117/12.816867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Can AM, Darling CL, Ho CM, Fried D. Non-destructive Assessment of Inhibition of Demineralization in Dental Enamel Irradiated by a λ=9.3-μm CO2 Laser at Ablative Irradiation Intensities with PS-OCT. Lasers in Surgery and Medicine. 2008;40:342–349. doi: 10.1002/lsm.20633. [DOI] [PubMed] [Google Scholar]
- 21.ten Cate JM, Arends J. Remineralization of artificial enamel lesions in vitro. Caries Res. 1977;11(5):277–286. doi: 10.1159/000260279. [DOI] [PubMed] [Google Scholar]
- 22.Bush J, Davis P, Marcus MA. All-Fiber Optic Coherence Domain Interferometric Techniques. Fiber Optic Sensor Technology II. 2000;4204:71–80. [Google Scholar]
- 23.Jones RS, Darling CL, Featherstone JDB, Fried D. Imaging artificial caries on occlusal surfaces with polarization sensitive optical coherence tomography. Caries Res. 2004;40(2):81–89. doi: 10.1159/000091052. [DOI] [PubMed] [Google Scholar]
- 24.Hirasuna K, Fried D, Darling CL. Near-IR imaging of developmental defects in dental enamel. J Biomed Opt. 2008;13(4):044011:1–7 . doi: 10.1117/1.2956374. [DOI] [PubMed] [Google Scholar]
- 25.Featherstone JDB, Barrett NA, Conners MG, Shariati M. A pH cycling model for assessing fluoride effects on root caries. J Dent Res. 1989;68:995. (Abstract) [Google Scholar]


