The purpose of this study was to examine the effects of nicotine, in gum form, on retinal responses in adult nonsmokers using the electroretinogram (ERG).
Abstract
Purpose.
To examine the effects of nicotine on responses from the human retina measured electrophysiologically.
Methods.
Electroretinogram (ERG) responses were obtained from ten healthy, visually normal adults who were nonsmokers. Nicotine (2 and 4 mg) and a placebo were administered in the form of gum 30 minutes before testing in two separate experiments. ERG responses were collected and analyzed using a full-field ERG system. Responses were recorded from one eye of each subject using a bipolar contact-lens electrode. Intensity–response curves were obtained under both dark- and light-adapted conditions. In experiment 1, both dark- and light-adapted tests were completed sequentially. In experiment 2, only light-adapted testing was performed. Intensity–response functions were analyzed using the Naka–Rushton equation.
Results.
In experiment 1, compared with placebo, dark-adapted b-wave amplitude responses decreased significantly after chewing gum containing both 2 and 4 mg of nicotine. Under light-adapted conditions, the peak b-wave amplitude was significantly decreased after chewing gum containing 4 mg of nicotine. In experiment 2, light-adapted b-wave amplitudes were increased after 4 mg nicotine. Oscillatory potentials were measured but no significant effects under nicotine were observed.
Conclusions.
To the knowledge of the authors, this is the first demonstration that nicotine by itself affects responses in the human retina. These data support reports of the expression of nicotinic acetylcholine receptors in rabbit and nonhuman primate retina.
Nicotine is an alkaloid found in tobacco plants that binds to and activates nicotinic acetylcholine receptors (nAChRs), which are members of the family of ligand-gated ion channels. nAChRs are pentameric receptors comprised of subunits α2–α6 and β2–β4 in α/β combinations, or of subunits α7–α9 in homomeric forms.1–3 The subunit composition of nAChRs has been shown to determine their pharmacologic and functional properties, including agonist/antagonist affinity, channel open time, and desensitization rate. nAChRs have been detected in cells of the retina, lateral geniculate nucleus, superior colliculus, and primary visual cortex in various species.2,4–10 In the retina of mice, chick, and rabbit, nAChR subtypes have been identified in bipolar, amacrine, and ganglion cells, including processes throughout the inner plexiform layer (IPL).8,10,11 Ligand binding studies in human retina revealed muscarinic and nicotinic binding sites in the IPL, although it was not entirely clear which cell types were involved.6 A recent immunohistochemical study of the retina of nonhuman primates also showed receptor expression in amacrine, bipolar, and ganglion cells.10
In the mammalian retina, there are subpopulations of amacrine cells, including starburst, dopaminergic, and AII amacrine cells. Each amacrine cell type uses a different neurotransmitter (i.e., acetylcholine, γ-aminobutyric acid [GABA], glycine, or dopamine). Results from earlier studies have shown that nicotinic agonists (nicotine and epibatidine) affect the release of neurotransmitters from these subpopulations of amacrine cells.12 The application of nicotine and epibatidine onto GABAergic amacrine cells increased the release of dopamine. However, Neal and colleagues12 determined this increase was an indirect effect of the nicotinic agonists. The increased dopamine release was a result of nicotine/epibatidine increasing the amount of GABA. This study revealed that nicotine acts on nAChRs in the retina to alter the function of the retinal cells.
Numerous studies on animals and humans have described the effects of cigarette smoking, nicotine, and/or byproducts of cigarette smoke such as carbon monoxide on vision.13–17 For example, Jünemann and Damaske14 reported a decrease in amplitude of the dark-adapted b-wave, after cigarette smoking, in subjects who were nonsmokers as well as smokers who had abstained from smoking. Since cigarette smoking influences blood flow, the authors concluded that a change in blood flow could explain their results.
Jurklies et al.13 studied electroretinogram (ERG) responses in the cat retina treated with either a cholinergic agonist (acetylcholine [ACh]) or a muscarinic ACh antagonist (scopolamine). Their results showed that ACh increased the dark-adapted b-wave across all concentrations examined (18–1600 μM), with maximal increases of the b-wave amplitude seen at lower concentrations (18–150 μM). ACh, in the same concentration range, induced an increase in the amplitude of the light-adapted b-wave across all concentrations. In addition, scopolamine decreased both the dark- and light-adapted b-waves across concentrations from 500 to 1000 μM. ERG a-waves and b-waves represent the electrical activity of photoreceptors and OFF- and ON-bipolar cells; therefore, Jurklies and colleagues13 hypothesized that the increase in amplitude of the light-adapted b-wave could be based on feedback mechanisms in the retina between the amacrine cells and ON-bipolar cells.
Other electrophysiologic studies have shown significant changes in retinal function in individuals who are smokers.16,17 Gundogan and colleagues17 and Holder18 used the pattern electroretinogram (PERG), which has contributions from inner retinal cells, retinal ganglion cells, and optic nerve head. Their results showed increased amplitudes and decreased latencies in the PERG after smoking for individuals who were smokers compared with individuals who were nonsmokers.17 The most recent study performed by this group compared multifocal electroretingram (mfERG) responses under photopic conditions obtained from smokers who had abstained for 12 hours. N1 and P1 components in the central retinal regions revealed increases in amplitudes and decreases in latencies.16 These two studies clearly demonstrated that smoking tobacco alters the responses of both the PERG and mfERG. However, there are many active compounds in cigarette smoke17 and the observed changes could not be unequivocally attributed to any one compound.
Cigarette smoking is a major risk factor for potentially blinding ocular diseases such as age-related macular degeneration (AMD) and glaucoma.19,20 Nicotine is thought to be the primary addictive substance in cigarettes.21 However, the number of active compounds in and the mechanisms underlying the correlation between inhalation of and/or exposure to cigarette smoke and eye diseases have not been clearly delineated. The purpose of our study was to observe the effects of nicotine, in gum form, on retinal ERG responses under both dark- and light-adapted conditions in nonsmoking adults.
Materials and Methods
Subjects
Ten subjects with no history of smoking participated in this study. Full comprehensive eye exams, including visual field tests, were used to determine ocular and retinal health. Exclusion criteria included any vision disorders that related to overall systemic health; ocular disorders such as glaucoma or diabetic retinopathy; health issues or prescription medications that contraindicated the use of nicotine; and refractive error of −3.00 D or higher, since high myopia has been shown to attenuate ERG responses.22 The subjects age ranged from 20 to 32 years (mean = 24.3 years). All our participants were males. Two females volunteered for the study, but were excluded on the basis of health issues and refractive error. Subject refractions ranged from +0.25 D to −2.50 D.
This study conformed to the tenets of the Declaration of Helsinki and was approved by the University of Alabama at Birmingham Institutional Review Board for Human Use. Written informed consent was obtained from all subjects.
ERG Procedure
One eye (the nondominant eye, determined subjectively by the participant) was tested. The pupil was dilated with tropicamide 1% (Alcon, Fort Worth, TX) before 30 minutes of dark adaptation. A bipolar lens electrode (Burian–Allen; Hansen Ophthalmic, Coralville, IA) was used to obtain the ERG recordings. The corneal surface was numbed with proparacaine 0.5% (Alcon) and a drop of lubricant eye drops (Celluvisc; Allergan, Inc., Irvine, CA) was applied to the electrode before placement. The ground electrode was placed behind the opposite ear on the skin of the mastoid process.
ERG responses were amplified (1–1000 Hz), displayed, digitized, and stored for later analysis using a full-field ERG system (Espion; Diagnosys, Lowell, MA). Oscillatory potentials (OPs) were filtered using a low-frequency cutoff of 100 Hz and a high-frequency cutoff of 300 Hz. Subjects were tested under both dark- and light-adapted conditions. Two to 15 responses were averaged for each condition, with a stimulus interval from 5 to 60 seconds. Responses that contained artifacts were manually rejected.
Dark-Adapted ERG.
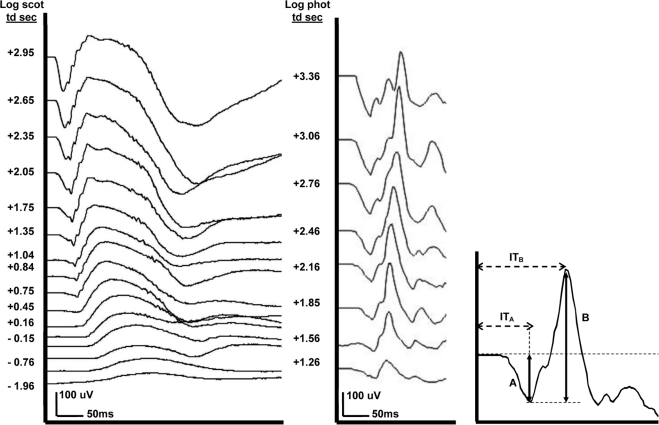
Subjects were dark adapted for 30 minutes. Responses were produced using a series of brief (≤1 ms), full-field 470-nm flashes, generated by an array of light-emitting diodes (presented in the ColorDome; Espion). Our retinal illuminance range was −1.96 to +2.95 log scotopic trolands, with 0.3-log unit steps. Responses were also obtained using a stimulus of 0.01 and 3.0 cd·s/m2, which is the International Society for Clinical Electrophysiology of Vision (ISCEV) standard for dark-adapted testing.23 Pupil diameter measurements ranged from 8 to 10 mm. The average pupil diameter of 9 mm was used to calculate trolands for both dark- and light-adapted conditions. OPs were obtained using the ISCEV standard maximal flash (3.0 cd·s/m2). Raw data from one subject are shown in Figure 1. Waveforms were measured from baseline to trough for the a-wave amplitude and trough to peak for the b-wave amplitude and b-wave implicit time (Fig. 1).
Figure 1.
Individual ERG responses for both scotopic and photopic intensity ranges. Left: Dark-adapted series with responses ranging over a 4.9 log unit range. Middle: Light-adapted series with a 2.1 log unit response range. Right: Representative ERG trace depicting measurements. (A) a-Wave amplitude from baseline to the tip of the first negative inflection. (B) b-Wave amplitude from the a-wave to the tip of the first positive peak; ITA: a-wave implicit time from time 0 to (A). ITB: b-wave implicit time from time 0 to (B).
Light-Adapted ERG.
Subjects were light-adapted for 10 minutes to a rod-saturating background (30 cd/m2). Responses were produced using a 630-nm light over a retinal illuminance range from +1.26 to +3.36 log photopic trolands incremented in 0.3-log unit steps. Xenon flashes were used for the highest retinal illuminance levels ranging from +3.05 to +3.36 log photopic trolands. OPs were obtained over the entire intensity range, as well as for the ISCEV standard light-adapted flash (3.0 cd·s/m2).
Administration of Nicotine
Two dosages (2 and 4 mg) of nicotine gum (GlaxoSmithKline Consumer Healthcare LP, Moon Township, PA) and one placebo gum (Laclede, Inc., Rancho Dominguez, CA) were used in this study. Nicotine gum (4 mg) has been shown to yield blood nicotine levels similar to those after smoking one cigarette.24 The placebo gum was chosen because of its similarity in taste and appearance to the nicotine gums. Testing sessions were at least 1 week apart. Order of testing sessions was randomized for both experiment 1 and experiment 2. The subject was masked to the testing condition. The experimenter was also masked to the testing condition during both data collection and initial analysis.
Experiment 1.
Subjects were tested in two separate sessions: one session with the placebo gum and the second session with either 2 or 4 mg nicotine gum. The same subjects were retested at two additional sessions with the alternate dosage of nicotine gum and another placebo session. ERGs were obtained under both dark-adapted and light-adapted conditions, which were completed sequentially. Gum was administered only during the 30-minute dark adaptation and was discarded before testing.
Experiment 2.
In experiment 2, we tested each subject in three separate sessions: 2 and 4 mg nicotine gum and placebo gum. Subjects were not dark-adapted and ERGs were recorded only under light-adapted conditions. Gum was administered 30 minutes before ERG recording and was discarded when recording started.
Data Analysis
b-Wave amplitude data were fit to the Naka–Rushton equation
where R is the response amplitude at stimulus intensity (I), Rmax is the maximal response amplitude, K is the stimulus intensity (I) that produces a response amplitude that is half of Rmax, and n is the constant that controls the slope of the function. The initial K value was chosen to be 100 and the n parameter was held at 1. The Rmax and K parameters were found using commercial software (PSI Plot; Poly Software International, Pearl River, NY). Individual data were normalized to the placebo Rmax values to minimize the variance. Within-subject comparisons among testing conditions (placebo and two levels of nicotine) were made by repeated-measures ANOVA (SPSS, Inc., Chicago, IL). Post hoc tests were performed using Student's t-test. The level of significance was set at P < 0.01 for all statistical tests.
Results
Individual ERG Responses
Dark- and light-adapted ERGs were recorded from ten subjects. Averaged amplitudes and implicit times produced by the ISCEV standard flash are shown in Table 1 for all testing conditions. Figure 1 presents both sets of placebo ERG data from a representative subject. As seen in Figure 1, under dark-adapted conditions, a-wave, b-wave, and OP amplitudes increase, whereas the implicit times of both components decrease with increasing stimulus retinal illuminance; under light-adapted conditions, the peak a- and b-wave amplitudes, increase up +3.06 log photopic trolands and then begin to decrease at higher intensities. OP amplitudes increase with increasing retinal illuminance across the entire range tested. b-Wave implicit times also increase with increasing retinal illuminance. Figure 2 compares the responses of a single subject for one stimulus retinal illuminance across all three nicotine conditions for experiment 1 and experiment 2 including OPs.
Table 1.
Dark- and Light-Adapted ERG Measures under ISCEV Standard Conditions: Placebo versus Nicotine
| Parameter/Condition | Experiment 1 |
Experiment 2 |
||||||
|---|---|---|---|---|---|---|---|---|
| Dark-Adapted |
Light-Adapted |
Light-Adapted |
||||||
| Rod Single (0.01 cd · s/m2) | Rod (15) (0.01 cd · s/m2) | Maximal Single (3 cd · s/m2) | Maximal (15) (3 cd · s/m2) | Single Flash (3 cs · s/m2) | Average (3 cd · s/m2) | Single Flash (3 cd · s/m2) | Average (3 cd · s/m2) | |
| a-Wave amplitude, μV | ||||||||
| Placebo/2 mg | −25.94 (8.6) | −17.21 (4.3) | −231.13 (17.7) | −186.76 (9.3) | −40.60 (3.2) | −40.44 (2.8) | −38.92 (5.3) | −35.06 (2.2) |
| Placebo/4 mg | −34.97 (20.9) | −26.20 (10.1) | −252.24 (21.4) | −228.97 (38.6) | −41.07 (3.3) | −39.15 (4.1) | ||
| 2 mg | −21.81 (13.7) | −20.65 (10.5) | −233.85 (27.3) | −166.15 (16.1) | −45.81 (6.2) | −38.11 (4.0) | −38.88 (6.0) | −34.96 (3.8) |
| 4 mg | −45.13 (13.0) | −18.88 (4.5) | −206.94 (46.7) | −199.75 (15.7) | −36.36 (4.3) | 39.96 (2.4) | −35.77 (5.8) | −40.29 (3.0) |
| a-Wave latency, ms | ||||||||
| Placebo/2 mg | 35.9 (1.1) | 35.9 (0.8) | 16.2 (0.2) | 17.2 (0.4) | 15.0 (0.3) | 15.4 (0.2) | 15.0 (0.3) | 15.0 (0.3) |
| Placebo/4 mg | 36.1 (1.0) | 35.9 (0.8) | 16.8 (0.7) | 20.0 (2.3) | 14.6 (0.6) | 15.2 (0.2) | ||
| 2 mg | 35.2 (1.6) | 40.8 (4.5) | 17.6 (1.6) | 18.0 (0.8) | 15.1 (0.4) | 15.4 (0.2) | 14.0 (1.1) | 14.8 (0.2) |
| 4 mg | 37.5 (1.8) | 33.6 (2.2) | 18.7 (1.1) | 18.1 (0.7) | 15.2 (0.3) | 15.4 (0.2) | 14.8 (0.2) | 15.0 (0.0) |
| b-Wave amplitude, μV | ||||||||
| Placebo/2 mg | 317.45 (36.9) | 302.48 (26.9) | 413.37 (35.9) | 351.27 (24.5) | 140.48 (16.5) | 145.33 (17.9) | 159.04 (13.6) | 159.82 (12.9) |
| Placebo/4 mg | 319.58 (47.1) | 321.90 (32.1) | 461.11 (48.5) | 321.12 (29.8) | 142.36 (14.3) | 144.75 (12.5) | ||
| 2 mg | 338.09 (61.9) | 255.28 (42.0) | 374.89 (33.0 | 356.14 (25.1) | 150.17 (10.8) | 158.51 (10.3) | 168.44 (18.2) | 170.16 (19.9) |
| 4 mg | 299.82 (33.1) | 328.93 (40.5) | 459.96 (73.8) | 385.85 (38.6) | 165.07 (20.1) | 163.39 (18.6) | 171.84 (17.3) | 175.26 (18.2) |
| b-Wave latency, ms | ||||||||
| Placebo/2 mg | 93.9 (4.0) | 86.6 (2.9) | 50.4 (0.5) | 46.3 (1.3) | 31.0 (0.3) | 30.5 (0.3) | 29.4 (0.2) | 29.6 (0.2) |
| Placebo/4 mg | 92.4 (3.4) | 87.4 (2.4) | 52.4 (1.9) | 43.7 (2.1) | 31.0 (0.3) | 31.0 (0.3) | ||
| 2 mg | 95.5 (5.1) | 94.9 (4.8) | 50.6 (1.1) | 48.5 (0.5) | 31.0 (0.4) | 31.0 (0.4) | 29.2 (0.2) | 29.2 (0.2) |
| 4 mg | 88.8 (3.0) | 88.2 (2.5) | 51.6 (1.3) | 46.9 (1.3) | 31.5 (0.4) | 31.1 (0.3) | 30.2 (0.4) | 29.4 (0.2) |
Values in parentheses indicate the SEM. ISCEV parameters: Rod Single, single flash; Rod (15), average of 15 trials; Maximal Single, single flash; Maximal (15), average of 15 trials. Experiment 1: Subject numbers were different for 2 and 4 mg nicotine conditions. Placebo/2 mg, placebo condition for 2 mg nicotine (n = 8). Placebo/4 mg, placebo condition for 4 mg condition (n = 9). Experiment 2: Subject numbers were equal across nicotine conditions (n =5). Placebo/2 mg, placebo condition for 2 mg nicotine (n = 8).
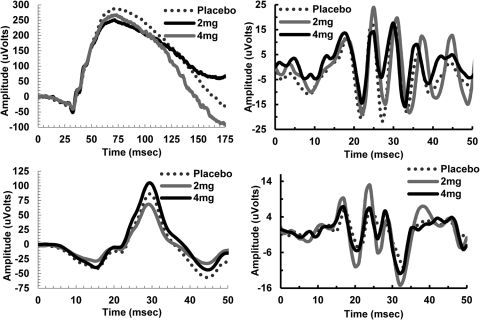
Figure 2.
Individual ERG responses for placebo, 2 mg nicotine gum, and 4 mg nicotine gum under both dark- and light-adapted conditions. Experiment 1, top left: dark-adapted waveforms measured at +0.45 log scotopic trolands (td). Top right: ISCEV dark-adapted OP waveform measured at +2.28 log scotopic td. Experiment 2, bottom left: light-adapted waveforms measured at +2.16 log photopic td. Bottom right: ISCEV light-adapted OP waveform measured at +2.28 log photopic td against a 30 cd/m2 background.
Effects of Nicotine on Dark-Adapted ERGs
Dark-adapted ERG responses (n = 8) were obtained after 30 minutes of dark adaptation; nicotine/placebo was administered during dark adaptation. a-Wave amplitudes were measured at a fixed time point (8 ms) to obtain some measure of photoreceptor activity since the leading edge of the a-wave is less contaminated by bipolar cell activity.25,26 Repeated-measures ANOVA did not indicate significant changes in timing or amplitudes for the a-wave component across conditions. b-Wave amplitudes were measured and fit to the Naka–Rushton equation. Rmax and K values are shown in Table 2. Using repeated-measures ANOVA, no significant changes between placebo and nicotine Rmax and K values were observed. Implicit times of the b-waves did not change across conditions. b-Wave amplitudes were normalized to the placebo Rmax and repeated-measures ANOVA indicated a significant effect of condition on the normalized dark-adapted b-wave responses with 2 mg (F1,98 = 7.60, P ≤ 0.01) and 4 mg (F1,112 = 7.53, P ≤ 0.01; Fig. 3). Summed OP amplitudes and latencies were not significantly different across conditions.
Table 2.
b-Wave Amplitude Naka–Rushton Fit Parameters for Dark- and Light-Adapted Conditions
| Condition | Rmax Mean (SD) | K Mean (SD) |
|---|---|---|
| Experiment 1: Dark-adapted | ||
| Placebo/2 mg | 397.95 ± 84.99 | 0.44 ± 0.09 |
| 2 mg | 394.80 ± 75.34 | 0.71 ± 0.71 |
| Placebo/4 mg | 429.72 ± 126.04 | 0.42 ± 0.07 |
| 4 mg | 424.26 ± 110.18 | 0.52 ± 0.20 |
| Experiment 1: Light-adapted | ||
| Placebo/2 mg | 133.75 ± 31.46 | 30.78 ± 9.88 |
| 2 mg | 135.62 ± 31.61 | 33.63 ± 10.48 |
| Placebo/4 mg | 143.96 ± 42.33 | 29.83 ± 9.08 |
| 4 mg | 132.30 ± 38.14 | 36.67 ± 12.76 |
| Experiment 2: Light-adapted | ||
| Placebo | 138.6 ± 31.3 | 27.2 ± 8.6 |
| 2 mg | 136.1 ± 37.7 | 26.4 ± 2.2 |
| 4 mg | 155.7 ± 30.2 | 27.8 ± 7.2 |
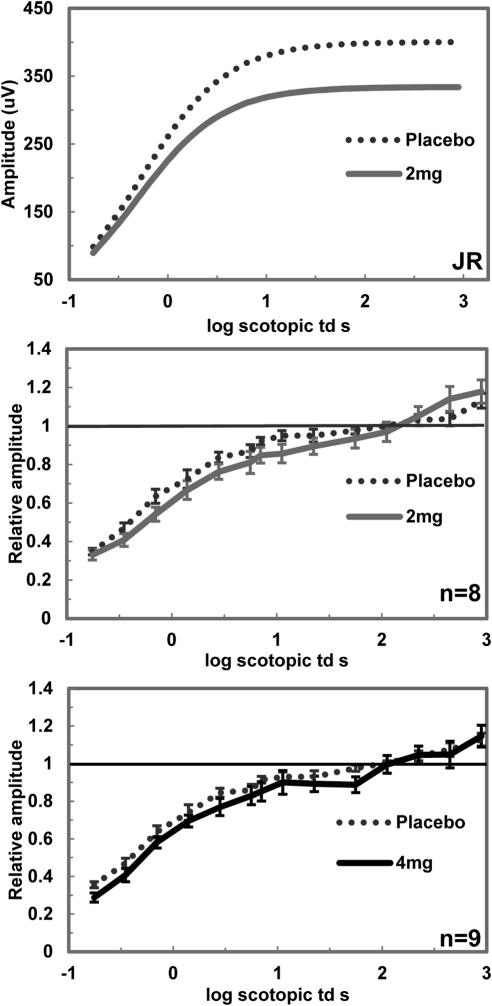
Figure 3.
Dark-adapted ERG values for placebo and nicotine conditions. ERGs were measured after 30 minutes of dark adaptation. Amplitudes are plotted against the log retinal illuminance measured by scotopic trolands (td). Top: individual b-wave amplitude responses curve fitted to the Naka–Rushton equation for placebo and 2 mg nicotine. Middle: normalized responses for b-wave amplitudes under 2 mg nicotine condition (n = 8). Bottom: normalized responses for b-wave amplitudes for 4 mg nicotine (n = 9). Significant amplitude decreases were seen with both 2 and 4 mg of nicotine (P ≤ 0.01). Error bars: ±SEM.
Effects of Nicotine on Light-Adapted ERGs
Experiment 1.
Light-adapted ERGs (n = 10) were obtained after 10 minutes of light adaptation immediately after dark-adapted testing. Placebo/nicotine had been administered during the 30-minute dark adaptation before dark-adapted testing. The a-wave mean amplitude values are listed in Table 1. No significant changes were seen across conditions. b-Wave amplitudes were fit to the Naka–Rushton equation and the Rmax and K values are reported in Table 2. Values of Rmax and K as well as implicit times for either a- or b-waves were not significantly different across conditions. Responses obtained in the 4 mg condition showed decreased amplitudes (Fig. 4). Repeated-measures analysis of the normalized b-wave amplitude responses showed a significant effect of 4 mg nicotine on light-adapted b-wave amplitudes (F1,63 = 6.68, P = 0.01), but did not show a significant effect with 2 mg nicotine (F1,49 = 0.07, P ≥ 0.05).
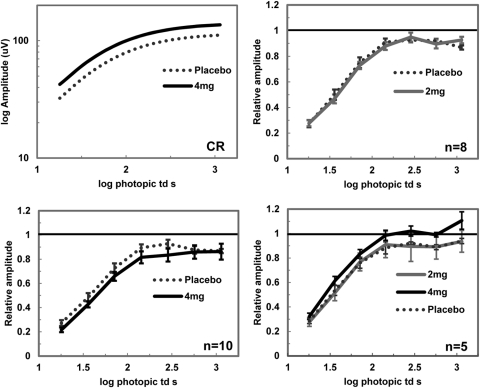
Figure 4.
Light-adapted ERG values for placebo and nicotine conditions. Amplitudes are plotted against log photopic trolands (td). Top left: individual b-wave amplitude responses for placebo and 4mg nicotine from experiment 2. Amplitude responses were increased with nicotine. Experiment 1, top right: normalized responses for b-wave amplitudes under 2 mg nicotine (n = 8). Bottom left: normalized responses for b-wave amplitudes for 4 mg nicotine (n = 10; P = 0.01). Experiment 2, bottom right: normalized responses for placebo and nicotine conditions (n = 5; P ≤ 0.01). Error bars: ±SEM.
Experiment 2.
Light-adapted ERG (n = 5) responses were obtained after 10 minutes of light adaptation. Nicotine and placebo gums were administered for a total of 30 minutes and were discarded immediately before testing. Amplitude and latency values are shown in Table 1. No significant differences were seen with either dosage of nicotine on a-wave amplitudes or a- and b-wave implicit times. b-Wave amplitudes were fit to the Naka–Rushton equation, and Rmax and K values are shown in Table 2. No significant changes were seen with the individual Rmax and K values. However, repeated-measures ANOVA on the normalized b-wave amplitude responses revealed a significant effect of condition (F1.20,33.57 = 6.09, P = 0.01). Post hoc pairwise comparisons indicated significant increases in the b-wave amplitudes under the 4 mg nicotine condition only (P ≤ 0.01) (Fig. 4). Repeated-measures ANOVA did not indicate any significant effect of condition on summed OP amplitudes and implicit times.
Discussion
Cigarette smoking causes a number of physiologic changes in humans that can directly and indirectly affect the retina. For example, smoking is known to change cardiovascular responses that, in turn, can affect retinal responses via altered blood flow. There are numerous additives (∼600)27 in cigarettes, some of which have been shown to alter electrophysiologic measures of brain activity (e.g., menthol and propylene glycol).27 Although it is reasonable to assume that the combination of chemicals from tobacco smoke affects the retina, it is all but impossible to isolate the effects of specific compounds. This study was designed to examine how nicotine in isolation, administered as gum, affects the human retina using ERG measurements. The key findings of this study are summarized in Table 3.
Table 3.
Overview of ERG Changes with Nicotine
| ERG Component | Results |
|---|---|
| Dark-adapted | |
| a-Wave | No changes |
| b-Wave | Decreased amplitudes with 2 and 4 mg nicotine |
| Light-adapted | |
| a-Wave | No changes |
| b-Wave | Increased amplitudes with 4 mg nicotine |
Under both dark- and light-adapted conditions, we observed changes in strength of the response as measured by b-wave amplitudes. The dark-adapted b-wave amplitude decreased with both dosages of nicotine. Previous studies have shown changes in the dark-adapted ERG with cigarette smoking and acetylcholine, a nicotinic agonist.13,14 Dmitrieva et al.28 studied the expression of α7 nicotinic acetylcholine receptors (α7nAChRs) in rabbit retina. Their data showed α7nAChR expression in a population of cone ON-bipolar cells, glycinergic and GABAergic amacrine cells, and ganglion cells. No expression was seen in rod bipolar cells or AII amacrine cells, which comprise the major rod pathway. However, the underlying mechanism for the changes we observed in the dark-adapted b-wave amplitudes could be attributed to the rod pathway that feeds into calbindin-positive cone ON-bipolar cells.28 Another possible underlying mechanism for the changes we observed in the dark-adapted b-wave amplitude is feedback mechanisms from amacrine cells onto rod and/or cone bipolar cells. Studies of rabbit retina have shown that nicotine and nicotinic agonists increase the release of dopamine and change the response properties of ganglion cells that express nicotinic receptors.12,29
Light-adapted ERGs were measured on two different occasions. In the first experiment, light-adapted testing began approximately 1 hour after the initiation of nicotine exposure. In the second experiment testing began 30 minutes after the initiation of nicotine exposure. The results from these two experiments revealed opposite changes with the 4 mg dose. In the first experiment, the b-wave amplitudes were significantly decreased, whereas in the second experiment, the b-wave amplitudes increased under the 4 mg condition. The 2 mg experiment showed little or no changes in either case. This discrepancy in our findings could be attributed to a couple of factors: (1) Based on our knowledge of maximal nicotine concentration, we believe the peak concentration of nicotine had declined in experiment 1, measured 1 hour postnicotine intake compared with 30 minutes postnicotine in experiment 2; and (2) potency, efficacy, and desensitization rate vary for different subtypes of nAChRs, which could explain our findings.30 Nonetheless, these data indicate that nicotine alters retinal function through the cone pathway, which is similar to that reported by Jurklies and colleagues13 and Gundogan and colleagues.16,17 In rabbit retina, α7 nicotine acetylcholine receptors (α7nAChRs) have been shown to be expressed on retinal neurons and processes in several types of neurons, including a class of cone bipolar cells.28 Nicotinic receptor expression in nonhuman primate retina also suggests that nicotine may affect the cone pathway.28 The observed changes from experiment 1 were unexpected and are inconsistent with previous findings, although they are suggestive of desensitization and/or the recovery of desensitization of nicotinic receptors.10,13,16
Published data indicate that nAChRs are expressed primarily in the inner retina, specifically amacrine and ganglion cells.6,8,10,28 Our analysis of the OPs derived from experiment 2 indicates very little to no change with summed OP amplitudes and latencies. Individual peak analysis did reveal changes in both dark- and light-adapted conditions. Pharmacologic studies indicate differing sensitivities of early and late OP peaks to dopamine, GABA, and glycine, with OPs diminishing in the presence of these neurotransmitter antagonists.31–34 Our results showed nicotine increased peak amplitudes of OP2 in dark-adapted conditions and OP2, -3, and -5 in light-adapted conditions (data not shown). These data would suggest an increase in inhibitory neurotransmitter release with nicotine based on the above-mentioned pharmacologic studies.
The results from this study show that nicotine changes the response properties of the retina, via nAChRs, in a naïve visual system that has no previous direct exposure to nicotine. What is unknown is exactly how nicotine and nAChRs interact to allow for the changes observed. We can hypothesize possible mechanisms based on the knowledge of prior studies investigating the effects of nicotine or nicotinic agonists on the retina of other species. Neal and colleagues12 investigated the role of nicotinic agonists on the activation of GABAergic amacrine cells in rabbit retina. Application of nicotine and/or epibatidine yielded an increase in the release of dopamine indirectly through GABAergic amacrine cells. They concluded that nicotine stimulates the release of GABA and indirectly stimulates the release of dopamine via inhibitory neurotransmission via GABA.12 nAChRs have been identified on amacrine cells and their processes in various species; in rabbit retina, nAChRs were identified specifically on GABAergic amacrine cells and terminals of ON-cone bipolar cells.8,10,28 It is possible that nicotine could initiate a process of disinhibition by increasing the release of glutamate from the cone bipolar terminals causing a positive feedback on the second-order neurons by increasing the release of GABA, leading to an increase in dopamine. Since dopaminergic amacrine cells interact with AII amacrines, an inhibitory feedback mechanism could be responsible for the changes observed in our dark-adapted conditions.
A limitation of this study is that we have no quantification of nicotine levels for our subjects. Ideally, we would be able to measure blood serum nicotine levels to quantify the amount of nicotine being absorbed through the gum. Without this information, the following three issues remain and cannot be evaluated against our response measures. First, we cannot definitively identify when nicotine concentrations reached their maximum. However, based on the investigation reported by Russell and colleagues24 into blood nicotine levels in cigarette smoking and nicotine gum, we can estimate when nicotine might reach the maximum level in our studies. Their study revealed maximum blood plasma nicotine levels 30 minutes after consumption of 4 mg nicotine gum, which was comparable to that of smoking one cigarette.24 Second, we have no information about the latency between nicotine ingestion and the point at which nicotine reaches levels sufficient to affect nAChRs. One study measured blood flow at the papilla and showed a decrease after cigarette smoking, although there is no other information related to this factor.35 Third, nicotine metabolism and uptake will vary across individuals based on their body mass index and other physiologic factors. We cannot quantitatively account for these individual differences, and a better understanding of the concentration of nicotine and its time course for individual participants would enhance the interpretation of our data.
Nevertheless, the results from this study show that nicotine, itself, affects the functional properties of retinal neurons. Additional research is required into the expression of nAChRs in the retina of both nonhuman primates and humans to better understand how nicotine alters visual processing. We plan to use psychophysical measures (e.g., contrast sensitivity), to explore the effects of nicotine at a behavioral level. Beneficial effects of nicotine have been observed in relieving symptoms and treatment of Parkinson's disease (PD).36,37 Janson and Møller36 have shown that nicotine acts as a neuroprotector in dopaminergic neurons in the brain of rats with PD. In relation to this study, Gottlob and colleagues38 showed decreased b-wave amplitudes in both dark- and light-adapted conditions in patients with PD, which is indicative of a disturbance in the inner retina, possibly with the dopaminergic system. Eventually, the information from our present study may lead to research into the role of nicotine in ocular diseases, such as AMD and glaucoma.
Footnotes
Supported in part by Clinical Research Advisory Committee funds awarded by the Department of Optometry, School of Optometry, University of Alabama at Birmingham, and National Institutes of Health Grants P30 EY003039 and R01 EY07845.
Disclosure: S.B. Varghese, None; J.C. Reid, None; E.E. Hartmann, None; K.T. Keyser, None.
References
- 1. McGehee DS. Molecular diversity of neuronal nicotinic acetylcholine receptors. Ann NY Acad Sci. 1999;868:565–577 [DOI] [PubMed] [Google Scholar]
- 2. Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry. 2001;49:166–174 [DOI] [PubMed] [Google Scholar]
- 3. Clementi F, Fornasari D, Gotti C. The structures of neuronal nicotinic receptors. In: Lindstrom J. ed. Handbook of Experimental Pharmacology. Berlin: Springer; 2000:101–162 [Google Scholar]
- 4. Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56:701–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74:1102–1111 [DOI] [PubMed] [Google Scholar]
- 6. Hutchins JB, Hollyfield JG. Acetylcholine receptors in the human retina. Invest Ophthalmol Vis Sci. 1985;26:1550–1557 [PubMed] [Google Scholar]
- 7. Keyser KT, Hughes TE, Whiting PJ, Lindstrom JM, Karten HJ. Cholinoceptive neurons in the retina of the chick: an immunohistochemical study of the nicotinic acetylcholine receptors. Vis Neurosci. 1988;1:349–366 [DOI] [PubMed] [Google Scholar]
- 8. Keyser KT, MacNeil MA, Dmitrieva N, Wang F, Masland RH, Lindstrom JM. Amacrine, ganglion, and displaced amacrine cells in the rabbit retina express nicotinic acetylcholine receptors. Vis Neurosci. 2000;17:743–752 [DOI] [PubMed] [Google Scholar]
- 9. Marritt AM, Cox BC, Yasuda RP, et al. Nicotinic cholinergic receptors in the rat retina: simple and mixed heteromeric subtypes. Mol Pharmacol. 2005;68:1656–1668 [DOI] [PubMed] [Google Scholar]
- 10. Liu J, McGlinn AM, Fernandes A, et al. Nicotinic acetylcholine receptor subunits in rhesus monkey retina. Invest Ophthalmol Vis Sci. 2009;50:1408–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cox BC, Marritt AM, Perry DC, Kellar KJ. Transport of multiple nicotinic acetylcholine receptors in the rat optic nerve: high densities of receptors containing α6 and β3 subunits. J Neurochem. 2008;105:1924–1938 [DOI] [PubMed] [Google Scholar]
- 12. Neal MJ, Cunningham JR, Matthews KL. Activation of nicotinic receptors on GABAergic amacrine cells in the rabbit retina indirectly stimulates dopamine release. Vis Neurosci. 2001;18:55–64 [DOI] [PubMed] [Google Scholar]
- 13. Jurklies B, Kaelin-Lang A, Niemeyer G. Cholinergic effects on cat retina in vitro: changes in rod- and cone-driven b-wave and optic nerve response. Vision Res. 1996;36:797–816 [DOI] [PubMed] [Google Scholar]
- 14. Jünemann G, Damaske E. The effect of smoking on the human electroretinogram (ERG) [in German]. Munch Med Wochenschr. 1968;110:586–590 [PubMed] [Google Scholar]
- 15. Ingenito AJ. Effects of cigarette smoke inhalation on the cat electroretinogram: comparisons with nicotine and carbon monoxide. J Pharmacol Exp Ther. 1979;211:647–655 [PubMed] [Google Scholar]
- 16. Gundogan FC, Erdurman C, Durukan AH, Sobaci G, Bayraktar MZ. Acute effects of cigarette smoking on multifocal electroretinogram. Clin Exp Ophthalmol. 2007;35:32–37 [DOI] [PubMed] [Google Scholar]
- 17. Gundogan FC, Durukan AH, Mumcuoglu T, Sobaci G, Bayraktar MZ. Acute effects of cigarette smoking on pattern electroretinogram. Doc Ophthalmol. 2006;113:115–121 [DOI] [PubMed] [Google Scholar]
- 18. Holder GE. The pattern electroretinogram. In: Heckenlively JR, Arden GB. eds. Principles and Practice of Clinical Electrophysiology of Vision. Cambridge, MA: MIT Press; 2006:341–352 [Google Scholar]
- 19. Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age-related macular degeneration: a review of association. Eye. 2005;19:935–944 [DOI] [PubMed] [Google Scholar]
- 20. Edwards R, Thornton J, Ajit R, Harrison RA, Kelly SP. Cigarette smoking and primary open angle glaucoma: a systematic review. J Glaucoma. 2008;17:558–566 [DOI] [PubMed] [Google Scholar]
- 21. Metz CN, Gregersen PK, Malhotra AK. Metabolism and biochemical effects of nicotine for primary care providers. Med Clin North Am. 2004;88:1399–1413, ix [DOI] [PubMed] [Google Scholar]
- 22. Yamamoto S, Nitta K, Kamiyama M. Cone electroretinogram to chromatic stimuli in myopic eyes. Vision Res. 1997;37:2157–2159 [DOI] [PubMed] [Google Scholar]
- 23. Marmor MF, Fulton AB, Holder GE, et al. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol. 2009;118:69–77 [DOI] [PubMed] [Google Scholar]
- 24. Russell MA, Feyerabend C, Cole PV. Plasma nicotine levels after cigarette smoking and chewing nicotine gum. Br Med J. 1976;1:1043–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robson JG, Saszik SM, Ahmed J, Frishman LJ. Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol. 2003;547:509–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hood DC, Birch DG. Measuring the health of the human photoreceptors with the leading edge of the a-wave. In: Heckenlively JR, Arden GB. eds. Principles and Practice of Clinical Electrophysiology of Vision. Cambridge, MA: MIT Press; 2006:487–501 [Google Scholar]
- 27. Rabinoff M, Caskey N, Rissling A, Park C. Pharmacological and chemical effects of cigarette additives. Am J Public Health. 2007;97:1981–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dmitrieva NA, Strang CE, Keyser KT. Expression of alpha 7 nicotinic acetylcholine receptors by bipolar, amacrine, and ganglion cells of the rabbit retina. J Histochem Cytochem. 2007;55:461–476 [DOI] [PubMed] [Google Scholar]
- 29. Strang CE, Amthor FR, Keyser KT. Rabbit retinal ganglion cell responses to nicotine can be mediated by beta2-containing nicotinic acetylcholine receptors. Vis Neurosci. 2003;20:651–662 [DOI] [PubMed] [Google Scholar]
- 30. Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356 [PubMed] [Google Scholar]
- 31. Ogden TE. The oscillatory waves of the primate electroretinogram. Vision Res. 1973;13:1059–1074 [DOI] [PubMed] [Google Scholar]
- 32. Wachtmeister L, Dowling JE. The oscillatory potentials of the mudpuppy retina. Invest Ophthalmol Vis Sci. 1978;17:1176–1188 [PubMed] [Google Scholar]
- 33. Wachtmeister L. Further studies of the chemical sensitivity of the oscillatory potentials of the electroretinogram (ERG) I. GABA- and glycine antagonists. Acta Ophthalmol (Copenh). 1980;58:712–725 [DOI] [PubMed] [Google Scholar]
- 34. Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res. 1998;17:485–521 [DOI] [PubMed] [Google Scholar]
- 35. Leitz-Partzsch A, Griesser SM, Flammer J, Haefliger IO. Decreased Heidelberg retina flowmeter (HRF) parameter “flow” at the papilla shortly after smoking a cigarette. Klin Monbl Augenheilkd. 2001;218:332–334 [DOI] [PubMed] [Google Scholar]
- 36. Janson AM, Møller A. Chronic nicotine treatment counteracts nigral cell loss induced by a partial mesodiencephalic hemitransection: an analysis of the total number and mean volume of neurons and glia in substantia nigra of the male rat. Neuroscience. 1993;57:931–941 [DOI] [PubMed] [Google Scholar]
- 37. Fagerström KO, Pomerleau O, Giordani B, Stelson F. Nicotine may relieve symptoms of Parkinson's disease. Psychopharmacology (Berl). 1994;116:117–119 [DOI] [PubMed] [Google Scholar]
- 38. Gottlob I, Schneider E, Heider W, Skrandies W. Alteration of visual evoked potentials and electroretinograms in Parkinson's disease. Electroencephalogr Clin Neurophysiol. 1987;66:349–357 [DOI] [PubMed] [Google Scholar]