Abstract
Background
HIV infected individuals have heightened cancer risk. With the advent of HAART, the frequency of some AIDS defining cancers (ADC) has decreased while certain non-AIDS defining cancers (NADC) are becoming more frequent. Cancers among HIV-infected individuals in Latin American and the Caribbean have not yet been carefully studied.
Methods
Cancer cases among the Caribbean, Central and South American network for HIV Research (CCASAnet) cohort were identified reviewing clinical records and preexisting databases.
Results
There were 406 cancers reported: 331 ADC (224 Kaposi´s sarcomas and 98 non Hodgkin lymphomas). Most frequent NADC (n=75) were Hodgkin lymphoma and skin cancers. Seventy-three percent of NADC and 45% of ADC were diagnosed >1 year after HIV diagnosis. 56% of ADC occurred before HAART start. Median time from HAART start until cancer diagnosis was 2.5 years for NADC and 0.5 years for ADC (p=<0.001). Within 3372 HAART starters, 158 were diagnosed with 165 cancers (82.4% ADC); 85 cases were previous to or concomitant with HAART initiation. Incidence of cancer after HAART initiation in 8080 person-years of follow-up was 7.2 per 1000 person-years (95%CI= 5.5–9.3) for ADC and 2.7 (95%CI= 1.8–4.1) for NADC; incidence was higher in the first two months, particularly for ADC (47.6). A pre-HAART ADC was a predictor of mortality after adjusting for age, sex, and CD4 at HAART initiation.
Conclusions
ADC were the most frequent cancers in this region and were often diagnosed close to HIV diagnosis and HAART start. Incidence of cancer was highest around HAART initiation.
Keywords: Neoplasms, HIV, Latin America, Caribbean, Cohort Studies
INTRODUCTION
Human immunodeficiency virus (HIV) infected people have heightened cancer risk due to immunosuppression. 1–4 Kaposi´s sarcoma (KS), non Hodgkin lymphoma (NHL) and invasive cervical cancer (ICC) have been included by the Centers for Disease Control and Prevention (CDC) as AIDS defining conditions because of their high prevalence among HIV infected individuals, thereby classifying them as AIDS defining cancers (ADC). 5
With the advent of highly active antiretroviral therapy (HAART), there has been a dramatic improvement in survival of HIV infected persons as well as a decline in the incidence of AIDS defining conditions. Morbidity and mortality patterns have changed, and non-AIDS defining cancers (NADC) have become more common. Several studies have documented a decline in ADC since HAART became available. In recent years, higher frequencies of NADC have been observed in HIV infected people and NADC have been reported as a major cause of death. These trends may reflect an aging population experiencing the effects of chronic inflammation and immunodeficiency due to HIV. 1, 6–10
Latin America and the Caribbean (LAC) represent approximately 6.5% of the world HIV infected population, with about 2.24 million people and an adult prevalence of 0.5– 1%. Nearly 85000 people in this region died due to AIDS related conditions during 2008. 11–13 Regional coverage of HAART is estimated to be 54% of those eligible. 14
According to the Panamerican Health Organization, cancer is the second most frequent cause of death in the Americas including 480,000 deaths in LAC. In general in South America, prostate, stomach and lung cancers are the most prevalent cancers observed in men. Cervical cancer (Andean region) and breast cancer (Southern Cone) are the most common cancers in women. 15
The cancer profile in the HIV Latin American and Caribbean epidemic has not yet been closely studied and there have been relatively few reports published on cancer conditions in HIV infected persons from this region. Several studies carried out by individual centers in the region have focused on KS and NHL. 16–22
The Caribbean, Central and South America Network for HIV Research (CCASAnet) collaboration includes sites from seven nations: Argentina, Brazil, Chile, Haiti, Honduras, Mexico, and Peru. CCASAnet established a region-wide registry of cancer-related information on HIV infected individuals seen at participating research sites in the network. In this manuscript we report observed cancers, treatments, outcomes, and incidence from seven CCASAnet sites.
METHODS
The CCASAnet cohort (http://ccasanet.vanderbilt.edu/) has been described elsewhere. 23 The collaboration was established in 2006 as Region 2 of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; www.iedea-hiv.org) with the goal of creating a shared repository of HIV data from Central and South America and the Caribbean to answer questions about the characteristics of the regional HIV epidemic. Seven sites participate in the cohort: Fundación Huésped in Buenos Aires, Argentina (FH-Argentina); Hospital Universitário Clementino Fraga Filho in Rio de Janeiro, Brazil (HUCFF-Brazil); Fundación Arriarán in Santiago, Chile (FA-Chile); Le Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes in Port-au-Prince, Haiti (GHESKIO-Haiti); Instituto Hondureño de Seguridad Social and Hospital de Especialidades in Tegucigalpa, Honduras (IHSS/HE-Honduras); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán in Mexico City, Mexico (INNSZ-Mexico); and Universidad Peruana Cayetano Heredia and Instituto de Medicina Tropical Alexander von Humboldt in Lima, Peru (IMTAvH-Peru). Cancer cases among HIV patients seeking medical attention at each of the sites were identified by review of clinical charts and preexisting local databases. Some sites also searched for cases in pathology reports (FH-Argentina and FA-Chile), oncology clinics (FH-Argentina, IHSS/HE-Honduras and FA-Chile), haematology clinics (FA-Chile) and proctology clinics (FH-Argentina). Data were collected retrospectively between 2007 and 2009 using a standardized case report form and then entered into a web-based database developed and hosted at the data coordinating center (DCC) at Vanderbilt University, Nashville, TN, USA. Data were checked for errors and inconsistencies.
All data were de-identified by local centers prior to entry into the database. Onsite data audits were performed by a team from the DCC. Institutional Review Board approval was obtained locally for each participating site as well as for the Vanderbilt DCC.
Cancer types were defined on a clinical, radiological, cytological or histological basis. Cancers were categorized as ADC (Kaposi´s sarcoma, non Hodgkin lymphoma or invasive cervical cancer) 5 or NADC (the remaining cancers). All known cases, before or after HIV diagnosis, were included for analysis. Preneoplastic lesions were also included but analyzed separately. Mild dysplasia and cervical and anal intraepithelial neoplasia type I (CIN/AIN I) were considered as low grade squamous intraepithelial lesions (LGSIL); moderate and severe dysplasia, cervical and anal intraepithelial neoplasia type II and III (AIN/ CIN II and AIN/ CIN III) were considered as high grade squamous intraepithelial lesions (HGSIL).
For the purpose of computing cancer incidence after HAART initiation, we incorporated data from a previously described CCASAnet cohort of HIV infected patients followed from the date of HAART initiation. 24, 25 Charts were reviewed in search of neoplastic or preneoplastic pathology for all patients in the HAART initiation analysis, except those from GHESKIO-Haiti.
Characteristics of ADC and NADC cases were compared using Chi-square or Wilcoxon rank sum tests, as appropriate. Cases from the same individual were considered independent. Time from cancer diagnosis/ HAART initiation until death was analyzed using Kaplan-Meier and Cox proportional hazards methods. Risk factors associated with incidence of cancer were assessed using Poisson regression. In multivariable analyses, missing CD4 values at HAART initiation (18%) were included using multiple imputation techniques. 26 Analysis scripts are available at http://ccasanet.vanderbilt.edu/files/public/ca-analysis.nw.
Persons with cancer were followed and treated at onsite and offsite facilities that varied among sites. Cancer treatment costs were covered by government (FH-Argentina, HUCFF-Brazil, FA-Chile, IHSS/HE-Honduras), social security (FH-Argentina, IHSS/HE-Honduras, IMTAvH-Peru), and private insurance sources (FH-Argentina, IMTAvH-Peru), as well as non-governmental organizations and/or foundations (INNSZ-Mexico, GHESKIO-Haiti) and sometimes by patient self-pay on subsidized prices (INNSZ-Mexico, IMTAvH-Peru).
GHESKIO-Haiti did not have a cancer diagnosis unit until July 2008 and cancer-specific data in medical records were scarce. Histopathological diagnosis was rarely available and cancer treatment was restricted. Therefore, it was not possible to identify or characterize all cancer cases in the GHESKIO-Haiti cohort. Their data are presented separately.
RESULTS
Characteristics of cancer cases
A total of 455 cancers or pre-cancerous lesions were reported from 428 unique patients. Table 1 shows numbers and types of recorded cancers/ pre-cancerous lesions by site. Of the 406 reported cancers, 82% were AIDS defining, predominantly Kaposi’s sarcoma (224) and non Hodgkin lymphoma (98). The most frequent NADC were Hodgkin lymphoma (15) and skin cancers (11, including 1 melanoma).
Table 1.
Cancers and preneoplasic lesions according to CCASAnet site
| Site | ||||||||
|---|---|---|---|---|---|---|---|---|
| FH- Argentina |
HUCFF- Brazil |
FA- Chile |
IHSS/HE- Honduras |
INNSZ- Mexico |
IMTAvH- Peru |
Combined | ||
| All Cancers and Preneoplastic Lesions | 142 | 40 | 122 | 47 | 62 | 42 | 455 | |
| AIDS Defining Cancers | 107 | 21 | 103 | 12 | 56 | 32 | 331 | |
| Kaposi Sarcoma | 81 | 7 | 71 | 5 | 42 | 18 | 224 | |
| Non Hodgkin lymphoma | 23 | 13 | 32 | 4 | 14 | 12 | 98 | |
| Invasive Cervical Carcinoma | 3 | 1 | 0 | 3 | 0 | 2 | 9 | |
| Non-AIDS Defining Cancers | 24 | 15 | 15 | 8 | 4 | 9 | 75 | |
| Hodgkin lymphoma | 2 | 5 | 6 | 0 | 1 | 1 | 15 | |
| Cervical Carcinoma in Situ | 3 | 0 | 0 | 1 | 1 | 6 | 11 | |
| Skin Cancer | 5 | 3 | 2 | 1 | 0 | 0 | 11 | |
| Anal Cancer | 3 | 2 | 1 | 0 | 1 | 1 | 8 | |
| Breast | 1 | 2 | 0 | 2 | 0 | 0 | 5 | |
| Lung | 4 | 0 | 0 | 0 | 0 | 1 | 5 | |
| Prostate | 2 | 0 | 1 | 1 | 1 | 0 | 5 | |
| Testicle | 1 | 0 | 3 | 0 | 0 | 0 | 4 | |
| Other * | 3 | 3 | 2 | 3 | 0 | 0 | 11 | |
| Preneoplastic Lesions | 11 | 4 | 4 | 27 | 2 | 1 | 49 | |
| Cervical HGSIL | 7 | 1 | 3 | 15 | 2 | 1 | 29 | |
| Cervical LGSIL | 2 | 1 | 1 | 12 | 0 | 0 | 16 | |
| Anal LGSIL | 1 | 1 | 0 | 0 | 0 | 0 | 2 | |
| Anal HGSIL | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Bowen Disease | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
Other cancers: bladder (1), conjunctiva epidermoid carcinoma (1), gastroesophageal union (1), larinx epidermoid carcinoma (1), leiomiosarcoma (1), multiple myeloma (1), not specified (2), rectum (1), sarcoma (1), vagina (1).
Approximately 70% of the cases were diagnosed by histology, 16% were diagnosed on a clinical basis (>90% of these were KS), 5% by cytology (all preneoplastic lesions except for two cervical carcinomas in situ), 1% by other methods, and method of diagnosis was unknown for 8%. NHL were known to be high grade in 71% of the cases, 1% were low grade and the rest were unknown. Stage at diagnosis was available in 24 cases, 15 being stage IV; twenty of the remaining cases had distant extension. KS involved only skin, as primary site, in 138 cases and mucosae and skin, in 42 cases.
The characteristics of the 406 recorded cancer cases are shown in Table 2. Of 331 ADC, 90% were observed in male patients compared to 68% of the 75 NADC. Persons with NADC tended to be older than those with ADC. Median CD4 count at cancer diagnosis was 92 cells/µL (IQR: 22–229). Most cancers were diagnosed after the year 2000 and the majority of NADC were diagnosed after 2005. Nearly half of all cancers were diagnosed prior to or within one year of HIV diagnosis, with a greater proportion of NADC being diagnosed more than 1 year after HIV diagnosis (74%) than ADC (44%). ADC were much more likely to occur in patients with no prior HAART. Among patients on HAART, the time from HAART initiation until their cancer diagnosis was longer for non-AIDS than AIDS defining cancers (median= 2.5 vs 0.5 years, p=<0.001). Excluding cancer diagnoses within the first two months after HAART initiation, this difference was still significant (median=2.9 and 1.2 years for NADC and ADC, respectively; p=0.002). About 90% of all cancer cases had “sexual” as possible HIV transmission route. ADC were more common in men who had sex with men and NADC were slightly more frequent in heterosexuals.
Table 2.
Characteristics of cancer cases
| Overall (n=406) |
AIDS Defining Cancers (n=331) |
Non-AIDS Defining Cancers (n=75) |
p- value |
||
|---|---|---|---|---|---|
| Male at birth | 350 (86%) | 299 (90%) | 51 (68%) | <0.001 | |
| Age at cancer diagnosis Median (IQR) | 37 (31–44) | 36 (31–43) | 41 (35–50) | <0.001 | |
| CD4 at cancer diagnosis a | <0.001 | ||||
| Median (IQR) | 92 (22–229) | 66 (18–178) | 268 (190–426) | ||
| Missing | 275 (68 %) | 222 (67 %) | 53 (71 %) | ||
| Year of cancer diagnosis | <0.001 | ||||
| <1996 | 8 (2%) | 7 (2%) | 1 (1%) | ||
| 1996–1999 | 56 (14%) | 50 (15%) | 6 (8%) | ||
| 2000–2004 | 175 (44%) | 153 (47%) | 22 (31%) | ||
| 2005–2008 | 159 (40%) | 117 (36%) | 42 (59%) | ||
| Time since HIV diagnosis | <0.001 | ||||
| Prior to diagnosis | 47 (12%) | 43 (13%) | 4 (5%) | ||
| 0–2 months | 62 (16%) | 57 (17%) | 5 (7%) | ||
| 2–12 months | 91 (23%) | 81 (25%) | 10 (14%) | ||
| >12 months | 200 (50%) | 145 (44%) | 55 (74%) | ||
| Time from HAART start to cancer b | <0.001c | ||||
| No prior HAART | 207 (51%) | 185 (56%) | 22 (30%) | ||
| 0–2 months | 51 (13%) | 45 (14%) | 6 (8%) | ||
| 2–12 months | 52 (13%) | 43 (13%) | 9 (12%) | ||
| >12 months | 92 (23%) | 55 (17%) | 37 (50%) | ||
| Smoker | 109 (54 %) | 90 (55 %) | 19 (50 %) | 0.69 | |
| Missing | 205 (50 %) | 168 (51 %) | 37 (49 %) | ||
| Probable Transmission Route | 0.88 | ||||
| Sexual | 370 (91%) | 302 (91%) | 68 (91%) | ||
| Heterosexual | 122 (33%) | 86 (28%) | 36 (53%) | <0.001d | |
| Homosexual | 243 (66%) | 215 (71%) | 28 (41%) | ||
| Unknown | 5 (1%) | 1 (0%) | 4 (6%) | ||
| Intravenous Drug Use | 8 (2%) | 6 (2%) | 2 (3%) | ||
| Other | 2 (0%) | 2 (1%) | 0 (0%) | ||
| Unknown | 26 (6%) | 21 (6%) | 5 (7%) | ||
3 months before until 1 month after the cancer diagnosis.
Among those who used HAART before cancer; if those diagnosed with cancer in the first 2 months after HAART start were excluded then p = 0.002. The rate of prior HAART was statistically higher among non-AIDS- defining cancers than AIDS-defining cancers (p <0.001).
Approximately 95% of ARV use was HAART. The rest had insufficient data to determine if antiretroviral treatment was HAART.
Among those whose probable transmission route was sexual.
Treatment varied according to type of cancer. Of the 224 KS cases, 57 received chemotherapy, 5 radiotherapy, 10 chemotherapy plus radiotherapy, 26 reported HAART alone as treatment, and 12 had other therapeutical approaches. Fifty percent started HAART two months before or after the KS diagnosis. Among the 98 NHL cases, 56 received chemotherapy, 2 radiotherapy, 17 chemotherapy plus radiotherapy, 2 chemotherapy plus surgery, 7 had no treatment, and treatment was unknown for 13. Of the 15 Hodgkin lymphoma cases, 10 received chemotherapy, 3 received chemotherapy plus radiotherapy, and treatment was unknown for 2. Nine out of the 11 skin cancer cases received surgery.
At patients' last visits, 185 (46%) of cases were cured, 136 (33%) of cases were persistent, and status was unknown for 85 (21%) cases. The percentage of resolved cases was similar between ADC and NADC (47% and 41%, respectively). Of the 398 cancer cases with follow-up information on vital status 96 died in a total of 1364 person-years of follow-up. The risk of death for those diagnosed with a NADC did not statistically differ from that of those diagnosed with ADC (hazard ratio=0.76; 95% CI=0.43–1.37; p=0.37).
Incidence estimates among subgroup followed from HAART initiation
In order to compute cancer incidence, we considered a cohort of 3372 HIV-infected, antiretroviral naive patients who were followed from the date of HAART initiation. 24, 25 Seventy four percent of the patients were male. At HAART initiation median age was 36 years (IQR=30–42), 45% of patients had AIDS and median CD4 count was 110 cells/µL (IQR=40–206). Half of the cancer cases (n=199) were found in patients in this cohort. Age at cancer diagnosis, sex, route of transmission, and smoking status were similar between patients belonging and not belonging to this cohort (p >0.1 for all). Patients included in this subanalysis tended to have a higher proportion of NADC (22% vs. 14%, p = 0.054) and to have cancer diagnosed later by calendar year (median=2005 vs. 2002, p <0.001), to have later dates of HIV diagnosis (median=2003 vs. 1999, p <0.001), and to have a later HAART start (median=2004 vs. 2001, p <0.001).
In this subanalysis, 158 of the 3372 patients (4.7%) were diagnosed with a total of 165 cancers prior to or during follow-up (30 cases occurred after the cohort follow-up period and 4 were missing a date of diagnosis). Eighty-five were diagnosed prior to or at HAART initiation (75 ADC, 10 NADC). Of the 80 cases diagnosed after HAART initiation, 29 were diagnosed within the first 2 months, 29 between 2–12 months, and 22 >12 months. Incidence of cancer in 8080 person-years of follow-up after HAART initiation (median=1.9, IQR=1–3.2 years) was 9.9 per 1000 person-years (95% CI= 7.9–12.3). It was higher in the first two months of treatment, particularly for ADC (Table 3).The median CD4 count at cancer diagnosis was 92 cells/µL (IQR=19–204), 80 (IQR=13–150) for ADC and 268 (IQR=172–316) for NADC (p=0.002).
Table 3.
Incidence of cancer after HAART initiation per 1000 person-years (95% CI)
| < 2 months | 2–12 months | > 12 months | Overall | |
|---|---|---|---|---|
| All cancers | 55.3 (38.4–79.5) | 12.4 (8.6–17.8) | 4.2 (2.8–6.4) | 9.9 (7.9–12.3) |
| AIDS Defining Cancers | ||||
| All | 45.7 (30.6–68.2) | 9.8 (6.5–14.8) | 2.1 (1.2–3.8) | 7.2 (5.5–9.3) |
| Kaposi´s sarcoma | 34.3 (21.6–54.4) | 4.7 (2.6–8.5) | 1.3 (0.6–2.8) | 4.5 (3.2–6.2) |
| Non Hodgkin Lymphoma | 11.4 (5.1–25.4) | 5.1 (2.9–9) | 0.4 (0.1–1.5) | 2.5 (1.6–3.8) |
| Non-AIDS Defining Cancers | 9.5 (4–22.9) * | 2.6 (1.1–5.7) | 2.1 (1.2–3.8) # | 2.7 (1.8–4.1) |
skin cancer (3), Hodgkin lymphoma (1), not specified lymphoma (1)
skin cancer (3), breast (2), cervix carcinoma in situ (1), conjunctiva epidermoid carcinoma (1), larynx epidermoid carcinoma (1),lung (1), prostate (1), testicle (1)
Table 4 shows the adjusted relative risks of cancer after HAART initiation. Patients initiating HAART with lower CD4 counts were more likely to have cancer: for a 100-cell-increase in CD4 at HAART initiation, the relative risk (RR) of cancer decreased 30% (95% CI 12–45%). CD4 at HAART initiation was not statistically associated with the incidence of cancer (AIDS or non-AIDS defining) after the first 2 months of HAART. Older age was associated with increased incidence of NADC.
Table 4.
Adjusted Relative Risks for Cancers after HAART initiation (95% Confidence Intervals)*
| Any Cancer | AIDS Defining Cancers |
Non-AIDS Defining Cancers |
|
|---|---|---|---|
| Cancer after HAART start | |||
| Age (per 10 years) | 1.21 (0.98–1.51) | 1.06 (0.81–1.39) | 1.67 (1.16–2.42) |
| Male | 1.36 (0.78–2.38) | 1.68 (0.82–3.47) | 0.76 (0.31–1.86) |
| CD4 (per 100 cells/µL) | 0.70 (0.55–0.88) | 0.69 (0.53–0.92) | 0.74 (0.49–1.13) |
| Cancer > 2 months after HAART start | |||
| Age (per 10 years) | 1.32 (1.02–1.72) | 1.15 (0.82–1.62) | 1.70 (1.12–2.58) |
| Male | 0.90 (0.48–1.69) | 1.14 (0.49–2.67) | 0.51 (0.19–1.35) |
| CD4 (per 100 cells/µL) | 0.79 (0.60–1.03) | 0.81 (0.59–1.13) | 0.79 (0.50–1.24) |
Adjusted for site.
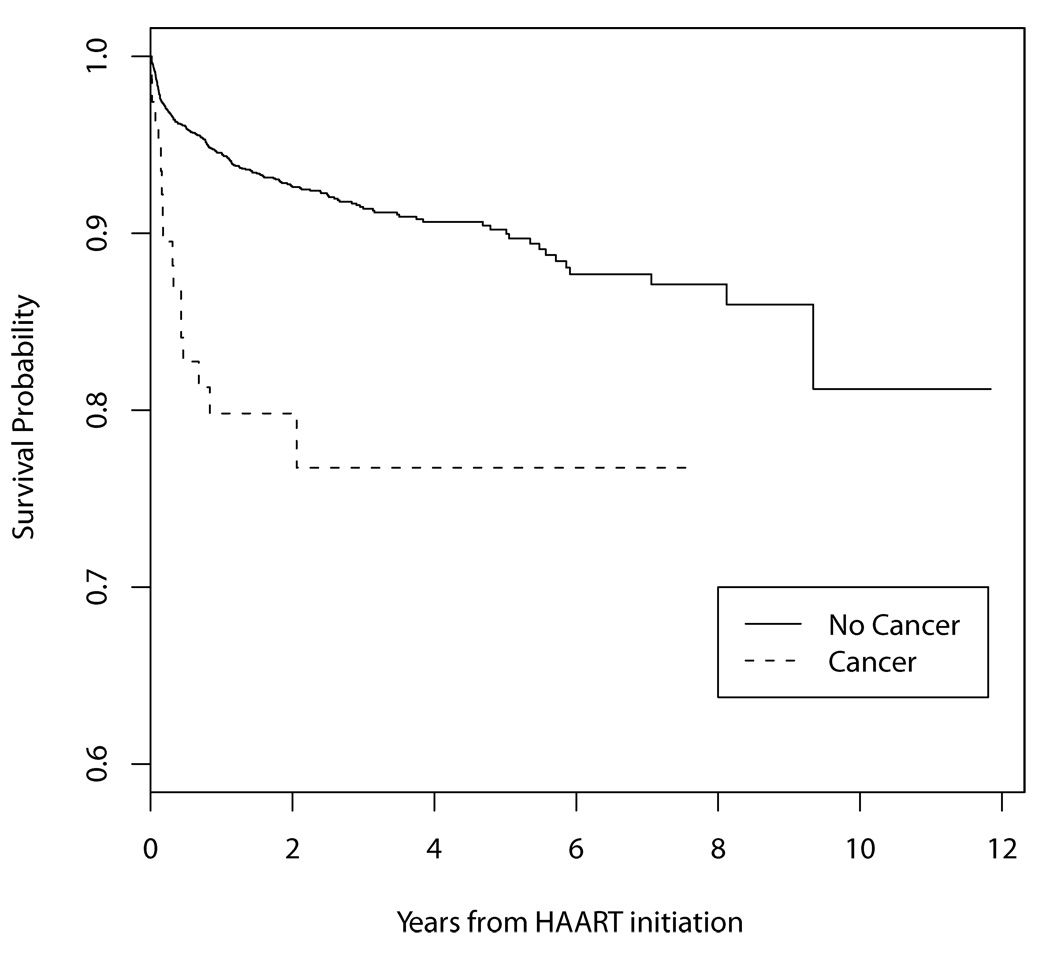
Those who started HAART with a prior or current cancer diagnosis were more likely to die than those without a baseline cancer diagnosis (p <0.0001) (Figure 1). The hazard ratio of death was higher for those with a pre-HAART ADC compared to those with no cancer (HR=4.02, 95% CI=2.34–6.88). Only 10 patients had a baseline NADC; the hazard ratio of death for those with a pre-HAART NADC was HR=1.72, 95% CI=0.42–7.06). A pre-HAART ADC was a strong predictor of mortality after adjusting for age, sex, and CD4 at HAART initiation (HR=3.45, 95% CI=2.01–5.93). The adjusted hazard ratios of death for those with KS and NHL prior to HAART initiation were 1.45 (95% CI 0.53–3.94) and 6.82 (95% CI 3.51–13.24), respectively. For a 100-cell-increase in the CD4 cell count at HAART initiation, the HR of death was 0.52 (95% CI=0.44–0.61).
Figure 1.
Survival probability for those with and without a cancer diagnosis when initiating HAART
GHESKIO-Haiti captured cases diagnosed between 2008 and 2009. Seventy-three cases in 73 patients (70% female) were recorded. Median age at cancer diagnosis was 42 years (IQR=36–45). All but one had sex as probable transmission route. Reported lesions were 27 ADC (18 KS, 8 invasive cervical cancer and 1 CNS lymphoma), 30 NADC and histologically undefined cancers including 7 breast cancers, 5 lymphomas, 2 thyroid cancers, 2 pancreas cancers, 2 throat cancers and 2 skin cancers; and 16 preneoplastic lesions in cervix. Of 38 cases with recorded date of HAART start and cancer diagnosis, 24 patients were on HAART at cancer diagnosis. Forty percent of 57 patients with cancer died, all with active cancer.
DISCUSSION
This is the first description of cancer in HIV infected people from Latin America and the Caribbean. The most frequent cancers in this population were AIDS related (82%). Previous reports from developed and developing countries have shown similar results among HIV-infected adults 20, 27 and when limited to people with AIDS. 28, 29
Consistent with other reports, KS and NHL were the most prevalent ADC in our cohort. 30, 31 KS was the most frequent ADC in all sites but HUCFF-Brazil, where NHL was most common. The most frequent NADC were Hodgkin lymphoma and skin cancer, similar to reports from the developed world. 3, 32, 33 As found in other studies, NADC were more likely to be diagnosed in older subjects. 31
In our cohort of patients starting HAART, 4.7% of patients were diagnosed with at least one cancer, similar to rates seen in other studies from the pre-HAART era. 34 The overall incidence of ADC was similar to previous reports but the overall incidence of NADC was lower. 31
Despite being one of the more common cancers among the general female population in the region 15 and its higher prevalence among HIV infected women 35, 36, few cases of invasive cervical cancer were reported. Possible explanations include the relatively short follow-up period and that, excluding GHESKIO-Haiti, the vast majority of the cohort was male. In GHESKIO-Haiti, with a 70% female study population, invasive cervical carcinoma and breast cancer were the second and third most frequent cancers after KS. We recorded several preneoplasic and in situ lesions, most of which were cervical and high grade. Advanced lesions were more likely to be diagnosed than early lesions, showing that screening programs still need to be improved. Our findings probably underestimate the actual problem since preneoplastic lesions, as well as cancers, might not be detected or recorded in clinical records. Guidelines for screening for cervical cancer are fairly standard, usually with Papanicolau smears, in both low and high resources countries while screening for anal dysplasia and cancer is not widely used in clinical practice. 30, 35, 37, 38
Almost all lesions were diagnosed in the HAART era and over 80% of cancers were diagnosed after year 2000. About 30% of recorded cancers were diagnosed prior to or in the first two months after HIV diagnosis and over 60% were diagnosed about the same time of HAART start, most of them ADC. Similarly, among our cohort of HAART initiators, about half were diagnosed prior to or at HAART initiation. The incidence of cancer, particularly ADC, was very high during the subsequent year following HAART initiation, especially in the first two months. This may represent late HIV diagnosis, late presentation to care, and/or late HAART initiation. These results also suggest that cancer may be what motivates some patients to first approach the healthcare system, prompting HIV testing and diagnosis.
Incidence of NADC was also high around HAART initiation but to a lesser extent. One year after HAART start, the incidence of ADC and NADC were the same. Studies from the developed world have shown a trend towards an increase in NADC and a decline in ADC in the HAART era. 10, 27, 33, 39, 40 Although this topic was not specifically addressed in our study, NADC were more likely to be diagnosed in recent calendar years, when HAART treatment was widely available in all CCASAnet sites. 25 Of note, about three quarters of NADC were found 1 year after HIV diagnosis and half of NADC after 1 year of starting HAART. Low nadir CD4 cell count has been associated with AIDS defining and certain NADC. 1, 10, 27, 33 Patients in our cohort initiating HAART with lower CD4 count were more likely to have any type of cancer.
Survival probability did not statistically differ between people diagnosed with ADC and NADC in the overall analysis. Not surprisingly, in the cohort of HAART starters those who had a diagnosis of cancer at or prior to starting HAART were more likely to die than those without a previous cancer. A pre-HAART ADC was a strong predictor of mortality after adjusting for age, sex, and CD4 at HAART initiation. The hazard of death was lower for KS than for NHL. 41, 42
Limitations
Our cohort comprises cohorts followed at network research sites and thus cannot be considered representative of the entire population in the Latin America and Caribbean region. Lack of national registries and mechanisms for systematic reporting of cancer cases currently precludes a more comprehensive population-based survey. Insufficient integration of different services taking care of HIV patients with cancer is likely to have generated some level of underreporting.
In conclusion, this is the first report addressing cancer among a cohort of HIV infected patients from Latin America and the Caribbean during the HAART era. AIDS defining cancers remain the most frequent in the region although the incidence of NADC equaled that of ADC after the first year of HAART. The large number of ADC diagnosed simultaneously with HIV diagnosis or HAART start, or shortly thereafter, demonstrates that there are barriers to HIV care that need to be addressed, including expanded HIV testing and HAART access. Cancer diagnosis and treatment resources should also be improved, and integration of services needs to be promoted, in order to better serve patients’ needs.
ACKNOWLEDGEMENTS
The CCASAnet collaboration gratefully acknowledges the many patients and collaborating site staff who made this project possible.
Funding for this work was provided in part by US National Institutes of Health Cooperative Agreement (1-U01-AI069923) from the National Institute of Allergy and Infectious Diseases and the National Cancer Institute and by a grant (1-UL1-RR024975) from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary data and partial results were presented on the following conferences:
11th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies (ICMAOI), Bethesda, October 6–7, 2008 [abstract 47]. Preliminary Findings on Cancer Incidence in HIV-Infected Persons from Six Countries in Central and South America and the Caribbean, Masys DR, Fang F, Fink V, Gotuzzo E, Padgett D, Pape JW, Cortes C, Bacon M, Schechter M. Proceedings published on Infectious Agents and Cancer 2009, 4(Suppl 2):O18
12th International Conference on Malignancies in AIDS and Other Acquired Immunodeficencies (ICMAOI), Bethesda, April 26–27, 2010. Cancer in HIV Patients in Latin America and the Caribbean: Characteristics in Seven Sites from the CCASAnet Cohort (IeDEA Region 2), Fink V, Shepherd B, Wehbe F, Cortes C, Crabtree B, Padgett D, Shafaee M, Schechter M, Gotuzzo E, Cesar C, Krolewiecki A, Bacon M, McGowan C, Cahn P, Masys D. Proceedings will be published on Infectious Agents and Cancer
XVIII International AIDS Conference, Vienna, July 13–18, 2010. Poster Exhibition: TUPE0142. Cancer and HIV in Latin America and the Caribbean: experience of seven sites (the Caribbean, Central and South America Network For HIV Research-CCASAnet Cohort), Valeria Fink, Bryan Shepherd, Firas Wehbe, Claudia Cortés, Brenda Crabtree, Denis Padgett, Maryam Shafaee, Mauro Schechter, Eduardo Gotuzzo, Carina Cesar, Alejandro Krolewiecki, Melanie Bacon, Catherine Mc Gowan, Pedro Cahn, Daniel Masys.
REFERENCES
- 1.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007 Jul 7;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 2.Grulich AE, Vajdic CM, Cozen W. Altered immunity as a risk factor for non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007 Mar;16(3):405–408. doi: 10.1158/1055-9965.EPI-06-1070. [DOI] [PubMed] [Google Scholar]
- 3.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. Jama. 2001 Apr 4;285(13):1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 4.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007 Jun 20;99(12):962–972. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 5.CDC. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1992;41:961–962. [PubMed] [Google Scholar]
- 6.Lewden C, Salmon D, Morlat P, et al. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005 Feb;34(1):121–130. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 7.Pantanowitz L, Schlecht HP, Dezube BJ. The growing problem of non-AIDS-defining malignancies in HIV. Curr Opin Oncol. 2006 Sep;18(5):469–478. doi: 10.1097/01.cco.0000239886.13537.ed. [DOI] [PubMed] [Google Scholar]
- 8.Grulich AE. Cancer: the effects of HIV and antiretroviral therapy, and implications for early antiretroviral therapy initiation. Curr Opin HIV AIDS. 2009 May;4(3):183–187. doi: 10.1097/COH.0b013e328329c5b2. [DOI] [PubMed] [Google Scholar]
- 9.Hessol NA, Pipkin S, Schwarcz S, Cress RD, Bacchetti P, Scheer S. The impact of highly active antiretroviral therapy on non-AIDS-defining cancers among adults with AIDS. Am J Epidemiol. 2007 May 15;165(10):1143–1153. doi: 10.1093/aje/kwm017. [DOI] [PubMed] [Google Scholar]
- 10.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008 May 20;148(10):728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 11.ONUSIDA. América Latina, Últimas tendencias epidemiológicas, Hoja de Datos. [Accessed 10 May 2010]; Available at: http://data.unaids.org/pub/FactSheet/2009/20091124_FS_latinamerica_es.pdf.
- 12.ONUSIDA. Caribe, Últimas tendencias epidemiológicas, Hoja de Datos. [Accessed 10 May 2010]; Available at: http://data.unaids.org/pub/FactSheet/2009/20091124_FS_caribbean_es.pdf.
- 13.UNAIDS. The global AIDS epidemic, Global Facts & Figures. [Accessed 10 May 2010]; Available at: http://data.unaids.org/pub/FactSheet/2009/20091124_FS_global_en.pdf.
- 14.WHO. TOWARDS UNIVERSAL ACCESS Scaling up priority HIV/AIDS interventions in the health sector 2009 Progress Report. [Accessed 06-May-2010];2009 Available at: http://www.who.int/hiv/pub/2009progressreport/en/index.html.
- 15.PAHO. PAHO Plan of Action for Cancer Prevention & Control: Cancer Stakeholders Meeting, Fact Sheet: Cancer in Latin America and the Caribbean. [Accessed 06-May-2010]; Available at: http://www.paho.org/english/ad/dpc/nc/pcc-fact-sheet-LAC.pdf.
- 16.Bacchi CE, Bacchi MM, Rabenhorst SH, et al. AIDS-related lymphoma in Brazil. Histopathology, immunophenotype, and association with Epstein-Barr virus. Am J Clin Pathol. 1996 Feb;105(2):230–237. doi: 10.1093/ajcp/105.2.230. [DOI] [PubMed] [Google Scholar]
- 17.Collins JA, Hernandez AV, Hidalgo JA, et al. High proportion of T-cell systemic non-Hodgkin lymphoma in HIV-infected patients in Lima, Peru. J Acquir Immune Defic Syndr. 2005 Dec 15;40(5):558–564. doi: 10.1097/01.qai.0000185135.54920.79. [DOI] [PubMed] [Google Scholar]
- 18.Ferrera A, Melchers WJ, Velema JP, Figueroa M. Association of infections with human immunodeficiency virus and human papillomavirus in Honduras. Am J Trop Med Hyg. 1997 Aug;57(2):138–141. doi: 10.4269/ajtmh.1997.57.138. [DOI] [PubMed] [Google Scholar]
- 19.Fink V, Cesar C, Ameri D, et al. Tumores y HIV en un centro de referencia de Buenos Aires: 5 años de experiencia. Actualizaciones en SIDA, vol 17 suppl 1: 64–75, pag 66. 2009 August;17 suppl 1:64–75. 66. [Google Scholar]
- 20.Laurido M, Uruena A, Vizzotti C, Bugarin G, Cassetti I. [Incidence variation in malignancies associated or not with AIDS at an outpatient care center, 1997–2005] Medicina (B Aires) 2007;67(3):243–246. [PubMed] [Google Scholar]
- 21.Osorio SG, Montenegro UC. Linfomas asociados a infección por virus de inmunodeficiencia humana en un complejo hospitalario de la Región Metropolitana, Chile: 1990–2002. Reporte de 14 casos y revisión. Rev Chil Infect. 2007;24(2):117–124. [PubMed] [Google Scholar]
- 22.Sampaio J, Brites C, Araujo I, et al. AIDS related malignancies in Brazil. Curr Opin Oncol. 2007 Sep;19(5):476–478. doi: 10.1097/CCO.0b013e3282c8c8eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGowan CC, Cahn P, Gotuzzo E, et al. Cohort Profile: Caribbean, Central and South America Network for HIV research (CCASAnet) collaboration within the International Epidemiologic Databases to Evaluate AIDS (IeDEA) programme. Int J Epidemiol. 2007 Oct;36(5):969–976. doi: 10.1093/ije/dym073. [DOI] [PubMed] [Google Scholar]
- 24.Tuboi SH, Schechter M, McGowan CC, et al. Mortality during the first year of potent antiretroviral therapy in HIV-1-infected patients in 7 sites throughout Latin America and the Caribbean. J Acquir Immune Defic Syndr. 2009 Aug 15;51(5):615–623. doi: 10.1097/QAI.0b013e3181a44f0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cesar C, Shepherd BE, Krolewiecki AJ, et al. Rates and reasons for early change of first HAART in HIV-1-infected patients in 7 sites throughout the Caribbean and Latin America. PLoS One. 2010;5(6):e10490. doi: 10.1371/journal.pone.0010490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schafer J, editor. Analysis of Incomplete Multivariate Data. London: United Kingdom: Chapman & Hall; 1997. [Google Scholar]
- 27.Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005 Mar 16;97(6):425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 28.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. Aids. 2006 Aug 1;20(12):1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 29.Galceran J, Marcos-Gragera R, Soler M, et al. Cancer incidence in AIDS patients in Catalonia, Spain. Eur J Cancer. 2007 Apr;43(6):1085–1091. doi: 10.1016/j.ejca.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan A, Levine AM. Malignancies in women with HIV infection. Womens Health (Lond Engl) 2008 Jul;4(4):357–368. doi: 10.2217/17455057.4.4.357. [DOI] [PubMed] [Google Scholar]
- 31.Long JL, Engels EA, Moore RD, Gebo KA. Incidence and outcomes of malignancy in the HAART era in an urban cohort of HIV-infected individuals. Aids. 2008 Feb 19;22(4):489–496. doi: 10.1097/QAD.0b013e3282f47082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgi A, Brodine S, Wegner S, et al. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer. 2005 Oct 1;104(7):1505–1511. doi: 10.1002/cncr.21334. [DOI] [PubMed] [Google Scholar]
- 33.Powles T, Robinson D, Stebbing J, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol. 2009 Feb 20;27(6):884–890. doi: 10.1200/JCO.2008.19.6626. [DOI] [PubMed] [Google Scholar]
- 34.Kiely B, O'Flaherty J, Surah S, et al. HIV-related malignancies pre- and post-highly active antiretroviral therapy: experiences in an inner city tertiary referral centre. Int J STD AIDS. 2010 May;21(5):332–336. doi: 10.1258/ijsa.2009.009486. [DOI] [PubMed] [Google Scholar]
- 35.Palefsky J. Human papillomavirus infection in HIV-infected persons. Top HIV Med. 2007 Aug–Sep;15(4):130–133. [PubMed] [Google Scholar]
- 36.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005 Apr 20;97(8):577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 37.PAHO. Estrategia y plan de acción regionales sobre la prevención y el control del cáncer cervocouterino. [Accessed 22 June 2010];142.a SESIÓN DEL COMITÉ EJECUTIVO. Available at: http://www.paho.org/spanish/gov/ce/ce142-10-s.pdf.
- 38.Ferenczy A, Coutlee F, Franco E, Hankins C. Human papillomavirus and HIV coinfection and the risk of neoplasias of the lower genital tract: a review of recent developments. Cmaj. 2003 Sep 2;169(5):431–434. [PMC free article] [PubMed] [Google Scholar]
- 39.Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. Aids. 2009 Jan 2;23(1):41–50. doi: 10.1097/QAD.0b013e328317cc2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bedimo R, Chen RY, Accortt NA, et al. Trends in AIDS-defining and non-AIDS-defining malignancies among HIV-infected patients: 1989–2002. Clin Infect Dis. 2004 Nov 1;39(9):1380–1384. doi: 10.1086/424883. [DOI] [PubMed] [Google Scholar]
- 41.Mocroft A, Sterne JA, Egger M, et al. Variable impact on mortality of AIDS-defining events diagnosed during combination antiretroviral therapy: not all AIDS-defining conditions are created equal. Clin Infect Dis. 2009 Apr 15;48(8):1138–1151. doi: 10.1086/597468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serraino D, De Paoli A, Zucchetto A, et al. The impact of Kaposi sarcoma and non-Hodgkin lymphoma on mortality of people with AIDS in the highly active antiretroviral therapies era. Cancer Epidemiol. 2010 Jun;34(3):257–261. doi: 10.1016/j.canep.2010.03.011. [DOI] [PubMed] [Google Scholar]