Abstract
Purpose
Roux-en-Y gastric bypass surgery is associated with an increased risk of nephrolithiasis but obesity itself is a known risk factor for kidney stones. To assess the mechanism(s) predisposing to nephrolithiasis after Roux-en-Y gastric bypass we compared urinary tract stone risk profiles in patients who underwent the procedure and normal obese individuals.
Materials and Methods
In this cross-sectional study urine and serum biochemistry was evaluated in 19 nonstone forming patients after Roux-en-Y gastric bypass and in 19 gender, age and body mass index matched obese controls without a history of nephrolithiasis.
Results
Compared with obese controls surgical patients had significantly higher mean ± SD urine oxalate (45 ± 21 vs 30 ± 11 mg daily, p = 0.01) and lower urine citrate (358 ± 357 vs 767 ± 307 mg daily, p <0.01). The prevalence of hyperoxaluria (47% vs 10.5%, p = 0.02) and hypocitraturia (63% vs 5%, p <0.01) was significantly higher in surgical patients, who also had significantly lower urine calcium than obese controls (115 ± 93 vs 196 ± 123 mg daily, p = 0.03). The calcium oxalate urine relative supersaturation ratio was not significantly different between the 2 groups.
Conclusions
Almost half of patients with Roux-en-Y gastric bypass without a history of nephrolithiasis showed hyperoxaluria or hypocitraturia. This prevalence was significantly higher than in body mass index matched controls. These risk factors were negated by lower urine calcium excretion in patients with Roux-en-Y gastric bypass.
Keywords: kidney, kidney calculi, gastric bypass, obesity, risk
Roux-en-Y gastric bypass surgery is popular for surgically treating morbid obesity.1 RYGB was popularized to circumvent the metabolic consequences of JI bypass, including hepatic failure, severe malabsorption, nephrolithiasis and renal insufficiency.2,3 Although it is generally safer and causes less severe malabsorption than JI bypass, RYGB still carries the risk of kidney stones, oxalate nephropathy and renal failure.4–8
Obesity itself increases the likelihood of kidney stones9,10 because of a number of metabolic risk factors, including low urine pH, hyperoxaluria and hypercalciuria.11–14 In a study of morbidly obese patients scheduled for gastric bypass 98% had at least 1 risk factor for kidney stone formation and 80% had 3 or more.15
To assess the mechanisms by which RYGB heightens the nephrolithiasis risk beyond the effect of obesity we compared urinary tract stone risk profiles in patients who underwent RYGB and obese individuals matched for BMI.
PATIENTS AND METHODS
Study Participants
Participants with RYGB were nonstone forming volunteers a mean ± SD of 3.5 ± 1.8 years (range 1 to 7) after bariatric surgery who had stable weight with less than a 10-pound weight change in the preceding 3 months. All had undergone open or laparoscopic RYGB with a 50 to 150 cm Roux limb (mean 106 ± 54). Mean preoperative body weight was 154 ± 44 kg and average postoperative weight loss was 50 ± 29 kg. Each patient with RYGB was matched with a single obese control from a cohort of healthy volunteers with BMI greater than 30 kg/m2 from an ongoing study at the mineral metabolism center at our institution. Controls were matched 1:1 with RYGB cases based on gender, BMI ± 5 kg/m2 and age ± 10 years. Excluded from study were pregnant women, individuals with creatinine clearance less than 70 ml per minute, hyperkalemia, hypercalcemia, metabolic alkalosis or treatment with calcium sparing diuretics, glucocorticoids or drugs for osteoporosis. The study was approved by the institutional review board at University of Texas Southwestern Medical Center, Dallas, Texas. All participants provided informed consent.
Study Protocol
This cross-sectional study was done in an outpatient setting. Study participants completed a 24-hour urine collection while maintaining the random home diet and a fasting blood sample was obtained. Body weight and height were measured at blood collection. Urine measurements included total volume, pH, creatinine, sodium, potassium, calcium, magnesium, oxalate, ammonium, citrate, sulfate, phosphorus, chloride and uric acid. A fasting blood sample was obtained to measure serum electrolytes, creatinine, uric acid and glucose.
Analysis
Urine pH was measured with a pH electrode. Urine creatinine was evaluated by the picric acid method. Urine sodium and potassium were assessed using flame emission method on a photometer. Oxalate was analyzed by a chromatography system using a carbonate-bicarbonate eluent and an anion column. Urine calcium and magnesium were analyzed by atomic absorption using a spectrometer system. Urine ammonium (NH4+) was measured by the glutamate dehydrogenase method. Urine citrate was determined enzymatically using reagents. Urine sulfate was determined by ion chromatography. Urine phosphorus was assessed on an automated analyzer using ammonium molybdate based reagent, which read a color change reaction spectrophotometrically at 340 nM. Uric acid was analyzed by the urate oxidase method using an alkalinized aliquot to prevent precipitation. Serum sodium, potassium, chloride, total carbon dioxide, glucose, blood urea nitrogen, uric acid and creatinine were measured as a part of systematic multichannel analysis using a chemistry analyzer. The urine calcium oxalate and brushite RSR, and the urine content of undissociated uric acid were determined with EQUIL2.16
Statistical Analysis
Demographic characteristics and biochemical features in the RYGB and obese control groups are shown as the mean ± SD and were compared with Student’s t test. Fisher’s exact test was used to compare gender distribution and the prevalence of lithogenic factors between the 2 groups. ANCOVA and linear models were used to examine the relationship between BMI and urine oxalate.
RESULTS
Demographics
A total of 38 individuals participated in this study, including 16 women and 3 men each in the RYGB and obese control groups. In the RYGB vs control groups mean age was 49 ± 11 vs 47 ± 10 years (p = 0.58), mean weight was 104 ± 25 vs 95 ± 17 kg (p = 0.19) and mean BMI was 38 ± 7 vs 36 ± 5 kg/m2. By design mean age and BMI were similar in the 2 groups.
Biochemical Characteristics
Urine profile
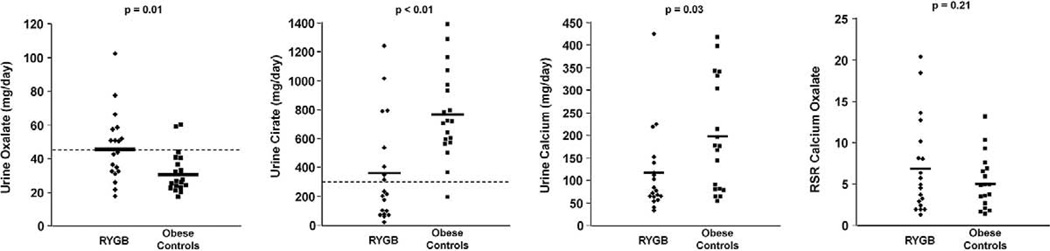
Table 1 shows contrasting 24-hour urine profiles. Between the 2 groups 24-hour urine volume, pH, sodium, chloride, magnesium, phosphorus and ammonium were not significantly different. Urine oxalate was significantly higher in the RYGB group than in obese controls (45 ± 21 vs 30 ± 13 mg daily, p = 0.01), urine citrate was significantly lower in the RYGB group than in controls (358 ± 357 vs 767 ± 307 mg daily, p <0.01) and urine calcium was significantly lower in the RYGB group than in controls (115 ± 93 vs 196 ± 123 mg daily, p = 0.03, fig. 1). The RYGB group had lower urine sulfate (30 ± 11 vs 43 ± 19 mEq daily, p = 0.02) and urine potassium (41 ± 21 vs 58 ± 15 mEq daily, p = 0.01). The ratio of ammonium to sulfate was significantly higher in the RYGB cohort (1.51 ± 0.84 vs 0.79 ± 0.32, p <0.01). Calcium oxalate RSR was not statistically significant between the groups (7.0 ± 5.7 vs 5.0 ± 3.2, p = 0.21, fig. 1). Brushite RSR was similar in the RYGB and control groups (1.1 ± 1.4 and 0.95 ± 0.9, respectively, p = 0.64).
Table 1.
Urine biochemistry in RYGB and control groups
| Mean ± SD RYGB | Mean ± SD Controls | p Value | |
|---|---|---|---|
| Vol (l/day) | 1.9 ± 0.9 | 2.1 ± 1.1 | 0.52 |
| pH | 5.89 ± 0.43 | 5.83 ± 0.44 | 0.66 |
| Creatinine (mg/day) | 1211 ± 334 | 1485 ± 447 | 0.04 |
| Oxalate (mg/day) | 45 ± 21 | 30 ± 13 | 0.01 |
| Calcium (mg/day) | 115 ± 93 | 196 ± 123 | 0.03 |
| Citrate (mg/day) | 358 ± 357 | 767 ± 307 | <0.01 |
| Sodium (mEq/day) | 174 ± 89 | 171 ± 69 | 0.90 |
| Chloride (mEq/day) | 161 ± 89 | 166 ± 67 | 0.85 |
| Potassium (mEq/day) | 41 ± 21 | 58 ± 15 | 0.01 |
| Magnesium (mg/day) | 96 ± 34 | 84 ± 41 | 0.33 |
| Ammonium (mEq/day) | 40 ± 18 | 37 ± 25 | 0.61 |
| Sulfate (mEq/day) | 30 ± 11 | 43 ± 19 | 0.02 |
| Ammonium/sulfate | 1.51 ± 0.84 | 0.79 ± 0.32 | <0.01 |
| Phosphorus (mg/day) | 807 ± 312 | 948 ± 401 | 0.23 |
| Uric acid (mg/day) | 436 ± 124 | 557 ± 193 | 0.03 |
| Calcium oxalate RSR | 7.0 ± 5.7 | 5.0 ± 3.3 | 0.21 |
| Brushite RSR | 1.1 ± 1.4 | 1.0 ± 0.9 | 0.64 |
Figure 1.
Comparison of urine oxalate, citrate, calcium and RSR for calcium oxalate between 19 patients in RYGB group and 19 obese controls. Horizontal bars indicate mean. Dotted lines indicate reference range.
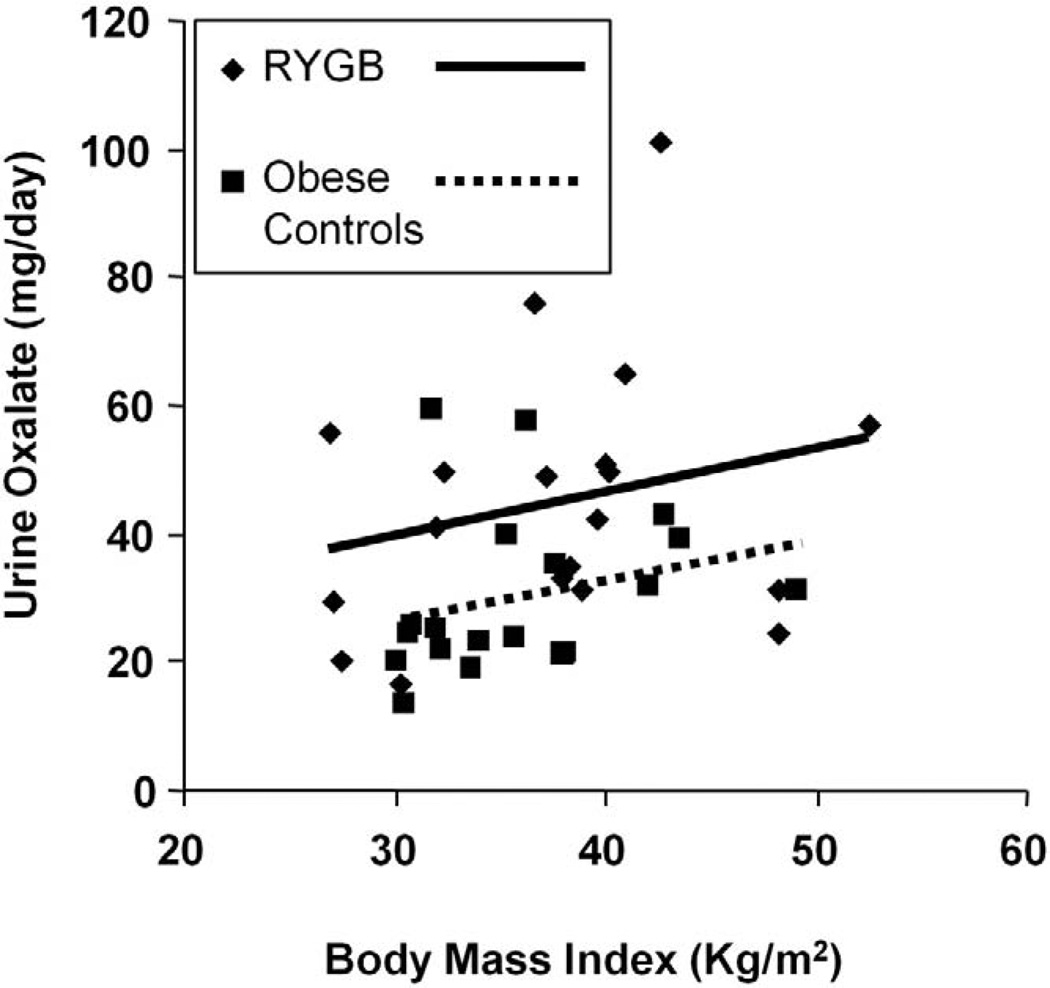
To determine the relationship between BMI and urine oxalate, we plotted the 2 variables in the 2 groups (fig. 2). A tendency toward greater urine oxalate with increasing BMI was observed in each group. Although the slope of urine oxalate-BMI regression lines was similar in the groups, the intercept was significantly greater in the RYGB group than in controls, such that urine oxalate was greater in the former at any given BMI.
Figure 2.
BMI vs 24-hour oxalate in 19 patients in RYGB group vs 19 obese controls. For given BMI urine oxalate was significantly higher in RYGB group than in obese controls (p = 0.01).
Hyperoxaluria, defined as urine oxalate greater than 45 mg daily, was more common in the RYGB group than in controls (47% vs 10.5%, p = 0.03). The rate of hypocitraturia, defined as urine citrate less than 320 mg daily, was significantly greater in the RYGB group than in controls (63% vs 5%, p <0.01). Hypercalciuria was less common in the RYGB group (5% vs 32%, p = 0.08).
Blood profile
Table 2 shows fasting serum biochemical profiles. Fasting blood was available in 18 patients with RYGB. Serum values were similar for potassium, calcium, chloride and bicarbonate. Serum sodium and phosphorus were higher in the RYGB group (141 ± 2 vs 138 ± 2 mEq/l, p <0.01 and 3.8 ± 0.7 vs 3.4 ± 0.6 mg/dl, p = 0.06, respectively).
Table 2.
Serum biochemistry in RYGB and control groups
| Mean ± SD RYGB | Mean ± SD Controls | p Value | |
|---|---|---|---|
| Sodium (mEq/l) | 141 ± 2 | 138 ± 2 | <0.01 |
| Potassium (mEq/l) | 4.3 ± 0.3 | 4.3 ± 0.3 | 0.64 |
| Chloride (mEq/l) | 106 ± 2 | 107 ± 3 | 0.38 |
| Bicarbonate (mEq/l) | 26 ± 6 | 26 ± 2 | 0.62 |
| Creatinine (mg/dl) | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.75 |
| Calcium (mg/dl) | 9.1 ± 0.5 | 9.3 ± 0.4 | 0.35 |
| Phosphorus (mg/dl) | 3.8 ± 0.7 | 3.4 ± 0.6 | 0.06 |
| Uric acid (mg/dl) | 5.1 ± 1.0 | 5.4 ± 1.2 | 0.46 |
DISCUSSION
Obesity and RYGB surgery are separately associated with an increased risk of kidney stones. We compared urinary tract stone risk profiles in patients after RYGB and obese controls to assess the mechanism(s) by which RYGB heightens the nephrolithiasis risk. We found significantly higher urine oxalate and lower urine citrate in RYGB cases than in BMI matched controls. The effects of these 2 lithogenic risk factors were negated by lower urine calcium excretion in the RYGB group.
In contrast to reports comparing patients who underwent RYGB to stone formers and healthy individuals,4,7 and prospective studies of patients before and after RYGB,17 of which none was weight matched, to our knowledge we are the first to compare urine parameters in patients with RYGB and BMI matched controls. This choice of control group is particularly important because obesity and weight gain are risk factors for nephrolithiasis10 and body size influences 24-hour urine composition.12,18 Higher BMI is inversely associated with urine pH11 and directly related to higher urine oxalate, sodium, phosphorus and uric acid excretion.12,18
Several recent reports of increased urine oxalate excretion in patients after bariatric surgery support our findings.4–7,17 JI bypass is well recognized as a major cause of calcium nephrolithiasis and oxalate induced nephropathy.3,19 However, oxalate nephropathy may also develop after RYGB, consequently increasing the risk of renal insufficiency.5,8 This suggests that the oxalate burden in some patients after RYGB may be high enough to increase the risk of kidney stone formation and also cause irreversible tissue damage. The exact causes of the varying degrees of hyperoxaluria after bariatric surgery have not been fully elucidated. Hyperoxaluria depends on the type of bariatric surgery since oxalate excretion is significantly greater after a biliopancreatic-duodenal switch than after RYGB.7 In contrast to that series, the relatively lower urine oxalate excretion in our patients with RYGB may in part be due to our exclusion of those who underwent duodenal switch surgery.
The degree of hyperoxaluria may also depend on the interval after surgery. One group noted more common hyperoxaluria at 12 than at 6 months after RYGB6 and another reported that oxalate excretion increased as early as 3 months after RYGB.17 In contrast, all of our patients with RYGB were evaluated at least 1 year after bypass. A potential mechanism for hyperoxaluria after RYGB is enhanced saponification of intestinal calcium with unabsorbed fatty acids due to fat malabsorption.20 The subsequent decrease in calcium oxalate complexation in the intestinal tract results in enhanced colonic oxalate absorption and subsequently in greater urine oxalate excretion. This has yet to be substantiated with stool fat measurement in patients after RYGB. An alternative mechanism may be a lower rate of intestinal oxalate degradation by Oxalobacter formigenes after RYGB.20 While a previous study in JI bypass cases showed decreased intestinal O. formigenes colonization,21 to our knowledge the colonization rate after RYGB has not been reported.
Mean urine citrate excretion was significantly lower in our patients with RYGB than in BMI matched controls with hypocitraturia in more than half of the patients.22 However, this was not uniformly observed in previous studies in patients after RYGB.4,7,17 Factors commonly associated with hypocitraturia such as overt metabolic acidosis, and excessive salt and/or animal protein intake23–25 were not observed in our RYGB cohort. Mild metabolic acidosis in our patients may have been masked by adaptive mechanisms, including alkali release from bone and lower urine excretion of citrate (a potential base) to maintain acid-base homeostasis.26 Although urine ammonium excretion did not differ between our 2 groups, the higher ammonium-to-sulfate ratio in the RYGB cohort indirectly supports the possibility of a subtle gastrointestinal alkali loss.
Calcium oxalate is the most common type of kidney stone after RYGB.6 Since urine calcium and oxalate contribute equally to calcium oxalate urine saturation,27 the relative hypocalciuria in patients with RYGB negated the effects of hyperoxaluria. As a result, urine calcium oxalate RSR was only marginally higher in our RYGB cohort. However, our results do not rule out the fact that patients with RYGB are at increased risk for kidney stones. A recent cross-sectional study showed a significantly higher prevalence of nephrolithiasis in patients with RYGB than in obese controls.28 In addition to the ion paring effect of calcium and citrate, which is accounted for by RSR, urine citrate influences the metastability limit.29 Thus, the minimum supersaturation needed to elicit calcium oxalate crystallization may have been lower in patients with RYGB than in normal obese controls. Whether this has a role in the prevalence of calcium oxalate stones in RYGB populations must be examined in detail. Furthermore, by only including individuals with normal renal function in our study, we may have excluded patients with RYGB who had severe oxalate nephropathy, thus decreasing mean urine oxalate and calcium oxalate RSRs in our RYGB population. Since the number of RYGB procedures grew exponentially in the United States in the last decade, the true incidence and prevalence of urine calcium oxalate supersaturation, the incidence of calcium oxalate nephrolithiasis and the relationship between these 2 factors must be fully elucidated.
Recent guidelines suggest annual 24-hour urine collection for a stone risk profile in patients who undergo biliopancreatic diversion with or without a duodenal switch but do not recommend specific screening after RYGB.30 In view of our findings and those of others of urine abnormalities in a significant proportion of patients after RYGB without nephrolithiasis, we advocate periodic 24-hour urine screening in this population. Optimal timing and frequency remain to be determined and would be dictated by available resources and proven treatment measures.
A study limitation is that net acid excretion was not measured since urine specimens were not collected under mineral oil, which precludes definitive conclusions about the cause of hypocitraturia. Also, our cross-sectional design does not provide insight into the time course of changes in urine parameters after RYGB. A prospective followup of the stone risk profile in this population would provide such information since the only published study with such a design has a short-term followup of 90 days.17 Another limitation is that our findings are based on a single 24-hour urine collection provided by each participant. Patel et al found significant variability in oxalate excretion in some patients after bariatric surgery who collected 2, 24-hour urine samples.7
CONCLUSIONS
Our study shows significantly higher urine oxalate and lower urine citrate excretion in patients with RYGB than in their BMI matched controls. However, relative hypocalciuria in patients with RYGB offsets these lithogenic risk factors. The true incidence of nephrolithiasis and optimal treatment for lithogenic risk factors in this population remain to be established.
Acknowledgments
Study received University of Texas Southwestern Medical Center institutional review board approval.
Supported by National Institutes of Health Grants P01-DK20543 and M01-RR00633.
Abbreviations and Acronyms
- BMI
body mass index
- JI
jejuno-ileal
- RSR
relative supersaturation ratio
- RYGB
Roux-en-Y gastric bypass
REFERENCES
- 1.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 2.Kirkpatrick JR. Jejunoileal bypass. A legacy of late complications. Arch Surg. 1987;122:610. doi: 10.1001/archsurg.1987.01400170116017. [DOI] [PubMed] [Google Scholar]
- 3.Requarth JA, Burchard KW, Colacchio TA, et al. Long-term morbidity following jejunoileal bypass. The continuing potential need for surgical reversal. Arch Surg. 1995;130:318. doi: 10.1001/archsurg.1995.01430030088018. [DOI] [PubMed] [Google Scholar]
- 4.Asplin JR, Coe FL. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol. 2007;177:565. doi: 10.1016/j.juro.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Nelson WK, Houghton SG, Milliner DS, et al. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:481. doi: 10.1016/j.soard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Sinha MK, Collazo-Clavell ML, Rule A, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72:100. doi: 10.1038/sj.ki.5002194. [DOI] [PubMed] [Google Scholar]
- 7.Patel BN, Passman CM, Fernandez A, et al. Prevalence of hyperoxaluria after bariatric surgery. J Urol. 2009;181:161. doi: 10.1016/j.juro.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasr SH, D’Agati VD, Said SM, et al. Oxalate nephropathy complicating Roux-en-Y Gastric Bypass: an underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol. 2008;3:1676. doi: 10.2215/CJN.02940608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curhan GC, Willett WC, Rimm EB, et al. Body size and risk of kidney stones. J Am Soc Nephrol. 1998;9:1645. doi: 10.1681/ASN.V991645. [DOI] [PubMed] [Google Scholar]
- 10.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 11.Maalouf NM, Sakhaee K, Parks JH, et al. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 12.Taylor EN, Curhan GC. Body size and 24-hour urine composition. Am J Kidney Dis. 2006;48:905. doi: 10.1053/j.ajkd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Negri AL, Spivacow FR, Del Valle EE, et al. Role of overweight and obesity on the urinary excretion of promoters and inhibitors of stone formation in stone formers. Urol Res. 2008;36:303. doi: 10.1007/s00240-008-0161-5. [DOI] [PubMed] [Google Scholar]
- 14.Lemann J, Jr, Pleuss JA, Worcester EM, et al. Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int. 1996;49:200. doi: 10.1038/ki.1996.27. [DOI] [PubMed] [Google Scholar]
- 15.Duffey BG, Pedro RN, Kriedberg C, et al. Lithogenic risk factors in the morbidly obese population. J Urol. 2008;179:1401. doi: 10.1016/j.juro.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 16.Werness PG, Brown CM, Smith LH, et al. EQUIL2: a BASIC computer program for the calculation of urinary saturation. J Urol. 1985;134:1242. doi: 10.1016/s0022-5347(17)47703-2. [DOI] [PubMed] [Google Scholar]
- 17.Duffey BG, Pedro RN, Makhlouf A, et al. Roux-en-Y gastric bypass is associated with early increased risk factors for development of calcium oxalate nephrolithiasis. J Am Coll Surg. 2008;206:1145. doi: 10.1016/j.jamcollsurg.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Siener R, Glatz S, Nicolay C, et al. The role of overweight and obesity in calcium oxalate stone formation. Obes Res. 2004;12:106. doi: 10.1038/oby.2004.14. [DOI] [PubMed] [Google Scholar]
- 19.Mole DR, Tomson CR, Mortensen N, et al. Renal complications of jejuno-ileal bypass for obesity. QJM. 2001;94:69. doi: 10.1093/qjmed/94.2.69. [DOI] [PubMed] [Google Scholar]
- 20.Lieske JC, Kumar R, Collazo-Clavell ML. Nephrolithiasis after bariatric surgery for obesity. Semin Nephrol. 2008;28:163. doi: 10.1016/j.semnephrol.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allison MJ, Cook HM, Milne DB, et al. Oxalate degradation by gastrointestinal bacteria from humans. J Nutr. 1986;116:455. doi: 10.1093/jn/116.3.455. [DOI] [PubMed] [Google Scholar]
- 22.Nicar MJ, Skurla C, Sakhaee K, et al. Low urinary citrate excretion in nephrolithiasis. Urology. 1983;21:8. doi: 10.1016/0090-4295(83)90113-9. [DOI] [PubMed] [Google Scholar]
- 23.Hamm LL, Hering-Smith KS. Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol Metab Clin North Am. 2002;31:885. doi: 10.1016/s0889-8529(02)00031-2. viii. [DOI] [PubMed] [Google Scholar]
- 24.Sakhaee K, Harvey JA, Padalino PK, et al. The potential role of salt abuse on the risk for kidney stone formation. J Urol. 1993;150:310. doi: 10.1016/s0022-5347(17)35468-x. [DOI] [PubMed] [Google Scholar]
- 25.Reddy ST, Wang CY, Sakhaee K, et al. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis. 2002;40:265. doi: 10.1053/ajkd.2002.34504. [DOI] [PubMed] [Google Scholar]
- 26.Alpern RJ, Sakhaee K. The clinical spectrum of chronic metabolic acidosis: homeostatic mechanisms produce significant morbidity. Am J Kidney Dis. 1997;29:291. doi: 10.1016/s0272-6386(97)90045-7. [DOI] [PubMed] [Google Scholar]
- 27.Pak CY, Adams-Huet B, Poindexter JR, et al. Rapid communication: relative effect of urinary calcium and oxalate on saturation of calcium oxalate. Kidney Int. 2004;66:2032. doi: 10.1111/j.1523-1755.2004.00975.x. [DOI] [PubMed] [Google Scholar]
- 28.Matlaga BR, Shore AD, Magnuson T, et al. Effect of gastric bypass surgery on kidney stone disease. J Urol. 2009;181:2573. doi: 10.1016/j.juro.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 29.Sakhaee K, Nicar M, Hill K, et al. Contrasting effects of potassium citrate and sodium citrate therapies on urinary chemistries and crystallization of stone-forming salts. Kidney Int. 1983;24:348. doi: 10.1038/ki.1983.165. [DOI] [PubMed] [Google Scholar]
- 30.Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic and Bariatric Surgery Medical Guidelines for Clinical Practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Surg Obes Relat Dis. 2008;4:S109. doi: 10.1016/j.soard.2008.08.009. [DOI] [PubMed] [Google Scholar]