Abstract
Cancer is the uncontrollable growth of cell, which may spread to other parts of the body. The interaction of cancer cells with extracellular matrix (ECM) is essential for metastasis, which is the principal cause of death in cancer patients. Disintegrins are naturally occurring low molecular weight peptides found in the venoms of many snakes. Disintegrins were first used to inhibit platelet aggregation, but more recently have been used to inhibit cancer cell growth, adhesion, migration, invasion and/or angiogenesis. The purpose of this study was to determine the anti-tumor properties of recombinant mojastin 1 (r-mojastin 1) and r-mojastin-GST, cloned from the venom glands of the Mohave rattlesnake (Crotalus scutulatus scutulatus). Human urinary bladder carcinoma (T24), human fibrosarcoma (HT-1080), human skin melanoma (SK-ML-28) and murine skin melanoma (B16F10) cell lines were used. r-Mojastin 1 inhibited SK-MEL-28 cell adhesion to fibronectin, but was not able to inhibit T24 cell adhesion to fibronectin. However, r-Mojastin-GST inhibited SK-MEL-28 and T24 cells adhesion to fibronectin. r-Mojastin-GST and r-mojastin 1 decreased the ability of SK-MEL-28 cells to migrate after 24 h of incubation but were not able to inhibit T24 cell migration. r-Mojastin 1 and r-mojastin-GST inhibited invasion of T24 and SK-MEL-28 cancer cells in vitro, and r-Mojastin 1 inhibited lung tumor colonization of B16F10 cells in mice in vivo. In conclusion, our studies suggest that r-mojastin could be a useful tool to develop novel anti-tumor agents by virtue of its ability to inhibit tumor cell adhesion, migration and invasion.
Keywords: Recombinant disintegrin, Mohave rattlesnake, Tumoral cells, Adhesion, Migration, Invasion
1. Introduction
Cancer cells interact with their surrounding cells and matrix proteins in order to replicate, gain nourishment, and migrate to a new location. The interaction of cancer cells with extracellular matrices (ECM) is essential for metastasis, which is the principle cause of death in cancer patients. Metastasis is the movement of tumor cells from a primary site to a secondary site, and is believed to be dependent on a number of physiological processes. Cancer cells need to detach from the primary tumor mass, migrate toward the lymphatic and blood vessels, penetrate into the vascular lumen, evade the immune system, adhere to the vascular endothelium, penetrate the vascular system, and grow in the invaded microenvironment (Felding-Habermann, 2003; McGary et al., 2002; Versteeg et al., 2004). Cancer cell movement is controlled by various factors produced by cancer cells and host cells, including growth and motility factors, cytokines, cell adhesion molecules and ECM proteins. Cell adhesion molecules play a crucial role in cell motility through cell–cell and cell–ECM interactions (Tsuji et al., 2002). The best-characterized cell adhesion receptors are the integrins. In many cases, integrins recognize a tripeptide binding site on the extracellular matrix proteins (Akiyama, 1996). Short synthetic or natural peptides containing this tripeptide binding site can inhibit tumor cell invasion in vitro and tumor dissemination in vivo (Ruoslahti, 1994).
Disintegrins are naturally occurring low molecular weight peptides found in venom of many venomous snakes. Snake venom disintegrins are classified as: short, medium, long, monomeric and dimeric molecules (Kim et al., 2005). Short disintegrins have 41–51 amino acids and 8 cysteines; medium size disintegrins are within the range of 70 amino acids and 12 cysteines; and long disintegrins are usually 84 amino acids with 14 cysteines (Olfa et al., 2005). Disintegrins are postulated to be synthesized from a metalloproteinase/disintegrin precursor and matures by cleavage from the precursor molecule (Okuda et al., 2002). Disintegrins contain a conserved cysteine configuration within their primary structure and their 3-D configuration is constructed primarily from disulfide linkages and interacts within the crevice of integrin receptors (Fujii et al., 2003). Most disintegrins posses an “RGD” binding motif located near the C-terminal. However, KGD, RTS, KTS, MGD, WGD and ECD binding domains have also been identified (McLane et al., 2004; Calvete et al., 2003). Disintegrins have not only been ascertained to inhibit platelet aggregation, but also cancer cell growth, adhesion, migration, invasion and/or angiogenesis (Corrêa et al., 2002; Moreno-Murciano et al., 2003; Sánchez et al., 2006, 2009; Yang et al., 2005; Yeh et al., 2001).
The cloning of disintegrins has been used to study anti-thrombotic and anti-tumor activity; however, only few have been reported with activity (Assakura et al., 2003; Fernandez et al., 2005; Sanz et al., 2005; Wang et al., 2004; Minea et al., 2005, 2010; Sánchez et al., in press).
Sánchez et al., (2006) isolated and characterized two disintegrins, mojastin 1 and mojastin 2, from the venom of Crotalus scutulatus scutulatus, that inhibited ADP-induced platelet aggregation in whole human blood both with an IC50 of 13.8 nM. Recently, recombinant mojastin 1 (r-mojastin 1), identical to mojastin 1, sub-cloned and expressed in Escherichia coli, inhibited ADP-induced platelet aggregation in whole blood and platelet rich plasma (PRP) with IC50 values of 46 and 119 nM, respectively. r-Mojastin 1 was also tested for its ability to inhibit platelet ATP release using PRP and platelet adhesion to fibronectin resulting with IC50s of 95.6 nM and 58.3 nM, respectively (Sánchez et al., in press).
In addition to their potent anti-platelet activity, studies of disintegrins have reported a new use in the diagnosis of cardiovascular diseases and the design of therapeutic agents in arterial thrombosis, osteoporosis, and angiogenesis-related tumor growth and metastasis (Yeh et al., 2001). The purpose of this study was to determine the effect of r-mojastin 1 on various cellular functional studies such as adhesion to extracellular matrices, migration and invasion, using the human urinary bladder carcinoma (T24), human fibrosarcoma (HT-1080), human skin melanoma (SK-ML-28) and murine skin melanoma (B16F10) cell lines.
2. Materials and methods
2.1. Preparation of native and recombinant mojastin
Native mojastin was used as a positive control to compare the activity with recombinant mojastin. Native mojastin was isolated from crude venom of C. s. scutulatus (Mohave rattlesnake), using a combination of three chromatographic steps consisting of reverse phase C18 (Vydac), size exclusion (WATERS Protein PAK60), and anion exchange (WATERS DEAE 5PW) as previously described (Sánchez et al., 2006).
Recombinant mojastin 1 was expressed in E. coli and further purified by two-step chromatography, using the method of Sánchez et al. (in press). Briefly, the cDNA was obtained and ligated into the pGEX-4T-1 expression vector (GE Healthcare Lifesciences) and transformed into E. coli DH5α competent cells. Recombinant plasmids containing r-mojastin 1 were purified and sequenced with disintegrin-specific primers. Once the sequence was obtained, in-frame r-mojastin 1-pGEX-4T-1 plasmids were transformed into E. coli BL21 cells. Culture were grown, inducted by 0.5 mM of isopropyl β-D thiogalactoside (IPTG) and centrifuged in order to obtain the bacterial cells. After bacterial cell disruption with a Branson Sonifier 450 (Danbury, CT), the cell debris was removed by centrifugation and the crude lysate was incubated with glutathione Sepharose 4B (GS4B) (Amersham Biosciences). r-Mojastin 1 proteins were cleaved and eluted from glutathione S-transferase (GST) bound to GS4B by thrombin cleavage. Thrombin was removed from r-mojastin 1 using a 1 mL HiTrap™ Benzamidine Sepharose 4 Fast Flow column (Amersham Biosciences). In order to obtain the r-mojastin-GST proteins, the crude lysate was not passed through the GS4B and benzamidine columns. Purity of native and recombinant mojastins (r-mojastin 1 and r-mojastin-GST) was determined by SDS-PAGE electrophoresis, and molecular weight was confirmed by mass spectrometric analysis (MALDI-TOF) (Sánchez et al., in press).
2.2. Cell line and culture conditions
The human urinary bladder carcinoma cell line (T24), human fibrosarcoma (HT-1080), the human skin melanoma (SK-Mel-28) and murine skin melanoma (B16F10) cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The T24 cells were maintained as a monolayer culture with McCoy’s 5A minimum essential medium, supplemented with 10% fetal calf serum (FBS) and 50 U/mL penicillin, 50 µg/mL streptomycin. The HT-1080 and SK-Mel-28 cell lines were maintained with Eagle’s minimum essential medium, supplemented with 10% fetal calf serum and 50 U/mL penicillin, 50 µg/mL streptomycin. The B16F10 cells were maintained with Dulbecco’s modified Eagle’s medium, supplemented with 10% fetal calf serum and 50 U/mL penicillin, 50 µg/mL streptomycin. The cells were maintained in a humidified 5% CO2 air incubator at 37 °C.
2.3. Cellular adhesion inhibition assay
Native disintegrins and two recombinant disintegrins (r-mojastin 1 and r-mojastin-GST) were used to inhibit the binding of T24 and SK-MEL-28 cells on fibronectin coated plate (Sánchez et al., 2009). Commercial echistatin at 0.1 mg/mL (SIGMA, Lot. 023K12301), a disintegrin that blocked binding of tumor cells to fibronectin, was used as a positive control (Sánchez et al., 2009). In the positive control, the T24 and SK-MEL-28 cells, failed to bind to fibronectin. The negative control consisted of T24 or SK-Mel-28 cells incubated with PBS. The negative controls allowed binding of cells to fibronectin. The percent inhibition was calculated by the following formula: [(absorbance of negative control − absorbance of cell/r-mojastin 1 sample) ÷ absorbance of negative control] × 100.
2.4. Wound healing
The migration of tumor cells was measured by cells being scraped from the bottom of the well and measuring the migration as described Galán et al., 2008. Commercial echistatin at 2.7 µM (final concentration), a disintegrin that blocked migration of T24 and SK-MEL-28 cells, was used as a positive control. The positive controls prevent tumor cells migration. The negative control consisted of T24 or SK-Mel-28 cells incubated with PBS, which allowed cell migration to occur. The cells were then incubated in a CO2 chamber and were only removed from the incubator for microscopy images at time 0, 3, 6 12 and 24 h. Percent of closure was calculated by the following equation: % Closure: [(C − E)/C] × 100, where C is the units of distance of cell edge (mm) at zero time for the control, and E is the distance from the cell edge (mm) at the final incubation time for the disintegrin.
2.5. Invasion assay
The T24, HT-1080 and SK-ML-28 invasion assays were performed using a modified method of Yang et al., (2005). Transwells (8.0-µm pore size; Corning) containing polycarbonate filters (Transwell inserts) were coated with Matrigel, a basement membrane matrix extracted from Engelbreth-Holm-Swarm mouse sarcoma, which consists of collagen type IV, heparan sulfate proteoglycan, entactin and laminin (Becton Dickinson). Matrigel was diluted to 2 mg/mL using serum-free McCoy’s 5A minimum essential medium or Eagle’s minimum essential medium at 4 °C and an aliquot (40 µL) of Matrigel (80 µg/well) was added to each filter insert and incubated for 30 min at 37 °C to form a uniform three-dimensional gel. Then, the lower chamber was filled with 0.6 mL of medium supplemented with 10% fetal calf serum. Tumor cells (7.5 × 105 cells/mL) were incubated with or without r-mojastin 1 (with GST and without GST) for 30 min and aliquots (200 µL) of cells were plated at the upper chamber of the Transwell. After 24-h incubation, all non-migrant cells were removed from the upper membrane of the Transwell with a cotton swab, and the migrant cells were fixed and stained with 0.5% toluidine blue in 4% paraformaldehyde. Invasion was quantified by counting the number of stained cells on the lower membrane per 40× field and photographed with a light microscope (Nixon, Japan).
2.6. Inhibition of lung tumors in vivo
Murine melanoma cells (B16F10), (5.0 × 106 cells/mL) were suspended in Dulbecco’s modified Eagle’s medium without FBS in the presence or absence of r-mojastin 1 at 1000 µg/kg per mouse and incubated 1 h at 37 °C. Control consisted of mice injected with B16F10 cells in Dulbecco’s modified Eagle’s medium without FBS and mice injected with medium alone. Two hundred microliter of cells/disintegrin mixture was injected intravenously (i.v) in the lateral tail vein of BALB/c mice (weight 18–20 g). Mice were sacrificed 19 days post injection, and lungs were examined for the presence of tumors. Lungs were visualized with a 4 × stereomicroscope. The tumors were counted for statistical analysis.
2.7. Statistical analysis
Results of wound healing and invasion were expressed as the mean ± standard deviation (n = 3), and analyzed using the one way-Anova test followed by Newman–Keuls Multiple Comparison Test, using the software program Graph Pad Prism. A two tailed t-test followed by Mann Witney test was used to determine the significance of r-mojastin 1 and the control in inhibiting the number of tumors in vivo. Differences were statistically significant if p was less than 0.05.
3. Results
3.1. Inhibition of cell adhesion
r-Mojastin 1 inhibited SK-MEL-28 cell adhesion to fibronectin, and had an IC50 of 94.6 nM r-Mojastin 1was not able to inhibit T24 cell adhesion to fibronectin. r-Mojastin-GST inhibited SK-MEL-28 and T24 cells adhesion to fibronectin in a dose-dependent manner with an IC50 of 16 and 200 nM, respectively. The inhibition of SK-MEL-28 cells was 12.5 times more effective when compared to T24 cells. The native mojastin inhibited SK-MEL-28 cell adhesion to fibronectin, with an IC50 of 113 nM, and native mojastin was not able to inhibit T24 cell adhesion to fibronectin. r-Mojastin-GST was 7.1 times more effective than native mojastin in inhibiting the SK-MEL-28 cell adhesion to fibronectin. There was no significant difference between r-mojastin 1 and native mojastin in inhibiting the SK-MEL-28 cells adhesion to fibronectin (Table 1). Commercial echistatin used as positive control at 1.8 µM completely inhibited SK-MEL-28 and T24 cells adhesion to fibronectin.
Table 1.
Adhesion of T24 and SK-MEL-28 cells to fibronectin in presence of mojastin disintegrins.
| Disintegrin | Inhibition of cell adhesion T24 cellsa |
Inhibition of cell adhesion SK-Mel-28 cellsa |
|---|---|---|
| GST-r-Mojastin | 200 nM | 16 nM |
| r-Mojastin 1 | NA | 95 nM |
| Native mojastin | NA | 113 nM |
NA = No activity.
Commercial echistatin used as positive control at 1.8 µM completely inhibited SK-MEL-28 and T24 cells adhesion to fibronectin.
The results are expressed as IC50
3.2. Cell migration
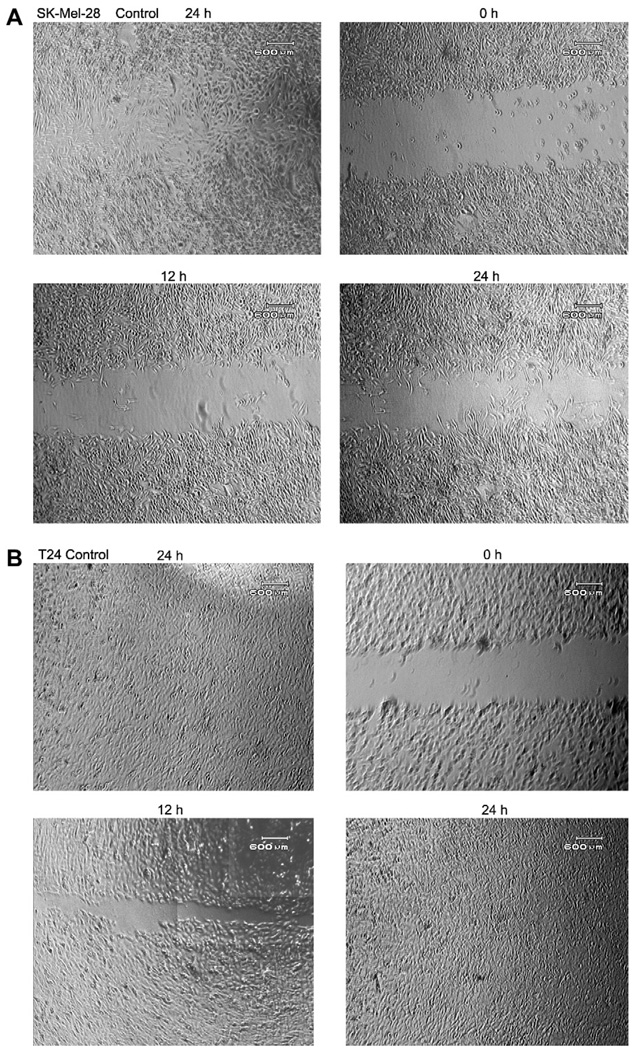
Cell migration and invasion is necessary for tumor metastasis, we therefore, investigated the effect of r-mojastin 1 on cell migration. T24 cells migrated into the wound, causing 100% closure in presence of r-Mojastin-GST and r-mojastin 1 at 5.3 µM after 24 h. However, SK-MEL-28 cells migrated by 40 and 32% after 24 h in presence of r-Mojastin-GST and r-mojastin 1, respectively at the same concentration. Commercial echistatin used as positive control at 2.7 µM allowed cell migration by 31 and 2% with T24 cells and SK-MEL-28 cells, respectively. In comparison to the negative control (SK-MEL-28 cells incubated with PBS buffer), a statistically significant difference between r-Mojastin-GST and r-mojastin 1 (p < 0.001) was observed. Native mojastin at 5.3 µM allowed T24 cells to migrate by 100%, and SK-MEL-28 cells to migrate by 38% after 24 h of incubation (Table 2 and Figs.1 and 2). There was no difference between recombinant disintegrins and native mojastin (p > 0.05).
Table 2.
Migration of T24 and SK-MEL-28 in presence of recombinant disintegrins.
| Time (h) |
T24 | SK-MEL-28 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (PBS) | r-Mojastin-GST | r-Mojastin 1 | nMojastin | Echistatin | Control (PBS) | r-Mojastin-GST | r-Mojastin 1 | nMojastin | Echistatin | |
| 3 | 22 ± 8 | 26 ± 10 | 11 ± 5 | 22 ± 0 | 4 ± 5 | 6 ± 5 | 5 ± 0 | 6 ± 3 | 5 ± 2 | 0 ± 0 |
| 6 | 56 ± 8 | 36 ± 15 | 26 ± 4 | 22 ± 0 | 8 ± 0 | 15 ± 9 | 20 ± 4 | 12 ± 2 | 10 ± 4 | 0 ± 0 |
| 12 | 100 ± 0 | 83 ± 12 | 63 ± 9 | 79 ± 0 | 28 ± 4 | 70 ± 0 | 28 ± 4 | 17 ± 4 | 20 ± 3 | 0 ± 0 |
| 24 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 31 ± 0 | 100 ± 0 | 40 ± 7 | 32 ± 7 | 38 ± 3 | 2 ± 4 |
Data are expressed as mean ± SD (n = 3). The results are expressed in percentage migration and calculated by the following equation: % Closure: [(C − E)/C] × 100, where C is the units of distance of cell edge (mm) for zero time of the control, and E is the units of distance of cell edge (mm) at the final incubation time for the disintegrin. The concentration of r-Mojastin 1, r-mojastin-GST and native mojastin used was 5.3 µM. Echistatin was used at 2.7 µM.
Fig. 1.
Inhibition cell migration of SK-MEL-28 (A) and T24 (B) cells were measured when r-mojastin 1 (5.3 µM) and r-mojastin-GST (5.3 µM) were added to tissue culture media. A confluent monolayer of cells was maintained in medium, and a line was scraped through the monolayer of cells with a plastic, sterile pipette tip. The cultures were allowed to migrate for 24 h at 37 °C in the presence or absence of recombinant disintegrins. The extent of wound closure was quantified by multiple measurements of the width of the scrape space for each cell line.
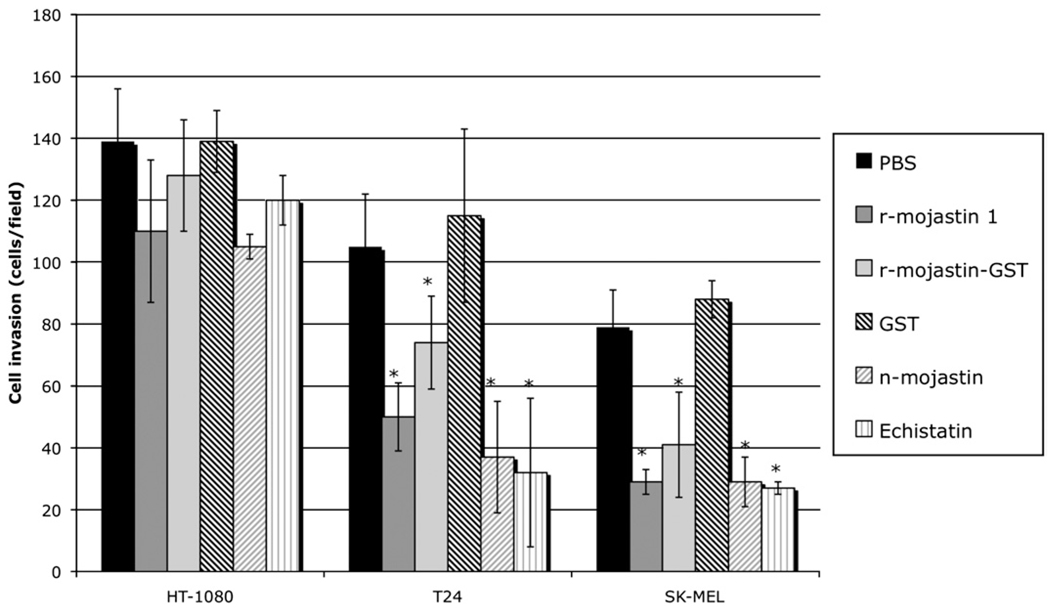
Fig. 2.
r-Mojastins inhibited the invasion of SK-MEL-28, T24 and HT-1080 cells. Tumor cells (7.5 × 105 cells/mL, 0.2 mL) were treated with 5.3 µM of r-mojastin 1 and r-mojastin-GST for 30 min at 37 °C, and then placed in the upper Boyden chamber containing a matrigel-coated filter membrane. Invasion was induced by adding McCoy’s 5A minimum essential medium and Eagle’s minimum essential medium with FBS to the lower chamber for 24 h. After fixation and removal of nonmigrated cells, cells that migrated to the underside of filter membrane were quantified by light microscope (3 fields/40×). *p < 0.0001.
3.3. Cell invasion
A modified Boyden chambers coated with three-dimensional matrigel was used to test recombinant mojastins ability to inhibit invasion of cancer cells through an artificial basement membrane. HT-1080, T24 and SK-MEL-28 cells were used in the Boyden chamber. All three cell lines, HT-1080, T24 and SK-MEL-28, migrated through matrigel toward the fetal bovine serum (Fig. 2). r-Mojastin 1 and r-mojastin-GST at 5.3 µM inhibited invasion of T24 cells by 57 and 36%, and invasion of SK-MEL-28 cells by 67 and 53%, respectively (p < 0.0001). In comparison with the negative control (cells incubated with PBS buffer), r-mojastin 1 was more potent than r-mojastin-GST in blocking T24 cells invasion. Native mojastin (5.3 µM) inhibited invasion of T24 and SK-Mel-28 cells by 68 and 67%, respectively. Positive control echistatin (1.8 µM) also inhibited invasion of T24 and SK-MEL-28 cells by 72 and 69%, respectively (p < 0.0001). r-Mojastin 1 and r-mojastin-GST were not able to inhibit invasion of HT-1080 (p > 0.05).
3.4. Inhibition of lung tumors in vivo
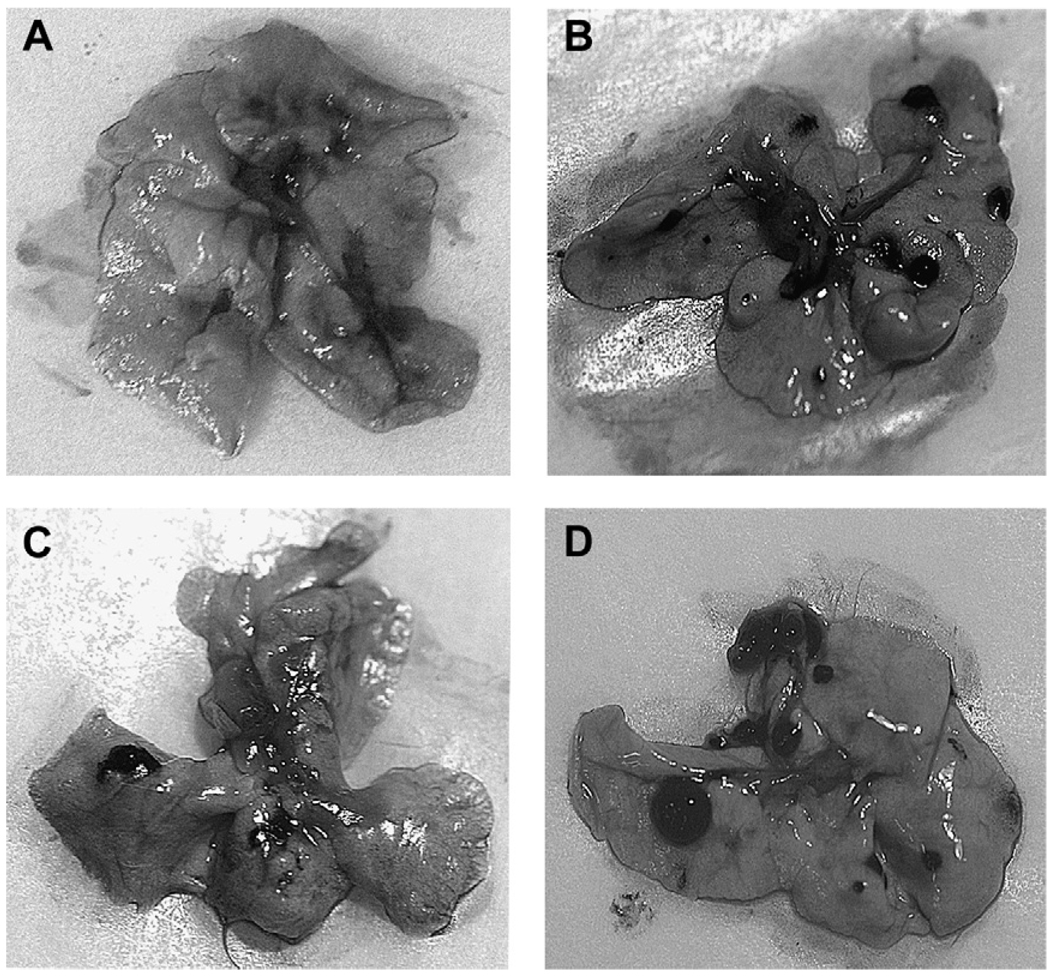
To investigate the inhibitory effect of r-disintegrins on lung colonization in vivo, r-mojastin 1 and r-mojastin-GST incubated with B16F10 melanoma cells (1 × 106) were injected i.v in BALB/c mice. r-Mojastin 1 inhibited lung colonization by 51.5% at a dose of 1000 µg/kg, compared to the control group (p-value = 0.0216, Table 3 and Fig. 3). r-Mojastin-GST was not able to inhibit tumor metastasis of B16F10 melanoma cells.
Table 3.
Comparative analysis of tumor foci per lung in BALB/c mice using B16F10 cells and r-mojastins at 1000 µg/kg compared to controls.
| Controla | r-Mojastin-GST | r-Mojastin 1 | |
|---|---|---|---|
| # mice | 9 | 6 | 5 |
| Minimum tumors | 12 | 1 | 4 |
| Maximum tumors | 29 | 42 | 13 |
| Mean tumors | 17 | 15 | 8 |
| Standard deviation | 6 | 15 | 4 |
| p-Value* | 0.2845 | 0.0216 |
p-Value as compared to the control. p < 0.05 = significant difference.
The control consisted of B16F10 tumoral cells in Dulbecco’s modified Eagle’s medium without FBS.
Fig. 3.
The effect of r-mojastin 1 on B16F10 lung tumor colonization in a BALB/c mice at 1000 µg/kg mouse. The B16F10 cells (1 × 106) were injected in the lateral tail vein of BALB/c mice in the absence or presence of r-mojastin 1. Lungs were isolated from BALB/c mice developed for experimental metastasis after 19 days. (A) Medium-treated mice. (B) B16F10 cells in medium. (C) 1000 µg r-mojastin 1/kg mouse. (D) 1000 µg r-mojastin-GST/Kg mouse.
4. Discussion
Snake disintegrins are potent and specific antagonists of various integrins. Integrins are a superfamily of cell adhesion receptors that are structurally related heterodimers consisting of a α and β subunits (Humphries, 2000; Hynes et al., 2002). Integrins participate in the complex biological process of embryonic development and maintenance of tissue integrity (Brown et al., 1993; Delon and Brown, 2009). They also function in wound healing and pathological processes such as inflammation and malignant transformation by affecting cellular activities like cell growth, differentiation, migration and apoptosis (Selistre-de-Araujo et al., 2005; Becchetti and Arcangeli, 2010). Over the last decade, snake disintegrins have been the subject of great interest as potent ligands to integrins (McLane et al., 2004). Recently, a disintegrin cloned from C. s. scutulatus named r-mojastin 1, was expressed in E. coli, which inhibited platelet functions. r-Mojastin 1 was 2–49 times stronger in inhibiting ADP-induced platelet aggregation using PRP when compared with other recombinant disintegrins such as r-adinbitor, r-echistatin and r-viplebedin-2 (Wang et al., 2004; Wierzbicka-Patynowski et al., 1999; Vija et al., 2009; Sánchez et al., in press). In this work r-mojastin 1 and r-mojastin-GST were evaluated for their role in preventing cancer cell adhesion to extracellular matrices, migration and invasion.
In order to survive, cells must be able to adhere to their surroundings. Cancer cell adhesion is supported by integrins and is essential for migration and invasion. Adhesion of tumor cells to the vascular extracellular matrix is a critical role in metastasis. Many integrins including αvβ3, αvβ5, αvβ6, α6β4 and α9β1 are highly expressed in metastatic cancer cells, and they are involved in the degradation of the basement membrane through interactions with proteolytic enzymes like MMP-2 and MMP-9 suggesting that certain integrins plays an important role in migration and invasion (Rathinam and Alahari, 2010).
In general, integrins recognize short linear peptide sequences on adhesion proteins, the most prevalent being the arginine-glycine-aspartic acid motif (RGD), which is found on many ECM proteins such as fibronectin, collagens and vitronectin, but also on many disintegrins (Akiyama, 1996). Snake disintegrins, which include contortrostatin, adinbitor, eristostatin, echistatin, and obstustatin to name a few, could be effective anti-cancer drugs.
For instance, contortrostatin (an RGD containing homodimeric disintegrin from Agkistrodon contortrix contortrix) disrupted adhesion of human bladder carcinoma (T24) and invasion of human ovarian cancer cells (OVCAR-5) (Zhou et al., 2000). Recombinant contortrostatin was expressed in a eukaryotic expression system using the Origami-gami B (DE3) pLysSas expression host. Recombinant contortrostatin also inhibited adhesion of breast cancer cell (MDA-MB-435) on fibronectin coated plated, with an IC50 almost identical to native contortrostatin (Minea et al., 2005). Similar to native contortrostatin, vicostatin (VCN) a chimeric disintegrin generated recombinantly by grafting the C-terminal tail end of the viperid snake venom disintegrin echistatin to the sequence of crotalid disintegrin contortrostatin (CN), inhibited significantly HUVEC, MDA-MB-231 and MDA-MB-435 cell invasion in a dose-dependent manner tested in a modified Boyden chamber assay (Minea et al., 2010). Another recombinant monomeric RGD containing disintegrin, r-Adinbitor, from Agkistrodon halys brevicaudus stejneger (Chinese snake) was able to inhibit basic fibroblast growth factor (bFGF)-induced proliferation of ECV304 cells with an IC50 of 890 nM (Wang et al., 2004). Disintegrins are also known to inhibit angiogenesis, a process mediated through integrins αvβ3, αvβ5, αvβ1 and α1β1 (Marcinkiewicz et al., 2003; Huang et al., 2001). The snake venom disintegrin, obstustatin (Vipera lebetina obtusa), inhibited angiogenesis via α1β1 using the chicken chorioallantoic membrane (CAM) assay (Marcinkiewicz et al., 2003).
In this work, r-mojastin 1 and r-mojastin-GST were used to study the inhibition of cancer cell adhesion, migration and invasion. The cloned disintegrin, r-mojastin 1, inhibited SK-ML-28 cell adhesion to fibronectin, but was not able to inhibit T24 cell adhesion to fibronectin. On the other hand, r-mojastin-GST inhibited SK-ML-28 and T24 cells adhesion to fibronectin. In a previous study, native mojastin (Sánchez et al., 2006) was also unable to inhibit T24 cell adhesion, which may indicate that the GST tag attached to the r-mojastin is playing a role in blocking the integrin(s) on T24 cells involved in binding fibronectin. Like r-mojastin 1, anti tumoral and anti-platelet activity has been reported in other recombinant disintegrins such as r-contortrostatin, robtustatin, r-jerdostatin, r-elengantin and r-kistrin which showed inhibitory activity toward αIIbβ3 (integrin found on platelets), and α5β1 and α1β1 integrin-mediated cell adhesion tested in different tumoral cell lines such as myelogenous leukemia (K562), human breast (MDA-MB-435) and Jurkat cells (Brown et al., 2009; Rahman et al., 1998; Sanz et al., 2005; Minea et al., 2005).
r-Mojastin disintegrins were also able to inhibit cell migration (Fig. 1 and Table 2). Cell migration plays an essential role in a wide variety of biological phenomena, which involve vascular diseases, chronic inflammatory diseases and tumor metastasis, the main cause of death from cancer. Many cell adhesion molecules including integrins have been implicated in cell migration and tumor invasion (Geiger and Peeper, 2009; Janik et al., in press). The integrin α3β1 is elevated in several types of metastatic tumors, and has been found to be associated with increased migration and invasion. It has been shown to recognize a variety of ECM proteins, including laminin-1, laminin-5, fibronectin, collagen, and entactin (Pochéc et al., 2006; Tsuji et al., 2002). Because it plays an essential role in angiogenesis and vascular remodeling, integrin αvβ3 is one of the best-characterized integrins (Rüegg and Alghisi, 2010). Other integrins including αvβ5, αvβ6, α6β4, and α9β1 exhibit high expression in cancer, and they are involved in the degradation and invasion of the basement membrane through interaction with proteolytic enzymes like MMP-2 and MMP-9 (Rathinam and Alahari, 2010).
Both r-mojastin-GST and r-mojastin 1 significantly decreased the ability of SK-MEL-28 cells to migrate after 24 h of incubation but were not able to inhibit T24 cell migration. The fact that the r-mojastin 1was more sensitive in inhibiting SK-MEL cells may be due to the presence of different integrins on both cell lines, which causes some cell lines to be more selective to certain ECM than others. This may be the case for r-mojastin 1, which may be more specific for certain integrins on SK-MEL cells and not to those integrins found on the T24 cells. Flow cytometry analysis and immunoprecipitation studies of T24 cells demonstrated the expression of α2, α3, α5, α6, αv, β1, β3, and β4 integrins subunits and the absence of α4 (Kuroda et al., 1993). In contrast, malignant melanomas like SK-MEL-28 demonstrate an increase in the expression of α4β1 (Johnson, 1999). The ligands of the α4α1 integrin include thrombospondin, fibronectin and the endothelial cell adhesion molecule VCAM-1. Expression of α4β1 by melanoma cells could allow the tumoral cells to migrate from the vascular system into any tissue in which VCAM endothelial expression has been induced, an important step in the establishment of metastatic lesions (Johnson, 1999). It is probable that r-mojastin 1 recognizes the α4β1 integrin on SK-Mel-28 and this integrin is not present on T24 cells, thus, explaining the higher potency with the SK-MEL-28 cell line (Sánchez et al., 2009).
In addition to the inhibition of adhesion and migration activities of native and recombinant mojastins, both inhibited the invasion of malignant cancer cells through an artificial basement membrane (Fig. 2). It has been reported that integrins αvβ3 and αvβ5 mediate cancer cell invasion, although the latter requires collaboration from certain growth factors (Zhou et al., 2000). Considering that mojastin 1 contains the sequence RGDW, a motif with a high affinity to αIIbβ3 receptors, but weak affinity to αvβ3 receptors in solid-phase ligand binding assays (Sánchez et al., 2006), it is probable that r-mojastin 1 recognizes the αvβ5 integrin blocking tumoral cell invasion.
One common disadvantage of cloned disintegrins is that their activities may be less than the native disintegrins (Sánchez et al., in press); however, it is worthwhile to note that in our cancer cell adhesion and migration studies the potency of r-mojastin 1 was similar to that of the native mojastin (Tables 1 and 2). This was not the case with inhibition of platelet aggregation studies, in which the recombinant disintegrins in some cases appear to have significantly lower activities than their native counterparts (Sánchez et al., in press). It is also important to note the increased potency observed with the r-mojastin-GST compared to r-mojastin 1. Therefore, it cannot be ignored that the GST molecule with a molecular weight of 26 kDa, which dominates the overall structure of the fusion protein, directly impacts the structure and activity of r-mojastin 1 (Sánchez et al., in press). It is possible that GST may affect disintegrin activity either by altering the folding of r-mojastin 1; and thus its specificity of binding to the integrin, and/or the GST which makes the r-mojastin 1 a larger molecule, resulting in an overhang that may block integrins. This could lead to increased activity observed in the adhesion assay with T24 and SK-MEL-28 cells (Table 1). In light of this, future research on structure–function relationships using cloned proteins should be conducted with the recombinant protein without the GST tag, which as shown in this study may affect the activity of recombinant proteins. To our knowledge, this is the first work comparing the anti tumoral activity of a recombinant protein with and without GST tag.
Disintegrins have also been shown to be effective anti-metastatic agents. Crotatroxin 2, a disintegrin isolated from the venom of Crotalus atrox inhibited lung tumor colonization of 66.3p cells at a dose of 1000 µg/kg tested in vivo using a BALB/c mouse model. Eristostatin inhibited MV3 cell experimental metastasis by interfering with α4β1 rather than α5β1 or αvβ3 (Galán et al., 2008; Danen et al., 1998). In vivo B16F10 melanoma experimental metastatsis, r-salmosin, a recombinant disintegrin derived from the Korean snake (A. h. brevicaudus) showed a remarkable inhibitory effect on lung tumor colonization in a concentration-dependant manner (Kang et al., 2000). In our present study, experimental inhibition of lung tumor colonization in vivo tested in a BALB/c mouse model was significantly inhibited by r-mojastin 1 at a dose of 1000 µg/kg permouse with respect to number of tumors that developed in absence of r-mojastin 1.
In conclusion, our studies suggest that after further characterization, r-mojastin 1 could be a useful tool in developing novel anti-tumor agents by virtue of its ability to inhibit tumor cell adhesion, migration and invasion. Taking account the importance of angiogenesis in metastasis, r-mojastin 1 is currently being used to study its effect on angiogenesis in endothelial cells, which are the main cells involve in vessel blood formation.
Acknowledgments
This work was funded by a grant from the National Institutes of Health (NIH/Viper Resource Center #5 P40 RR018300-07). We are thankful to the students of the Summer Research Program at Texas A&M University-Kingsville, USA, for their assistance. We are grateful to the staff of the National Natural Toxins Research Center (NNTRC).
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical statement
This research was approved by Texas A&M University-Kingsville Human Subjects Committee.
References
- Akiyama SK. Integrins in cell adhesion and signaling. Hum. Cell. 1996;3:181–186. [PubMed] [Google Scholar]
- Assakura MT, Silva CA, Mentele R, Camargo ACM, Serrano SMT. Molecular cloning and expression of structural domains of bothropasin, a P-III metalloproteinase from the venom of Bothrops jararaca. Toxicon. 2003;41:217–227. doi: 10.1016/s0041-0101(02)00279-9. [DOI] [PubMed] [Google Scholar]
- Becchetti A, Arcangeli A. Integrins and ion channels in cell migration: implications for neuronal development, wound healing and metastasis spread. Adv. Exp. Med. Biol. 2010;674:107–123. doi: 10.1007/978-1-4419-6066-5_10. [DOI] [PubMed] [Google Scholar]
- Brown MC, Eble JA, Calvete JJ, Marcinkiewicz C. Structural requirements of KTS- disintegrins for inhibition of α1β1 integrin. Biochem. J. 2009;417:95–101. doi: 10.1042/BJ20081403. [DOI] [PubMed] [Google Scholar]
- Brown NH, Bloor JW, Dunin-Borkowski O, Martín-Bermudo MD. Integrins and morphogenesis. Dev. Suppl. 1993:177–183. [PubMed] [Google Scholar]
- Calvete JJ, Moreno-Murciano MP, Theakston RD, Kisiel DG, Marcinkiewicz C. Snake venom disintegrins: novel dimeric disintegrins and structural diversification by disulphide bond engineering. Biochem. J. 2003;15:725–734. doi: 10.1042/BJ20021739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa MC, Jr, Maria DA, Moura-da-Silva AM, Pizzocaro KF, Ruiz IRG. Inhibition of melanoma cells tumorigenicity by the snake venom toxin jararhagin. Toxicon. 2002;40:739–748. doi: 10.1016/s0041-0101(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Danen EH, Marcinkiewicz C, Cornelissen IM, Van KA, Pachter JA, Ruiter DJ, Niewiarowski S, Van MG. The disintegrin eristostatin interferes with integrins alpha 4 beta 1 function and with experimental metastasis of human melanoma cells. Exp. Cell Res. 1998;238:188–196. doi: 10.1006/excr.1997.3821. [DOI] [PubMed] [Google Scholar]
- Delon I, Brown NH. The integrin adhesion complex changes its composition and function during morphogenesis of an epithelium. J. Cell Sci. 2009;1:4363–4374. doi: 10.1242/jcs.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding-Habermann B. Targeting tumor cell-platelet interaction in breast cancer metastasis. Pathophysiol. Haemost. Thromb. 2003;33:56–58. doi: 10.1159/000073295. [DOI] [PubMed] [Google Scholar]
- Fernandez JH, Silva CA, Assakura MT, Camargo ACM, Serrano SMT. Molecular cloning, functional expression, and molecular modeling of bothrostatin, a new highly active disintegrin from Bothrops jararaca venom. Biochem. Biophys. Res. Commun. 2005;329:457–464. doi: 10.1016/j.bbrc.2005.01.148. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Okuda D, Fujimoto Z, Horii K, Morita T, Mizuno H. Crystal structure of trimestatin, a disintegrin containing a cell adhesion recognition motif RGD. J. Mol. Biol. 2003;332:1115–1122. doi: 10.1016/s0022-2836(03)00991-4. [DOI] [PubMed] [Google Scholar]
- Galán JA, Sánchez EE, Rodríguez-Acosta A, Soto JG, Bashir S, McLane MA, Paquette-Straub C, Pérez JC. Inhibition of lung tumor colonization and cell migration with the disintegrin crotatroxin 2 isolated from the venom of Crotalus atrox. Toxicon. 2008;51:1186–1196. doi: 10.1016/j.toxicon.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger TR, Peeper DS. Metastasis mechanisms. Biochim. Biophys. Acta. 2009;2:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Huang TF, Yeh CH, Wu WB. Viper venom components affecting angiogenesis. Haemostasis. 2001;31:192–206. doi: 10.1159/000048063. [DOI] [PubMed] [Google Scholar]
- Humphries MJ. Integrin structure. Biochem. Soc. Trans. 2000;28:311–339. [PubMed] [Google Scholar]
- Hynes RO, Lively JC, McCarty JH, Taverna D, Francis SE, Hodivala-Dilke K, Xiao Q. The diverse roles of integrins and their ligands in angiogenesis. Cold Spring Harb. Symp. Quant Biol. 2002;67:143–153. doi: 10.1101/sqb.2002.67.143. [DOI] [PubMed] [Google Scholar]
- Janik ME, Litynska A, Vereecken P. Cell migration-The role of integrin glycosylation. Boichim Biophys Acta. doi: 10.1016/j.bbagen.2010.03.013. in press. [DOI] [PubMed] [Google Scholar]
- Johnson JP. Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Metastasis Rev. 1999;18:345–357. doi: 10.1023/a:1006304806799. [DOI] [PubMed] [Google Scholar]
- Kang IC, Kim DS, Jang Y, Chung KH. Suppressive mechanism of salmosin, a novel disintegrin in B16 melanoma cell metastasis. Biochem. Biophys. Res. Commun. 2000;275:169–173. doi: 10.1006/bbrc.2000.3130. [DOI] [PubMed] [Google Scholar]
- Kim J, Hong S, Park H, Kim D, Lee W. Structure and function of RGD peptides derived from disintegrin proteins. Mol. Cells. 2005;19:205–211. [PubMed] [Google Scholar]
- Kuroda K, Brown E, Telle WB, Russell DG, Ratliff TL. Characterization of the internalization of bacillus calmette-Guerin by human bladder tumor cells. J. Clin. Invest. 1993;91:69–76. doi: 10.1172/JCI116202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz C, Weinreb PH, Calvete JJ, Kisiel DG, Mousa SA, Tuszynski GP, Lobb RR. Obtustatin: a potent selective inhibitor of α1β1 integrin in vitro and angiogenesis in vivo. Cancer Res. 2003;63:2020–2023. [PubMed] [Google Scholar]
- McGary EC, Chelouche D, Bar-Eli M. Cellular adhesion pathways and metastatic potencial of human melanoma. Cancer Biol. Ther. 2002;1:459–465. doi: 10.4161/cbt.1.5.158. [DOI] [PubMed] [Google Scholar]
- McLane MA, Sánchez EE, Wong A, Paquette-Straub C, Pérez JC. Disintegrins. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004;4:327–355. doi: 10.2174/1568006043335880. [DOI] [PubMed] [Google Scholar]
- Minea R, Helchowski CM, Zidovetzki SJ, Costa FK, Swenson SD, Markland FS. Vicrostatin-an anti-invasive multi-integrin targeting chimeric disisntegrin with tumor anti-angiogenic and pro-apoptotic activities. PLoS ONE. 2010;5(6):e10929. doi: 10.1371/journal.pone.0010929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minea R, Swenson S, Costa F, Chen TC, Markland FS. Development of a novel recombinant disintegrin, contortrostatin, as an effective anti-tumor and anti-angiogenic agent. Pathophysiol. Haemost. Thromb. 2005;34:177–183. doi: 10.1159/000092419. [DOI] [PubMed] [Google Scholar]
- Moreno-Murciano MP, Monleón D, Calvete JJ, Celda B, Marcinkiewicz C. Amino acid sequence and homology modeling of obtustatin, a novel non-RGD-containing short disintegrin isolated from the venom of Vipera lebetina obtusa. Protein Sci. 2003;12:366–371. doi: 10.1110/ps.0230203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda D, Koike H, Morita T. A new disintegrin gene structure of the disintegrin family: a subunit of dimeric disintegrin has a short coding region. Biochemistry. 2002;41:14248–14254. doi: 10.1021/bi025876s. [DOI] [PubMed] [Google Scholar]
- Olfa K, Jose L, Salma D, Amine B, Najet SA, Nicolas A, Maxime L, Raoudha Z, Kamel M, Jacques M, Jean-Marc S, Mohamed EA, Naziha M. Lebestatin, a disintegrin from Macrovipera venom, inhibits integrin-mediated cell adhesion, migration and angiogenesis. Lab. Invest. 2005;85:1507–1516. doi: 10.1038/labinvest.3700350. [DOI] [PubMed] [Google Scholar]
- Pochéc E, Litynska A, Bubka M, Amoresano A, Casbarra A. Characterization of the oligosaccharide component of α3β1 integrin from human bladder carcionoma cell line T24 and its role in adhesion and migration. Eur. J. Cell Biol. 2006;85:47–57. doi: 10.1016/j.ejcb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rahman S, Aitken A, Flynn G, Formstone C, Savidge G. Modulation of RGD sequence motifs regulates disintegrin recognition of αIIbβ3 and α5β1 integrin complexes. Biochem. J. 1998;335:247–257. doi: 10.1042/bj3350247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- Rüegg C, Alghisi GC. Vascular integrins: therapeutic and imaging targets of tumor angiogenesis. Recent Results Cancer Res. 2010;180:83–101. doi: 10.1007/978-3-540-78281-0_6. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Cell adhesion and tumor metastasis. Princess Takamatsu Symp. 1994;24:99–105. [PubMed] [Google Scholar]
- Sánchez EE, Galán JA, Russell WK, Soto JG, Russell DH, Pérez JC. Isolation and characterization of two disintegrins inhibiting ADP-induced human platelet aggregation from the venom of Crotalus scutulatus scutulatus (Mohave rattlesnake) Toxicol. Appl. Pharmacol. 2006;212:59–68. doi: 10.1016/j.taap.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Rodriguez AA, Palomar R, Lucena SE, Bashir S, Soto JG, Pérez JC. Colombistatin: a disintegrin isolated from the venom of the south American snake (Bothrops colombiensis) that effectively inhibits platelet aggregation and SK-MEL-28 cell adhesion. Arch. Toxicol. 2009;83:271–279. doi: 10.1007/s00204-008-0358-y. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Lucena SE, Reyes S, Soto JG, Cantu E, Lopez-Johnston JC, Guerrero B, Salazar AM, Rodríguez-Acosta A, Galán JA, Tao WA, Pérez JC. Cloning, expression, and hemostatic activities of a disintegrin, r-mojastin 1, from the Mohave rattlesnake (Crotalus scutulatus scutulatus) Thromb Res. doi: 10.1016/j.thromres.2010.06.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L, Chen R, Pérez A, Hilario R, Juárez P, Marcinkiewicz C, Monleón D, Celda B, Xiong Y, Pérez-Payá E, Calvete JJ. cDNA cloning and functional expression of jerdostatin, a novel RTS-disintegrin from Trimeresurus jerdonii and a specific antagonist of the α1β1 integrins. J. Biol. Chem. 2005;280:40714–40722. doi: 10.1074/jbc.M509738200. [DOI] [PubMed] [Google Scholar]
- Selistre-de-Araujo HS, Cominetti MR, Terruggi CHB, Mariano-Oliveira A, De Freitas MS, Crepin M, Figueiredo CC, Morandi V. Alternagin-C, a disintegrin-like protein from the venom of Bothropss alternatus, modulates α2β1 integrin-mediated cell adhesion, migration and proliferation. Braz. J. Med. Biol. Res. 2005;38:1505–1511. doi: 10.1590/s0100-879x2005001000007. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Kawada Y, Kai-Murozono M, Komatsu S, Han SA, Takeuchi K, Mizushima H, Miyazaki K, Irimura T. Regulation of melanoma cell migration and invasion by laminin-5 and α3β1 integrin (VLA-3) Clin. Exp. Metastasis. 2002;19:127–134. doi: 10.1023/a:1014573204062. [DOI] [PubMed] [Google Scholar]
- Versteeg HH, Spek CA, Peppelenbosch MP, Richel D. Tissue factor and cancer metastasis: the role of intracellular and extracellular signaling pathways. Mol. Med. 2004;10:6–11. doi: 10.2119/2003-00047.versteeg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vija H, Samel M, Siigur E, Aaspõllu A, Tõnismägi K, Trummal K, Subbi J, Siigur J. VGD and MLD-motifs containing heterodimeric disintegrin viplebedin-2 from Vipera lebetina snake venom. purification and cDNA cloning. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2009;153:253–260. doi: 10.1016/j.cbpb.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Wang JH, Wu Y, Ren F, Lu L, Zhao BC. Cloning and characterization of adinbitor, a novel disintegrin from the snake venom of Agkistrodon halys brevicaudus stejneger. Acta Biochem. Biophys. Sin. 2004;36:425–429. doi: 10.1093/abbs/36.6.425. [DOI] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Niewiarowski S, Marcinkiewicz C, Calvette JJ, Marcinkiewicz MM, McLane MA. Structural requirements of echistatin for the recognition of alpha (v) beta (3) and alpha (5) beta (1) integrins. J. Biol. Chem. 1999;274:37809–37814. doi: 10.1074/jbc.274.53.37809. [DOI] [PubMed] [Google Scholar]
- Yang R, Tang C, Chuang W, Huang T, Peng H, Huang T, Fu W. Inhibition of tumor formation by snake venom disintegrin. Toxicon. 2005;45:661–669. doi: 10.1016/j.toxicon.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Yeh C, Peng H, Yang R, Huang T. Rhodostomin, a snake venom disintegrin, inhibits angiogenesis tumor growth by a selective αvβ3 blockage of endothelial cells. Mol. Pharmacol. 2001;59:1333–1342. doi: 10.1124/mol.59.5.1333. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Nakada MT, Brooks PC, Swenson SD, Ritter MR, Argounova S, Arnold C, Markland FS. Contortrostatin, a homodimeric disintegrin, binds to Integrin αvβ5. Biochem. Biophys. Res. Commun. 2000;267:350–355. doi: 10.1006/bbrc.1999.1965. [DOI] [PubMed] [Google Scholar]