Abstract
Objectives
Our aim was to examine the independent and combined associations of changes in fitness and fatness with the subsequent incidence of the cardiovascular disease (CVD) risk factors of hypertension, metabolic syndrome, and hypercholesterolemia.
Background
The relative and combined contributions of fitness and fatness to health are controversial, and few studies are available on the associations of changes in fitness and fatness with the development of CVD risk factors.
Methods
We followed 3,148 healthy adults who received at least three medical examinations. Fitness was determined by a maximal treadmill test. Fatness was expressed by percent body fat and body mass index. Changes in fitness and fatness between the first and second examinations were categorized into loss, stable, or gain groups.
Results
During the 6-year follow-up after the second examination, 752, 426, and 597 adults developed hypertension, metabolic syndrome, and hypercholesterolemia, respectively. Maintaining or improving fitness was associated with lower risk of developing each outcome, whereas increasing fatness was associated with higher risk of developing each outcome, after adjusting for possible confounders and fatness or fitness for each other (all p for trend <0.05). In the joint analyses, the increased risks associated with fat gain appeared to be attenuated, although not completely eliminated, when fitness was maintained or improved. In addition, the increased risks associated with fitness loss were also somewhat attenuated when fatness was reduced.
Conclusions
Both maintaining or improving fitness and preventing fat gain are important to reduce the risk of developing CVD risk factors in healthy adults.
Keywords: cardiorespiratory fitness, body fatness, hypertension, metabolic syndrome, hypercholesterolemia
Cardiovascular disease (CVD) accounts for one third of U.S. mortality, and the high prevalence of hypertension, metabolic syndrome, and hypercholesterolemia is a major factor.(1) Cardiorespiratory fitness (hereafter fitness) and body fatness are strong predictors for CVD risk factors,(2–4) as well as CVD morbidity and mortality.(5–8)
Given the diverse combinations of both fitness and fatness in adult populations,(9) the relative and combined contributions of fitness and fatness to health are controversial.(3,4,8,10,11) Some studies indicate that fitness can eliminate the harmful effect of fatness,(12,13) suggesting fat but fit persons do not have excess health problems. Others report that higher levels of fitness or physical activity are beneficial and attenuate, but do not completely eliminate, the negative effect of fatness.(10,14) One possible reason for the discrepancy among these studies is the inaccurate measurement of fitness and fatness.(11) Many population studies used self-reported physical activity rather than objectively-measured fitness, and fatness was measured by body mass index (BMI), a crude estimate of body fatness.(15) In addition, previous studies were conducted mostly on mortality outcomes, but little is known about the combined associations of fitness and fatness with the development of CVD risk factors.
Fitness and fatness change over time, and the patterns of change vary among individuals.(16) However, most previous studies were based on a single baseline assessment of either fitness or fatness with health outcomes. Combined associations of simultaneous changes in fitness and fatness with subsequent incident CVD risk factors remain uncertain. The purpose of this study was to examine the independent and combined associations of changes in fitness and fatness with the development of CVD risk factors, focusing on hypertension, metabolic syndrome, and hypercholesterolemia, in healthy adults, using objectively-measured fitness and fatness.
Methods
Study Population
The Aerobics Center Longitudinal Study (ACLS) is a prospective observational study of individuals who received extensive preventive medical examinations at the Cooper Clinic in Dallas, Texas. More than 95% of participants are non-Hispanic whites from middle-to-upper socioeconomic strata.(17) Participants are self-referred or are referred by their employers or physicians. Our current analyses included men and women aged 18 years or older at baseline who received at least three medical examinations during 1979 to 2006. We used the first (baseline) and second examinations to assess changes in fitness and fatness, and followed participants for incident CVD risk factors from the second through their final examinations. All participants achieved at least 85% of their age-predicted maximal heart rate (220 minus age in years) on the maximal exercise test at the first and second examinations.(17,18)
We excluded participants with CVD, cancer, diabetes, or abnormal resting or exercise electrocardiogram at the first and/or second examinations (n=1,525). For the analyses of incident hypertension, metabolic syndrome, and hypercholesterolemia as study outcomes, we also excluded participants with any of these conditions at either examination (n=4,197). Additionally, we excluded participants who answered “Yes” to the question about “unexplained weight loss or gain” at the second examination (n=24). These exclusion criteria eliminated many participants. However, this conservative approach was important to minimize potential bias due to underlying or preexisting disease on changes in fitness, body weight, and subsequent health risk.(19,20) There were no women who were pregnant at the baseline or second examination. Our final sample included 3,148 health adults (2,622 men and 526 women). This study was reviewed and approved annually by the Cooper Institute Institutional Review Board. All participants gave written informed consent for the examinations and follow-up study.
Clinical Examination
All participants completed comprehensive clinical examinations by a physician. Blood chemistries were analyzed after at least 12 hours overnight fast with automated bioassays in the Cooper Clinic laboratory. Resting blood pressure was measured by standard auscultatory methods after at least 5 minutes of seated rest.(21) Waist circumference was measured at the umbilicus level. Smoking status, alcohol consumption, leisure-time physical activity, and physician-diagnosed CVD, cancer, hypertension, diabetes, and hypercholesterolemia were obtained from a standardized medical history questionnaire.
Cardiorespiratory Fitness, Percent Body Fat, and Body Mass Index
Maximal treadmill testing using a modified Balke protocol was utilized to assess fitness in maximal metabolic equivalents( METs), as previously described.(17,22) Participants were encouraged to give maximal effort, and the test was terminated upon volitional exhaustion or medical reasons determined by physician. Maximal METs was estimated based on the final treadmill speed and grade using the following formula from the American College of Sports Medicine: [3.5 + (0.1 X speed) + (1.8 X speed X grade)] / 3.5.(23) Percent body fat was determined using seven-site skinfold measurement (chest, axilla, triceps, subscapula, abdomen, supra-iliac, and thigh) with a skinfold fat caliper. Body fatness was estimated using a generalized body density equation that is highly correlated (r>0.90) with percent body fat from hydrodensitometry (underwater weighing).(24) Detailed procedures for this percent body fat assessment have been reported previously.(12,24) BMI was calculated as measured weight in kilograms divided by the square of measured height in meters.
Changes in fitness and fatness as continuous variables were calculated as the differences in maximal METs, percent body fat, and BMI between the first and second examinations, divided by number of years between examinations. Because the intervals between examinations vary among individuals in this cohort, we used changes in fitness and fatness per year. Approximately half of the participants showed increases in maximal METs (54%), percent body fat (52%), and BMI (53%), and others showed a decrease (or no change). Based on these approximately equal distributions, changes in fitness and fatness were categorized into thirds for simplifying the complicated combined associations of changes in fitness and fatness with three incident CVD risk factors. The lower thirds of changes in maximal METs, percent body fat, and BMI showing annual mean (range) decreases of –0.58 (–3.56 to -0.03) METs, –2.76 (–16.91 to –0.70) %, and –0.84 (–9.93 to –0.18) kg/m2, respectively, were categorized as “loss”; the middle thirds showing small changes of 0.16 (–0.03 to 0.46) METs, 0.05 (–0.70 to 0.72) %, and 0.03 (–0.18 to 0.21) kg/m2 were categorized as “stable”; and the upper thirds showing increases of 1.22 (0.46 to 5.64) METs, 2.11 (0.72 to 13.09) %, and 0.64 (0.21 to 7.40) kg/m2 were categorized as “gain”. For the joint analyses, we created nine combinations from each of the three fitness and percent body fat change categories.
Cardiovascular Disease Risk Factors
Hypertension was defined as resting systolic or diastolic blood pressure at least 140/90 mm Hg or physician-diagnosed hypertension. Metabolic syndrome was defined as the presence of three or more of the following criteria: waist circumference greater than 102 cm in men and 88 cm in women, triglycerides at least 150 mg/dL, high-density lipoprotein (HDL) cholesterol less than 40 mg/dL in men and 50 mg/dL in women, blood pressure at least 130/85 or physician-diagnosed hypertension, and fasting glucose at least 100 mg/dL or physician-diagnosed diabetes according to the National Cholesterol Education Program Adult Treatment Panel III.(25) Hypercholesterolemia was defined as total cholesterol at least 240 mg/dL or physician-diagnosed hypercholesterolemia. Type 2 diabetes was not included in this analysis due to a small number of events (n=43). Follow-up was calculated from the second examination to the first event of each CVD risk factor or the last examination through 2006.
Statistical Analysis
We used Cox proportional hazards regression to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of developing each CVD risk factor across changes in fitness and fatness. Linear trends across changes in fitness and fatness were tested using general linear models in Table 1, and Cox regression models in Table 2 using the exposure categories as linear variables. Since changes in lifestyle may distort the true relation between changes in fitness or fatness and incident CVD risk factors, analyses were adjusted for dummy variables for changes in each lifestyle characteristic (smoking status, alcohol intake, and physical activity). Each lifestyle change was categorized into four categories, remained non-smokers, became nonsmokers, became smokers, or remained smokers; remained non-heavy drinkers, became non-heavy drinkers, became heavy drinkers, or remained heavy drinkers; and remained active, became active, became inactive, or remained inactive. We next explored how and to what extent changes in fitness or fatness associated with simultaneous changes in each component of CVD risk factors, such as blood pressure and lipids, between the first and second examinations using Pearson partial correlation coefficients after adjusting for age, sex, and change in fatness or fitness for each other. To test effect modification by sex on the associations between changes in fitness or fatness and incident CVD risk factors, we checked interaction terms in the Cox regressions and compared risk estimates in the sex-stratified analyses. We found similar trends in the development of CVD risk factors between men and women, and no significant interactions were observed, thus we presented the results of pooled analyses. There were also no significant interactions between change in fitness and change in fatness on developing CVD risk factors, using interaction terms in the Cox regression. The proportional hazards assumptions were met by comparing the log-log survival plots. We used SAS software (version 9.2) for all statistical analyses, and 2-sided p values <0.05 were deemed significant.
Table 1.
Baseline Characteristics by Changes in Fitness and Fatness
| Fatness change | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fitness change | ||||||||||||
| % body fat change | Body mass index change | |||||||||||
| Characteristics |
Loss (n=78 5) |
Stable (n=1,3 33) |
Gain (n=1,0 30) |
P Value * |
Loss (n=1,0 51) |
Stable (n=1,0 48) |
Gain (n=1,0 49) |
P Value * |
Loss (n=1,0 49) |
Stable (n=1,0 50) |
Gain (n=1,0 49) |
P Value * |
| Age, yrs | 42.3 (9.0) |
43.0 (8.8) |
41.4 (8.2) |
<0.00 1 |
43.5 (8.7) |
41.0 (8.7) |
42.4 (8.5) |
0.009 | 43.3 (8.8) |
41.8 (8.8) |
41.8 (8.3) |
0.009 |
| Maximal METs, | 13.5 (2.5) |
12.7 (2.3) |
12.3 (2.3) |
<0.00 1 |
12.4 (2.3) |
13.0 (2.4) |
12.8 (2.4) |
<0.00 1 |
12.4 (2.4) |
13.0 (2.4) |
12.9 (2.4) |
<0.00 1 |
| Unfit† | 1.0 | 2.6 | 5.4 | <0.00 1 |
4.4 | 2.1 | 2.9 | <0.00 1 |
4.1 | 2.1 | 3.2 | <0.00 1 |
| Body mass index, kg/m2 | 24.0 (2.7) |
24.0 (2.7) |
24.4 (2.9) |
<0.00 1 |
24.6 (2.9) |
23.9 (2.7) |
23.9 (2.6) |
<0.00 1 |
25.0 (2.8) |
23.7 (2.6) |
23.8 (2.6) |
<0.00 1 |
| 18.5–24.9 | 66.4 | 65.9 | 61.7 | 0.06 | 57.4 | 68.3 | 68.3 | 0.03 | 53.5 | 69.5 | 71.0 | 0.03 |
| 25.0–29.9 | 31.7 | 32.2 | 33.8 | 0.60 | 3.80.60 | 29.4 | 344 | 0.34 | 41.5 | 29.4 | 26.9 | 0.34 |
| ≥30 | 1.9 | 1.9 | 4.5 | <0.00 1 |
4.6 | 2.3 | 1.3 | <0.00 1 |
5.0 | 1.1 | 2.1 | <0.00 1 |
| Percent body fat, % | 19.0 (5.6) |
19.6 (5.5) |
19.7 (5.6) |
0.007 | 21.5 (5.4) |
18.8 (5.5) |
18.2 (5.2) |
0.004 | 21.0 (5.4) |
18.8 (5.4) |
18.7 (5.6) |
0.004 |
| Waist circumference, cm | 84.2 (10.5) |
84.9 (10.4) |
86.2 (10.9) |
<0.00 1 |
86.8 (10.7) |
84.3 (10.5) |
84.3 (10.5) |
<0.00 1 |
87.6 (10.2) |
83.6 (10.7) |
84.3 (10.5) |
<0.00 1 |
| SBP, mm Hg | 112 (10) |
113 (10) |
113 (10) |
0.03 | 113 (10) |
113 (10) |
112 (10) |
0.008 | 114 (10) |
112 (10) |
112 (9) |
0.008 |
| DBP, mm Hg | 75(7) | 75(7) | 75(7) | 0.42 | 75(7) | 75(7) | 75(7) | 0.19 | 76(7) | 75(7) | 75(7) | 0.19 |
| Fasting glucose, mg/dL | 94.2 (7.9) |
95.2 (8.6) |
94.6 (8.5) |
0.03 | 95.2 (8.6) |
94.8 (8.5) |
94.4 (8.1) |
0.37 | 95.4 (8.8) |
94.4 (8.2) |
94.4 (8.2) |
0.38 |
| Total cholesterol, mg/dL | 186.3 (26.2) |
187.2 (26.5) |
188.8 (26.3) |
0.11 | 190.9 (26.8) |
185.2 (26.4) |
186.4 (25.6) |
0.04 | 190.7 (26.7) |
187.0 (25.6) |
184.8 (26.4) |
0.04 |
| Triglycerides, mg/dL | 83.5 (43.4) |
85.5 (39.5) |
90.9 (42.7) |
<0.00 1 |
89.4 (41.0) |
84.2 (39.4) |
86.8 (44.3) |
<0.00 1 |
92.3 (46.1) |
84.1 (38.8) |
83.9 (39.2) |
<0.00 1 |
| HDL cholesterol, mg/dL | 52.3 (13.0) |
51.7 (13.0) |
49.9 (12.4) |
<0.00 1 |
51.0 (12.9) |
50.7 (12.7) |
52.1 (12.8) |
<0.00 1 |
50.4 (13.1) |
51.5 (12.4) |
51.9 (12.9) |
<0.00 1 |
| Current smoker | 13.3 | 14.6 | 12.3 | 0.28 | 13.3 | 12.4 | 14.8 | 0.49 | 12.3 | 13.8 | 14.4 | 0.49 |
| Heavy drinker‡ | 16.7 | 16.0 | 15.9 | 0.89 | 17.6 | 15.4 | 15.4 | 0.68 | 15.9 | 17.3 | 15.2 | 0.68 |
| Physically inactive§ | 12.7 | 13.7 | 15.0 | 0.39 | 15.4 | 14.3 | 11.8 | 0.17 | 15.6 | 13.8 | 12.1 | 0.17 |
Data are mean (SD) or %.
p value for linear trend.
Defined as the lower 20% in each age- and sex-specific distribution of maximal treadmill exercise test duration from the entire ACLS population.
Defined as alcohol drinks >14 per week for men and >7 drinks per week for women.
Defined as no leisure-time physical activity in the 3 months before the examination.
DBP = Diastolic blood pressure; HDL = high-density lipoprotein; MET = metabolic equivalent; SBP = Systolic blood pressure.
Table 2.
Hazard Ratios of Incident Cardiovascular Disease Risk Factors by Changes in Fitness and Fatness
| Hypertension | Metabolic syndrome | Hypercholesterolemia | ||||
|---|---|---|---|---|---|---|
| Model 1* | Model 2† | Model 1* | Model 2† | Model 1* | Model 2† | |
| Hazard Ratios (95 percent confidence intervals) | ||||||
| Fitness change | ||||||
| Loss | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Stable | 0.74 (0.62–0.89) | 0.76 (0.63–0.91) | 0.58 (0.45–0.75) | 0.62 (0.48–0.79) | 0.74 (0.60–0.91) | 0.75 (0.61–0.93) |
| Gain | 0.72 (0.59–0.88) | 0.77 (0.62–0.95) | 0.48 (0.37–0.63) | 0.59 (0.44–0.78) | 0.70 (0.56–0.88) | 0.74 (0.59–0.94) |
| p for linear trend | 0.003 | 0.02 | <0.001 | <0.001 | 0.003 | 0.02 |
| Per 1-MET increase | 0.93 (0.87–0.99) | 0.96 (0.89–1.03) | 0.78 (0.71–0.85) | 0.84 (0.75–0.93) | 0.88 (0.81–0.95) | 0.89 (0.82–0.97) |
| Fatness change | ||||||
| % body fat change | ||||||
| Loss | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Stable | 1.20 (1.00–1.44) | 1.18 (0.98–1.43) | 1.26 (0.99–1.62) | 1.16 (0.90–1.50) | 1.37 (1.11–1.69) | 1.33 (1.07–1.65) |
| Gain | 1.27 (1.05–1.53) | 1.24 (1.02–1.51) | 1.71 (1.34–2.19) | 1.52 (1.17–1.97) | 1.48 (1.20–1.83) | 1.41 (1.13–1.76) |
| p for linear trend | 0.01 | 0.03 | <0.001 | 0.002 | <0.001 | 0.003 |
| Per 1-% increase | 1.04 (1.01–1.06) | 1.03 (1.01–1.06) | 1.10 (1.06–1.13) | 1.08 (1.05–1.12) | 1.05 (1.02–1.08) | 1.04 (1.01–1.07) |
| BMI change | ||||||
| Loss | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Stable | 1.28 (1.07–1.53) | 1.28 (1.06–1.53) | 1.19 (0.93–1.53) | 1.12 (0.86–1.44) | 1.17 (0.96–1.44) | 1.16 (0.94–1.43) |
| Gain | 1.28 (1.07–1.54) | 1.27 (1.05–1.54) | 1.90 (1.50–2.42) | 1.74 (1.35–2.24) | 1.51 (1.23–1.85) | 1.46 (1.18–1.81) |
| p for linear trend | 0.002 | 0.006 | <0.001 | <0.001 | <0.001 | <0.001 |
| Per 1–kg/m2 increase | 1.16 (1.08–1.24) | 1.15 (1.08–1.24) | 1.37 (1.26–1.48) | 1.34 (1.23–1.47) | 1.18 (1.10–1.27) | 1.17 (1.09–1.26) |
Model 1 was adjusted for age, sex, examination year at baseline, and baseline systolic blood pressure for hypertension, baseline metabolic syndrome components (systolic blood pressure, fasting glucose, triglyceride, HDL cholesterol, and waist circumference) for metabolic syndrome, or baseline total cholesterol for hypercholesterolemia, and baseline maximal METs for fitness change, baseline % body fat for % body fat change, or baseline BMI for BMI change, and lifestyle changes (smoking status, alcohol intake, and physical activity) between the baseline and second examinations.
Model 2 was adjusted as for Model 1 plus baseline % body fat and % body fat change for fitness change or baseline maximal METs and maximal MET change for fatness change.
BMI = body mass index; MET = metabolic equivalent.
Results
Among 3,148 healthy participants, 752, 426, and 597 adults developed hypertension, metabolic syndrome, and hypercholesterolemia during the mean (interqurtile range) follow-up of 6.1 (2.1–8.7), 6.6 (2.1–9.4), and 6.3 (2.1–9.0) years, respectively. The corresponding incidence rates were 39.2, 20.5, and 30.1 per 1,000 person-years. The mean (interquartile range) interval between the first and second examinations was 2.1 (1.0–2.2) years with a minimum of 5 months. In general, participants were middle-aged (mean age 42.3 years), relatively fit, normal weight, and healthy at baseline (Table 1).
Participants who maintained or improved fitness had 26% and 28% lower risk of incident hypertension, 42% and 52% lower risk of metabolic syndrome, and 26% and 30% lower risk of hypercholesterolemia, respectively, compared with those who lost fitness (Table 2), after adjusting for possible confounders and baseline fitness levels (Model 1). However, those who gained percent body fat had 27%, 71%, and 48% higher risk of incident hypertension, metabolic syndrome, and hypercholesterolemia, respectively, compared with those who lost percent body fat. Similar results were observed in BMI change. When we additionally adjusted for fatness (baseline and change in percent body fat) for fitness change, or fitness (baseline and change in maximal METs) for fatness change (Model 2), the observed associations were slightly attenuated but remained significant for all three CVD risk factors (all p for trend <0.05). Every 1-MET improvement in fitness between the baseline and second examinations was associated with 7%, 22%, and 12% lower risk of subsequent incidence of hypertension, metabolic syndrome, and hypercholesterolemia, respectively. Every unit increase in percent body fat or BMI was associated with 4%, 10%, and 5%, and 16%, 37%, and 18% higher risk of developing corresponding CVD risk factors, after adjusting for confounders and baseline levels of each exposure (Model 1). Additional adjustment for fatness or fitness for each other (Model 2) did not alter the associations, except for the association between each 1-MET increase and hypertension, which did not reach statistical significance.
Because waist circumference is correlated with fatness, we additionally excluded waist circumference from the metabolic syndrome criteria. However, the associations between fatness change and incident metabolic syndrome were similar (data not shown). Thus, we decided to retain waist circumference as a metabolic syndrome component because waist circumference, a marker of abdominal obesity, has independent effects on CVD. In additional analyses, we examined how change in abdominal fatness (waist circumference) related to incident CVD risk factors. The associations between change in abdominal fatness and incident CVD risk factors were very similar to the associations between change in total body fatness (percent body fat) and incident CVD risk factors (data not shown).
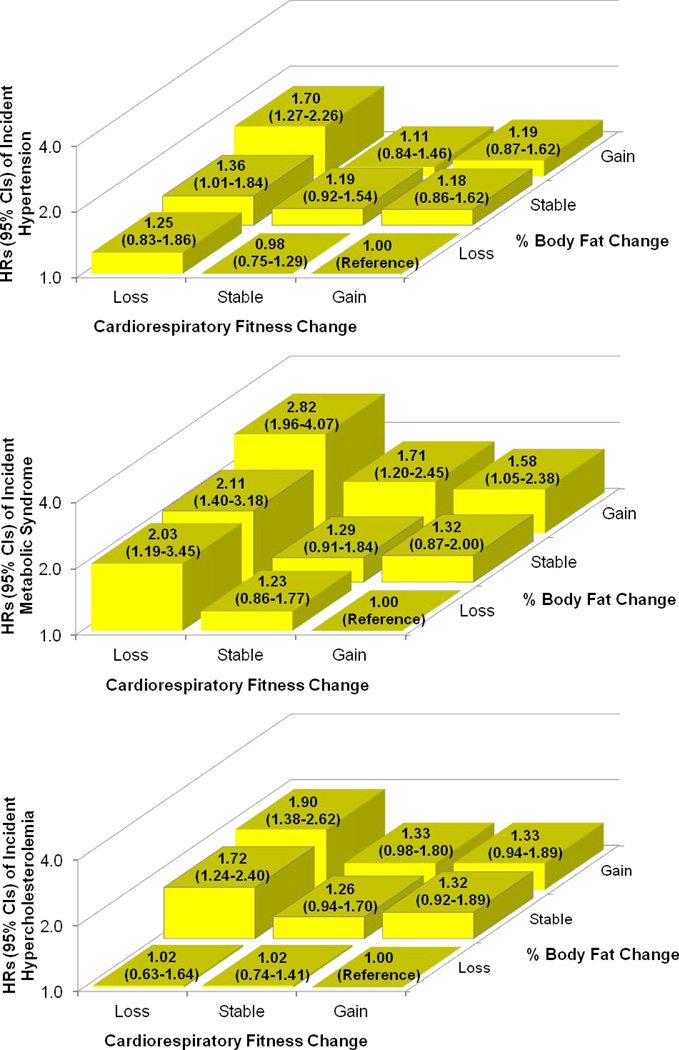
In the joint analyses (Figure 1), fitness loss, combined with stable or increased fatness, was associated with a higher risk of developing hypertension and hypercholesterolemia, compared with the reference, fitness gain and fatness loss group. However, both losing fitness regardless of fatness change, and gaining fatness regardless of fitness change, were associated with a higher risk of developing metabolic syndrome. In the development of each CVD risk factor, the increased risks associated with fat gain appeared to be attenuated when fitness was maintained or improved. Similarly, the increased risks associated with fitness loss also appeared to be attenuated when fatness was reduced, particularly in hypertension and hypercholesterolemia. We observed similar results in the combined associations of changes in fitness and BMI with incident CVD risk factors (data not shown).
Figure 1. Hazard Ratios (95% Confidence Intervals) of Incident Cardiovascular Disease Risk Factors by Combined Categories of Changes in Cardiorespiratory Fitness and Percent Body Fat.
Adjusted for age, sex, examination year, maximal METs, % body fat at baseline, and baseline systolic blood pressure for hypertension, baseline metabolic syndrome components (systolic blood pressure, fasting glucose, triglyceride, HDL cholesterol, and waist circumference) for metabolic syndrome, or baseline total cholesterol for hypercholesterolemia, and lifestyle changes (smoking status, alcohol intake, and physical activity) between the baseline and second examinations. The number of participants in the fitness loss, stable, and gain groups were 130, 401, and 520 in the % body fat loss group; 305, 488, and 255 in the stable % body fat group; and 350, 444, and 255 in the % body fat gain group, respectively. HR = hazard ratio; CI = confidence interval.
Fitness change was negatively associated with blood pressure, waist circumference, triglycerides, and total cholesterol and positively associated with HDL cholesterol (Table 3). Fatness change was positively associated with blood pressure, waist circumference, fasting glucose, triglycerides, and total cholesterol and negatively associated with HDL cholesterol. These significant correlations (all p values <0.05) were adjusted for baseline age, sex, and change in fatness or fitness for each other. In general, the correlations for change in fitness with components of CVD risk factors were as strong as the change in fatness. Changes in both fitness and fatness had relatively higher correlations with change in waist circumference. The correlation coefficient between the change in fitness and the change in fatness (% body fat) was - 0.37 (p <0.001).
Table 3.
Correlations Between Changes in Fitness or Fatness and Changes in Components of Cardiovascular Disease Risk Factors Between Baseline and Second Examinations
| Fitness change | Fatness change | |||||
|---|---|---|---|---|---|---|
| % body fat change | BMI change | |||||
| CVD risk factor components | r* | p Value | r* | p Value | r* | p Value |
| Unadjusted | ||||||
| Systolic blood pressure change | −0.08 | <0.001 | 0.10 | <0.001 | 0.14 | <0.001 |
| Diastolic blood pressure change | −0.05 | 0.003 | 0.06 | 0.002 | 0.09 | <0.001 |
| Waist circumference change | −0.21 | <0.001 | 0.35 | <0.001 | 0.38 | <0.001 |
| Fasting glucose change | 0.01 | 0.43 | 0.05 | 0.006 | 0.010 | <0.001 |
| Triglycerides change | −0.15 | <0.001 | 0.16 | <0.001 | 0.23 | <0.001 |
| HDL cholesterol change | 0.11 | <0.001 | −0.12 | <0.001 | −0.11 | <0.001 |
| Total cholesterol change | −0.12 | <0.001 | 0.21 | <0.001 | 0.22 | <0.001 |
|
Adjusted for age, sex, and % body fat change for fitness change or maximal MET change for fatness change |
||||||
| Systolic blood pressure change | −0.05 | 0.006 | 0.07 | <0.001 | 0.12 | <0.001 |
| Diastolic blood pressure change | −0.04 | 0.045 | 0.04 | 0.04 | 0.07 | <0.001 |
| Waist circumference change | −0.14 | <0.001 | 0.37 | <0.001 | 0.42 | <0.001 |
| Fasting glucose change | 0.03 | 0.08 | 0.06 | 0.002 | 0.10 | <0.001 |
| Triglycerides change | −0.10 | <0.001 | 0.12 | <0.001 | 0.19 | <0.001 |
| HDL cholesterol change | 0.08 | <0.001 | −0.09 | <0.001 | −0.08 | <0.001 |
| Total cholesterol change | −0.05 | 0.008 | 0.17 | <0.001 | 0.18 | <0.001 |
Values are Pearson correlation coefficients in the unadjusted model and Pearson partial correlation coefficients in the adjusted model.
BMI = body mass index; HDL = high-density lipoprotein; MET = metabolic equivalent.
Discussion
Changes in both fitness and fatness were significantly associated with the development of hypertension, metabolic syndrome, and hypercholesterolemia in healthy adults. These associations were found after accounting for possible baseline confounders, changes in lifestyle, and simultaneous change in fatness or fitness for each other. Significant correlations between changes in fitness or fatness and each component of CVD risk factors, such as blood pressure, lipids, and fasting glucose, supported these results. In the joint analyses, maintaining or improving fitness appeared to attenuate, although not completely eliminated, some of the negative effects of fat gain. Also, reducing body fat was likely to counteract some of the increased risk of developing CVD risk factors associated with fitness loss.
The Coronary Artery Risk Development in Young Adults (CARDIA) study found that improving fitness was associated with reduced risk for developing type 2 diabetes and metabolic syndrome, but was not associated with hypertension or hypercholesterolemia.(2) However, after further adjustment for baseline BMI and weight change, the HRs were no longer significant for any CVD risk factors. The inconsistency between the CARDIA and the current study may be due to the younger population in the CARDIA study (mean age 25), resulting in lower incident rates for CVD risk factors. Also, the CARDIA study excluded participants with only a given CVD risk factor at baseline from the incident analysis of that CVD risk factor, and fitness change over the 7 years was the first half of the 15 years of follow-up period for incident CVD risk factors.
There is convincing evidence from clinical trials and epidemiologic studies that improving physical activity or, especially, fitness is beneficial,(2,26,27) whereas gaining weight or fatness is detrimental,(7,28–30) for developing hypertension, metabolic syndrome, and hypercholesterolemia. Also, improving physical activity or fitness is correlated with favorable changes in components of CVD risk factors such as blood pressure, lipid profiles and waist circumference,(31–34) and gaining weight or fatness is correlated with unfavorable changes in such components.(29,33,35) However, most previous studies have examined the associations between changes in either fitness or fatness and simultaneous changes in such CVD risk factors during the same time interval. Thus, the causal relationships among changes in fitness, fatness, and CVD risk factors are uncertain because CVD risk factors may also affect changes in fitness and fatness. Many studies on changes in fitness or fatness also have not mutually adjusted for each other, although both are strong independent risk factors on developing CVD risk factors.
Whether physical activity or fitness can compensate for the health hazards of overweight or obesity, comprising of two-thirds of the U.S. adult population,(36) and understanding the relative contributions among these factors, has clinical and public health significance. Several prospective studies found both physical activity or fitness and obesity are independent predictors for the development of CVD risk factors.(18,37,38) Although study results vary depending on study population, health status, assessment of physical activity or fitness, and study outcome,(11) objectively-measured fitness results in stronger associations with health outcomes than does self-reported physical activity.(39) Most previous studies examined the combined associations of fitness and fatness at one time point with subsequent incidence of outcomes. Our results support that not only baseline fitness and fatness but also changes in fitness and fatness are significantly associated with incident CVD risk factors.
It is postulated that improving fitness or physical activity by exercise training may reduce blood pressure through reductions in catecholamines and total peripheral resistance, and alterations in vasodilators and vasoconstrictors.(40) Exercise-induced changes in metabolic factors, such as improvements in glucose metabolism, insulin sensitivity, lipoprotein subfraction profiles,(32) and reductions in inflammation markers, visceral and liver fat, even in the absence of weight loss,(31) may serve as links between improving fitness and the lower risk for metabolic syndrome and hypercholesterolemia independent of fatness change. For mechanisms between fatness and CVD risk factors, increases in sympathetic nervous system activity, renal sodium retention, and systemic vascular resistance have been considered to play a role in obesity-induced hypertension.(41) Several proposed mechanisms linking obesity, visceral fat in particular, to metabolic syndrome and hyperlipidemias include insulin resistance, atherogenic dyslipidemia, oxidative stress, elevated fatty acids, inflammation, and ectopic fat deposition.(42,43)
Study Limitations
Limitations of our study include a population that consists primarily of well-educated, non-Hispanic white from middle-to-upper socioeconomic strata, thus it is possible that the results may be different in other populations. However, fitness and other physiologic characteristics in this cohort are similar to representative North American populations,(17) and the socioeconomic homogeneity can reduce potential confounding of education, income, and ethnicity. We do not have information on whether changes in fitness and fatness were intentional, thus cautious interpretation of these results is necessary. However, to minimize potential confounding by unintentional changes in fitness and fatness due to disease, we excluded individuals with various chronic diseases and subclinical conditions before the outcome follow-up. In fact, we observed positive correlations between changes in fitness and physical activity (r=0.22, p value <0.001), and negative correlations between changes in fatness and physical activity (r=−0.14, p value <0.001). The lack of data on medications and diet information may have biased the results through the misclassification of the hypertension, metabolic syndrome, and hypercholesterolemia and potential effects on changes in fatness and the development of CVD risk factors.
This prospective study expands knowledge about the effects of fitness and fatness on CVD morbidity and mortality by exploring the independent and combined associations of changes in these exposures with the development of CVD risk factors over a wide age range of healthy adults, using objective measures for fitness and fatness. Given the concern over the strong confounding effect of ill health on these associations,(19) extensive exclusion of preexisting and subclinical conditions strengthens our results.
Conclusions
We found that changes in both fitness and fatness, even after controlling for each other, are significantly associated with the development of CVD risk factors of hypertension, metabolic syndrome, and hypercholesterolemia, in healthy adult population. In addition, maintaining or improving fitness is likely to counteract, although not completely eliminate, some of the adverse effects of fat gain. Similarly, reducing body fat appears to compensate for some of the increased risk of developing CVD risk factors associated with fitness loss.
Acknowledgments
The authors thank the Cooper Clinic physicians and technicians for collecting the baseline data, staff at the Cooper Institute for data entry and data management, and Gaye Christmus for editorial assistance.
This study was supported by the National Institutes of Health grants (AG06945, HL62508, and DK088195), and an unrestricted research grant from The Coca-Cola Company. The authors have reported that they have no relationships to disclose.
Abbreviations and Acronyms
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- HR
hazard ratio
- MET
metabolic equivalent
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR, Jr, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290:3092–3100. doi: 10.1001/jama.290.23.3092. [DOI] [PubMed] [Google Scholar]
- 3.Christou DD, Gentile CL, DeSouza CA, Seals DR, Gates PE. Fatness is a better predictor of cardiovascular disease risk factor profile than aerobic fitness in healthy men. Circulation. 2005;111:1904–1914. doi: 10.1161/01.CIR.0000161818.28974.1A. [DOI] [PubMed] [Google Scholar]
- 4.Diaz VA, Player MS, Mainous AG, III, Carek PJ, Geesey ME. Competing impact of excess weight versus cardiorespiratory fitness on cardiovascular risk. Am J Cardiol. 2006;98:1468–1471. doi: 10.1016/j.amjcard.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 5.Berrington de GA, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 7.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 8.Wessel TR, Arant CB, Olson MB, et al. Relationship of physical fitness vs body mass index with coronary artery disease and cardiovascular events in women. JAMA. 2004;292:1179–1187. doi: 10.1001/jama.292.10.1179. [DOI] [PubMed] [Google Scholar]
- 9.Duncan GE. The "fit but fat" concept revisited: population-based estimates using NHANES. Int J Behav Nutr Phys Act. 2010;7:47. doi: 10.1186/1479-5868-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 11.Lee DC, Sui X, Blair SN. Does physical activity ameliorate the health hazards of obesity? Br J Sports Med. 2009;43:49–51. doi: 10.1136/bjsm.2008.054536. [DOI] [PubMed] [Google Scholar]
- 12.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 13.Sui X, LaMonte MJ, Laditka JN, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298:2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens J, Cai J, Evenson KR, Thomas R. Fitness and fatness as predictors of mortality from all causes and from cardiovascular disease in men and women in the lipid research clinics study. Am J Epidemiol. 2002;156:832–841. doi: 10.1093/aje/kwf114. [DOI] [PubMed] [Google Scholar]
- 15.Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 16.Jackson AS, Sui X, Hebert JR, Church TS, Blair SN. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169:1781–1787. doi: 10.1001/archinternmed.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blair SN, Kannel WB, Kohl HW, Goodyear N, Wilson PW. Surrogate measures of physical activity and physical fitness. Evidence for sedentary traits of resting tachycardia, obesity, and low vital capacity. Am J Epidemiol. 1989;129:1145–1156. doi: 10.1093/oxfordjournals.aje.a115236. [DOI] [PubMed] [Google Scholar]
- 18.Lee DC, Sui X, Church TS, Lee IM, Blair SN. Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care. 2009;32:257–262. doi: 10.2337/dc08-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonsen MK, Hundrup YA, Obel EB, Gronbaek M, Heitmann BL. Intentional weight loss and mortality among initially healthy men and women. Nutr Rev. 2008;66:375–386. doi: 10.1111/j.1753-4887.2008.00047.x. [DOI] [PubMed] [Google Scholar]
- 20.Wannamethee SG, Shaper AG, Walker M. Weight change, body weight and mortality: the impact of smoking and ill health. Int J Epidemiol. 2001;30:777–786. doi: 10.1093/ije/30.4.777. [DOI] [PubMed] [Google Scholar]
- 21.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 22.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 23.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 8th. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. p. 158. [Google Scholar]
- 24.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell MS, Goslin BR, Gellish RL, et al. Metabolic syndrome status changes with fitness level change: a retrospective analysis. Metab Syndr Relat Disord. 2008;6:8–14. doi: 10.1089/met.2007.0013. [DOI] [PubMed] [Google Scholar]
- 27.Williams PT. Incident hypercholesterolemia in relation to changes in vigorous physical activity. Med Sci Sports Exerc. 2009;41:74–80. doi: 10.1249/MSS.0b013e3181831417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen PC, Sung FC, Su TC, Chien KL, Hsu HC, Lee YT. Two-year change in body mass index and subsequent risk of Hypertension among men and women in a Taiwan community. J Hypertens. 2009;27:1370–1376. doi: 10.1097/HJH.0b013e32832af6d4. [DOI] [PubMed] [Google Scholar]
- 29.Hillier TA, Fagot-Campagna A, Eschwege E, et al. Weight change and changes in the metabolic syndrome as the French population moves towards overweight: the D.E.S.I.R. cohort. Int J Epidemiol. 2006;35:190–196. doi: 10.1093/ije/dyi281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams PT. Changes in body weight and waist circumference affect incident hypercholesterolemia during 7 years of follow-up. Obesity (Silver Spring) 2008;16:2163–2168. doi: 10.1038/oby.2008.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamer M, O'Donovan G. Cardiorespiratory fitness and metabolic risk factors in obesity. Curr Opin Lipidol. 2010;21:1–7. doi: 10.1097/MOL.0b013e328331dd21. [DOI] [PubMed] [Google Scholar]
- 32.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 33.Rainwater DL, Mitchell BD, Comuzzie AG, VandeBerg JL, Stern MP, MacCluer JW. Association among 5-year changes in weight, physical activity, and cardiovascular disease risk factors in Mexican Americans. Am J Epidemiol. 2000;152:974–982. doi: 10.1093/aje/152.10.974. [DOI] [PubMed] [Google Scholar]
- 34.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 35.Norman JE, Bild D, Lewis CE, Liu K, West DS, Cardia S. The impact of weight change on cardiovascular disease risk factors in young black and white adults: the CARDIA study. Int J Obes Relat Metab Disord. 2003;27:369–376. doi: 10.1038/sj.ijo.0802243. [DOI] [PubMed] [Google Scholar]
- 36.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 37.Hu G, Barengo NC, Tuomilehto J, Lakka TA, Nissinen A, Jousilahti P. Relationship of physical activity and body mass index to the risk of hypertension: a prospective study in Finland. Hypertension. 2004;43:25–30. doi: 10.1161/01.HYP.0000107400.72456.19. [DOI] [PubMed] [Google Scholar]
- 38.Sui X, Hooker SP, Lee IM, et al. A prospective study of cardiorespiratory fitness and risk of type 2 diabetes in women. Diabetes Care. 2008;31:550–555. doi: 10.2337/dc07-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee DC, Sui X, Ortega FB, et al. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br J Sports Med. 2011;45:504–510. doi: 10.1136/bjsm.2009.066209. [DOI] [PubMed] [Google Scholar]
- 40.Pescatello LS, Franklin BA, Fagard R, et al. American College of Sports Medicine position stand. Exercise and Hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 41.Bogaert YE, Linas S. The role of obesity in the pathogenesis of Hypertension. Nat Clin Pract Nephrol. 2009;5:101–111. doi: 10.1038/ncpneph1022. [DOI] [PubMed] [Google Scholar]
- 42.Carmena R, Ascaso JF, Real JT. Impact of obesity in primary hyperlipidemias. Nutr Metab Cardiovasc Dis. 2001;11:354–359. [PubMed] [Google Scholar]
- 43.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]