Abstract
Sepsis is a major cause of death worldwide. The associated risks and mortality are known to significantly increase on exposure to alcohol (chronic or acute). The underlying mechanisms of the association of acute ethanol ingestion and poor prognosis of sepsis are largely unknown. The study described here was designed to determine in detail the role of ethanol and TLR4 in the pathogenesis of the sepsis syndrome. The effects of acute ethanol exposure and TLR4 on bacterial clearance, spleen cell numbers, peritoneal macrophage numbers, and cytokine production were evaluated using wild type and TLR4 hypo-responsive mice treated with ethanol and then challenged with a non pathogenic strain of Escherichia. coli (E. coli). Ethanol treated mice exhibited a decreased clearance of bacteria and produced lesser amounts of most pro-inflammatory cytokines in both strains of mice at two hours after challenge. Neither ethanol treatment nor a hypo-responsive TLR4 had significant effects on the cell numbers in the peritoneal cavity and spleen 2 hours post infection. The suppressive effect of acute ethanol exposure on cytokine and chemokine production was more pronounced in the wild type mice, but the untreated hyporesponsive mice produced less of most cytokines than untreated wild type mice. The major conclusion of this study is that acute ethanol exposure suppresses pro-inflammatory cytokine production and that a hypo-responsive TLR4 (in C3H/HeJ mice) decreases pro-inflammatory cytokine levels but the cytokines and other mediators induced through other receptors are sufficient to ultimately clear the infection but not enough to induce lethal septic shock. In addition, results reported here demonstrate previously unknown effects of acute ethanol exposure on LIF (leukemia inhibitory factor) and eotaxin and provide the first evidence that IL-9 is induced through TLR4 in vivo.
Keywords: Acute alcohol, sepsis, pro-inflammatory response, cytokines, ethanol, TLR4
Introduction
Systemic infection and the system wide inflammatory response associated with it is called sepsis (Bone et al., 1992). It has a complex etiology resulting from interactions between the host and the causative microbe. There are about 751, 000 cases of severe sepsis annually leading to about 215,000 deaths in the United States (Angus et al., 2001) making it the 10th leading cause of death (Statistics, 2007) despite the availability of antibiotic and supportive therapy. The incidence of sepsis increases about 100 fold with age (>85 years) (Angus et al., 2001). As the population ages, the incidence of sepsis and the number of sepsis related deaths in United States is projected to further increase in the coming years (Angus et al., 2001; Martin et al., 2003). Studies in both humans and animal models indicate that acute ethanol consumption is a significant risk factor for mortality in sepsis (Huttunen et al., 2007; McGill et al., 1995; Pruett et al., 2004b; Woodman et al., 1996). The molecular mechanisms underlying this association are not clearly known. The systemic inflammatory response, the hallmark of sepsis is caused by an exaggerated pro-inflammatory response; while a hyper pro-inflammatory response is detrimental the host still needs protection from the invading microorganism which requires the initiation of an innate immune response. The inhibition of TLR signaling (Dai et al., 2005; Dolganiuc et al., 2006; Goral and Kovacs, 2005; Pruett et al., 2004b; Szabo et al., 2007a) and cytokine production (Happel et al., 2007; Mason et al., 2000; Nelson et al., 1989; Pruett et al., 2003; Pruett et al., 2004b; Szabo et al., 1999) by acute ethanol ingestion is implicated as a major mechanism by which acute ethanol decreases resistance to sepsis.
Our previous study indicated that acute ethanol exposure adversely affected the outcome of sepsis caused by E. coli (injected intraperitoneally) by significantly decreasing pro-inflammatory cytokine production, which probably is responsible for the decreased bactericidal activity of the macrophages and the neutrophils. The role of TLR4 in the innate immune response to sepsis was also evaluated. In hypo-responsive TLR4 mutant mice, purified lipopolysaccharide (LPS) induced only a small fraction of the level of cytokines as compared to wild type mice. Because ethanol also inhibits TLR4 mediated cytokine production, we expected that the outcome of sepsis would be similar in TLR4 mutant mice and wild type mice treated with ethanol, because cytokine production is decreased in both. However, in our previous study we found that a higher percentage of mice with a hypo-responsive TLR4 survived than those with a normal TLR4 response (Pruett et al., 2010). It is intriguing that although acute ethanol ingestion is known to inhibit cytokine production in both humans and animal models, similar decreases in mice with defective TLR4 did not decrease resistance to sepsis (Pruett et al., 2010). The present study was designed to determine if effects reported previously for a few cytokines in serum were also applicable to a broad range of pro- and anti- inflammatory cytokines and chemokines in the peritoneal fluid early in the infection. In addition, this study was designed to determine if the spread of bacteria systemically during the early stages of infection is affected by ethanol and if this effect is different in TLR4 wild type and mutant mice.
Material and methods
Mice
Two different strains of mice, C3H/HeJ and C3H/HeOuJ obtained from Jackson Labs (Bar Harbor, Maine) were used in the study. C3H/HeJ mice have a mutant TLR4 gene which yields a protein that is essentially non-responsive to bacterial lipopolysaccharide, which is the naturally occurring ligand for TLR4. The C3H/HeOuJ mice match the C3H/HeJ strain at virtually every locus other than TLR4. Female mice were used because males fight when group housed and this causes stress, which can affect the results. The mice were allowed to acclimate and to recover from the shipping stress for at least 2 weeks before use in experiments at 8-12 weeks of age. All the mice were housed in filter-top shoebox cages with four mice per cage in a temperature (70-78°F) and humidity (40-60%) controlled environment. The food, water and bedding for the mice were autoclaved before use. Sentinel mice housed in the same room as the mice used in this study were negative for common infectious agents during the period of this study. The laboratory animal facility and animal research program at Mississippi State University are accredited by the American Association for Accreditation of Laboratory Animal Care. Mice were housed and used in accordance with the National Institute of Health and Mississippi State University regulations.
Administration of ethanol
This mouse model of binge drinking used in this study has been developed and thoroughly characterized in our laboratory (Carson and Pruett, 1996). Briefly, ethanol was administered as a 32% v/v (volume/volume) solution in tissue culture-grade water by gavage using an 18-guage stainless steel gavage needle. The naive mice were treated with vehicle control (water) given by gavage as a control for handling and dosing related stress. All the other mice were treated with a dosage of 6 g/kg ethanol. This dose of alcohol yields a peak blood ethanol concentration of ∼0.4% (∼87 mM) which is not particularly rare in humans (Urso et al., 1981). Although this blood concentration represents the high-end of the range typically observed in ethanol-dependent persons or binge drinkers, concentrations in this range occur in a surprising number of cases (95 cases were reported by John and Holmgren) (Jones and Holmgren, 2009). Moreover, ethanol is cleared about 2-3 times faster in mice than in humans so the area under the curve at a peak value of 0.4% in mice is similar to that in humans at a considerably lower peak level (Wu et al., 1994). We recently reported that all the immunological changes in response to E. coli challenge caused by ethanol at 6 g/kg were also significant at 4 g/kg (Pruett et al., 2010). The peak blood level at 4g/kg is ∼0.2%, which is not unusual in human binge drinkers.
Administration of E. coli
A non pathogenic isolate of E. coli (characterized by the College of Veterinary Medicine Clinical Microbiology Laboratory) isolated from the colon of one of the mice in our specific pathogen free colony was used for the study. Each mouse was injected in the peritoneal cavity with 2 × 108 bacterial cells suspended in a phosphate buffered saline solution. The bacteria were harvested while they were growing in log phase (determined by OD at 650nm) as described previously (Pruett et al., 2010).
Acute ethanol exposure significantly increases the risk of infection in patients with penetrating abdominal trauma (Gentilello et al., 1993). The mouse model used in this study is expected to be representative of sepsis in humans that begins with the loss of gastrointestinal barrier function which is usually caused by trauma, appendicitis, diminished liver function, or alcohol abuse. Although these kinds of infections begin as polymicrobial, in most cases only a single species of bacteria predominates in human sepsis originating from peritonitis. In approximately half of the cases, E. coli is the only species that isolated from blood cultures (De Waele et al., 2008; Mbopi Keou et al., 1992). In addition, administration of a single strain of indigenous E. coli in our model allows more controlled conditions and minimal differences within groups as compared to cecal ligation and puncture but it yields peritonitis and sepsis similar to that observed in humans.
Experimental design
Experiments were designed with a group size of 4 mice for cytokine analysis and 3 or 4 mice for all other assays. Both the strains of mice were divided into 3 groups each on the basis of treatment administered. The first group of mice in each strain remained untreated and was referred to as the naive group. This group was used to define the baseline cytokine concentrations to confirm that the anticipated inflammatory changes were induced by E. coli. The second group in each strain was treated with E. coli only and the third group was treated with both E. coli and ethanol. Mice were treated by gavage with ethanol or water and challenged with intraperitoneal injection of E. coli after 30 min. The mice were anesthetized by inhalation of halothane at 2 hours after E. coli challenge for collection of peritoneal lavage fluid and spleen.
Sampling of peritoneal lavage and spleen and procedures
After euthanasia by inhalation of halothane, peritoneal lavage was performed by injection of 1ml of PBS. The abdominal area was massaged to distribute the fluid and to mix the contents of the peritoneal cavity. The skin was dissected away to reveal the peritoneum and a sample from the peritoneal cavity was withdrawn using a syringe with a 25-guage needle. Spleens from all the mice were also collected and weighed and stored in different vials at -80 C.
A part of the peritoneal fluid and spleen cells were used for bacterial counts by making serial dilutions in LB agar, and counting CFU (colony forming units) after overnight incubation at 37°C.
The number of nucleated cells in peritoneal fluid as well as spleen was determined by using a Coulter Z1 particle counter (Hialeah, FL). Manual lysing reagent was added to lyse the cytoplasmic membrane, leaving only nuclei to be counted.
The differential cell count of peritoneal fluid was done by making cytospin preparations from the peritoneal fluid samples which were then stained with Wright Giemsa stain and visualized at 600× magnification under an oil immersion lens. The rest of the supernatant from peritoneal fluid was stored frozen at -20 C until needed for cytokine and chemokine assay.
The percentage of selected subpopulations in the spleen was determined using a BD Biosciences FACS Calibur flow cytometer, a multicolor bench top flow cytometry system capable of both analyzing and sorting. The different markers used include CD4, CD8, NK1.1 and MHC II. The cells labeled by these markers are T helper lymphocytes (CD4), cytotoxic T lymphocytes (CD8), NK cells (NK1.1), B lymphocytes (MHC II) and macrophages (MHC II).
Cytokine assays
The concentrations of a panel of 25 cytokines and chemokines (IFN-γ, IL-1α, IL-1β, IL-6, IL-9, IL-10, 1L-13, IL-15, IL-12p40, IL-12p70, IL-17, MIP-2, TNF-α, eotaxin, GM-CSF, RANTES, M-CSF, G-CSF, MIG, MIP-1α, MIP-1β, LIF, MCP-1, LIX and IP-10) Cytokine and chemokine concentrations in peritoneal lavage fluid were quantified using Milliplex AP Assay kits which are based on the Luminex xMAP technology (Millipore Corporation, Billierica, Massachusetts) using standards for each cytokine and chemokine provided by the manufacturer.
Statistical analysis
The data analysis was done using Prism 5.0 software (Graph Pad, San Diego, CA). Data with continuous variables were analyzed by analysis of variance (ANOVA), followed by Newman-Keuls post-hoc test to identify differences in group means. A P value of 0.05 or less was considered significant.
Results
Acute administration of ethanol decreased the clearance of bacteria and suppressed the immune response in both the wild type (C3H/HeOuJ) and TLR4 mutant (C3H/HeJ) mice. The results in Table 1 indicate the average number of viable E. coli isolated 2 hours after challenge from the peritoneal cavity and spleen of wild type and mutant mice. The results demonstrate that ethanol significantly suppressed the clearance of bacteria from the peritoneal cavity of both strains of mice and from the spleen in wild type mice. As expected, the bacterial counts in spleen were much lower than those observed in peritoneal fluid as the bacterial challenge was administered intraperitoneally and the CFU counts were performed at an early time point of 2 hours. It has been reported previously that the differences in bacterial numbers get larger over time, most of the bacteria in the E. coli treated mice were cleared by 21 hours; the bacterial numbers continue to increase significantly in the mice exposed to ethanol before E. coli treatment (Pruett et al., 2010). The differences in bacterial numbers at 2 hours indicate that mechanisms involved in bacterial clearance were already inhibited by 2 hours. Two hours was chosen for the present study, because it seemed that events that occurred at this time were important in the ultimate outcome of infection, and because we have already reported the basic kinetics of disease progression with and without ethanol in this model (Pruett et al., 2010). To determine whether the decreased bacterial clearance in ethanol treated mice was caused by ethanol's effect on the cells of the peritoneal fluid and spleen, we determined the cell counts of these samples from both the mouse strains. There was no significant effect of ethanol on cell number in either strain of mouse, (Table 1). In addition to the total cell counts, differential cell counts were also performed using a microscopic analysis of stained cytospin preparations of the peritoneal fluid samples from both strains of mice. Most of the cells in the peritoneal cavity 2 hours post infection were macrophages and there were small percentages of lymphocytes present in both the control as well as mutant mice. Neither E. coli nor ethanol had a significant effect on the percentages of these cells. There was also no significant difference between strains (Table 1). The differential cell population of the spleen samples from the all the groups was also evaluated using flow cytometry. Ethanol did not significantly affect the percentage of any of these cells types (Table 1). Macrophages and B lymphocytes were detected in the spleen by expression of MHC II and no significant changes were mediated by ethanol. However, macrophages constitute only about 5% of cells in the spleen, and it is possible that changes in macrophage numbers would not be detectable. This should be considered in interpreting these results.
Table 1.
Effect of ethanol on bacterial counts and cell counts of peritoneal lavage fluid and spleen samples isolated from wild type (C3H/HeOuJ) and TLR4 mutant (C3H/HeJ) mice.
| Treatment | Peritoneal fluid | Spleen | |||
|---|---|---|---|---|---|
| C3H/HeOuJ [n=3] (Mean±SEM) |
C3H/HeJ [n=3] (Mean±SEM) |
C3H/HeOuJ [n= 3] (Mean±SEM) |
C3H/HeJ [n=3] (Mean±SEM) |
||
| Bacterial counts (Number of bacteria × 103) | Naïve | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| E. coli | 16700.00 ± 14.70 | 21600.00 ± 7.09 | #31.00 ± 9.28 | #55.25 ± 12.17 | |
| E. coli + EtOH | *20766.00 ± 4.90 | *27333.00 ± 5.45 | #*150.50 ± 27.11 | #91.25 ± 10.93 | |
| Cell counts (Number of cells × 106) | Naïve | 5.02 ± 0.39 | 4.26 ± 0.41 | 62.80 ± 0.05 | 53.60 ± 0.65 |
| E. coli | 5.78 ± 0.44 | 4.75 ± 0.23 | 58.90 ± 0.86 | 56.60 ± 0.35 | |
| E. coli + EtOH | 4.66 ± 0.63 | 3.81 ± 0.72 | 49.20 ± 0.71 | 52.60 ± 0.15 | |
| Macrophage count (% of total cells) | Niave | 81.33 ± 2.02 | 82.66 ± 1.76 | ND | ND |
| E. coli | 87.33 ± 1.76 | 85.00 ± 2.07 | ND | ND | |
| E. coli + EtOH | 84.67 ± 2.72 | 84.67 ± 2.40 | ND | ND | |
| Lymphocytes (% of total cells) | Niave | 18.66 ± 2.02 | 17.33 ± 1.76 | ND | ND |
| E. coli | 12.67 ± 1.76 | 15.00 ± 2.07 | ND | ND | |
| E. coli + EtOH | 15.33 ± 2.72 | 15.33 ± 2.70 | ND | ND | |
| CD 4 positive cells (% of total cells) | Niave | ND | ND | 12.11 ± 0.83 | 17.60 ± 0.76 |
| E. coli | ND | ND | 13.20 ± 0.13 | 16.79 ± 1.19 | |
| E. coli + EtOH | ND | ND | 14.25 ± 1.16 | 16.44 ± 0.45 | |
| CD8 positive cells (% of total cells) | Niave | ND | ND | 5.09 ± 0.40 | 7.68 ± 0.80 |
| E. coli | ND | ND | 5.75 ± 0.62 | 8.49 ± 0.53 | |
| E. coli + EtOH | ND | ND | 5.41 ± 0.72 | 7.00 ± 0.48 | |
| NK 1.1 positive cells (% of total cells) | Niave | ND | ND | 2.62 ± 0.17 | 3.44 ± 0.06 |
| E. coli | ND | ND | 2.33 ± 0.21 | 2.17 ± 0.29 | |
| E. coli + EtOH | ND | ND | 2.76 ± 0.17 | 2.39 ± 1.37 | |
| MHC II positive cells (% of total cells) | Niave | ND | ND | 12.74 ± 0.86 | 17.84 ± 0.43 |
| E. coli | ND | ND | 13.43 ± 0.33 | 16.76 ± 1.10 | |
| E. coli + EtOH | ND | ND | 14.63 ± 1.16 | 16.42 ± 0.34 | |
SEM- Standard error of mean, EtOH- ethanol,
Significantly decreased as compared to the same strain of mice treated with E. coli, ND- not determined,
n=4 for this experiment.
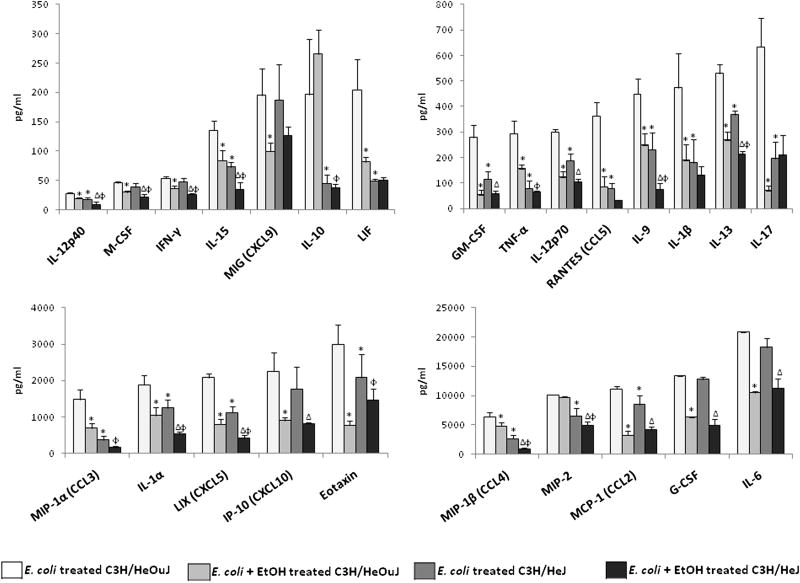
The observed effects of ethanol on the bacterial counts seen in this study and the decreased survival in the presence of ethanol and a functional TLR4 gene observed in our previous study (Pruett et al., 2010) were not caused by a decrease in cell number or type in the peritoneal cavity. We speculated that these effects might be caused by functional defects in these cell types. To test this hypothesis we evaluated the effects of ethanol on a panel of 25 cytokine and chemokine in the peritoneal fluid at a time point of 2 hours after E. coli challenge. The cytokines and chemokines chosen for this study are known to play a role in sepsis in animals or humans. The results of the cytokine assays are described in Fig.1. Most of these cytokines are likely to be secreted by the peritoneal macrophages which are the cells that form the first line of defense at this site, although some of the cytokines might have originated from other sources and reached the peritoneal fluid through circulation. However, we previously reported that cytokines from lysed peritoneal cells (mostly macrophages) exhibited a pattern of cytokine production very similar to that observed in peritoneal lavage fluid. This was observed in two separate studies involving different chemical compounds, and it supports the idea that the cytokines measured in peritoneal lavage fluid originated primarily from peritoneal cells (Pruett et al., 2004a; Pruett et al., 2005). However, it is still possible that other cellular sources contribute significantly to cytokines and chemokines in the peritoneal cavity.
Fig. 1.
Average concentration of cytokines and chemokines in pg/ml in peritoneal lavage fluid of wild type (C3H/HeOuJ) (n=4) and TLR4 hypo-responsive (C3H/HeJ) mice (n=4) treated with E. coli (2×108 cells) intraperitoneally or E. coli and ethanol (6g/Kg) measured 2 hours after E. coli challenge. The error bars represent the standard error of mean. *Significantly decreased as compared to C3H/HeOuJ mice treated with E. coli, ΔSignificantly decreased as compared to C3H/ HeJ mice treated with E. coli, ϕsignificantly decreased as compared to C3H/HeOuJ mice treated with E. coli + EtOH. The significance was determined by one way ANOVA followed by Newman-Keuls post hoc test.
The production of all cytokines and chemokines tested, except IL-10 and MIP-2, were significantly decreased by ethanol treatment in wild type mice challenged with E. coli (Fig. 1). In contrast, several cytokines and chemokines (IL-1β, IL-10, IL-17, TNF-α, Eotaxin, RANTES, MIG, MIP-1α and LIF) were not significantly altered by ethanol in TLR4 mutant mice (Fig. 1). These results indicate that the concentrations of many cytokines and chemokines induced through receptors other than TLR4 are not significantly decreased by ethanol (Fig. 1).
The TLR4 mutation in C3H/HeJ mice significantly decreased production of most of the 25 cytokines and chemokines tested both in the presence and absence of ethanol (Fig. 1). Only IFN-γ, IL-6, M-CSF, G-CSF, MIG and IP-10 were not significantly different in mutant as compared to wild type mice (Fig. 1).
Discussion
Alcohol consumption has long been recognized as a risk factor for infections including sepsis (Nelson and Kolls, 2002; Rehm et al., 2010). Previous studies in our laboratory (Dai et al., 2005; Pruett et al., 2010; Pruett et al., 2003; Pruett et al., 2004b) and by others (Dolganiuc et al., 2006; Goral and Kovacs, 2005; Happel et al., 2007; Mason et al., 2000; Nelson et al., 1989; Szabo et al., 1999; Szabo et al., 2007b) have clearly demonstrated that acute ethanol treatment interferes with innate immune responses. The results presented here demonstrate that mice treated with ethanol and then challenged intraperitoneally with non-pathogenic E. coli show decreased clearance of bacteria in both the peritoneal cavity and the spleen 2 hours after challenge. The production of most pro-inflammatory cytokines and chemokines was also suppressed. These results indicate that ethanol inhibits the initial inflammatory response to E. coli, which, in turn, decreases the clearance of bacteria in the first few hours after the challenge. Overall, these results are consistent with our previous studies in which we found that ethanol decreased resistance to E. coli and decreased other indicators of inflammation as well (Dai et al., 2005; Pruett et al., 2010; Pruett et al., 2003; Pruett et al., 2004b). However, in the present study, bacterial counts in the spleen as well as the peritoneal cavity were measured, and many more cytokines and chemokines were analyzed. More importantly, the cytokine and chemokine measurements in the present study are from peritoneal lavage fluid and are expected to be more indicative of the response occurring at the initial site of infection. Both wild type and TLR4 hyporesponsive mice were used in this study as in our previous study (Pruett et al., 2010), and this allowed us to more thoroughly assess the role of TLR4 early in the response to bacteria.
Penetrating abdominal trauma is associated with a significantly increased risk of infection in persons with acute ethanol exposure (Gentilello et al., 1993). Excessive alcohol consumption also substantially increases the risk of peritonitis. Although alcoholic cirrhosis seems to be involved in the initial translocation of bacteria from the gastrointestinal tract, ethanol per se is necessary for increased risk of infection (Rosa et al., 2000). The model of acute ethanol exposure and subsequent E. coli challenge used in our study is expected to represent patient populations in which sepsis is caused by microbial contamination of the peritoneal cavity. Peritonitis may begin as a multi-bacterial infection, but progression to sepsis is typically associated with a single bacterium and in about 50% of cases it is E. coli (De Waele et al., 2008; Mbopi Keou et al., 1992). Our finding of viable bacteria in the spleen suggests sepsis occurred in the present study, but the spleen lies in the peritoneal cavity, so some of the bacterial counts from spleen samples might reflect bacteria adhered to its surface rather than those coming through circulation. Even so, the high concentrations of cytokines in the serum we have previously reported (Pruett et al., 2010) strongly suggest systemic stimulation, as would be expected in sepsis. In any case, acute ethanol exposure significantly suppressed the clearance of bacteria from both the spleen and the peritoneal cavity.
Most of the cells in the peritoneal cavity at 2 hours were macrophages (∼80%) and about 15% were lymphocytes, demonstrating that neutrophils have not been recruited. This is consistent with a report indicating that chemotactic attraction of neutrophils by intraperitoneal inoculation of E. coli is delayed and not evident by 2 hours (Haziot et al., 1996). Cell number and percentages of each type remained unaffected by the absence of a fully functional TLR4 or the presence of acute ethanol. The temporal response of the effect of ethanol on peritoneal cells evaluated in our previous study indicate that the percentage of macrophages with bacteria increases from 1 to 4h in E. coli treated mice, after 4 hours this percentage decreases as bacteria were phagocytosed. On the other hand, ethanol increases the percentage of macrophages throughout the time course of 21 hours probably because of a dysfunctional neutrophil chemotaxis (Pruett, 2010). As with peritoneal cells, ethanol and TLR4 mutation did not have a significant effect on the total or differential cell counts of spleen.
Because ethanol did not affect the total or differential counts of the immune cells responsible for bacterial clearance at early time points, but was still associated with suppressed bacterial clearance, it seemed likely that the observed differences in bacterial clearance were caused by a functional defect in these cells. Since cytokines play a central role in activating macrophages to high levels of phagocytic and microbicidal activity, we evaluated the concentration of peritoneal cytokines and chemokines.
Most pro-inflammatory cytokines and chemokines were suppressed by ethanol and anti-inflammatory mediators were not. The results from the cytokine and chemokine study also showed various patterns in response to the different treatments in both the control and the mutant mice. Whereas the concentration of most of the cytokines and chemokines was significantly altered by ethanol in the wild type mice, a group of cytokines and chemokines (IL-1β, IL-10, IL-17, TNF-α, Eotaxin, RANTES, MIG, MIP-1α and LIF) were not significantly affected by the ethanol treatment in TLR4 mutant mice. Similar results were recently reported by Pascual et al., (Pascual et al., 2011). In addition to TLR4, inflammatory responses to E. coli are mediated through other TLR's (Roger et al., 2009). Moreover, there are several other receptors both cytoplasmic and membrane bound which respond to E. coli components (Lorenz et al., 2001). Our results demonstrate that the concentrations of several cytokines and chemokines induced through receptors other than TLR4 are not significantly decreased by ethanol. This may represent compensatory induction of cytokines or chemokines mediated by receptors that bind E. coli components other than LPS and are not sensitive to ethanol. The findings of the current study further support our previous findings that ethanol suppresses the induction of some but not all cytokines induced through various TLRs (Pruett et al., 2004b).
The concentration of a group of cytokines (IFN-γ, IL-6, M-CSF, G-CSF, MIG, IP-10) was suppressed by ethanol in both strains of mice, and the absence of a fully functional TLR4 did not have a major effect on their production. In fact, the concentration of these cytokines and chemokines produced by both the strains of mice for the same treatments were similar. This indicates that some receptors or signaling pathways in addition to TLR4 are adversely affected by ethanol, which is consistent with our previous study (Pruett et al., 2004b).
The concentration of most cytokines and chemokines (IL-1α, IL-9, IL-13, IL-15, IL-12p40, MIP-2, MIP-1β and LIX) was substantially lower in TLR4 mutant mice as compared to wild type mice. Suppression due to ethanol treatment in both strains indicates that LPS is the predominant inducer of these cytokines and chemokines and that compensatory upregulation of other receptors does not restore production to wild type levels. In addition, the results indicate that the receptors that mediate low level production of these cytokines and chemokines are sensitive to inhibition by ethanol.
The effect of ethanol on the serum concentrations of four cytokines (TNF-α, IL-6, MIP-1α and IL-10) in wild type (C3H/HeOuJ) and TLR4 hypo-responsive (C3H/HeJ) mice was evaluated in our previous study (Pruett et al., 2010). A comparison of the serum cytokine levels with the concentration of the cytokines in the peritoneal fluid (used in this study) showed that the trends remained the same, but the effect of ethanol was more pronounced for serum cytokines as compared to cytokines in peritoneal fluid. This reaffirms our previous finding that ethanol had varying effects on cytokines derived from different cell types (Pruett et al., 2004b). As noted in our previous study (Pruett et al., 2004b), the concentrations of the cytokines in the peritoneal lavage fluid might not reflect the effective concentrations in vivo as the injection of 1 ml of lavage fluid dilutes the peritoneal fluid by several fold so the actual concentration of the cytokines in the peritoneal fluid is higher than determined by these assays. Nevertheless, the relative concentrations used to compare groups are still valid.
The assessment of cytokines and chemokines here provided new information about some cytokines. The results provided the first direct evidence for induction of IL-9 through TLR4 in vivo, although induction of IL-9 from whole blood incubated with E. coli (Brekke et al., 2008) and mast cells incubated with LPS (Stassen et al., 2001) has been previously reported. Eotaxin (CCL11) has been shown to be a negative regulator of neutrophil recruitment in a murine model of endotexemia (Cheng et al., 2002). Eotaxin is associated with sepsis in humans (Osuchowski et al., 2006; Punyadeera et al., 2010). The results of our study indicate that acute ethanol exposure significantly suppressed eotaxin levels in the wild type mice and that there was no significant effect of ethanol on this chemokine in the mutant mice. To the best of our knowledge the effect of acute ethanol on eotaxin has not been previously reported, however chronic ethanol exposure followed by LPS injection leads to an increase in hepatic eotaxin mRNA levels in rats (Pennington et al., 1998). However, acetaldehyde which is a metabolite of ethanol, does not have an effect on eotaxin levels in airway epithelial cells (Machida et al., 2003). Another cytokine whose response to ethanol exposure has not previously been reported is the leukemia inhibitory factor (LIF), which, like eotaxin, was significantly suppressed by acute ethanol exposure in wild type mice. LIF has been shown to play an important protective role in animal models of sepsis (Jansen et al., 1996; Mayer et al., 1993; Waring et al., 1995; Weber et al., 2005). The role of early inhibition of LIF and eotaxin production by ethanol on survival in our sepsis model is not clear. Although results from other studies indicate that elevated eotaxin late in the course of sepsis is associated with poor prognosis (Osuchowski et al., 2006; Punyadeera et al., 2010), this does not necessarily mean that decreased eotaxin early in our model acts to enhance survival. Timing of enhanced or inhibited inflammatory mediators is a critical and often overlooked factor in survival in sepsis (Latifi et al., 2002). It might be expected that inhibition of LIF production by ethanol would diminish its protective effects in sepsis, but this effect may only be important late in the course of sepsis, so it is not clear how the early inhibition reported here would affect survival. This is an important issue that will be addressed in future studies.
In conclusion, the results of this study indicate that the decreased bacterial clearance in ethanol treated mice at early stages of sepsis (2 hours) is not caused by defects in immune cell numbers in the peritoneal cavity. However, decreased bacterial clearance does correlate with decreases in pro-inflammatory cytokines, which at later times after challenge leads to poor killing of phagocytosed bacteria (Pruett et al., 2010). The evaluation of cytokines in the peritoneal cavity in this study gives more direct evidence than the serum cytokines evaluated in our previous study because the peritoneal cytokines directly influence the key phagocytic cells in the peritoneal fluid, which act as the first line of defense in this particular model. The results suggest that ethanol suppresses the pro–inflammatory response to a level that no longer leads to effective bacterial clearance, whereas a sufficient pro-inflammatory response occurs in mice with a hyporesponsive TLR4 to clear bacteria. However, this response is not sufficient to cause septic shock and death. Results presented here also are consistent with the idea that ethanol treatment has different effects on cytokines induced through different TLRs. A relatively thorough assessment of cytokines and chemokines here provided the first direct evidence for induction of IL-9 through TLR4 in vivo. The study also demonstrated the previously unknown effects of acute ethanol exposure on LIF (leukemia inhibitory factor) and eotaxin. Both of these cytokines are known play an important role in animal models of sepsis and in studies conducted on sepsis patients (Cheng et al., 2002; Jansen et al., 1996; Presneill et al., 2000; Punyadeera et al., 2010; Waring et al., 1995; Weber et al., 2005).
Acknowledgments
This work was supported by R01AA009505 from the National Institute of Alcohol Abuse and Alcoholism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Brekke OL, Christiansen D, Fure H, Pharo A, Fung M, Riesenfeld J, Mollnes TE. Combined inhibition of complement and CD14 abolish E. coli-induced cytokine-, chemokine- and growth factor-synthesis in human whole blood. Mol Immunol. 2008;45:3804–3813. doi: 10.1016/j.molimm.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Carson EJ, Pruett SB. Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol Clin Exp Res. 1996;20:132–138. doi: 10.1111/j.1530-0277.1996.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Cheng SS, Lukacs NW, Kunkel SL. Eotaxin/CCL11 is a negative regulator of neutrophil recruitment in a murine model of endotoxemia. Exp Mol Pathol. 2002;73:1–8. doi: 10.1006/exmp.2002.2439. [DOI] [PubMed] [Google Scholar]
- Dai Q, Zhang J, Pruett SB. Ethanol alters cellular activation and CD14 partitioning in lipid rafts. Biochem Biophys Res Commun. 2005;332:37–42. doi: 10.1016/j.bbrc.2005.04.088. [DOI] [PubMed] [Google Scholar]
- De Waele JJ, Hoste EA, Blot SI. Blood stream infections of abdominal origin in the intensive care unit: characteristics and determinants of death. Surg Infect (Larchmt) 2008;9:171–177. doi: 10.1089/sur.2006.063. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Bakis G, Kodys K, Mandrekar P, Szabo G. Acute ethanol treatment modulates Toll-like receptor-4 association with lipid rafts. Alcohol Clin Exp Res. 2006;30:76–85. doi: 10.1111/j.1530-0277.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Gentilello LM, Cobean RA, Walker AP, Moore EE, Wertz MJ, Dellinger EP. Acute ethanol intoxication increases the risk of infection following penetrating abdominal trauma. J Trauma. 1993;34:669–674. doi: 10.1097/00005373-199305000-00009. [DOI] [PubMed] [Google Scholar]
- Goral J, Kovacs EJ. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J Immunol. 2005;174:456–463. doi: 10.4049/jimmunol.174.1.456. [DOI] [PubMed] [Google Scholar]
- Happel KI, Rudner X, Quinton LJ, Movassaghi JL, Clark C, Odden AR, Zhang P, Bagby GJ, Nelson S, Shellito JE. Acute alcohol intoxication suppresses the pulmonary ELR-negative CXC chemokine response to lipopolysaccharide. Alcohol. 2007;41:325–333. doi: 10.1016/j.alcohol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, Stewart CL, Goyert SM. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- Huttunen R, Laine J, Lumio J, Vuento R, Syrjanen J. Obesity and smoking are factors associated with poor prognosis in patients with bacteraemia. BMC Infect Dis. 2007;7:13. doi: 10.1186/1471-2334-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen PM, de Jong IW, Hart M, Kim KJ, Aarden LA, Hinshaw LB, Taylor FB, Jr, Hack CE. Release of leukemia inhibitory factor in primate sepsis. Analysis of the role of TNF-alpha. J Immunol. 1996;156:4401–4407. [PubMed] [Google Scholar]
- Jones AW, Holmgren A. Age and gender differences in blood-alcohol concentration in apprehended drivers in relation to the amounts of alcohol consumed. Forensic Sci Int. 2009;188:40–45. doi: 10.1016/j.forsciint.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Latifi SQ, O'Riordan MA, Levine AD. Interleukin-10 controls the onset of irreversible septic shock. Infect Immun. 2002;70:4441–4446. doi: 10.1128/IAI.70.8.4441-4446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz E, Jones M, Wohlford-Lenane C, Meyer N, Frees KL, Arbour NC, Schwartz DA. Genes other than TLR4 are involved in the response to inhaled LPS. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1106–1114. doi: 10.1152/ajplung.2001.281.5.L1106. [DOI] [PubMed] [Google Scholar]
- Machida I, Matsuse H, Kondo Y, Kawano T, Saeki S, Tomari S, Fukushima C, Shimoda T, Kohno S. Acetaldehyde induces granulocyte macrophage colony-stimulating factor production in human bronchi through activation of nuclear factor-kappa. B Allergy Asthma Proc. 2003;24:367–371. [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Mason CM, Dobard E, Kolls JK, Nelson S. Ethanol and murine interleukin (IL)-12 production. Alcohol Clin Exp Res. 2000;24:553–559. [PubMed] [Google Scholar]
- Mayer P, Geissler K, Ward M, Metcalf D. Recombinant human leukemia inhibitory factor induces acute phase proteins and raises the blood platelet counts in nonhuman primates. Blood. 1993;81:3226–3233. [PubMed] [Google Scholar]
- Mbopi Keou FX, Bloch F, Buu Hoi A, Lavril M, Belec L, Mokbat JE, Petite JP, Acar JF. Spontaneous peritonitis in cirrhotic hospital in-patients: retrospective analysis of 101 cases. Q J Med. 1992;83:401–407. [PubMed] [Google Scholar]
- McGill V, Kowal-Vern A, Fisher SG, Kahn S, Gamelli RL. The impact of substance use on mortality and morbidity from thermal injury. J Trauma. 1995;38:931–934. doi: 10.1097/00005373-199506000-00019. [DOI] [PubMed] [Google Scholar]
- Nelson S, Bagby GJ, Bainton BG, Summer WR. The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. J Infect Dis. 1989;160:422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- Nelson S, Kolls JK. Alcohol, host defence and society. Nat Rev Immunol. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- Pascual M, Fernandez-Lizarbe S, Guerri C. Role of TLR4 in ethanol effects on innate and adaptive immune responses in peritoneal macrophages. Immunol Cell Biol. 2011 doi: 10.1038/icb.2010.163. [DOI] [PubMed] [Google Scholar]
- Pennington HL, Wilce PA, Worrall S. Chemokine and cell adhesion molecule mRNA expression and neutrophil infiltration in lipopolysaccharide-induced hepatitis in ethanol-fed rats. Alcohol Clin Exp Res. 1998;22:1713–1718. [PubMed] [Google Scholar]
- Presneill JJ, Waring PM, Layton JE, Maher DW, Cebon J, Harley NS, Wilson JW, Cade JF. Plasma granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor levels in critical illness including sepsis and septic shock: relation to disease severity, multiple organ dysfunction, and mortality. Crit Care Med. 2000;28:2344–2354. doi: 10.1097/00003246-200007000-00028. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Cheng B, Glover M, Tan W, Deng X. Innate immunity and inflammation in sepsis: mechanisms of suppressed host resistance in mice treated with ethanol in a binge-drinking model. Toxicol Sci. 2010;117:314–324. doi: 10.1093/toxsci/kfq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q. Acute ethanol administration profoundly alters poly I:C-induced cytokine expression in mice by a mechanism that is not dependent on corticosterone. Life Sci. 2003;72:1825–1839. doi: 10.1016/s0024-3205(02)02507-9. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Schwab C, Zheng Q, Fan R. Suppression of innate immunity by acute ethanol administration: a global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J Immunol. 2004a;173:2715–2724. doi: 10.4049/jimmunol.173.4.2715. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol. 2004b;33:147–155. doi: 10.1016/j.alcohol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Schwab C, Fan R. Sodium methyldithiocarbamate inhibits MAP kinase activation through toll-like receptor 4, alters cytokine production by mouse peritoneal macrophages, and suppresses innate immunity. Toxicol Sci. 2005;87:75–85. doi: 10.1093/toxsci/kfi215. [DOI] [PubMed] [Google Scholar]
- Punyadeera C, Schneider EM, Schaffer D, Hsu HY, Joos TO, Kriebel F, Weiss M, Verhaegh WF. A biomarker panel to discriminate between systemic inflammatory response syndrome and sepsis and sepsis severity. J Emerg Trauma Shock. 2010;3:26–35. doi: 10.4103/0974-2700.58666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, Burns K, Riederer BM, Akira S, Calandra T. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A. 2009;106:2348–2352. doi: 10.1073/pnas.0808146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa H, Silverio AO, Perini RF, Arruda CB. Bacterial infection in cirrhotic patients and its relationship with alcohol. Am J Gastroenterol. 2000;95:1290–1293. doi: 10.1111/j.1572-0241.2000.02026.x. [DOI] [PubMed] [Google Scholar]
- Stassen M, Muller C, Arnold M, Hultner L, Klein-Hessling S, Neudorfl C, Reineke T, Serfling E, Schmitt E. IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-kappa B is decisively involved in the expression of IL-9. J Immunol. 2001;166:4391–4398. doi: 10.4049/jimmunol.166.7.4391. [DOI] [PubMed] [Google Scholar]
- Statistics, N.C.f H. Health, United States, 2007: With Chartbook on Trends in the Health of Americans. Report No.: 2007-1232. 2007. [PubMed] [Google Scholar]
- Szabo G, Chavan S, Mandrekar P, Catalano D. Acute alcohol consumption attenuates interleukin-8 (IL-8) and monocyte chemoattractant peptide-1 (MCP-1) induction in response to ex vivo stimulation. J Clin Immunol. 1999;19:67–76. doi: 10.1023/a:1020518703050. [DOI] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A, Dai Q, Pruett SB. TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007a;178:1243–1249. doi: 10.4049/jimmunol.178.3.1243. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Oak S, Mayerle J. Effect of ethanol on inflammatory responses. Implications for pancreatitis. Pancreatology. 2007b;7:115–123. doi: 10.1159/000104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sci. 1981;28:1053–1056. doi: 10.1016/0024-3205(81)90752-9. [DOI] [PubMed] [Google Scholar]
- Waring PM, Waring LJ, Billington T, Metcalf D. Leukemia inhibitory factor protects against experimental lethal Escherichia coli septic shock in mice. Proc Natl Acad Sci U S A. 1995;92:1337–1341. doi: 10.1073/pnas.92.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MA, Schnyder-Candrian S, Schnyder B, Quesniaux V, Poli V, Stewart CL, Ryffel B. Endogenous leukemia inhibitory factor attenuates endotoxin response. Lab Invest. 2005;85:276–284. doi: 10.1038/labinvest.3700216. [DOI] [PubMed] [Google Scholar]
- Woodman GE, Fabian TC, Beard JD, Proctor KG. Actions of acute ethanol intoxication on cardiopulmonary function after an endotoxin challenge. Surgery. 1996;120:80–92. doi: 10.1016/s0039-6060(96)80245-5. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Wolcott RM, Pruett SB. Ethanol decreases the number and activity of splenic natural killer cells in a mouse model for binge drinking. J Pharmacol Exp Ther. 1994;271:722–729. [PubMed] [Google Scholar]