Abstract
Previously, we have shown that A2A adenosine receptor (A2AAR) knockout mice (KO) have increased contraction to adenosine. The signaling mechanism(s) for A2AAR is still not fully understood. In this study, we hypothesize that, in the absence of A2AAR, ω-hydroxylase (Cyp4a) induces vasoconstriction through mitogen-activated protein kinase (MAPK) via upregulation of adenosine A1 receptor (A1AR) and protein kinase C (PKC). Organ bath and Western blot experiments were done using isolated aorta from A2AKO and corresponding wild-type (WT) mice. Isolated aortic rings from WT and A2AKO mice were precontracted with submaximal dose of phenylephrine (10−6 M), and concentration responses for selective A1AR, A2AAR agonists, angiotensin II and cytochrome P-450-epoxygenase, 20-hydroxyeicosatrienoic acid (20-HETE) PKC, PKC-α, and ERK1/2 inhibitors were obtained. 2-p-(2-Carboxyethyl)-phenethylamino-5′-N-ethylcarboxamidoadenosine hydrochloride (CGS-21680, A2AAR agonist) induced concentration-dependent relaxation in WT, which was blocked by methylsulfonyl-propargyloxyphenylhexanamide (cytochrome P-450-epoxygenase inhibitor; 10−5 M) and also with removal of endothelium. A1 agonist, 2-chloro-N6-cyclopentyladenosine (CCPA) produced higher contraction in A2AKO aorta than WT (49.2 ± 8.5 vs. 27 ± 5.9% at 10−6 M, P < 0.05). 20-HETE produced higher contraction in A2AKO than WT (50.6 ± 8.8 vs. 21.1 ± 3.3% at 10−7 M, P < 0.05). Contraction to CCPA in WT and A2AKO aorta was inhibited by PD-98059 (p42/p44 MAPK inhibitor; 10−6 M), chelerythrine chloride (nonselective PKC blocker; 10−6 M), Gö-6976 (selective PKC-α inhibitor; 10−7 M), and HET0016 (20-HETE inhibitor; 10−5 M). Also, contraction to 20-HETE in WT and A2AKO aorta was inhibited by PD-98059 and Gö-6976. Western blot analysis indicated the upregulation of A1AR, Cyp4a, PKC-α, and phosphorylated-ERK1/2 in A2AKO compared with WT (P < 0.05), while expression of Cyp2c29 was significantly higher in WT. CCPA (10−6 M) increased the protein expression of PKC-α and phosphorylated-ERK1/2, while HET0016 significantly reduced the CCPA-induced increase in expression of these proteins. These data suggest that, in the absence of A2AAR, Cyp4a induces vasoconstriction through MAPK via upregulation of A1AR and PKC-α.
Keywords: adenosine receptors, relaxation, contraction, cytochrome p4504a, extracellular signal-regulated kinase-1/2 kinases
adenosine regulates vascular tone through four adenosine receptor (AR) subtypes i.e., A1, A2A, A2B, and A3 (42). The response produced in vasculature depends, in part, on the subtype of receptor activated, leading to either vasoconstriction or vasorelaxation. Activation of the A1AR and A3AR causes contraction, whereas A2AAR and A2BAR cause relaxation (2, 10, 12, 17, 25, 41, 43, 44). We have shown that the lack of A2AAR markedly lowers adenosine-mediated vascular relaxation and that cytochrome P-450 (CYP) epoxygenases play a role in A2A-induced vascular relaxation (27, 32).
Metabolites of arachidonic acid (AA), produced by the endothelium, are known to regulate vascular tone. Epoxyeicosatrienoic acids (EETs) are metabolites of AA, formed through the action of CYP epoxygenases, including Cyp2c29, which have been proposed to be an endothelium-derived hyperpolarizing factor (EDHF) (9). Activation of A2AAR is related to increased activity of CYP epoxygenase that results in vasorelaxation (6, 27). We also found an upregulation of Cyp2c29 associated with the presence of A2AAR that caused endothelium-dependent relaxation (27).
On the other hand, 20-hydroxyeicosatrienoic acid (20-HETE), a ω-hydroxylation product of AA catalyzed by Cyp4a, is a potent vasoconstrictor (36). Several studies have shown a link between vascular 20-HETE generation and responses in various vascular beds, including renal, cerebral, skeletal, and mesenteric (7, 8, 18). Increase in intracellular Ca2+ in smooth muscle causes an increase in 20-HETE (13), and, subsequent to this, 20-HETE inhibits Ca2+-dependent K+ channels, resulting in increase in vascular tone (14).
Previously, we have found that, in A2A knockout (KO) aorta, the use of A1-selective antagonist blunted the contraction response, suggesting a role for A1AR-mediated contraction (32). A1AR is coupled to Gi/o proteins, and its activation results in a decrease in cyclic AMP through inhibition of adenylate cyclase (20). Studies have also shown that A1AR activates p42/44 MAPK (ERK1/2) in vascular tissue and different cell lines (4, 16, 37). This activation may involve protein kinase A, protein kinase C (PKC), Src tyrosine kinase, or Ras activation (30, 38).
Studies from this laboratory have also found that several PKC isoforms are upregulated in porcine coronary artery smooth muscle cells through A1AR upregulation, which is blocked by pertussis toxin (19, 21). More recently, our laboratory has published reports that indicate that A1AR causes PKC-α activation, leading to p42/p44 MAPK phosphorylation in mouse coronary artery smooth muscle cells from wild-type (WT) and A1KO that also causes vascular contraction in mesenteric arteries (4). 20-HETE has also been linked with the activation of PKC (14, 36). Therefore, we hypothesize that, in the absence of A2AAR, Cyp4a-derived metabolite, 20-HETE induces vasoconstriction through MAPK via upregulation of A1AR and PKC-α.
MATERIALS AND METHODS
Animals.
A2AKO mice (originally from C. Ledent, Universite Libre de Bruxelles, Brussels, Belgium) were obtained from Dr. Stephen Tilley, University of North Carolina Chapel Hill, on C57BL/6J background. C57BL/6J (WT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). A2AKO mice were backcrossed 12 generations to the C57BL/6J background. A2AKO mice were generated and genotyped by polymerase chain reaction. Males and females 14–18 wk old were used in our studies. All experimental animals used in this study were under a protocol approved by the Institutional Animal Care and Use Committee of West Virginia University.
Preparation of isolated mouse aorta and isometric force measurement.
Mice were killed by anesthesia with pentobarbital sodium (65 mg/kg ip), followed by thoracotomy and removal of aorta that was then cut transversely into 3- to 4-mm rings. The rings were mounted vertically between two stainless steel wire hooks and then suspended in 10-ml organ baths containing Krebs-Henseleit buffer. The Krebs-Henseleit buffer (pH 7.4), containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 11 mM glucose, and 2.5 mM CaCl2, was maintained at 37°C with continuous bubbling of 95% O2 and 5% CO2. For measurement of isometric force response, aortic rings were equilibrated for 90 min with a resting force of 1 g and change of the bathing solution at 15-min interval. The resting force of 1 g has been used earlier in our laboratory and was determined using a length-tension relationship (2, 31, 43). At the end of the equilibration period, tissues were contracted with 50 mM KCl to check the contractility of individual aortic rings twice, which were washed out with Krebs-Henseleit buffer. Aortic rings were then constricted with phenylephrine at submaximal concentration (PE, 10−6 M) to obtain a steady contraction, and changes in tension were monitored continuously with fixed range precision force transducer (TSD, 125 C, BIOPAC system) connected to the differential amplifier (DA 100B, BIOPAC system). The data were recorded using MP100, BIOPAC digital acquisition system and analyzed using Acknowledge 3.5.7 software (BIOPAC system).
Contraction/relaxation experiments.
After equilibration, the responsiveness and stability of individual rings were checked by successive administration of PE (10−6 M). The integrity of the vascular endothelium was assessed pharmacologically by acetylcholine (10−6 M) to produce relaxation of PE precontracted rings. Aortic rings were then washed several times with Krebs-Henseleit solution and allowed to equilibrate for 30 min before the experimental protocol began. In experiments in which the involvement of endothelium was investigated, endothelium was removed mechanically before mounting in the bath, using a thin stainless steel wire to scrape the lumen of the aorta.
Experimental protocol.
The concentration-response to 2-p-(2-carboxyethyl)-phenethylamino-5′-N-ethylcarboxamidoadenosine hydrochloride (CGS-21680; 10−11-10−6 M), 2-chloro-N6-cyclopentyladenosine (CCPA; 10−11-10−6 M), and angiotensin II (10−11-10−5 M) were run in parallel in aortic rings from WT and A2AKO mice. In all cases, drug was added to yield the next higher concentration, only when the response to the earlier dose reached a steady state. For 20-HETE, a single concentration (10−7 M) was used. In experiments in which the effect of an antagonist was studied, it was added 30 min before contraction of the tissue with PE and was present throughout the experiments. Contraction/relaxation responses were expressed as a percentage increase/decrease in the contraction with respect to PE (alone) in response to each concentration of agonist used. The effect of methylsulfonyl-propargyloxyphenylhexanamide (MS-PPOH; 10−5 M) was studied in PE-precontracted tissues using this protocol. The concentration of drugs was chosen on the basis of previous studies (4, 27, 31).
Effects of chelerythrine chloride (PKC blocker), Gö-6976 (selective PKC-α inhibitor), PD-98059 (p42/p44 MAPK inhibitor), and HET0016 (20-HETE inhibitor) on CCPA-induced concentration response.
The effects of chelerythrine chloride (10−6 M), 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)carbazole (Gö-6976; 10−7 M), PD-98059 (10−6 M), and HET0016 (10−5 M) on CCPA-response were studied in PE-precontracted tissues in parallel, as described above. These drugs were added 30 min before contraction of the tissue with PE and were present throughout the experiments.
Effect of PD-98059 and Gö-6976 on 20-HETE-induced response.
The effect of PD-98059 (10−6 M) and Gö-6976 (10−7 M) on 20-HETE-induced response was studied in PE-precontracted tissues in parallel, as described above. The drug was added 30 min before contraction of the tissue with PE and was present throughout the experiments.
Western blot analysis.
Aorta from WT and A2AKO mice were isolated and divided into three groups: control (nontreated), CCPA (10−6 M; A1 agonist), and CCPA + HET0016 (10−5 M; Cyp4a inhibitor)-treated groups. Tissues were treated with 10−6 M CCPA at 37°C for 60 min. For experiments with the Cyp4a inhibitor, tissues were first incubated for 30 min with HET0016 (10−5 M), followed by treatment with CCPA (10−6 M) for 60 min. Each sample was homogenized with 130 μl RIPA buffer on wet ice. The samples were transferred to dry ice for 5 min and then thawed on wet ice. After thawing, the samples were vortexed and centrifuged for 5 min at 12,000 rpm at 4°C. Supernatants were sonicated and stored at −80°C. Protein was measured using Bio-Rad assay based on the Bradford dye procedure with bovine serum albumin as a standard. The protein mixture was divided into aliquots and stored at −80°C. At the time of analysis, samples were thawed, and 30 μg of total protein per lane were loaded on a slab gel. Proteins were separated by SDS-PAGE using 10% acrylamide gels (1-mm thick). After electrophoresis, the proteins on the gel were transferred to nitrocellulose membrane (Hybond-ECL) by electroelution. Protein transfer was confirmed by employing prestained molecular weight markers (Bio-Rad Laboratories, Hercules, CA). Following blocking with either 5% nonfat dry milk or BSA, the nitrocellulose membranes were incubated with primary antibody (A1AR, Cyp4a, PKC-α, and p-ERK 1/2). 1:1,000 primary antibody concentrations were used for A1AR, Cyp4a, and PKC-α, while 1:500 dilutions were used for total ERK and phosphorylated-ERK1/2 (p-ERK1/2). For Cyp2c29, a 1:5,000 antibody concentration was used. β-Actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used as an internal control to normalize the target protein expression in each lane. The secondary antibody was a horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG. The membranes were developed using enhanced chemiluminescence (Amersham BioSciences) and exposed to X-ray film for the appropriate time. The data are presented as the normalized ratio (%change) of target protein to WT protein expression.
Drugs and chemicals.
Acetylcholine and PE were dissolved in distilled water. DPCPX, CGS-21680, CCPA, chelerythrine chloride, angiotensin II, PD-98059, Gö-6976 (Calbiochem, La Jolla, CA), as well as 20-HETE and HET0016 (Cayman Chemical, Ann Arbor, MI), were dissolved in 100% DMSO as a 10 mM stock solution. MS-PPOH (Cayman Chemical, Ann Arbor, MI) was dissolved 100% ethanol as a 10 mM stock solution. The final concentration of DMSO in organ bath (10 ml) had no effect by itself on the aortic rings (2). PKC-α monoclonal antibody was purchased from BD Transduction Laboratories (San Diego, CA). ERK and β-actin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Cyp4a antibody (which possibly recognizes Cyp4a1, Cyp4a10, Cyp4a14, and Cyp4a3 isoforms, according to the manufacturer) was obtained from Abcam (Cambridge, MA), and Cyp2c29 antibody was obtained from Dr. Darryl Zeldin (National Institute of Environmental Health Sciences/National Institutes of Health). Unless stated otherwise, all chemicals were of the highest grade available and were purchased from Sigma Chemicals (St. Louis, MO).
Statistical analysis.
The data are expressed as means ± SE. Comparisons among different groups were analyzed by either one-way or two-way ANOVA (analysis of variance), followed by Tukey's multiple-comparison test or Bonferroni's post hoc test, respectively. Comparison between two groups was assessed by unpaired t-test. A P value of <0.05 was considered as the level of significance for all statistical tests. All of the statistical analyses were performed using GraphPad Prism statistical package.
RESULTS
Acetylcholine-dependent response in WT and A2AKO mouse aorta.
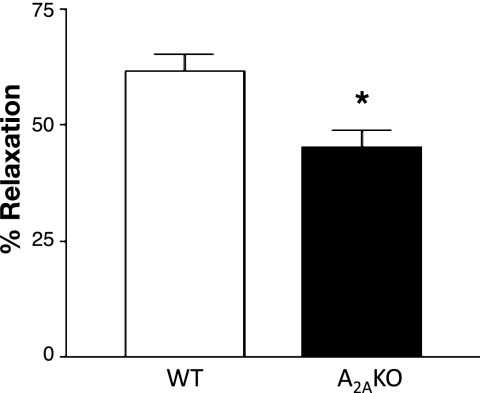
A2AKO mice had significantly lower vascular relaxation (∼49%) to acetylcholine compared with respective WT on a C57BL/6J background (∼62%; P < 0.05; Fig. 1). This is similar to what has been reported (on CD-1 background strain) from this laboratory earlier (27, 31).
Fig. 1.
Endothelial-dependent responses to acetylcholine (10−6 M) in wild-type (WT) and A2A knockout (KO) mouse aorta (C57BL/6J background). Values are means ± SE (n = 6 mice). *P < 0.05 compared with WT.
Effect of CYP epoxygenase inhibitor (MS-PPOH) on CGS-21680-induced vascular response.
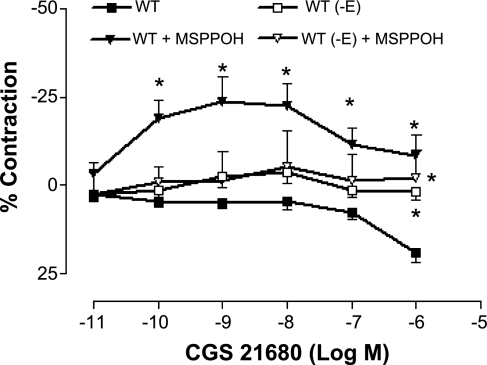
The vascular response to CGS-21680 (selective A2AAR agonist) was studied in WT and A2AKO mice aorta. Maximum response (20% relaxation) to CGS-21680 was observed in WT aorta at 10−6 M (Fig. 2), while A2AKO did not respond at all (data not shown). We also determined the contribution of the endothelium to A2AAR-mediated relaxation, by performing concentration response with CGS-21860 in WT in which the endothelium has been removed (-E). Removal of the endothelium abolished CGS-21860-induced relaxation, suggesting that endothelium was involved in A2A receptor-mediated relaxation.
Fig. 2.
Concentration response to 2-p-(2-carboxyethyl)-phenethylamino-5′-N-ethylcarboxamidoadenosine hydrochloride (CGS-21860) in WT and the effect of methylsulfonyl-propargyloxyphenylhexanamide (MS-PPOH; 10−5 M) in mouse aorta, with (+E) and without endothelium (-E). Values are means ± SE (n = 5 mice). On y-axis, positive and negative values indicate relaxation and contraction, respectively. *P < 0.05 compared with WT (+E).
In the intact endothelium of WT, the relaxation to CGS-21680 was significantly inhibited by MS-PPOH (10−5 M), and the response changed from relaxation of 19.02 ± 2.75% to contraction of 8.31 ± 5.9% at 10−6 M (P < 0.05; Fig. 2). MS-PPOH had no effect in the absence of endothelium. These data suggest that the involvement of endothelium through CYP epoxygenases causes A2A-mediated relaxation and confirmed our laboratory's previous findings (26, 31), which were observed in a different strain (CD-1) of A2AKO and WT mice.
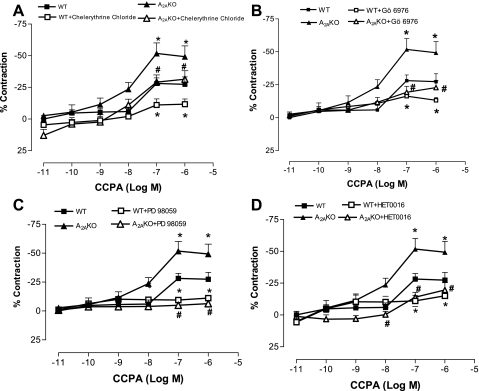
Effects of PKC blocker (chelerythrine chloride), selective PKC-α inhibitor (Gö-6976), p42/p44 MAPK inhibitor (PD-98059), and 20-HETE inhibitor (HET0016) on CCPA-induced vascular response.
The present data also showed a concentration-dependent response to CCPA (selective A1AR agonist) in both A2AKO and WT aorta, with the KO having significantly higher contraction than WT (Fig. 3). The maximum contraction produced by CCPA (10−7 M) was 51.7 ± 8.27% in KO compared with WT (29.01 ± 4.3%; P < 0.05; Fig. 3). The responses to CCPA in aorta were comparable to those obtained using mesenteric artery responses (contraction) in WT (30.02 ± 11.61% at 10−7 M) and A2AKO (52.96 ± 21.81% at 10−7 M; Dr. Teng, unpublished personal communication). The contraction to CCPA (10−7 M) was significantly reduced by chelerythrine chloride (PKC blocker; 10−6 M; Fig. 3A) from 29.01 ± 4.3 to 11 ± 4% (P < 0.05) in WT and from 51.7 ± 8.27 to 29.1 ± 5.6% (P < 0.05) in KO. For examining the specific PKC isoform involved in CCPA-mediated contraction, we used selective PKC-α inhibitor Gö-6976, which significantly lowered the contraction response from 29.01 ± 4.3 to 16.2 ± 1.84% (P < 0.05) in WT and from 51.7 ± 8.27 to 19.3 ± 4.51% (P < 0.05) in KO (Fig. 3B). Similarly, PD-98059 (p42/p44 MAPK inhibitor; 10−6 M) reduced CCPA-induced contraction from 29.01 ± 4.3 to 9.4 ± 1.37% (P < 0.05) in WT and from 51.7 ± 8.27 to 4.88 ± 1.8% (P < 0.05, Fig. 3C) in KO. Also, HET0016 (20-HETE inhibitor; 10−5 M) significantly lowered the CCPA-induced contraction from 29.01 ± 4.3 to 10.92 ± 4.3% (P < 0.05) in WT and from 51.7 ± 8.27 to 13.9 ± 3.8% (P < 0.05, Fig. 3D) in KO. These results indicate that the CCPA-mediated contraction most likely involves PKC-α and p42/p44 MAPK (ERK1/2) signaling through Cyp4a.
Fig. 3.
Effect of PKC blocker chelerythrine chloride (10−6 M; A), selective PKC-α inhibitor 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)carbazole (Gö-6976; 10−7 M; B), p42/p44 MAPK inhibitor PD-98059 (10−6 M; C), and 20-hydroxyeicosatrienoic acid (20-HETE) inhibitor HET0016 (10−5 M; D) on concentration response to 2-chloro-N6-cyclopentyladenosine (CCPA) in aorta from WT and A2AKO. The control responses are the same for A–D. Values are means ± SE (n = 4 mice). On y-axis, positive and negative values indicate relaxation and contraction, respectively. *P < 0.05 compared with WT. #P < 0.05 compared with A2AKO.
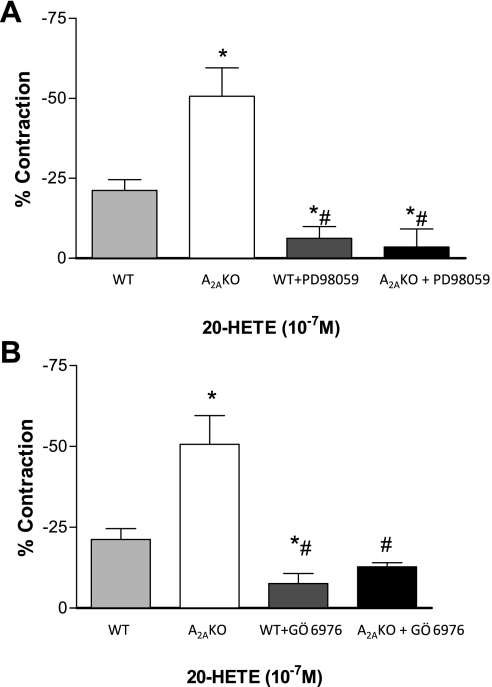
Effect of PD-98059 and Gö-6976 on 20-HETE-induced response.
To gauge the effect of 20-HETE in WT and A2AKO mouse aorta, we used 20-HETE in organ bath experiments. 20-HETE (10−7 M) produced significantly higher contraction in KO than WT (50.6 ± 8.80 vs. 21.1 ± 3.7%, P < 0.05; Fig. 4). Contraction to 20-HETE (10−7 M) in aorta was significantly reduced with PD-98059 (MAPK inhibitor, 10−6 M) from 21.1 ± 3.7 to 6.1 ± 3.7% (P < 0.05) in WT and from 50.6 ± 8.80 to 3.5 ± 5.8% (P < 0.05, Fig. 4A) in KO. Gö-6976 (selective PKC-α inhibitor, 10−7 M) also lowered 20-HETE-induced contraction significantly from 21.1 ± 3.7 to 7.51 ± 3.14% (P < 0.05) in WT and from 50.6 ± 8.80 to 12.72 ± 1.23% (P < 0.05, Fig. 4B) in KO. These results indicate that contraction mediated by 20-HETE involves PKC-α and MAPK signaling pathway.
Fig. 4.
Contraction response in aorta from WT and A2AKO to 20-HETE (10−7 M) and the effect of p42/p44 MAPK inhibitor PD-98059 (10−6 M; A) and selective PKC-α inhibitor Gö-6976 (10−7 M; B). The control responses are the same for A and B. Values are means ± SE (n = 4 mice). On y-axis, negative values indicate contraction. *P < 0.05 compared with WT. #P < 0.05 compared with A2AKO.
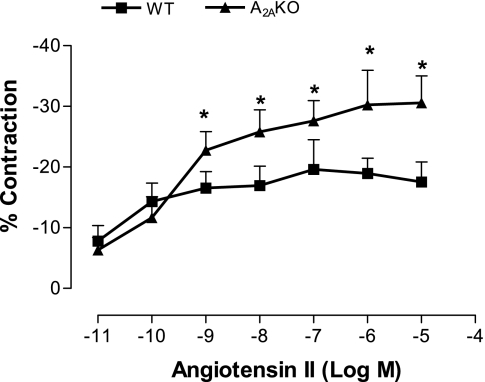
Angiotensin II concentration response in WT and A2AKO mouse aorta.
The aortic response to angiotensin II was studied in aortas from WT and A2AKO mice. Angiotensin II is known to activate the MAPK/PKC signaling pathways, and hence this experiment was done to support the involvement of MAPK/PKC signaling in our experiments. We found that angiotensin II produced concentration-dependent contraction in both WT and KO aorta, with A2AKO showing significantly higher contraction than WT (P < 0.05; Fig. 5).
Fig. 5.
Concentration response to angiotensin II in WT and A2AKO aorta. Values are means ± SE (n = 4 mice). On y-axis, negative values indicate contraction. *P < 0.05 compared with WT.
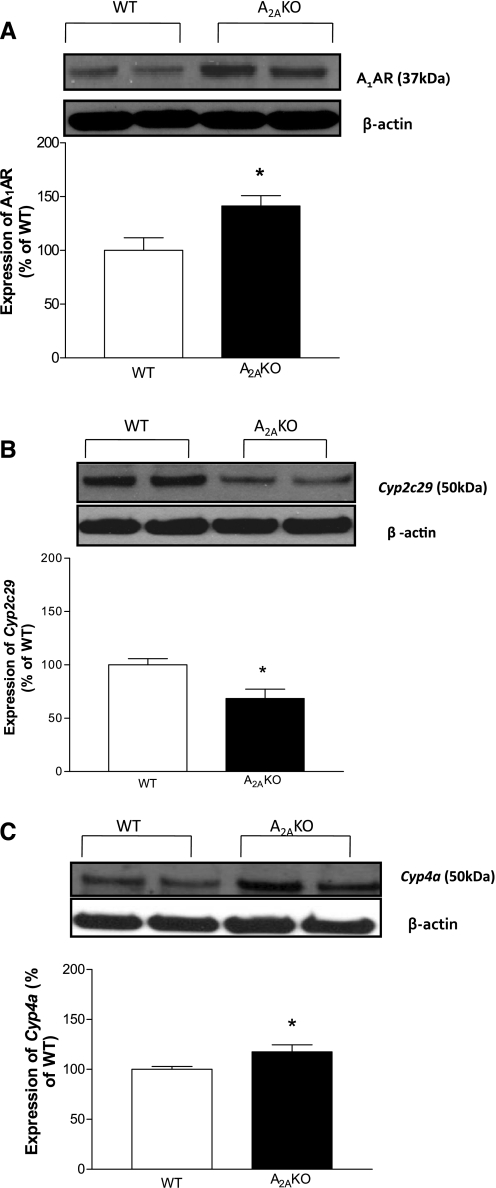
Expression of A1AR, Cyp2c29, and Cyp4a proteins in A2AKO and WT mouse aorta.
The expression of A1AR (∼37 kDa; Fig. 6A) was ∼41% higher in A2AKO (141.23 ± 9.67%; P < 0.05) compared with WT (100 ± 11.81%). The expression of Cyp2c29 (∼50 kDa; Fig. 6B) was ∼32% lower (68.44 ± 8.83% in A2AKO; P < 0.05) compared with WT (100 ± 5.95%). Also, Cyp4A (∼50 kDa; Fig. 5C) was ∼18% higher in A2AKO (117.6 ± 6.94%; P < 0.05) compared with WT (100 ± 2.98%).
Fig. 6.
A: expression of A1 adenosine receptor (AR) in WT and A2AKO aorta and densitometric analysis. B: expression of Cyp2c29 in WT and A2AKO aorta and densitometric analysis. C: expression of Cyp4a in WT and A2AKO aorta and densitometric analysis. All of the data (A–C, representative blots) are presented as means (%ratio to WT) ± SE (n = 5-6 mice). *P < 0.05 compared with WT.
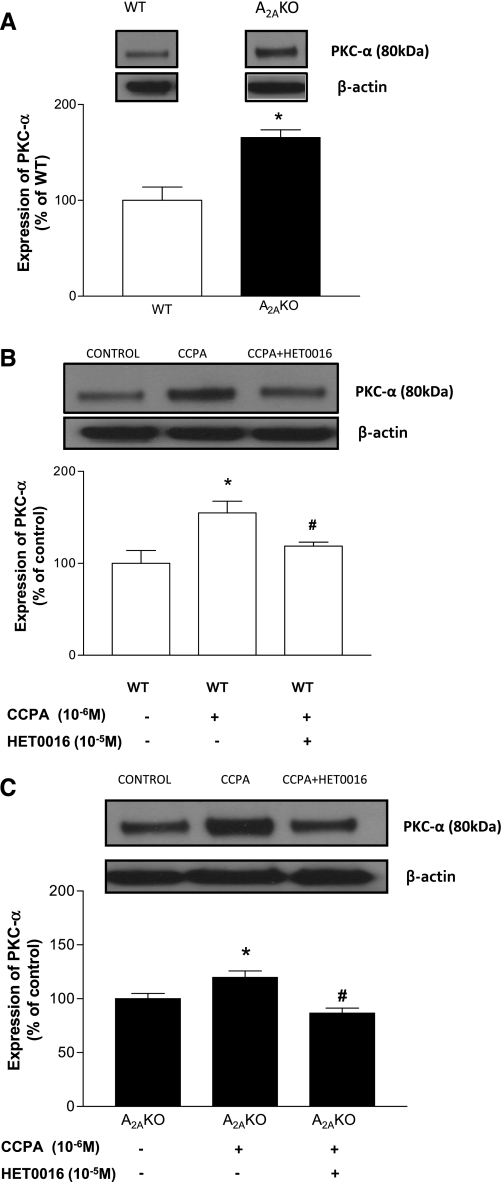
Protein expression of PKC-α in A2AKO and WT mouse aorta.
The expression of PKC-α (∼80 kDa) was ∼66% higher in A2AKO (165.6 ± 8.05%; P < 0.05) compared with WT (100 ± 13.99%; Fig. 7A). CCPA (10−6 M) increased PKC-α expression in WT by ∼55% (from 100 ± 13.99 to 154.84 ± 12.81%; P < 0.05) while HET0016 (10−5 M) reduced the CCPA-induced PKC-α expression by ∼36% (from 154.84 ± 12.81 to 118.77 ± 4.35%; P < 0.05; Fig. 7B). Similarly, in A2AKO, CCPA increased expression of PKC-α by ∼20% (from 100 ± 4.86 to 119.7 ± 6%; P < 0.05), while HET0016 reduced the CCPA-induced PKC-α expression by ∼33% (from 119.7 ± 6 to 86.66 ± 4.65%; P < 0.05; Fig. 7C). We also studied the effect of HET0016 alone and found there was no significant difference in expression of PKC-α between control and HET0016 treated aorta (WT: 100 ± 26.61% vs. WT+HET0016 treated: 93.32 ± 35.95%; n = 4; A2AKO: 100 ± 30.61% vs. A2AKO+HET0016 treated: 92.53 ± 22.98%; n = 4).
Fig. 7.
A: expression of PKC-α in WT and A2AKO aorta and densitometric analysis. *P < 0.05 compared with WT. Data shown for WT are the same control as in B (lane 1), and data shown for A2AKO are the same control as in C (lane 1). B: effect of CCPA and HET0016 on expression of PKC-α in WT. *P < 0.05 compared with WT control. #P < 0.05 compared with WT + CCPA. C: effect of CCPA and HET0016 on expression of PKC-α in A2AKO. *P < 0.05 compared with A2AKO control. #P < 0.05 compared with A2AKO + CCPA. All of the data (A–C, representative blots) are presented as means (%ratio to respective control) ± SE (n = 5–6 mice).
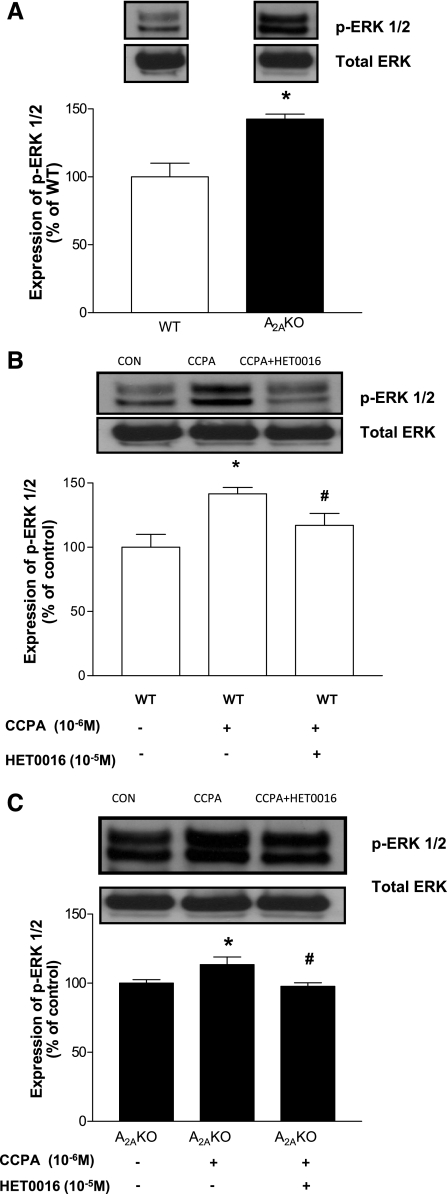
Protein expression of p-ERK1/2 in A2AKO and WT mouse aorta.
The expression of p-ERK1/2 (42/44 kDa; Fig. 8A) was ∼43% higher in A2AKO (142.51 ± 3.72%; P < 0.05) compared with WT (100 ± 10%). CCPA (10−6 M) increased p-ERK1/2 expression in WT by ∼42% (from 100 ± 10% in control to 141.6 ± 4.92%; P < 0.05 compared with WT control), while HET0016 (10−5 M) inhibited the CCPA-induced increase in p-ERK1/2 expression by ∼25% (from 141.6 ± 4.92 to 117.08 ± 9.17%; Fig. 8B). Similarly in A2AKO, CCPA increased expression of p-ERK1/2 by 13% (from 100 ± 2.61% in control to 113.44 ± 5.5%; P < 0.05), while HET0016 reduced the CCPA-induced increase in expression by ∼16% (from 113.44 ± 5.5% to 97.73 ± 2.56%; P < 0.05; Fig. 8C). HET0016 by itself had no effect in expression of p-ERK1/2 in aorta (WT: 100 ± 23.51% vs. WT + HET0016 treated: 92.68 ± 24.66%; n = 4; A2AKO: 100 ± 16.95% vs. A2AKO + HET0016 treated: 102.33 ± 18.93%; n = 4).
Fig. 8.
A: expression of phosphorylated ERK1/2 (p-ERK1/2) in WT and A2AKO aorta and densitometric analysis. *P < 0.05 compared with WT. Data shown for WT are the same control as in B (lane 1), and data shown for A2AKO are the same control as in C (lane 1). B: effect of CCPA and HET0016 on expression of p-ERK1/2 in WT. *P < 0.05 compared with WT control. #P < 0.05 compared with WT + CCPA. C: effect of CCPA and HET0016 on expression of PKC-α in p-ERK1/2. *P < 0.05 compared with A2AKO control. #P < 0.05 compared with A2AKO + CCPA. All of the data (A–C, representative blots) are presented as means (%ratio to respective control) ± SE (n = 5–6 mice).
DISCUSSION
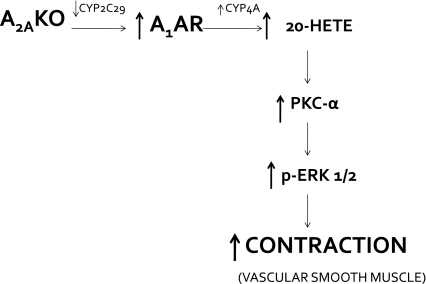
The principal finding of this study supports the involvement of Cyp4a (via 20-HETE) through upregulation of A1AR, leading to the activation of PKC-α and MAPK (ERK1/2) and ultimately causing vascular contraction in A2AAR KO mice (Fig. 9).
Fig. 9.
Schematic representation of hypothesis tested and proposed pathway for A1AR signaling in A2AKO mice.
The endothelium plays a vital role in adenosine-mediated vascular responses, in both contraction and relaxation (2, 3, 25, 27, 32). Many studies have reported that these effects are either completely endothelium-dependent/independent, or partially endothelium-dependent in various isolated vessels (2, 3, 29, 39, 46). It has been found that the A2AAR contributes to vascular relaxation through an endothelium-dependent mechanism in aorta and other vascular tissues (1, 23–25, 27, 32, 34, 35). Upregulation of Cyp2c29 is also associated with A2AAR in the endothelium-dependent relaxation to adenosine (adenosine 5′-N-ethylcarboxamide and CGS-21680) in aorta (27). In our study, we found that A2AKO had significantly lower response to acetylcholine compared with WT (Fig. 1), as well as lower expression of Cyp2c29 (Fig. 6B), confirming our laboratory's previous findings (27, 32) that were obtained using a different strain of mice (CD1). WT aorta had a maximum response of 20% relaxation at the highest concentration of CGS-21680 (Fig. 2), while A2AKO aorta did not respond (data not shown). Removal of the endothelium abolished the CGS-21680-mediated response (Fig. 2). Repetition of some of these experiments was essential to establish baseline responses in C57BL/6J mice (background strain for A2AKO).
One of the possible factors that may cause the endothelium-dependent response is CYP epoxygenase, which generates AA-derived metabolites in the form of EETs in the endothelium. These EETs have been proposed to act as EDHFs (9). To determine whether CYP epoxygenase plays any role in the CGS-21680-induced response, we used MS-PPOH, a selective CYP epoxygenase inhibitor. CGS-21860-induced relaxation in WT aorta changed to contraction with MS-PPOH, while it had no effect when the endothelium was removed (Fig. 2). These data confirmed that endothelial epoxygenases were involved in CGS-21680-induced relaxation. Similar findings have been reported by other laboratories as well as us (5, 27, 28).
The A1 receptor is believed to mediate contraction in mouse aorta (33, 44). In our laboratory's previous study, we found that the treatment of A2AKO aorta with a selective A1AR antagonist, DPCPX, lowered the magnitude of contraction to adenosine 5′-N-ethylcarboxamide, which is a nonselective adenosine analog (32). This indicated a role for A1AR in increased vascular contraction that was observed in A2AKO aorta. In the present study, we examined the effects of CCPA, a selective A1 agonist in WT and A2AKO aorta (Fig. 3). CCPA induced significantly higher contraction in A2AKO aorta compared with WT. Western blot analysis also confirmed higher basal A1AR protein expression in A2AKO (Fig. 6A).
Studies from other laboratories have shown the involvement of MAPK (ERK1/2) signaling pathway downstream to A1AR activation (14, 31). Recently, our laboratory has also shown a possible role for PKC-α upstream of ERK1/2 signaling in A1-mediated vascular contraction (4).
To determine whether PKC played any role in CCPA-induced contraction in A2A KO and WT mice, we treated aorta with a nonspecific PKC blocker chelerythrine chloride, which inhibits all PKC isoforms, and then performed concentration response to CCPA. The PKC blocker significantly lowered contraction in both WT and A2AKO tissues, suggesting that PKC may play a role in the signaling cascade downstream of A1AR activation (Fig. 3). We then used a selective PKC-α inhibitor Gö-6976 to determine whether this particular isoform was involved in CCPA-induced contraction and found that inhibition of PKC-α significantly attenuated the contraction response to CCPA in both WT and A2AKO aorta. Our data indicated that PKC-α is involved in A1AR-mediated aortic contraction.
To confirm the role of p42/p44 MAPK (ERK1/2), we used PD-98059 (p42/p44 MAPK inhibitor) to pretreat tissues, followed by concentration response to CCPA. Response to CCPA was completely blocked with the use of PD-98059 in both WT and A2AKO aorta, suggesting that ERK1/2 is involved in A1-mediated contraction. Inhibition of CCPA-induced contraction with chelerythrine chloride was less compared with PD-98059, suggesting the involvement of other signaling molecules upstream of ERK1/2, in addition to PKC.
20-HETE is an AA-derived metabolite synthesized in the presence of Cyp4a, and it is a potent vasoconstrictor (35). HET0016 blocks Cyp4a activity and thereby inhibits the synthesis of 20-HETE in vasculature (15). We determined the effect of HET0016 on CCPA-induced vascular response in A2AKO and WT aorta and found that CCPA-induced vascular contraction was significantly reduced (Fig. 3). These findings suggest that Cyp4a was involved in mediating the vasoconstriction through A1AR. Western blot analysis confirmed these findings and showed that A2AKO mice had higher basal protein expression for both Cyp4a and A1AR compared with WT (Fig. 6, A and C). These data, along with lowered Cyp2c29 expression in A2AKO (Fig. 6B), as well as the findings reported earlier by Nayeem et al. (27), suggest that, in the absence of A2AAR, there is an imbalance between CYP epoxygenases and ω-hydroxylases that leads to greater vascular contraction through upregulation of A1AR in A2AKO.
We also investigated the effect of 20-HETE itself on vascular tone in A2AKO and WT tissues (Fig. 4) to determine whether aortic responses to the ω-hydroxylation product were different. We used a single concentration of 20-HETE, based on literature (11). A2AKO had significantly higher contraction to 20-HETE than WT (Fig. 4). Our next aim was to determine whether 20-HETE was linked to ERK1/2 and PKC-α signaling. We pretreated aortas taken from A2AKO and WT mice with ERK1/2 inhibitor PD-98059 (Fig. 4A) and selective PKC-α inhibitor Gö-6976 (Fig. 4B). The 20-HETE response was blocked by PD-98059 and Gö-6976, respectively, suggesting that there is a link between Cyp4a, PKC-α, and ERK1/2.
Angiotensin II is known to activate MAPK/PKC pathway (22), and we performed angiotensin II concentration response in WT and A2AKO mouse aortas to confirm the possibility of upregulation of MAPK/PKC in A2AKO aorta (Fig. 5). We found that A2AKO had significantly higher angiotensin II-mediated contraction compared with WT. These data confirm the downstream signaling mechanisms being similar to angiotensin II and A1AR-mediated contraction. Taken together with the Western blot data that showed significantly higher basal levels of p-ERK1/2 (Fig. 8A) and PKC-α (Fig. 7A) in control A2AKO aorta compared with WT, these data suggest that there is upregulation of MAPK/PKC signaling in A2AKO aorta.
ARs are coupled to many signaling pathways through G proteins, which include MAPKs. ERK1/2 is part of the MAPK family that regulates several physiological functions, like cell growth and differentiation, through various classes of receptors, including G protein-coupled receptors (30, 38). ERK1/2 is activated by all four ARs (40), and our laboratory has shown that the activation of ERK1/2 is dependent on PKC-α activity through A1AR (4). We performed Western blot experiments using nontreated, CCPA-treated, and CCPA + HET0016-treated A2AKO and WT aortas. Based on the data from the current investigation and previous studies (4, 14, 26, 36), we probed for PKC-α and p-ERK1/2 proteins (Figs. 7 and 8). Studies have reported that 20-HETE is linked to PKC activation (14), and the exogenous application of 20-HETE increases the phosphorylation of PKC-α (36). PKC-α is also linked to A1AR-mediated signaling, as our laboratory has reported earlier (4).
The basal protein expression of PKC-α and p-ERK1/2 was higher in A2AKO compared with WT. Furthermore, we have shown, in the present study, that CCPA-treated A2AKO and WT mouse aorta had a significant increase in p-ERK1/2 and PKC-α proteins, suggesting the involvement of ERK1/2 and PKC signaling in response to A1AR activation in A2AKO (Figs. 7 and 8). The data regarding the involvement of ERK1/2 and PKC isoforms through A1AR activation are supported by the use of PD-98059, chelerythrine chloride, and Gö-6976 (PKC-α inhibitor) in our organ bath studies, which blocked the CCPA-induced vascular response in A2AKO and WT mouse aorta. HET0016 inhibited the CCPA-induced increase in protein expressions (PKC-α and p-ERK1/2) in both WT and A2AKO mice. The HET0016-treated protein expression data, along with the functional (organ bath) studies using 20-HETE, suggest a relationship exists among A1AR, Cyp4a, PKC, and MAPK signaling.
Perspectives and Significance
The present study shows that the deletion of A2AAR results in an upregulation of A1AR and Cyp4a, accompanied by downregulation of CYP epoxygenase (Cyp2c29). The A2AKO mouse aorta has lower endothelial function compared with WT and has higher contraction to A1AR agonist and 20-HETE. The data from this study also suggest that PKC-α and p42/p44 MAPK play important roles in the signaling pathway, leading to vasoconstriction through upregulation of A1AR and Cyp4a in A2AKO mice. In the present study, we used mouse aorta, which is a conduit vessel. This is a limitation of our study. However, in another unpublished study from our laboratory, the preliminary data showed no significant differences between the aortic vs. mesenteric responses with CCPA in A2AKO compared with WT mice (45).
AA-derived metabolite, 20-HETE, has a role in vasoconstriction, whereas A2AAR plays a role in vasodilation. Both of these factors have roles in the regulation of vascular tone, and hence understanding how these two interact could give important insights into pathways involved in vascular signaling. Due to the heterogeneity of the vascular tissue (smooth muscle and endothelial cells) used in our studies, further investigations will be necessary to confirm the detailed signaling mechanisms by which the absence of A2AAR upregulates A1AR, which modulates vasoconstriction, probably with the use of isolated endothelial and smooth muscle cells from the vascular tissue. A better understanding of these signaling pathways may lead to better approaches for the therapeutic interventions in vascular disorders.
ACKNOWLEDGMENT
We are thankful to Daniel Fil for technical help with the Western blots.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-027339, HL-094447 (S. J. Mustafa), and HL-071802 (S. L. Tilley), and National Institute of Environmental Health Sciences Z01 ES025034 (D. C. Zeldin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.S.P., M.A.N., S.S.K., and S.J.M. conception and design of research; D.S.P. and S.S.K. performed experiments; D.S.P. and S.S.K. analyzed data; D.S.P., M.A.N., S.S.K., and S.J.M. interpreted results of experiments; D.S.P. prepared figures; D.S.P. drafted manuscript; D.S.P., M.A.N., and S.J.M. edited and revised manuscript; D.S.P., M.A.N., S.S.K., S.L.T., D.C.Z., C.L., and S.J.M. approved final version of manuscript.
REFERENCES
- 1. Abebe W, Makujina SR, Mustafa SJ. Adenosine receptor-mediated relaxation of porcine coronary artery in presence and absence of endothelium. Am J Physiol Heart Circ Physiol 266: H2018–H2025, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Ansari HR, Nadeem A, Talukder MA, Sakhalkar S, Mustafa SJ. Evidence for the involvement of nitric oxide in A2B receptor-mediated vasorelaxation of mouse aorta. Am J Physiol Heart Circ Physiol 292: H719–H725, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Ansari HR, Nadeem A, Tilley SL, Mustafa SJ. Involvement of COX-1 in A3 adenosine receptor-mediated contraction through endothelium in mice aorta. Am J Physiol Heart Circ Physiol 293: H3448–H3455, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Ansari HR, Teng B, Nadeem A, Roush KP, Martin KH, Schnermann J, Mustafa SJ. A(1) adenosine receptor-mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol 297: H1032–H1039, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation 107: 769–776, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol 141: 441–448, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frisbee JC, Roman RJ, Falck JR, Krishna UM, Lombard JH. 20-HETE contributes to myogenic activation of skeletal muscle resistance arteries in Brown Norway and Sprague-Dawley rats. Microcirculation 8: 45–55, 2001 [PubMed] [Google Scholar]
- 8. Frisbee JC, Roman RJ, Krishna UM, Falck JR, Lombard JH. 20-HETE modulates myogenic response of skeletal muscle resistance arteries from hypertensive Dahl-SS rats. Am J Physiol Heart Circ Physiol 280: H1066–H1074, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Gauthier KM, Edwards EM, Falck JR, Reddy DS, Campbell WB. 14,15-Epoxyeicosatrienoic acid represents a transferable endothelium-dependent relaxing factor in bovine coronary arteries. Hypertension 45: 666–671, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Grbovic L, Radenkovic M. Analysis of adenosine vascular effect in isolated rat aorta: possible role of Na+/K+-ATPase. Pharmacol Toxicol 92: 265–271, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Hama-Tomioka K, Kinoshita H, Azma T, Nakahata K, Matsuda N, Hatakeyama N, Kikuchi H, Hatano Y. The role of 20-hydroxyeicosatetraenoic acid in cerebral arteriolar constriction and the inhibitory effect of propofol. Anesth Analg 109: 1935–1942, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Hansen PB, Castrop H, Briggs J, Schnermann J. Adenosine induces vasoconstriction through Gi-dependent activation of phospholipase C in isolated perfused afferent arterioles of mice. J Am Soc Nephrol 14: 2457–2465, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Harder DR, Campbell WB, Roman RJ. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res 32: 79–92, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Harder DR, Lange AR, Gebremedhin D, Birks EK, Roman RJ. Cytochrome P450 metabolites of arachidonic acid as intracellular signaling molecules in vascular tissue. J Vasc Res 34: 237–243, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Inoue K, Sodhi K, Puri N, Gotlinger KH, Cao J, Rezzani R, Falck JR, Abraham NG, Laniado-Schwartzman M. Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. Am J Physiol Renal Physiol 297: F875–F884, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee HT, Emala CW. Characterization of adenosine receptors in human kidney proximal tubule (HK-2) cells. Exp Nephrol 10: 383–392, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Lewis CD, Hourani SM, Long CJ, Collis MG. Characterization of adenosine receptors in the rat isolated aorta. Gen Pharmacol 25: 1381–1387, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Ma YH, Gebremedhin D, Schwartzman ML, Falck JR, Clark JE, Masters BS, Harder DR, Roman RJ. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res 72: 126–136, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Marala RB, Mustafa SJ. Adenosine A1 receptor-induced upregulation of protein kinase C: role of pertussis toxin-sensitive G protein(s). Am J Physiol Heart Circ Physiol 269: H1619–H1624, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Marala RB, Mustafa SJ. Direct evidence for the coupling of A2-adenosine receptor to stimulatory guanine nucleotide-binding-protein in bovine brain striatum. J Pharmacol Exp Ther 266: 294–300, 1993 [PubMed] [Google Scholar]
- 21. Marala RB, Ways K, Mustafa SJ. 2-Chloroadenosine prevents phorbol ester-induced depletion of protein kinase C in porcine coronary artery. Am J Physiol Heart Circ Physiol 264: H1465–H1471, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Meijering BD, van der Wouden EA, Pelgrom V, Henning RH, Sharma K, Deelman LE. TGF-beta inhibits Ang II-induced MAPK p44/42 signaling in vascular smooth muscle cells by Ang II type 1 receptor downregulation. J Vasc Res 46: 459–468, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Mustafa S, Abebe W. Coronary vasodilation by adenosine receptor subtypes and mechanism of action. Drug Dev Res 39: 308–313, 1996 [Google Scholar]
- 24. Mustafa SJ, Askar AO. Evidence suggesting an Ra-type adenosine receptor in bovine coronary arteries. J Pharmacol Exp Ther 232: 49–56, 1985 [PubMed] [Google Scholar]
- 25. Mustafa SJ, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol: 161–188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nayeem MA, Mustafa SJ. Protein kinase C isoforms and A1 adenosine receptors in porcine coronary smooth muscle cells. Vascul Pharmacol 39: 47–54, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS, Ansari HR, Mustafa SJ. Role of CYP epoxygenases in A2A AR-mediated relaxation using A2A AR-null and wild-type mice. Am J Physiol Heart Circ Physiol 295: H2068–H2078, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nayeem MA, Ponnoth DS, Boegehold MA, Zeldin DC, Falck JR, Mustafa SJ. High-salt diet enhances mouse aortic relaxation through adenosine A2A receptor via CYP epoxygenases. Am J Physiol Regul Integr Comp Physiol 296: R567–R574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Newman WH, Becker BF, Heier M, Nees S, Gerlach E. Endothelium-mediated coronary dilatation by adenosine does not depend on endothelial adenylate cyclase activation: studies in isolated guinea pig hearts. Pflügers Arch 413: 1–7, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22: 153–183, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Ponnoth DS, Nadeem A, Mustafa SJ. Adenosine-mediated alteration of vascular reactivity and inflammation in a murine model of asthma. Am J Physiol Heart Circ Physiol 294: H2158–H2165, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ponnoth DS, Sanjani MS, Ledent C, Roush K, Krahn T, Mustafa SJ. Absence of adenosine-mediated aortic relaxation in A(2A) adenosine receptor knockout mice. Am J Physiol Heart Circ Physiol 297: H1655–H1660, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prentice D, Boon K, Hourani S. Relaxation of mouse isolated aorta to adenosine and its analogues does not involve adenosine A(1), A(2) or A(3) receptors. Eur J Pharmacol 415: 251–255, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 35. Ramgopal MV, Chitwood RW, Jr, Mustafa SJ. Evidence for an A2A adenosine receptor in human coronary arteries. Eur J Pharmacol 151: 483–486, 1988 [DOI] [PubMed] [Google Scholar]
- 36. Randriamboavonjy V, Kiss L, Falck JR, Busse R, Fleming I. The synthesis of 20-HETE in small porcine coronary arteries antagonizes EDHF-mediated relaxation. Cardiovasc Res 65: 487–494, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Robinson AJ, Dickenson JM. Regulation of p42/p44 MAPK and p38 MAPK by the adenosine A(1) receptor in DDT(1)MF-2 cells. Eur J Pharmacol 413: 151–161, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68: 320–344, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rubanyi G, Vanhoutte PM. Endothelium-removal decreases relaxations of canine coronary arteries caused by beta-adrenergic agonists and adenosine. J Cardiovasc Pharmacol 7: 139–144, 1985 [DOI] [PubMed] [Google Scholar]
- 40. Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal 15: 813–827, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Shepherd RK, Linden J, Duling BR. Adenosine-induced vasoconstriction in vivo. Role of the mast cell and A3 adenosine receptor. Circ Res 78: 627–634, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Tabrizchi R, Bedi S. Pharmacology of adenosine receptors in the vasculature. Pharmacol Ther 91: 133–147, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Talukder MA, Morrison RR, Jacobson MA, Jacobson KA, Ledent C, Mustafa SJ. Targeted deletion of adenosine A(3) receptors augments adenosine-induced coronary flow in isolated mouse heart. Am J Physiol Heart Circ Physiol 282: H2183–H2189, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol 288: H1411–H1416, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Teng B, Tilley S, Ledent C, Mustafa SJ. Functional profile of adenosine receptor subtypes in mouse mesenteric artery (Abstract). FASEB J 25: 816.812, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yen MH, Wu CC, Chiou WF. Partially endothelium-dependent vasodilator effect of adenosine in rat aorta. Hypertension 11: 514–518, 1988 [DOI] [PubMed] [Google Scholar]