Abstract
The paraventricular nucleus (PVN) of the hypothalamus is involved in the neural control of sympathetic drive, but the precise mechanism(s) that influences the PVN is not known. The activation of the PVN may be influenced by input from higher forebrain areas, such as the median preoptic nucleus (MnPO) and the subfornical organ (SFO). We hypothesized that activation of the MnPO or SFO would drive the PVN through a glutamatergic pathway. Neuroanatomical connections were confirmed by the recovery of a retrograde tracer in the MnPO and SFO that was injected bilaterally into the PVN in rats. Microinjection of 200 pmol of N-methyl-d-aspartate (NMDA) or bicuculline-induced activation of the MnPO and increased renal sympathetic activity (RSNA), mean arterial pressure, and heart rate in anesthetized rats. These responses were attenuated by prior microinjection of a glutamate receptor blocker AP5 (4 nmol) into the PVN (NMDA − ΔRSNA 72 ± 8% vs. 5 ± 1%; P < 0.05). Using single-unit extracellular recording, we examined the effect of NMDA microinjection (200 pmol) into the MnPO on the firing activity of PVN neurons. Of the 11 active neurons in the PVN, 6 neurons were excited by 95 ± 17% (P < 0.05), 1 was inhibited by 57%, and 4 did not respond. The increased RSNA after activation of the SFO by ANG II (1 nmol) or bicuculline (200 pmol) was also reduced by AP5 in the PVN (for ANG II − ΔRSNA 46 ± 7% vs. 17 ± 4%; P < 0.05). Prior microinjection of ANG II type 1 receptor blocker losartan (4 nmol) into the PVN did not change the response to ANG II or bicuculline microinjection into the SFO. The results from this study demonstrate that the sympathoexcitation mediated by a glutamatergic mechanism in the PVN is partially driven by the activation of the MnPO or SFO.
Keywords: glutamate, angiotensin II, sympathetic drive, cardiovascular
the paraventricular nucleus (PVN) of the hypothalamus is involved in the neural control of the cardiovascular system (10, 23). Previous researchers from this laboratory (7, 23, 25) and others (4, 5) have observed that the activity of PVN neurons associated with sympathoexcitation is due to an increase in glutamatergic mechanisms within the PVN. Specifically, we have shown that the parvocellular neurons of the PVN are involved in mediating the neural component of cardiovascular reflexes by influencing renal sympathetic nerve activity (RSNA) (7, 23, 25). We have also demonstrated that the PVN is involved in baroreflex regulation of lumbar sympathetic nerve activity (23). Neuroanatomically, the parvocellular neurons of the PVN have been shown to connect to the nucleus of the solitary tract (10), as well as project to sympathetic preganglionic neurons within the intermediolateral cell column (IML) of the spinal cord directly or via the rostral ventrolateral medulla (33). Taken together, these results demonstrate that a central component of sympathoexcitation in cardiovascular regulation is mediated through a glutamatergic mechanism in the PVN.
Although the activation of the PVN has been implicated in this sympathetic pathway and much is known about the organization and neurotransmission within the PVN (10, 29), the details of the source of this activation and the specific neurotransmitters involved in the excitation of the PVN to mediate sympathoexcitation are not entirely clear. That is, does the PVN receive projections from other neurons, or is it self-driven? Neuronal activation in the PVN may be mediating input from higher forebrain areas, such as the median preoptic nucleus (MnPO) and the subfornical organ (SFO). A variety of lesion and stimulation studies have indicated the importance of areas of the anteroventral region of the third ventricle (AV3V), specifically the MnPO and SFO, in cardiovascular and fluid-balance regulation (9, 19, 20). Additionally, there is substantial neuroanatomical and electrophysiological evidence indicating that the circumventricular organs, such as the organum vasculosum of lamina terminalis and SFO project to the MnPO and PVN (3, 21, 30–32, 35, 36, 38). Specific neuronal pathways from the AV3V were investigated by injecting agglutinin-horseradish peroxidase into the AV3V region (30). The conjugate was identified and was linked to the regions of the SFO, MnPO, and PVN (30). Another anatomical study used viral transneuronal labeling to identify sympathetically related neurons after pseudorabies virus injections into either the superior cervical ganglion, stellate ganglion, celiac ganglion, or adrenal gland of rats (36). The organum vasculosum of lamina terminalis, SFO, AV3V, and MnPO were identified by the viral neuronal label, and they were found to be a part of the descending sympathetic pathway linked to cardiovascular control (36). In addition to the anatomical evidence, the stimulation studies showed that electrical stimulation of the SFO produces action potentials in efferent neurons of the PVN (3). These circumventricular organs may play an important role in the sympathoexcitatory pathway by providing information regarding the circulating humoral milieu, since their neuronal cell bodies are positioned at the site of weak blood-brain barrier and can thereby interact with molecules in the circulation, such as ANG II (8, 27, 35). Investigation has shown that intracarotid artery injection of ANG II activated some MnPO neuronal projections to the PVN (35). Additionally, lesion studies using electrolytic or ibotenic acid have investigated the role of the MnPO and circulating ANG II on blood pressure. While ANG II infusion increases blood pressure in normal rats, those that had electrolytic or ibotenic acid lesions in the MnPO demonstrated attenuated hypertensive responses to ANG II infusion (27, 28). Additionally, there is evidence that ANG II acts as a neurotransmitter from the SFO to the PVN (3, 18). Li and Ferguson (18) first recorded magnocellular PVN neurons and observed the response of electrical stimulation or direct application of ANG II in the SFO. The electrical stimulation in the SFO caused depolarization of magnocellular neurons in the PVN (mPVN), but some responses were delayed. Pretreatment with ANG II type 1 (AT1) receptor blocker, losartan (Los), in the PVN attenuated the depolarization response of PVN neurons to SFO electrical stimulation (18). Therefore, they suggested that ANG II might be acting as a neurotransmitter in the SFO-mPVN projection. In support of their theory, they found that the mPVN neurons depolarized in response to the local administration of ANG II in the PVN (18). Because of the short latency of the pressor response to SFO stimulation, Bains and Ferguson (3) conducted an experiment in which the SFO was electrically stimulated and IML-projecting parvocellular neurons in the PVN (pPVN) were recorded (3). They found that the majority (78%) of the neurons that responded to SFO stimulation displayed short-duration excitations in the pPVN neurons that could not be blocked by intravenous injection of Los, while the remaining (22%) pPVN neurons exhibited a long-duration response that could be blocked by Los (3). These results suggest that although ANG II may be a player in the SFO-pPVN-IML pathway, there are likely other neurotransmitters involved.
Although the higher forebrain areas, such as the MnPO and SFO, have been implicated in the central cardiovascular control pathway, the specific mechanism driving sympathoexcitation via the PVN has not been identified. The purpose of this study was to determine whether activation of the MnPO and SFO drives sympathetic output. Secondly, the study was designed to investigate the specific neurotransmitter mechanism(s) within the PVN by which MnPO and SFO influence sympathoexcitation. It was hypothesized that activation of the MnPO and SFO would drive sympathetic outflow and that this response is mediated, in part, by glutamatergic mechanisms in the PVN.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats weighing 180–200 g (Sasco Breeding Laboratories, Omaha, NE) were fed and housed according to institutional guidelines. Protocols were approved by the University of Nebraska Institutional Animal Care and Use Committee and are in accordance with the American Physiological Society's Guiding Principles in the Care and Use of Laboratory Animals. Rats were given rat chow and water ad libitum and were housed in a room with a 12:12-h light-dark cycle. Rats were allowed to acclimatize for 1 wk prior to research studies.
Retrograde labeling of neurons projecting to the PVN.
Brain sections of the MnPO and SFO were processed with a retrograde tracer to identify MnPO and SFO neurons that project into the PVN (n = 4). Specifically, the fluorescent tracer LatexGreen was injected into the parvocellular region of the PVN (4%, 100 nl) 7 days prior to death. This allowed the tracer enough time to be transported from the PVN to the neurons that project to the PVN. After death, the brain was removed from each rat, postfixed at 4°C for 4 h in 4% paraformaldehyde solution, and then placed in 20% sucrose for 24 h. The brain was locked in a coronal plane and sectioned at 20-μm thickness in a cryostat to obtain sections of the SFO and MnPO. The sections were mounted with fluo-remounting and observed under a microscope with corresponding filters to identify LatexGreen in neurons.
Hemodynamic and RSNA measurements.
For the preparation of hemodynamic and RSNA measurements, rats were anesthetized with urethane (0.75 g/kg ip) and α-chloralose (70 mg/kg ip). The left femoral vein was cannulated with polyethylene tubing (PE-50). The left femoral artery was also cannulated and connected to PowerLab by a pressure transducer (Gould P23 1D) to record mean arterial blood pressure (MAP) and heart rate (HR). The absolute change in MAP and HR from baseline (prior to microinjection) to peak was calculated for each microinjection experiment.
Then, the left kidney and renal nerves were exposed via a retroperitoneal flank incision. A branch of the renal nerve was isolated from fat and connective tissue, and it was placed onto bipolar platinum electrodes. The electrode was fixed to the nerve and electrically insulated (Wacker Silgel mixture - 604 and 601). The electrical signal was amplified with high- and low-frequency cutoffs of 1,000 Hz and 100 Hz, respectively (Grass amplifier). The rectified output (RC filtered, time constant, 0.5 s) was then recorded and integrated using PowerLab (8si, ADInstruments, Sydney, Australia). At the beginning of each experiment (prior to microinjection), baseline RSNA was recorded for 1 min. The background noise of the recording was assessed by the nerve activity at the end of the experiment after hexamethonium (30 mg/kg iv). RSNA during the experiment was calculated by subtracting the background noise from the recorded value. The peak RSNA response to microinjection of drugs into the MnPO or SFO was expressed as a percent change from the baseline RSNA value.
Extracellular single-unit recording in the PVN after activation of the MnPO.
The stereotaxic coordinates for the PVN were determined according to Paxinos and Watson's atlas (26), as described below. A single-unit extracellular recording of a neuron was obtained using a single-micropipette (resistance 5–15 MΩ) filled with 0.5 M sodium acetate dissolved in 2% pontamine sky blue. The glass micropipettes were advanced using a microdrive controller (Type 860; Hugo Sachs Elektronik, March, Germany) into the PVN. The spontaneous action potentials of neurons were amplified (gain: 1000) with an AC/DC differential amplifier (model IX1, Dagan Corporation, Minneapolis, MN) with low-frequency cutoff at 30 Hz and high-frequency cutoff at 3 kHz. The neuronal discharge was recorded on a PowerLab data acquisition system (8/30; ADInstruments). The frequency of the neuronal discharge was analyzed with special software (SpikeHistogram, ADInstruments). The pontamine sky blue was iontophoresed (−15 μA, 10 min) to mark the location of recording site.
Microinjections into the MnPO, SFO, and PVN.
For microinjection into the MnPO, SFO, and PVN, the anesthetized rat was placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). A longitudinal incision was made on the head, and the bregma was exposed. A small burr hole was made in the skull to allow access for microinjection into the MnPO, SFO, and PVN. The coordinates of these sites were determined using the Paxinos and Watson atlas (26). Specific coordinates for the three regions are MnPO, 0.3 mm posterior to bregma, and 8.7 mm ventral to the dura; SFO, 0.9 mm posterior to bregma and 5.6 mm ventral to the dura; PVN, 1.5 mm posterior to bregma, 0.4 mm lateral to midline, and 7.8 mm ventral to the dura. A thin needle (0.2 mm OD) connected to a 0.5 μl microsyringe (Hamilton, Reno, NV) was lowered into the three sites and manually microinjected into these nuclei. A volume of 50–100 nl was injected into the MnPO, SFO, or PVN. Microinjections were administered approximately every 30 min, totaling 3–6 injections per site. At the end of the experiment, Chicago blue dye (Sigma-Aldrich, St. Louis, MO) was injected, and the sites were confirmed by histological analysis. Missed injection sites were not included in the results.
To identify the neurotransmitters and receptors that propagate nerve signaling through the MnPO, SFO, and PVN, specific exogenous drugs, as described below, were microinjected to activate the MnPO and SFO and to determine their influence on RSNA, MAP, and HR by way of the PVN.
Activation of the MnPO-PVN pathway.
The MnPO was activated by microinjection of a glutamate analog N-methyl-d-aspartic acid (NMDA; 200 pmol; Calbiochem, La Jolla, CA) into the MnPO. RSNA, MAP, and HR responses to NMDA were recorded. Then, RSNA, MAP, and HR were monitored and allowed to return to basal levels. To determine whether the glutamatergic signaling was mediated by the PVN, 4 nmol of an NMDA receptor blocker, 2-amino-5-phosphonopentanoic acid (AP5; Sigma-Aldrich, St. Louis, MO) was microinjected bilaterally into the PVN to prevent glutamatergic signaling through the PVN. Then, NMDA was injected into the MnPO and again RSNA, MAP, and HR were recorded. In a separate group of rats, extracellular single-unit recording was carried out to observe PVN neuronal firing activity after microinjection of 200 pmol of NMDA into the MnPO.
Another group of rats was used to activate the MnPO by blockade of GABA-mediated inhibition in the MnPO. A GABAA antagonist, bicuculline (Bic; Sigma-Aldrich, St. Louis, MO), was used to disinhibit the MnPO. A microinjection of 200 pmol of Bic was made into the MnPO, and the RSNA, MAP, and HR responses were recorded. To determine whether NMDA signaling was mediated through the PVN, 4 nmol of AP5 was microinjected bilaterally into the PVN. Then, Bic (200 pmol) was microinjected into the MnPO and RSNA, MAP, and HR were recorded.
Activation of the SFO-PVN pathway.
The SFO is a highly vascularized circumventricular organ that lacks a blood-brain barrier and likely comes into contact with circulating molecules, such as ANG II. Therefore, ANG II was used to stimulate the SFO to examine PVN-mediated changes in RSNA, MAP, and HR. ANG II (1 nmol; Sigma-Aldrich, St. Louis, MO) was injected into the SFO, and the RSNA, MAP, and HR responses were recorded. After returning to a basal level, 4 nmol of AP5 was microinjected bilaterally into the PVN. ANG II (1 nmol) was again injected into the SFO, and RSNA, MAP, and HR were recorded. In a separate group of rats, a similar experiment was carried out; however, 4 nmol of an AT1 receptor blocker, Los (Sigma-Aldrich, St. Louis, MO), was microinjected bilaterally into the PVN to block angiotensinergic signaling. This dose of Los was confirmed to adequately inhibit the sympathoexcitatory response to ANG II in the PVN (17). In a preliminary study, increasing doses of Los were tested to determine the maximal blocking dose. Specifically, the change in RSNA after 1 nmol of ANG II microinjection into the PVN (47.2 ± 8.7%) was blunted by 4 nmol Los (9.0 ± 1.9%, P < 0.05). Concentrations higher than this maximal dose of Los did not further alter the response to ANG II.
Alternatively, the SFO was also activated by blockade of GABAA receptors by Bic. The SFO was disinhibited by microinjection of 200 pmol of Bic, and the RSNA, MAP, and HR responses were recorded. To determine whether the response was mediated through the PVN, 4 nmol of AP5 was microinjected bilaterally into the PVN. Then, Bic (200 pmol) was again injected into the SFO and RSNA, MAP, and HR were recorded. In a separate group of rats, a similar experiment was conducted, with the exception that Los (rather than AP5), was microinjected bilaterally into the PVN to block angiotensinergic signaling.
Statistical analysis.
Data are presented as means ± SE. Standard t-tests were used to compare percent change in RSNA, as well as absolute change in MAP and HR before and after injecting a blocking agent into the PVN. Statistical significance was inferred by a value of P < 0.05.
RESULTS
Retrograde labeling of neurons projecting to the PVN.
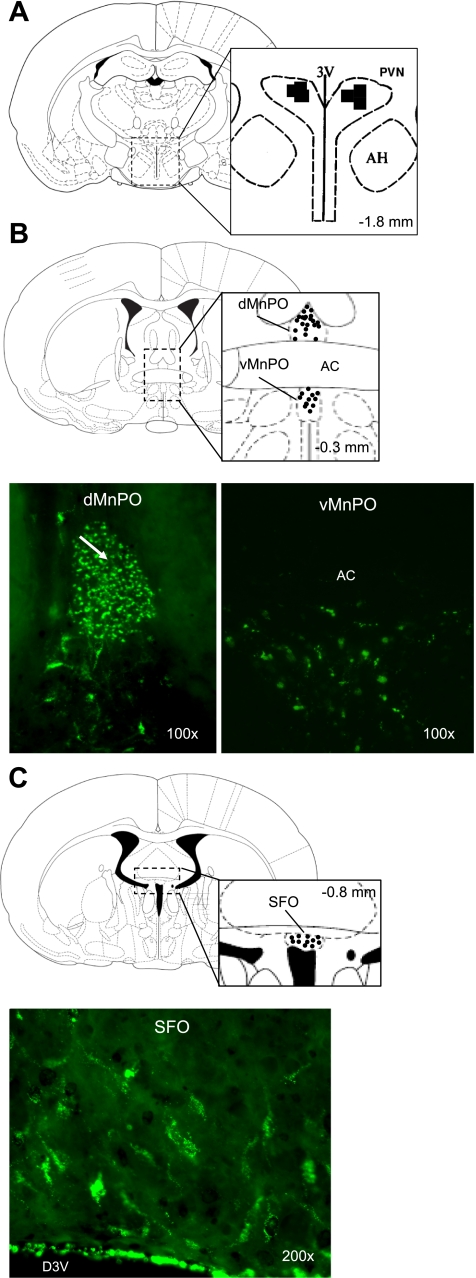
Fluorescent retrograde tracer LatexGreen that was injected into the PVN was recovered in the brain sections of the MnPO and SFO 7 days after microinjection (Fig. 1). The tracer was localized within the boundaries of the MnPO and SFO nuclei, demonstrating the specificity of the projections. These images illustrate that the neurons originating in the MnPO and SFO project to the PVN. Similar localization of the tracer within the MnPO and SFO was observed in all four rats after PVN injections.
Fig. 1.
Coronal brain sections illustrating the neuroantatomical connection between the subfornical organ (SFO), median preoptic nucleus (MnPO), and paraventricular nucleus (PVN). A: bilateral injection of the retrograde marker latex green was made into the PVN. Retrograde marker was recovered in neuron cell bodies within the boundaries of the dorsal and ventral MnPO (B) and the SFO (C). AC, anterior commissure.
Activation of the MnPO.
Microinjection of NMDA into the MnPO increased RSNA (72.4 ± 7.6% of basal value), MAP (15.9 ± 2.4 mmHg), and HR (34.4 ± 5.1 beats/min). Prior microinjection of glutamate receptor blocker AP5 into the PVN attenuated the RSNA (4.6 ± .7%), MAP (2.7 ± 1.1 mmHg), and HR (4.0 ± 1.2 beats/min) response to activation of the MnPO with NMDA (Fig. 2, P < 0.05). These results show that the majority (94%) of the sympathoexcitatory response to NMDA was blocked by AP5. Therefore, the activation of NMDA receptors on neurons within the MnPO results in sympthoexcitation and increases in MAP and HR by activation of glutamatergic receptors within the PVN.
Fig. 2.
A: representative raw tracings of the responses to N-methyl-d-aspartic acid (NMDA) in the MnPO alone and with prior AP5 in the PVN. Activation of MnPO neurons with NMDA (200 pmol; n = 7) (A and B) or Bic (200 pmol, n = 5) (C) increased renal sympathetic activity (RSNA), mean arterial pressure (MAP), and heart rate (HR). The responses to NMDA and Bic were also recorded after prior microinjection of AP5 (4 nmol) into the PVN. iRSNA, integrated RSNA. *P < 0.05 vs. before AP5.
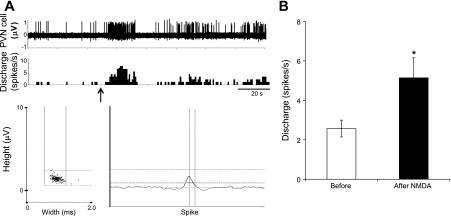
Using single-unit extracellular recording, we also examined the effect of NMDA microinjection into the MnPO on the firing activity of PVN neurons. Of the 11 spontaneously active neurons in the PVN, 6 neurons were excited by 95 ± 17% (P < 0.05), 1 was inhibited by 57%, and 4 did not respond (Fig. 3). These results corroborate that activation of neurons by NMDA in the MnPO excites neurons in the PVN. These data demonstrate the neuronal connection between the MnPO and PVN; however, they do not confirm their glutamatergic mode of transmission.
Fig. 3.
The firing response of PVN neurons to NMDA in MnPO. A: segments of original recordings show firing activity of one PVN neuron increases after NMDA microinjection (200 pmol) into the MnPO (arrow). B: mean data of changes in discharge rate after NMDA microinjection into the MnPO. *P < 0.05 vs. before NMDA.
Disinhibition of the MnPO by microinjection of Bic into the MnPO increased RSNA (35.8 ± 3.4% of basal value), MAP (12.1 ± 1.9 mmHg), and HR (33.8 ± 4.5 beats/min). Prior microinjection of AP5 into the PVN attenuated the RSNA (15.6 ± 2.9%), BP (3.8 ± .8 mmHg), and HR (13.8 ± 4.1 beats/min) responses to Bic-induced activation of the MnPO (Fig. 2C, P < 0.05). These data illustrate that the activation of neurons within the MnPO by disinhibition produces sympathoexcitation and increases in MAP and HR via activation of glutamate receptors within the PVN.
Activation of the SFO.
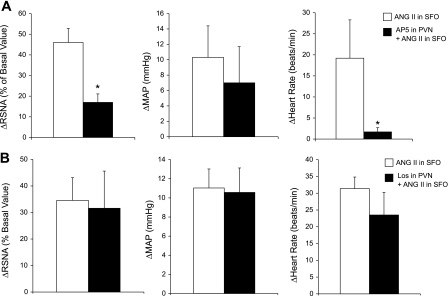
ANG II microinjection into the SFO increased RSNA (46.0 ± 6.8%), MAP (10.3 ± 4.1 mmHg), and HR (19.2 ± 9.1 beats/min). Prior microinjection of AP5 into the PVN reduced the RSNA (17.0 ± 4.1%) and HR (1.7 ± 1 beats/min) responses, but not MAP (7.0 ± 4.7 mmHg) response to activation of the SFO by ANG II (Fig. 4A, P < 0.05). Thus, the activation of neurons within the SFO with ANG II produces sympathoexcitation and increases in HR by activation of glutamate receptors within the PVN. In a separate experiment, prior microinjection of Los into the PVN did not change the RSNA, MAP, or HR response to activation of the SFO with ANG II (Fig. 4B). These data suggest that AT1 receptor activation within the PVN is not necessary for the sympathoexcitation produced by ANG II administration into the SFO.
Fig. 4.
Activation of SFO neurons with ANG II (1 nmol) increased RSNA, MAP, and HR. A: this response was attenuated with prior blocking of the PVN with AP5 (4 nmol, n = 5). B: response to ANG II microinjection into the SFO with prior microinjection of losartan (Los) (4 nmol) into the PVN (n = 5). *P < 0.05 vs. before AP5.
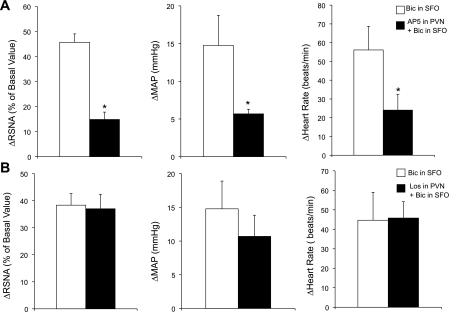
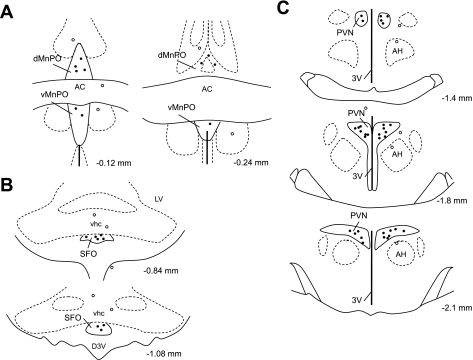
Disinhibition of the SFO by microinjection of Bic resulted in an increased RSNA (45.7 ± 3.4%), MAP (14.8 ± 4.0 mmHg), and HR (56.1 ± 12.6 beats/min). Prior microinjection of AP5 into the PVN attenuated the RSNA (14.9 ± 2.9%), MAP (5.7 ± 0.6 mmHg), and HR (24.1 ± 8.5 beats/min) response to Bic-induced activation of the SFO (Fig. 5A; P < 0.05). Prior microinjection of Los into the PVN did not change the RSNA, MAP, or HR response to Bic-induced activation of the SFO (Fig. 5B). These results demonstrate that the activation of neurons within the SFO by disinhibition produces sympathoexcitation and increased MAP and HR via activation of glutamate receptors, but not AT1 receptors, within the PVN. In addition, in the cases when the injections missed the site (i.e., MnPO or SFO), no changes in RSNA, MAP, or HR were observed after NMDA microinjection (200 pmol). Confirmed injection sites from these experiments are shown in Fig. 6.
Fig. 5.
Activation of SFO neurons with Bic (200 pmol) increased RSNA, MAP, and HR. A: this response was attenuated with prior blocking of the PVN with AP5 (4 nmol, n = 5). B: the response to bicuculline (Bic) into the SFO after prior microinjection of Los (4 nmol) into the PVN (n = 5). *P < 0.05 vs. before AP5.
Fig. 6.
Representative injection sites in the brain. Solid circles denote hits; open circles denote misses. A: confirmed injection sites from the experiments shown in Fig. 2, NMDA microinjection into the MnPO. B: confirmed injection sites from the experiments shown in Fig. 4, ANG II microinjection into the SFO. C: confirmed injection sites from the experiments shown in Fig. 2: AP5 bilateral microinjection into the PVN. dMnPO, dorsal median preoptic nucleus; AC, anterior commissure; vMnPO, ventral medial preoptic nucleus; PVN, paraventricular nucleus; AH, anterior hypothalamus; 3V, third ventricle.
DISCUSSION
The results of this study demonstrate in a systematic way that the activation of the MnPO and the SFO influence PVN-mediated sympathetic activity by a glutamatergic mechanism. Specifically for the SFO, this neurotransmission to the PVN does not seem to be mediated by ANG II, as has been previously suggested. To confirm the neuroanatomical connections of these sites, a fluorescent retrograde tracer was injected into the PVN and was then identified in the neurons of the MnPO and SFO. These data are consistent with previous anatomical studies that demonstrate that MnPO and SFO neurons project to the PVN (30, 36). Furthermore, the results of this study demonstrate that activating the MnPO or SFO increases RSNA, MAP, and HR. The activation of the MnPO was also shown to increase activity of neurons in the PVN. Additionally, this excitatory response was found to be mediated by a glutamatergic mechanism within the PVN, as blocking the glutamatergic receptors with AP5 in the PVN abrogated the MnPO- and SFO-mediated increase in RSNA, MAP, and HR.
The response to activation of the MnPO by microinjection of NMDA was attenuated with prior microinjection of glutamate blocker AP5 into the PVN. These results are consistent with a recent electrophysiological study that reports a glutamatergic mechanism being responsible for neuronal excitability of the MnPO in brain slices from rats (13). Using whole-cell patch-clamp techniques, they found that glutamate receptor agonists induced an excitatory current in MnPO neurons (13). Additionally, our electrophysiology results showed that PVN neurons were excited by microinjection of NMDA into the MnPO. These data support the idea that activation of the MnPO causes activation of the PVN. Electrophysiological studies show that baroreceptor stimulation/inhibition via intravenous injection of phenylephrine or sodium nitroprusside, as well as hyperosmotic challenge and circulating ANG II-induced activation of MnPO neurons, signals the PVN to discharge (35). In addition, supportive functional evidence demonstrated an increased RSNA after electrical stimulation of the MnPO (37). Subsequently, the increase in RSNA was blocked by lidocaine microinjection into the PVN, preventing PVN neuron depolarization by blocking sodium channels (37). Collectively, this evidence supports the results of this study that activation of the MnPO drives sympathoexcitation via the PVN. In our study, activation of neurons in the MnPO by disinhibition of the MnPO with Bic was also attenuated with prior AP5 in the PVN. These results suggest that under normal conditions, the neurons in the MnPO may be inhibited by a strong tonic GABAergic input. Consistent with our results from activation of the MnPO with NMDA, disinhibition of MnPO neurons signals the activation of the PVN presympathetic neurons via a glutamatergic mechanism.
This study also demonstrated that the activation of the SFO drives sympathoexcitation via the PVN. Microinjection of ANG II into the SFO increased RSNA, MAP, and HR. This response shows that the SFO is activated by elevated exogenous ANG II. Because of the circumventricular location of the SFO and the lack of a blood-brain barrier in this area, circulating ANG II may be similarly activating the SFO under normal physiological conditions (22). Previous lesion studies have also indicated that ANG II-induced hypertension is mediated by the activation of the SFO (8). Furthermore, the excitatory sympathetic response elicited by ANG II microinjection into the SFO was attenuated with prior microinjection of glutamatergic blocker into the PVN. It is important to note that the MAP response was not significantly attenuated with prior injection of AP5 in the PVN. These data suggest that other neurotransmitters or pathways may be influencing overall MAP besides as a glutamate neurotransmitter at the level of the PVN in response to ANG II stimulation in the SFO. Alternatively, there may be a differential sympathetic outflow to different peripheral beds that are not necessarily excitatory like those to the renal circulation (1, 2, 6). Nonetheless, these results show that neurons activated by ANG II in the SFO project to the PVN and drive RSNA by releasing glutamate. Disinhibition of the SFO with a GABAA receptor blocker generated a sympathoexcitatory response comparable to activation by microinjection of ANG II. Therefore, under normal conditions, the neurons in the SFO appear to be tonically inhibited by the GABAergic mechanism similar to those in MnPO. Subsequently, the sympathoexcitatory response was abrogated by prior blockade of glutamate receptors in the PVN. Similar to the ANG II-induced activation, these results demonstrate that the PVN receives glutamatergic inputs produced by activated SFO neurons.
Further examination into the specificity of the neurotransmitter mechanism within the PVN revealed that sympathoexcitation mediated from the SFO induced by ANG II or Bic was not changed by prior microinjection of Los into the PVN. This seminal observation suggests that the sympathoexcitation produced by activating the SFO and mediated by the PVN does not use ANG II as a primary neurotransmitter. Rather, glutamatergic activation of the PVN is primarily driving the increase in sympathetic nerve activity. Although, this finding appears to be in contrast to electrophysiological evidence that ANG II acts as a neurotransmitter between the SFO and the PVN (3, 18). Specifically, the results in their study showed that electrical stimulation of the SFO produced a depolarization of magnocellular neurons in the mPVN (18). In addition, they found that pretreatment with Los in the mPVN attenuated the increased neuronal firing response to SFO electrical stimulation. Therefore, they suggested that ANG II is a neurotransmitter in the synaptic connection between SFO and mPVN. In support of their conclusions, they found that the mPVN neurons had increased neuronal firing after local administration of ANG II in the PVN (18). However, the short latency of the pressor response to SFO stimulation suggested that the SFO-mPVN-vasopressin pathway did not mediate this response. Therefore, Bains and Ferguson (3) conducted an experiment in which the SFO was electrically stimulated and IML-projecting pPVN neurons were recorded. They found that 78% (14 neurons) of the neurons that responded to SFO stimulation displayed short-duration excitations in the pPVN neurons, while 22% (4 neurons) exhibited a long-duration response. In a subset of these neurons (5 neurons), the short-duration-responsive neurons were not blocked by prior intravenous injection of Los, while the excitations from long-duration responsive neurons were abolished in three neurons (3). It is important to note that the majority of the pPVN neurons responsive to SFO stimulation was of the short-duration excitation type, and could not be blocked by Los. Additionally, it is not clear whether the long-duration responses that could be blocked by Los were mediated by a direct or indirect mechanism, as the Los was administered systemically, not locally at the level of the pPVN. Therefore, these results suggest that although ANG II may be a player in the SFO-pPVN pathway (for a few neurons), there are other neurotransmitters involved for the major component of the response. The current study demonstrates that glutamate, not ANG II, is the major excitatory neurotransmitter that signals from neurons in the SFO to the PVN to drive sympathoexcitation. Alternatively, the previous research concludes that ANG II is the excitatory neurotransmitter driving activation of SFO to the magnocellular neurons in the PVN. Recently, investigators have used antegrade and retrograde tracers to identify the connections between the SFO and the PVN (11). They have demonstrated that there are two regions of the SFO, dorsolateral, peripheral, and ventromedial core, which project differently to the magnocellular and parvocellular regions of the PVN, respectively (11). As these groups of neurons are anatomically distinct in the SFO, as well as their projections to the PVN, it is possible that their excitatory end pathways may also be different. Therefore, it is conceivable that our findings of a glutamatergic pathway from the SFO to the PVN are in contrast to the previous work that identified an angiotensinergic pathway from the SFO to the PVN, because the neurons may originate from two different parts of the SFO and project to different regions of the PVN. It is recognized that PVN neuronal firing increased in response to the local administration of ANG II in the PVN (14). These studies suggest that ANG II can excite PVN neurons that project to the RVLM. However, the current study showed that stimulation of the SFO does not use this pathway to any significant effect. Perhaps the local endogenous ANG II system may also dictate the level of sympathoexcitation, or it may be driven by other mechanisms. For example, the neurons in the PVN may be self-driven, such that they create an environment of activation that acts upon nearby neurons. Additionally, there is a possibility that interneurons are involved in the connection between the MnPO-PVN or SFO-PVN. Thereby, more than one neurotransmission mechanism may be involved if there are multiple synapses between these regions. These questions remain to be examined further.
The contribution of various central mechanisms to the overall activation of sympathoexcitation is not well understood; however, these studies aid in understanding the role of higher brain centers (MnPO and SFO) in the sympathoexcitation pathway via the PVN. The results from this study illustrate that neurons in the MnPO can be activated by the glutamatergic pathway and inhibited by GABAergic inputs. Similarly, the neurons in the SFO are activated by ANG II stimulation and inhibited by the GABAergic pathway. Subsequently, some of these activated neurons in the MnPO and SFO project to the PVN and release glutamate to propagate the nerve signal and ultimately increase sympathetic nerve activity. Future studies should examine the other areas of the brain to which MnPO and SFO neurons may project, as the sympathetic response to activating these areas was not completely abolished with the prevention of PVN activation in this study. Additionally, it is necessary to understand the signaling mechanism between the two groups of neurons in the MnPO and SFO to fully understand their neural connectivity, and their individual and collective inputs to the PVN. Recent whole-cell patch-clamp studies have demonstrated the important role of glutamate and GABA in the synapse of SFO projections to the MnPO (12, 13). However, the contribution of the MnPO and SFO to the PVN and to sympathoexcitation had not been as well established. Finally, as some of the neurotransmitter mechanisms and their possible pathways in the regulation of cardiovascular control have been presented in this study, these mechanisms should be investigated in pathological conditions, where there is altered central control of sympathetic outflow. Specifically, it would be useful to determine the differences in MnPO and SFO activation of sympathoexcitation in hypertensive or heart failure conditions where RSNA is elevated. For example, under heart failure conditions, the MnPO and SFO may become more or less sensitive to a given electrical or chemical stimulus. These investigations would provide important information to elucidate the role of the MnPO and SFO in the regulation of sympathetic outflow and, thus, cardiovascular control in healthy and diseased conditions, such as hypertension and heart failure.
Perspectives and Significance
In conclusion, sympathoexcitation occurs after activation of the MnPO and/or SFO. The results from this study demonstrate that activation of neurons in the MnPO and SFO contribute to sympathoexcitation via the PVN by a glutamatergic mechanism. Although it has been previously thought that ANG II was the neurotransmitter involved in the SFO-PVN neurotransmission for sympathoexcitatory responses, the current findings suggest that glutamate is the primary neurotransmitter acting at the level of the PVN after activation of the SFO. Although it is clear that ANG II within the PVN also produces a sympathoexcitation, it does not appear to contribute to any large extent in the SFO-PVN sympathoexcitation. Identification of glutamate as the major component of the SFO-PVN sympathoexcitatory pathway provides insight into possible candidate transmitters or therapeutic/pharmacological targets within the PVN that may be manipulated in disease states, such as heart failure (16) and hypertension (15).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.L., H.Z., and K.P.P. conception and design of research; T.L., X.L., and B.X. performed experiments; T.L., X.L., B.X., and K.P.P. analyzed data; T.L., H.Z., X.L., B.X., and K.P.P. interpreted results of experiments; T.L., X.L., B.X., and K.P.P. prepared figures; T.L. and K.P.P. drafted manuscript; T.L., H.Z., B.X., and K.P.P. edited and revised manuscript; T.L., H.Z., X.L., B.X., and K.P.P. approved final version of manuscript.
ACKNOWLEDGMENTS
This study was supported by the UNMC Skala Fellowship (T. L. Llewellyn) and National Institutes of Health Grant HL62222.
REFERENCES
- 1. Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol 28: 95–99, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Badoer E. Neurons in the hypothalamic paraventricular nucleus that project to the rostral ventrolateral medulla are not activated by hypotension. Brain Res 801: 224–227, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol Regul Integr Comp Physiol 268: R625–R633, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Chen QH, Haywood JR, Toney GM. Sympathoexcitation by PVN-injected bicuculline requires activation of excitatory amino acid receptors. Hypertension 42: 725–731, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol 285: R1231–R1239, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol 90: 169–173, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Haselton JR, Goering J, Patel KP. Parvocellular neurons of the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J Auton Nerv Syst 50: 1–11, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Hendel MD, Collister JP. Contribution of the subfornical organ to angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 288: H680–H685, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Johnson AK, Cunningham JT, Thunhorst RL. Integrative role of the lamina terminalis in the regulation of cardiovascular and body fluid homeostasis. Clin Exp Pharmacol Physiol 23: 183–191, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Kannan H, Yamashita H. Connections of neurons in the region of the nucleus tractus solitarius with the hypothalamic paraventricular nucleus: their possible involvement in neural control of the cardiovascular system in rats. Brain Res 329: 205–212, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Kawano H, Masuko S. Region-specific projections from the subfornical organ to the paraventricular hypothalamic nucleus in the rat. Neuroscience 169: 1227–1234, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Kolaj M, Bai D, Renaud LP. GABAB receptor modulation of rapid inhibitory and excitatory neurotransmission from subfornical organ and other afferents to median preoptic nucleus neurons. J Neurophysiol 92: 111–122, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Kolaj M, Renaud LP. Metabotropic glutamate receptors in median preoptic neurons modulate neuronal excitability and glutamatergic and GABAergic inputs from the subfornical organ. J Neurophysiol 103: 1104–1113, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Li DP, Pan HL. Angiotensin II attenuates synaptic GABA release and excites paraventricular-rostral ventrolateral medulla output neurons. J Pharmacol Exp Ther 313: 1035–1045, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension 49: 916–925, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res 93: 990–997, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Li YF, Wang W, Mayhan WG, Patel KP. Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Regul Integr Comp Physiol 290: R1035–R1043, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Li Z, Ferguson AV. Subformical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol Regul Integr Comp Physiol 265: R302–R309, 1993 [DOI] [PubMed] [Google Scholar]
- 19. McKinley MJ, Gerstberger R, Mathai ML, Oldfield BJ, Schmid H. The lamina terminalis and its role in fluid and electrolyte homeostasis. J Clin Neurosci 6: 289–301, 1999 [DOI] [PubMed] [Google Scholar]
- 20. McKinley MJ, Pennington GL, Oldfield BJ. Anteroventral wall of the third ventricle and dorsal lamina terminalis: headquarters for control of body fluid homeostasis? Clin Exp Pharmacol Physiol 23: 271–281, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Miselis RR. The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res 230: 1–23, 1981 [DOI] [PubMed] [Google Scholar]
- 22. O'Neill TP, Brody MJ. Role for the median preoptic nucleus in centrally evoked pressor responses. Am J Physiol Regul Integr Comp Physiol 252: R1165–R1172, 1987 [DOI] [PubMed] [Google Scholar]
- 23. Patel KP, Schmid PG. Role of paraventricular nucleus (PVH) in baroreflex-mediated changes in lumbar sympathetic nerve activity and heart rate. J Auton Nerv Syst 22: 211–219, 1988 [DOI] [PubMed] [Google Scholar]
- 25. Patel KP, Zhang PL. Reduced renal responses to volume expansion in streptozotocin-induced diabetic rats. Am J Physiol Regul Integr Comp Physiol 257: R672–R679, 1989 [DOI] [PubMed] [Google Scholar]
- 26. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Orlando: Academic, 1986 [DOI] [PubMed] [Google Scholar]
- 27. Ployngam T, Collister JP. Role of the median preoptic nucleus in chronic angiotensin II-induced hypertension. Brain Res 1238: 75–84, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Ployngam T, Katz SS, Collister JP. Role of the median preoptic nucleus in the chronic hypotensive effect of losartan in sodium-replete normal rats. Clin Exp Pharmacol Physiol 37: e7–e13, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat 38: 197–208, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Saper CB, Levisohn D. Afferent connections of the median preoptic nucleus in the rat: anatomical evidence for a cardiovascular integrative mechanism in the anteroventral third ventricular (AV3V) region. Brain Res 288: 21–31, 1983 [DOI] [PubMed] [Google Scholar]
- 31. Sawchenko PE, Swanson LW. The organization and biochemical specificity of afferent projections to the paraventricular and supraoptic nuclei. Prog Brain Res 60: 19–29, 1983 [DOI] [PubMed] [Google Scholar]
- 32. Sawchenko PE, Swanson LW. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol 218: 121–144, 1983 [DOI] [PubMed] [Google Scholar]
- 33. Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801: 239–243, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Stocker SD, Toney GM. Median preoptic neurones projecting to the hypothalamic paraventricular nucleus respond to osmotic, circulating Ang II and baroreceptor input in the rat. J Physiol 568: 599–615, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Westerhaus MJ, Loewy AD. Sympathetic-related neurons in the preoptic region of the rat identified by viral transneuronal labeling. J Comp Neurol 414: 361–378, 1999 [PubMed] [Google Scholar]
- 37. Yasuda Y, Honda K, Negoro H, Higuchi T, Goto Y, Fukuda S. The contribution of the median preoptic nucleus to renal sympathetic nerve activity increased by intracerebroventricular injection of hypertonic saline in the rat. Brain Res 867: 107–114, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Zardetto-Smith AM, Thunhorst RL, Cicha MZ, Johnson AK. Afferent signaling and forebrain mechanisms in the behavioral control of extracellular fluid volume. Ann NY Acad Sci 689: 161–176, 1993 [DOI] [PubMed] [Google Scholar]