Abstract
Circadian rhythms influence a variety of physiological and behavioral processes; however, little is known about how circadian rhythms interact with the organisms' ability to acquire and retain information about their environment. These experiments tested whether rats trained outside their endogenous active period demonstrate the same rate of acquisition, daily performance, and remote memory ability as their nocturnally trained counterparts in tasks of sustained attention and spatial memory. Furthermore, we explored how daily task training influenced circadian patterns of activity. We found that rats demonstrate better acquisition and performance on an operant task requiring attentional effort when trained during the dark-phase. Time of day did not affect acquisition or performance on the Morris water maze; however, when animals were retested 2 wk after their last day of training, they showed better remote memory if training originally occurred during the dark-phase. Finally, attentional, but not spatial, task performance during the light-phase promotes a shift toward diurnality and the synchronization of activity to the time of daily training; this shift was most robust when the demands on the cognitive control of attention were highest. Our findings support a theory of bidirectional interactions between cognitive performance and circadian processes and are consistent with the view that the circadian abnormalities associated with shift-work, aging, and neuropsychiatric illnesses may contribute to the deleterious effects on cognition often present in these populations. Furthermore, these findings suggest that time of day should be an important consideration for a variety of cognitive tasks principally used in psychological and neuroscience research.
Endogenous circadian oscillators are responsible for daily changes in both physiological and behavior systems. The role of circadian rhythms in physiological processes has been well-characterized and includes daily regulation of genes important for metabolic homeostasis (Rutter et al. 2002), immune function (Oishi et al. 2003), cell development and proliferation (Meerlo et al. 2009), and cell signaling (Barnes et al. 1977). Furthermore, circadian dysregulation has been linked to a variety of systemic pathologies that have profound influences on human health and cognitive function (Folkard and Akerstedt 2004; Waage et al. 2009; Lange et al. 2010). While much of the basic physiology under control of circadian pacemakers has been well-studied, the interactions between these processes and cognitive behavior have been relatively unexplored. Although there is evidence that performance and learning may be influenced by circadian processes (for reviews, see Daan 2000; Gerstner and Yin 2010), we have little information about how regularly timed cognitive processes impact circadian rhythms; in particular, can rhythms be modified by experience to optimize task acquisition or augment performance?
The role of circadian effects on learning and memory has long been of interest to researchers. Early findings by Holloway and Wansley demonstrated that passive avoidance performance was optimized periodically at 24-h intervals following learning (Holloway and Wansley 1973a,b; Wansley and Holloway 1976), and it was later determined that this periodic performance was dependent upon an intact suprachiasmatic nucleus (SCN) (Stephan and Kovacevic 1978). Investigators have also examined how SCN-driven biological rhythms interact with performance through time-of-day studies on learning. For example, habituation to auditory cues in pigeons (Valentinuzzi and Ferrari 1997) and habituation to spatial novelty in mice (Valentinuzzi et al. 2000) were more robust during the animal's endogenous active phase. Hoffmann and Balschun (1992) illustrated that mice, trained on an alternating T-maze, produced fewer errors and faster rates of acquisition when training occurred during the dark-phase; and in studies of contextual and cued fear conditioning, time-of-day effects have been reported in acquisition, recall, and extinction learning (Chaudhury and Colwell 2002; Eckel-Mahan et al. 2008).
The series of experiments were designed to determine how daily cognitive task performance at different times of day modifies patterns of activity and if the strength of this modification predicts future performance in two different cognitive tasks. The first was a discrimination-based operant task requiring sustained periods of attentional effort and is dependent upon the basal forebrain cholinergic system for above chance levels of performance (McGaughy et al. 1996). Sustained attention can be defined as an individual's readiness to detect the presence of a rarely occurring signal over a prolonged period of time and their ability to correctly discriminate the presence or absence of this signal from nonsignal events or “noise” (Sarter et al. 2001). The sustained attention task (SAT) requires animals to discriminate a brief and unpredictable cue over a prolonged period and report the presentation or absence of the cue through a lever response for a water reward. Training takes several weeks with animals advancing through two shaping stages that establish the operant associations necessary for performing the final version of the task. In the final version of the task, illumination of the testing chamber increases demands on cognitive control and requires animals to constrain their behavior toward the reward panel during the variable inter-trial interval to optimize performance (see Materials and Methods).
The second task was the Morris water maze (MWM), a commonly used task of hippocampal-dependent spatial learning in rodents (Morris 1984). We chose two tasks that were cognitive in nature but otherwise very different. The SAT is appetitive and requires the discrimination of an unpredictable signal for a reward (McGaughy and Sarter 1995), while the MWM is aversive, produces a stress response, and relies on an intrinsic motivation of animals to escape the water (Morris 1984; Brandeis et al. 1989; Hodges 1996; Aguilar-Valles et al. 2005; Harrison et al. 2009). The SAT requires sustained periods of attentional effort and is cholinergic-dependent (McGaughy et al. 1996). The MWM does not appear to be dependent on basal forebrain cholinergic signaling (Baxter et al. 1995, 1996; Baxter and Gallagher 1996), although it may sequester brief periods of attentional control for the augmentation of performance (Brandner and Schenk 1998; Parent and Baxter 2004). The MWM is additionally spatial, dependent on an intact hippocampus, and requires the use of visual and proprioceptive feedback for the animals to reach their objective (Morris et al. 1982). The SAT is critically dependent on associative areas, including the prefrontal cortex, and the basal forebrain cholinergic system to maintain attentional performance (McGaughy et al. 1996). Both tasks are similar in that they are fairly challenging under our training conditions: the SAT requires several stages of associative learning and takes many weeks to achieve criterion performance on the final version of the task, while water maze training was limited to a single training trial each day to enhance the dependence on 24-h retention memory and to increase the level of task difficulty. Our goal was to select divergent cognitive tasks, representing a broad spectrum of cognitive learning and performance that would best allow us to characterize the interaction of cognition and circadian systems.
We trained groups of animals housed in a 12:12-h light–dark (LD) schedule 4 h after the onset of the dark-phase (ZT16) or 4 or 10 h after the onset of the light-phase (ZT4 and ZT10, respectively) (see Fig. 1A). We measured whether the rate of acquisition and level of performance was influenced by time of day as has been described in some performance tasks. Secondly, we tested how timed daily task performance influenced daily patterns of activity acutely and how these patterns of activity changed over long periods of continuous daily training. Finally, we explored whether the strength of cognitive entrainment, as measured by phase markers of activity to the time of task training, are predictive of future task performance.
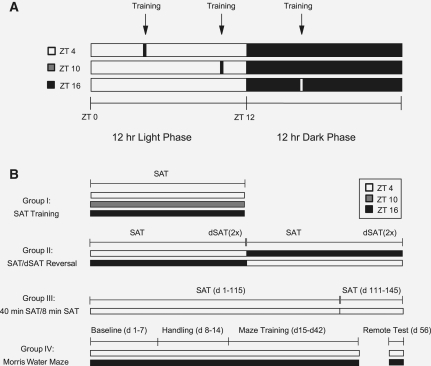
Figure 1.
Study design and timeline. (A) Training schedule for animals training on the sustained attention task or Morris water maze. All animals were maintained on a 12:12-h LD schedule with animals training at one of three training times (ZT4: 4 h after lights-on; ZT10: 10 h after lights-on; and ZT16: 4 h after lights-off). (B) Design for four experimental groups used in this study: Group I animals consisted of three different time-of-day SAT training groups. White bars represent training at ZT4, gray bars represent training at ZT10, black bars represent training at ZT16. Group II and Group IV animals consisted of animals training at ZT4 and ZT16 only. Group III animals trained on the SAT at ZT4 only. Group II animals had their training times reversed after reaching asymptotic performance. dSAT represents a challenge session where the middle 54 trials of a training session are performed in the presence of a flashing house-light as a distracter (see Materials and Methods and Fig. 3D, below, for illustration). Group III animals were trained to criterion on the SAT on the full version of the task (162 trials, ∼40 min). After reaching criterion, animals continued training for 30 additional days on an 8-min version of the task. Group IV animals were trained on the Morris water maze for a period of 28 d. Following training, animals were housed in constant darkness until the remote memory test 14 d later. Sustained attention task (SAT), distracter sustained attention task (dSAT).
Here, we report that time of day did not affect acquisition or performance on the Morris water maze as has been demonstrated previously (Valentinuzzi et al. 2004); however, we did find that remote memory for platform location, when tested 2 wk after the last day of training, is significantly better if acquisition had originally occurred during the dark-phase. Sustained attention task performance during the light-phase synchronizes activity to the time of daily training, producing a diurnal phenotype. This change in activity was most robust when animals were advanced to the final version of the task that coincides with the period where the demands on the cognitive control of attention are the highest. In contrast, hippocampal-dependent spatial training on the water maze had almost no effect on daily activity rhythms. In conclusion, our findings support the theory that cognitive performance and circadian processes can interact in a bidirectional manner and are consistent with the view that the deficits on cognition associated with aging, neuropsychiatric illnesses, and shift-work may be related to abnormalities in circadian function.
Results
An experimental outline for this study is presented in Figure 1. Groups I, II, and III reflect training groups comprised of animals that underwent SAT operant training. Group IV animals represent Morris water maze-trained animals. All animals were randomly assigned to groups following their arrival from the supplier (see Materials and Methods for details).
Acquisition rate and performance in a task of sustained attention is dependent on time of day
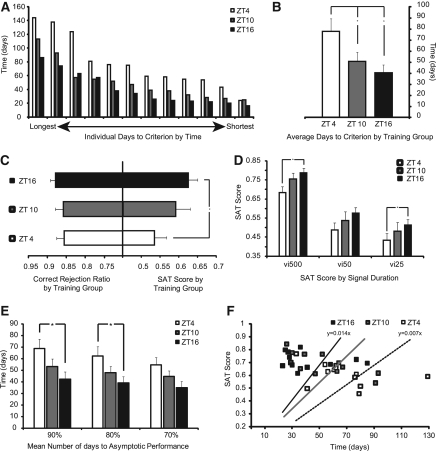
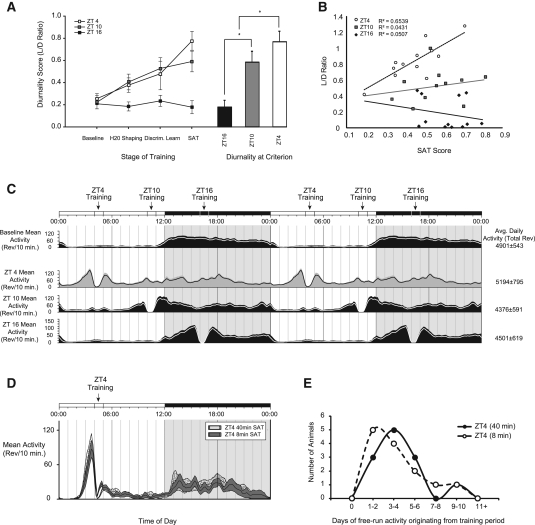
All SAT-trained animals from Group I reached criterion performance within 144 d of training, demonstrating the ability to acquire the task regardless of the time of day that training occurs. Time of daily training, however, substantially influenced the rate of acquisition (Fig. 2A,B). Criterion performance was defined as three consecutive sessions with performance >70% for all nonsignal trials and >70% for the longest signal trials (500 msec) on the final stage of training (see Materials and Methods). Differences in acquisition were driven entirely by acquisition during the last stage of training where signal trials are shortened and the house-light is illuminated, augmenting demands for attentional control. Acquisition did not significantly differ across the two brief stages of shaping designed to introduce animals to lever pressing for reward and discrimination of signal and nonsignal trial types. Figure 2A denotes absolute time to criterion for each animal plotted along the x-axis from first to last for all 36 animals trained (n = 12 per group), sorted by time of daily training. Animals trained at ZT16 consistently showed fewer days to reach criterion, with animals trained at ZT4 taking the longest time to achieve criterion, and animals trained at ZT10 falling in between. ZT16 animals, on average, showed the fastest acquisition rate (40.2 ± 6.8 d), followed by ZT10 (50.5 ± 7.8 d) and ZT4 (77.6 ± 11.0 d). There was a significant main effect of training time on acquisition as defined by time to criterion performance (F(2,33) = 4.847, P = 0.014). Post-hoc analysis revealed significant differences in the number of days to reach criterion between ZT4 and ZT10 (P = 0.036) and between ZT4 and ZT16 (P = 0.013) but not between ZT10 and ZT16 (Fig. 2B). This basic pattern was conserved for all animals in this study, with the slowest ZT16 animals generally reaching criterion faster than the slowest ZT4 animals, and the ZT10 animals in the middle, as demonstrated in Figure 2A. These data demonstrate that, in tasks requiring sustained levels of attention, animals trained outside of their endogenously driven period of activity show deficiencies in their rate of acquisition.
Figure 2.
Acquisition and criterion performance for Group I. (A) Days to criterion for individual animals plotted from longest to shortest for all SAT-trained animals by time of daily training (n = 36; 12 animals/group). (B) Mean days to criterion by training time ± SEM, (*) P < 0.05. Criterion performance is defined as the time needed for animals to reach 70% accuracy on nonsignal trials (correct rejections) and 70% accuracy on the longest signal duration trials (hits: 500 msec). (C) Correct rejection ratio and overall SAT score by training time ± SEM, (*) P < 0.05. SAT score provides a composite measure of performance that includes both signal and nonsignal trials across all three signal durations. A score of 0 represents chance performance or a complete lack of ability to discriminate between signal and nonsignal events, while a score of 1 represents perfect performance, with animals providing correct responses on every trial (see Materials and Methods). (D) SAT score by signal duration across all three training groups ± SEM, (*) P < 0.05. (SAT score = vigilance index [vi] at 500-msec, 50-msec, and 25-msec signal durations). (E) Mean number of days for animals to reach 90%–70% of their own asymptotic performance by group ± SEM, (*) P < 0.05. (F) Rate of acquisition relative to individual asymptotic performance. Figure plots number of days for animals to reach 90% of their asymptotic performance (x-axis) plotted against their SAT score on that date (y-axis). Slope represents the rate of acquisition for each population.
We also assessed whether animals trained at different times of day would show relative differences in peak performance on the well-trained task. Post-criterion performance of ZT16 animals was significantly better than that of the ZT4 training groups and marginally better than that of the ZT10 training group (Fig. 2C,D). There was an expected within-subjects effect of signal duration as animals performed better on longer signal trials (F(2,64) = 372.097, P < 0.001) and a between-subjects main effect of time of daily training on performance as characterized by the vigilance score (F(2,32) = 3.371, P < 0.047) (Fig. 2D). The vigilance score is a composite value combining signal and nonsignal trial performance at each signal duration into one metric (see Materials and Methods). Post-hoc analysis revealed ZT16 animals showed significantly better performance on the longest and shortest signal trials when compared to animals training at ZT4 (500 msec: P = 0.009; 50 msec: P = 0.092; 25 msec: P = 0.047). This was driven primarily by differences in hit rates on signal trials (F(2,32) = 3.551, P = 0.040), as no significant effects on nonsignal trial responses were observed (Correct Rejection Ratio; F(2,32) = 0.592, P = 0.559) (Fig. 2C). This suggests that animals training at ZT4 have more difficulty detecting unpredictable signals when they do occur. ZT10 animals had intermediate performance at all signal durations that did not differ significantly from the ZT4 or ZT16 training groups.
Because ZT4 animals show lower maximal levels of performance, as demonstrated in Figure 2C, we were concerned that by applying the same threshold for criterion across all training groups (70% correct on nonsignal trials and 70% correct on longest-duration signal trials), we may be biasing our measure of acquisition rate. Therefore, we also looked at acquisition rate relative to the established asymptotic level of performance for each animal. Our results revealed a significant main effect of time of daily training on the rate of acquisition for time to 90% of asymptotic performance (F(2,32) = 3.492, P = 0.042), time to 80% of asymptotic performance (F(2,32) = 3.312, P = 0.049), and near-significance for time to 70% of asymptotic performance (F(2,32) = 3.141, P = 0.057). Post-hoc analysis revealed significant differences in the number of days to reach criterion between ZT4 and ZT16 (90%; P = 0.013, 80%; P = 0.016); however, all other comparisons were nonsignificant (Fig. 2E). The acquisition rate for time to 90% of asymptotic performance is presented in Figure 2F. A line has been fit from the first day of training (chance performance) to the mean first three days of 90% of maximal performance, with the slope representing the rate of acquisition for the population by training time. Note that animals training at ZT16 show more rapid acquisition than ZT10 or ZT4 training groups even when criterion standards are adjusted to reflect relative levels of asymptotic performance.
Task performance in well-trained animals is dynamic
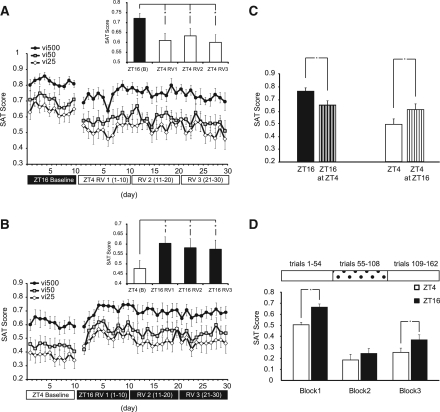
In addition to testing how rate of acquisition and absolute performance differed by time of day for the SAT, we evaluated how performance by animals would be affected by reversing the time of daily training. After reaching stable performance, Group II animals were removed from training and allowed to return to a stable nocturnal pattern of entrainment (34 d). Afterward, animals were switched to training during the phase opposite of their previous training time: ZT4 animals were trained at ZT16, and ZT16 animals were trained at ZT4. The goal was to determine if asymptotic performance was influenced by time of training once the acquisition period was over. We determined that under either condition, there was a significant main effect of reversal on performance as measured by vigilance score (F(1,19) = 8.844, P = 0.008) (Fig. 3A,B). ZT16 animals showed a robust decrease in performance when trained at ZT4 (F(1,10) = 7.927, P = 0.018) (Fig. 3A). ZT4 animals showed robust increases in performance when trained at ZT16 (F(1,9) = 11.277, P = 0.008) (Fig. 3B). Interestingly, we noted that animals originally trained at ZT4, when trained at ZT16, were incapable of reaching the same performance level as animals that were originally trained at ZT16 (t(1,19) = 2.738 P = 0.013; ZT4 animals later trained at ZT16: SAT score = 0.586 ± 0.127; ZT16: SAT score = 0.721 ± 0.079) (Fig. 3C). This time-of-day acquisition effect also impacted those animals trained at ZT16 originally; their performance when training at ZT4 never fell to the level of animals that were originally trained at ZT4 (t(1,19) = 2.527 P = 0.021; ZT16 trained at ZT4: SAT score = 0.615 ± 0.113; ZT4: SAT score = 0.476 ± 0.131) (Fig. 3C). This finding reveals a training history effect that may play an important role in future performance and suggests that deficits associated with rest phase (daytime) task acquisition may have long-lasting or even permanent effects on performance.
Figure 3.
Time-of-day reversal training and dSAT performance for Group II. (A) SAT score of ZT16-trained animals by signal duration switched to training at ZT4. Baseline period consists of final 10 d of training at asymptotic performance. Interval between baseline and restarting training for both groups was 34 d. Reversal training continued for 30 d. (RV1) Reversal days 1–10, (RV2) reversal days 11–20, (RV3) reversal days 21–30. (Inset) Overall SAT score by 10-d periods ± SEM, (*) P < 0.05. (B) Baseline, (RV) reversal period. (B) SAT score of ZT4-trained animals by signal duration switched to training at ZT16. (C) History effect comparing performance of animals from before and after reversal of training time. SAT score by baseline and reversal periods 1–3 ± SEM, (*) P < 0.05. (D) dSAT performance for animals in Group II by block ± SEM, (*) P < 0.05. Blocks 1 and 3 consist of standard trial types. Block 2 consists of trials where distracter is present.
Distracter performance is time-of-day sensitive
We also compared how animals training at different phases of the light–dark cycle performed on trials with unexpected challenge sessions. Two “distracter” sessions (dSAT) were administered before the reversal in training time, separated by several days; followed by two additional sessions, separated by several days, at the conclusion of the 30-d reversal training period. The challenge session consists of trials where the house-light cycles on and off at 0.5 Hz during the middle block (54 trials) of the 162-trial training session. Sustained attention performance under challenging conditions (e.g., distracter presentation or fatigue) is thought to emphasize top-down optimization of input processing for the maintenance of performance (Sarter et al. 2005, 2006; Kozak et al. 2006; Parikh et al. 2007; St. Peters et al. 2011). There was no interaction of order by training time on dSAT performance; therefore, dSAT sessions from before and after the reversal were binned together by training time. Testing at ZT16 resulted in significantly better performance compared to performance scores when animals were tested at ZT4: SAT score (t(1,40) = −4.130, P < 0.001) (Fig. 3D). There was a within-subjects effect of training block and a main effect of training time on performance (F(1,40) = 4.138, P < 0.049). Paired t-test analysis revealed that this effect was significant at all signal durations—500 msec: P < 0.006, 50 msec: P < 0.001, 25 msec: P = 0.020)—suggesting that under conditions of extreme attentional challenge, animals, when tested at ZT16, performed better and recovered faster (Block 3 performance) than when they were tested at ZT4.
Daily SAT performance entrains activity rhythms and entrainment predicts performance
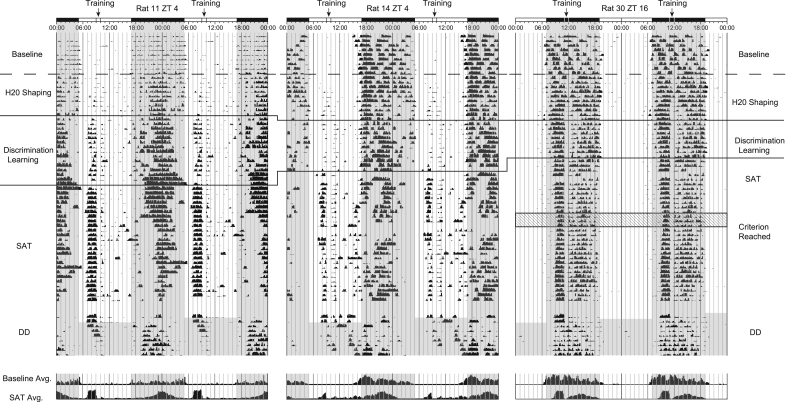
We quantified the change in diurnal activity at each stage of training to assess how task acquisition influences daily distribution of activity rhythms. Animals trained at ZT16 show a decrease in light–dark (LD) activity ratio relative to their pretraining baseline and a condensing of activity during the lights-off portion of the 24-h day as animals advanced through each stage of training (i.e., activity begins several hours later into the dark-phase and ends earlier relative to the onset of the light-phase) (Figs. 4, 6C, below). ZT4 and ZT10 animals both showed increases in LD ratio as training progressed across each phase of training concurrent with an increase in synchronized activity, or entrainment, beginning several hours before training (Figs 4, 6A,C, below). Unexpectedly, we discovered sharp changes in activity patterns at transition points during task acquisition that have not previously been reported. Both daytime-trained groups showed modest increases in diurnal activity during water shaping and lever training (Fig. 6A, below); however, a profound shift in light–dark ratio was correlated with the transition to the final stage of training that requires orienting toward and maintaining attentional focus on the intelligence panel (SAT). During the final stage of training, because of the illumination of the house-light, animals are required to attend to the signal cue directly, as orienting away from the intelligence panel increases the likelihood of missing a signal cue. Figure 4 depicts representative actograms spanning 60 d from two ZT4 (Rat 11, Rat 14; early-day)-trained animals and one ZT16 (Rat 30; early-night)-trained animal. Baseline data collected during the first 10-d period demonstrates the nocturnal pattern of activity endogenous to all animals prior to training. Animals training at ZT4 generally adopt one of two strategies for daytime training, as depicted in Figure 4: Rat 11 provides an example of a delayed activity onset phenotype, where animals extend their late-night activity through the time of task performance and delay the onset of their nighttime activity until several hours after lights-off; the more common phenotype, represented by rat 14, is to increase activity before task onset and remain somewhat day-active until several hours after lights-off. In addition, these animals generally show decreased late-night/early-morning activity. Neither rat 11 nor rat 14 reached criterion within the 60-d period shown in the figure—criterion performance was met on day 91 for rat 11 and day 80 for rat 14. The last 9 d of Figure 4 show the last 2 d of SAT training after animals reached criterion and the first 7 d of activity under conditions of total darkness after training had stopped. The presence of activity at the time of day that training would have normally occurred under conditions of total darkness (DD) confirms that training on the SAT produces a stable circadian relationship for the zeitgeber event (i.e., SAT), as reported previously for light-phase-trained animals (Gritton et al. 2009). Time histograms at the bottom of Figure 4 provide 10-d averages of the daily activity shown above for the baseline and SAT phases of training.
Figure 4.
Representative double-plotted actograms for three animals training on the SAT. SAT training is relative to the topmost LD bar (where dark bar = lights-off), and the training marker represents the ∼40-min period in which animals are absent from their home cages during SAT training. Superimposed gray shading over the actogram marks periods where lights are off. Actograms are separated into two phases: The first phase represents a continuous 60 d, with the baseline period reflecting the first 10 plotted days. The dashed line denotes the onset of the water deprivation and a 3- or 4-d shaping phase (learning to press levers). The solid lines denote a variable training phase during which animals are introduced to discriminating between signal and nonsignal trials with the house-light off (discrimination learning). The following period represents training on the final version of the SAT and is characterized by a robust increase in anticipation for the daily training session and changes in nocturnal activity distribution. The hatched area represents the 3-d period in which this animal reaches criterion performance (see Results and Materials and Methods). The second phase of the actogram illustrates the final 2 d of SAT training and the first 7 d of activity under constant conditions after training had ceased (total darkness: DD). Note that activity continues at the time training would have normally occurred for several days after training had stopped under constant conditions. Time histograms at the bottom of each actogram represent averages of the daily bins of activity shown above from both baseline and SAT phases of training, with 48 h per line.
Figure 5.
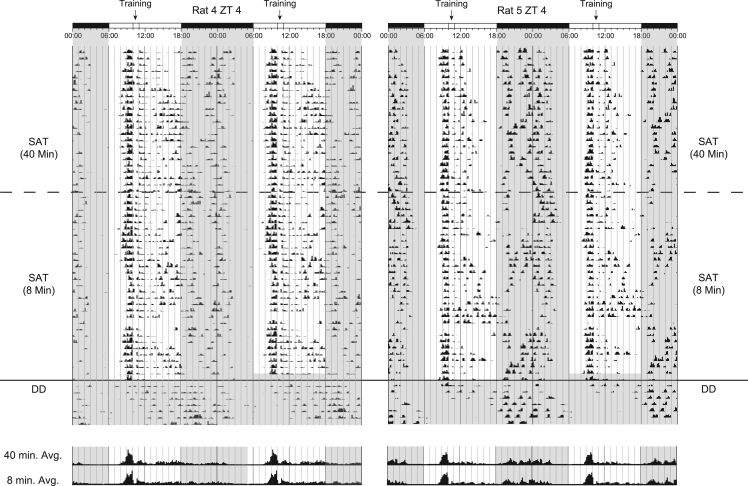
Double-plotted actograms for two Group III SAT-trained animals. Actograms represent the final 53 d of training and the first 7 d of activity after training had ceased under constant conditions. After reaching criterion on the full version of the task (40-min version), training was reduced to one block of trials (∼8-min version) and continued for 30 d. The dashed line denotes the onset of the abbreviated training paradigm. The solid line denotes the onset of the first 7 d of activity under constant conditions after training had ceased (total darkness: DD). Rat 4 showed particularly strong entrainment to the time of training with activity originating at the time training would have occurred over all 7 d shown. Rat 5 shows a more representative level of entrainment with activity persisting from the time training would have occurred for 3 d under constant conditions. Time histograms at the bottom of each actogram represent averages of the daily bins of activity shown above for the final 10 d of training under the full training paradigm (40 min) and the abbreviated training paradigm (8 min). Note the similarities in activity under either training condition suggesting duration of training is not a key factor in determining the amplitude of light-phase activity.
Figure 6.
Mean activity ratios and effects of entrainment on performance. (A) Light–dark ratio of activity from animals across distinct stages of SAT training (left) and at criterion (right) for Group I animals (LD ratio ± SEM, (*) P < 0.05). (B) Correlation between performance (SAT score) at criterion and LD ratio. (C) Mean double-plotted histograms for all animals from Group I ± SEM: x-axis is plotted in zeitgeber time (ZT) and y-axis (left) represents wheel revolutions with activity grouped into 10-min bins. Mean total daily counts ± SEM are expressed on the right. Baseline activity represents activity from all animals prior to training. SAT training is relative to the topmost LD bar (dark bar = lights-off). ZT4 (white), ZT10 (gray), and ZT16 (black) mean histograms are taken from the last 10 d of the experiment with all animals at criterion performance. The inner line represents mean activity and upper shading represents SEM. (D) Mean histograms for animals from Group III ± SEM training at ZT4: x-axis is zeitgeber time and y-axis (left) is wheel revolutions in bins of 10 min. Hatched shading is mean daily activity for all animals from last 10 d of training on the 40-min version of the task ± SEM. Gray shading is mean daily activity from the same animals for the last 10 d of training on the abbreviated version of the task ± SEM. (E) Histogram demonstrating the distribution of free-run activity originating from the time training would have occurred under constant conditions for all animals within each training group (40 min vs. 8 min). All animals showed a minimum of at least 1 d of activity with one animal from each group showing as many as 9–10 d of activity (40-min mean = 3.83 ± 0.69 d; 8-min mean = 3.67 ± 0.74 d).
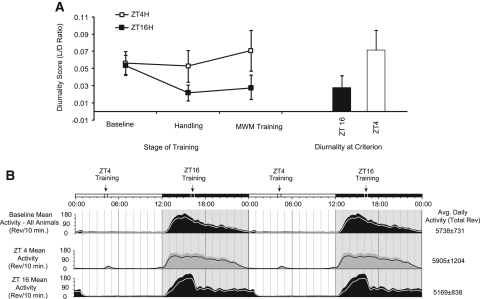
The Figure 6C (below) baseline period shows the average collective histogram distribution of activity for all 36 animals from this experimental group. ZT4, ZT10, and ZT16 activity distributions during the final stages of SAT training for all animals within each respective group are plotted below the baseline plot and represent the average activity profile for animals from each training time.
We quantified the changes in diurnal activity by analyzing the ratio of light-phase activity/dark-phase activity (LD ratio). ANOVA revealed a significant main effect of time of daily training on the LD ratio (F(2,33) = 10.448, P < 0.001) (Fig. 6A, below) and revealed a group by stage-of-training interaction (F(6,99) = 13.947, P < 0.001). Post-hoc analysis determined that the ZT4 training group differed from the ZT16 training group at all points other than baseline (H20 Shaping, P = 0.029; Discrimination Learning, P = 0.024; SAT, P = 0.018). ZT4 animals did not differ from ZT10 animals until the final stage of training (P = 0.048) (Fig. 6A, below).
We also assessed the relationship between diurnality and performance when animals reached criterion on the SAT. Figure 6B (below) plots performance as measured by overall SAT score vs. LD ratio for all animals. Animals trained at ZT4 show a significant positive correlation between diurnality and performance (Pearson correlation = 0.809, P < 0.001). This relationship was also positive for animals trained at ZT10 and negative for animals trained at ZT16, although neither correlation was significant (ZT10: Pearson correlation = 0.208, P = 0.540; ZT16: Pearson correlation = −0.225, P = 0.482). These findings indicate that entrainment, as measured by the LD ratio, is predictive of performance for ZT4 trained animals and suggests that entrainment is a necessary component of cognitive performance in animals training outside of their endogenous active period.
Brief periods of daily SAT performance are sufficient to entrain activity rhythms
In a final experiment involving animals trained on the SAT, we compared how time on task (in minutes) each day influences entrainment. Our goal was to distinguish if duration of cognitive training is an essential element in maintaining entrainment, as measured by activity distribution and the onset of activity in anticipation of time of training. In order to answer this question, we trained a group of animals at ZT4 to criterion on the full version of the task (∼40 min; long-version [LV]). Following acquisition, animals continued daily training on an abbreviated version of the task (8 min; short-version [SV]) for 30 additional days. Figure 5 consists of representative actograms spanning 60 d from two animals from this training group. We compared both LD ratio and the onset of anticipatory activity relative to SAT training time for the last 10 d of activity under each stage of training (i.e., 40 min and 8 min). Paired t-test analysis revealed no significant differences in LD ratio between the long and short versions of the task (t(1,11) = −0.556, P = 0.589; LV = 1.13 ± 0.28; SV = 1.12 ± 0.27) or the onset of activity phase relative to training time (t(1,11) = 0.540, P = 0.600; LV = 63.3 ± 9.1 min; SV = 60.8 ± 5.7 min). In addition, we compared across treatment groups, total bins of activity (BOA), BOA during the light-phase, and BOA during the dark-phase to assess if the change in the duration of training had any effect on animal locomotor distribution. Statistical analysis was carried out for the final 10 d of activity records under both the LV and SV of task training. Paired t-tests revealed no significant differences in total activity (t(1,11) = 0.390, P = 0.704; LV = 31.33 ± 2.87 BOA; SV = 31.08 ± 2.70 BOA), activity during the light-phase (t(1,11) = 0.368, P = 0.720; LV = 16.65 ± 1.45 BOA; SV = 16.40 ± 1.55 BOA), or activity during the dark-phase (t(1,11) = 0.009, P = 0.993; LV = 14.68 ± 2.06 BOA; SV = 14.67 ± 2.00 BOA). The plots in Figure 6D confirm the statistical findings and reveal that the collective distribution of activity for animals from the two training conditions does not differ substantially. These results reveal that the duration of SAT training is not a key driver in maintaining a diurnal activity distribution.
Finally, we assessed how entrainment, as measured by days of continuous activity originating from the time training would have normally occurred, persisted under conditions of total darkness. We compared the days of free-run activity from Group I animals trained at ZT4 (40 min) with those of Group III animals trained for 30 d under the abbreviated training condition (8 min). This data is represented in the distribution histogram shown in Figure 6E. Our analysis revealed no significant differences in free-run activity between groups (F(1,22) = 0.164, P = 0.871; (40-min: 3.83 ± 0.69 d; 8-min: 3.67 ± 0.74 d). We also assessed entrainment under conditions of constant darkness by comparing bins of activity from the period that would have corresponded to the light-phase across both training groups. Repeated-measures ANOVA revealed a substantial within-subjects effect of day (F(9,198) = 6.190 P < 0.001), confirming that animals show more diurnal behavior on the first day of constant conditions than on subsequent days. However, our analysis revealed no between-subjects effects of training duration on measures of activity from the corresponding light-phase period (F(1,22) = 0.662, P = 0.425). These analyses suggest that duration of task performance is not correlated to the strength of SAT entrainment as assessed by activity in constant conditions.
Water maze acquisition rate and performance are time-of-day insensitive while remote memory is time-of-day sensitive
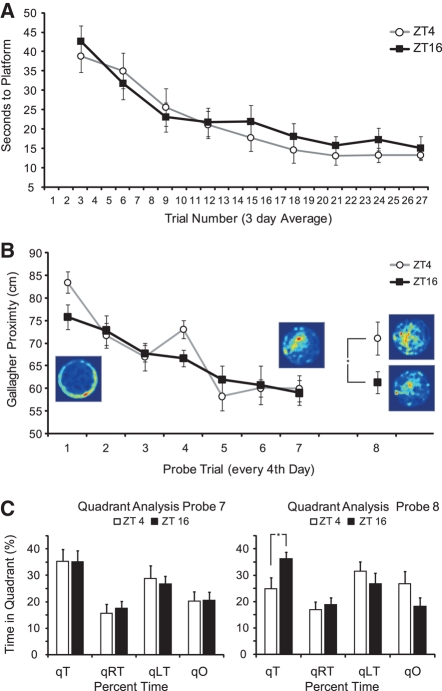
In addition to testing the effects of cognitive learning on time-of-day performance in a sustained attention task, we tested how performance in a hippocampal-dependent spatial memory task was impacted by time of day. We found no significant differences in task acquisition by treatment group in time to platform for the animals trained in the MWM (F(1,14) = 0.606, P = 0.449) (Fig. 7A). Repeated-measures ANOVA also revealed no significant main effect of treatment group for swim speed (F(1,14) = 0.513 P = 0.486) or path length (F(1,14) = 0.751, P = 0.401). Probe trial analysis did not reveal a main effect of training time during the acquisition phase on Gallagher proximity (F(1,14) = 0.261, P = 0.618) (Fig. 7B) or quadrant analysis (F(1,14) = 0.064, P = 0.805).
Figure 7.
Morris water maze acquisition and remote memory. (A) Acquisition rate as measured by time to platform for all animals in the study grouped by time of training (ZT4, n = 8; ZT16, n = 8). Data were binned across 3 d and represents time in sec ± SEM. No significant differences were found between groups. (B) Probe trial performance as measured by average distance to platform during the 60-sec probe trial (Gallagher proximity) ± SEM, (*) P < 0.05. (Insets) Three-dimensional (3D) heat maps represent relative location density during probe trial (warm colors = higher density of activity, cooler colors = little or no time spent in that location). Heat maps for probe trials 1 and 7 combine location density for all animals (n = 16). Heat maps for probe trial 8 are separated by group: (top) ZT4 (n = 8), (bottom) ZT16 (n = 8). (C) Quadrant analysis during probe trials 7 and 8 ± SEM, (*) P < 0.05. Note nonsignificant differences for treatment groups for time spent in target quadrant on probe trial 7. During the remote memory test (probe trial 8), ZT4 animals returned to chance performance (∼25%), while ZT16 animals did not differ from their probe trial 7 performance. (qT) Target quadrant, (qO) quadrant opposite target, (qRT) quadrant to right of target, (qLT) quadrant to left of target.
We also assessed how time-of-day training may have impacted remote memory. Following the last training trial on day 28, rats were released into constant darkness where they remained for 14 d. Animals were, thus, given the remote memory test under free-running conditions. Differences in remote memory were made by comparing performance between probe trials 7 and 8. Paired samples t-tests revealed a significant change in performance as assayed by time spent in the target quadrant (qT) after the 2 wk without training for ZT4 (t(1,7) = 2.901, P = 0.023) but not for ZT16 animals (t(1,7) = −0.414, P = 0.691). Further analysis revealed significant differences in overall performance for time spent in platform location between groups (t(1,14) = −2.274, P = 0.039; ZT4 qT = 24.84 ± 4.3%; ZT16 qT = 36.30 ± 2.6%) on probe trial 8 despite nonsignificant differences in performance on probe trial 7 (t(1,14) = 0.025, P = 0.980; ZT4 qT = 35.26 ± 4.4%; ZT16 qT = 35.11 ± 4.0%) (Fig. 7C). Similar results were found for Gallagher proximity with ZT4 animals showing impairment following the retention period (t(1,7) = −3.740, P = 0.007) and significant differences between groups for performance on probe trial 8 only (t(1,14) = 2.249, P = 0.041) (Fig. 7B).
Water maze training does not alter daily patterns of activity
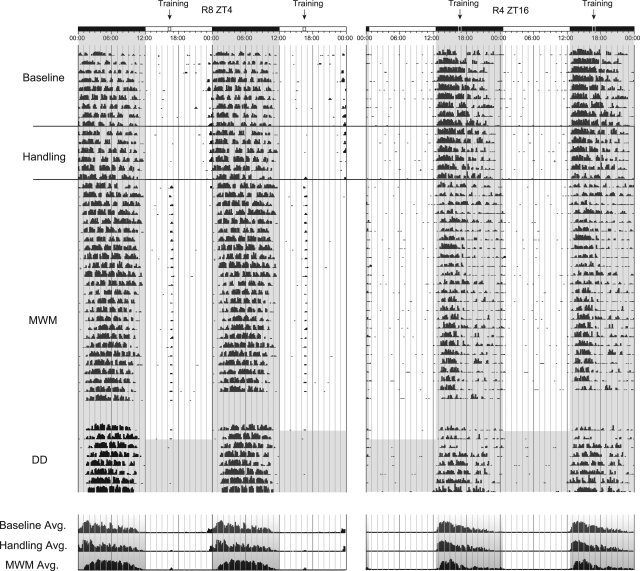
Animals trained on the Morris water maze showed slight changes in LD ratio in response to daily training. Similar to ZT16 animals on the SAT, ZT16 MWM-trained animals showed a slight decrease in light–dark ratio and a condensing of activity during the early portion of the lights-off phase of the LD cycle (Fig. 8). ZT16 animals also showed an increase in activity in anticipation for daily water maze training relative to handling alone (Fig. 9A). ZT4 animals showed subtle increases in activity in the light-phase as a result of daily handling and water maze training. Figure 8 shows representative actograms spanning 45 d from a subjective daytime (ZT4)- and nighttime (ZT16)-trained animal. Baseline data indicates strong nocturnal patterns of activity in both animals prior to training. The 7-d period that follows represents 1 wk of timed daily handling at ZT4 or ZT16, respectively, prior to the initiation of MWM training on day 16 of the actogram. Remaining activity denotes the daily MWM training portion of the experiment and represents the first 24 d of the 28-d training period. The bottom portion of activity in Figure 8 shows the last 2 d of MWM training and the subsequent period of activity under conditions of total darkness. Note the lack of activity in both amplitude and persistence relative to the time training would have occurred, compared to animals training on the SAT (Fig. 4). Time histograms at the bottom of Figure 8 represent the averaged activity for the baseline period, handling period, and MWM periods shown above.
Figure 8.
Representative double-plotted actograms for animals training on the MWM. Training is relative to the topmost LD bar (where dark bar = lights-off) and the training marker represents the ∼20-min MWM training session in which animals are absent from their home cages (animals are moved to and from the testing area in groups of eight and tested one at a time). Superimposed gray shading over the actogram marks periods where lights are off. Actograms are separated into two phases: The first phase represents a continuous 40 d, with the baseline period reflecting the first 10 plotted days. The solid lines denote 1 wk of timed daily handling, with the remaining activity representing the first 24 d of the 28-d-long MWM training phase. The second phase of the actogram illustrates the final 2 d of MWM training and the first 7 d of activity under constant conditions after training had stopped (total darkness: DD). Time histograms at the bottom provide averages of the daily bins of activity shown above from both baseline and MWM training phases above.
Figure 9.
Mean activity ratios across task training for animals on the MWM. (A) Light–dark activity ratio from animals during the baseline condition, 1 wk of timed daily handling, and 28 d of MWM training ± SEM (left). No significant differences were noted in LD ratio across the three phases tested. LD ratio for the MWM phase of testing is plotted on the right (±SEM). (B) Mean double-plotted histograms for all animals from MWM experiments (Group IV): x-axis is plotted in zeitgeber time (ZT) and y-axis represents wheel revolutions with activity grouped into 10-min bins (left). Mean total daily counts ± SEM are expressed on the right. Baseline activity represents activity from all animals prior to training. MWM training is relative to the topmost LD bar (dark bar = lights-off). ZT4 (white) and ZT16 (black) mean histograms are taken from the last 10 d of water maze training. Inner line represents mean activity and upper shading represents SEM.
Although ZT4 animals displayed increases in activity at the onset of training that extended throughout the 30-d training period, these changes were minimal compared to animals training on the SAT and were not significantly different from animals training at ZT16 on the MWM (F(1,14) = 1.228, P = 0.286) (Fig. 9A). Figure 9B represents the collective activity profile of all animals by training time over the final 10 d of water maze training (ZT4, n = 8; ZT16, n = 8). Baseline data include all 16 animals in the study.
Discussion
The purpose of this study was to address three questions: (1) Is there a time-of-day effect on the acquisition, daily performance, and remote memory ability of animals across multiple tasks of cognitive learning? (2) Does the timed daily performance of these tasks influence daily patterns of activity acutely and do markers of activity and entrainment change over the course of task acquisition? (3) Does the underlying level of task synchronization (i.e., based on measures of anticipatory activity in advance of daily training and LD ratio) correlate with task performance?
Differences in acquisition or performance associated with time of day occur for some tasks in rodents and humans (Hoffmann and Balschun 1992; Chaudhury and Colwell 2002; Allen et al. 2008; Hogan et al. 2009) but not for others (Devan et al. 2001; Ralph et al. 2002; Cain et al. 2004; Valentinuzzi et al. 2004). Our results indicate that for cognitive tasks requiring sustained periods of attentional effort, there is a clear and robust impact of time of daily training on acquisition and performance (Fig. 2A–F). Animals trained daily 4 h after lights-off (ZT16) reached criterion performance twice as fast as animals trained 4 h after lights-on (ZT4) and demonstrated higher asymptotic performance than both daytime training groups (ZT4, ZT10).
When we compared differences in post-criterion performance, effects of training time were not limited to the standard versions of the task, as ZT16-trained animals performed better on unexpected dSAT challenge sessions when compared to ZT4 animals (Fig. 3D). In order to dissociate performance deficits associated with daytime training from daytime task acquisition, a second group of animals underwent reversal training once they reached asymptotic performance. The deleterious effect of daytime training was not limited to the phase of the light–dark cycle during which the animals originally acquired the task, as animals trained during the dark-phase when shifted to daytime training showed consistently worse performance in every metric measured (Fig. 3A). Correspondingly, light-phase-trained animals, when switched to dark-phase training, showed substantial improvements in performance (Fig. 3B). Reversal training occurred over 30 d, and most animals established their new ceiling performance in 3–6 d, with animals whose SAT training was shifted from ZT16 to ZT4 taking slightly longer on average to reach their new performance level. It is unlikely that continued training at the new time would have allowed the eventual return to previous performance, as no animal showed even minimal changes in performance after day 7 of training. Somewhat surprising were the differences in asymptotic performance between the two training groups based on training history: ZT4 animals, when training at ZT16, never performed as well as animals originally trained at ZT16. In fact, their performance when training at ZT16 was equivalent to ZT16 animals training at ZT4, as shown in Figure 3C. This finding presents a compelling argument for an interaction between time of acquisition and future performance and suggests that the conditions under which training is acquired has consequences for performance long after the initial learning has occurred.
This dissociation was in contrast to our findings with daily training in the MWM: groups training at opposite times of day showed no differences in acquisition or performance over the 28-d MWM acquisition period (Fig. 7A,B). Only when remote memory was tested 2 wk after the last day of continuous training did animals show a significant difference in platform location memory, dependent on the time of day training originally occurred (Fig. 7B,C).
It is possible that the time-of-day differences on performance and acquisition between the two tasks can be explained by the reliance on different neural networks utilized for task performance and the attentional demands placed on the animal as a result of task performance. The SAT requires focused attentional effort that remains elevated throughout the testing period and results in increases of cortical acetylcholine (ACh) of 140% or more throughout the duration of the task (Arnold et al. 2002; Kozak et al. 2006; St. Peters et al. 2011). In contrast, the MWM is a hippocampal-dependent spatial learning task that requires a relatively brief application of cognitive effort, particularly for well-trained animals, and no longer than 60 sec, assuming the animal is integrating proprioceptive information relative to external spatial cues throughout the duration of the training trial. Under these conditions, water maze trials with a fixed platform location across multiple training days may not evoke the same level of attentional effort required by the SAT. The largest time-of-day effects on performance in human subjects have been reported for cognitive tasks that challenge attentional processes or executive functions requiring prefrontal areas (Yoon et al. 1999), which the SAT recruits in both humans and rodents (Sarter et al. 2005, 2006; Kozak et al. 2006; Parikh et al. 2007; Demeter et al. 2008, 2011; St. Peters et al. 2011). Based on these results, we can infer that if cognitive capacity is modulated by time of day, it is possible that the relatively small reliance on cognitive effort in our variant of the Morris water maze was insufficiently taxing to produce time-of-day effects on acquisition or performance. Potentially, if animals had been trained with varying across-day platform locations designed to increase their dependence on spatial working memory that more effectively taxed cognitive effort, time-of-day effects on performance might have been observed. In support of this theory, Winocur and Hasher (2004) showed a time-of-day effect on performance between early dark-phase- and late dark-phase-tested animals on a working memory non-matching-to-sample (NMTS) variant of the water maze.
In response to the question of cognitive effects on circadian rhythms, we demonstrated that SAT training produces a robust change in activity rhythms that was not observed for water maze training. We had previously demonstrated that training on the SAT during the light-phase (ZT10) produces a robust and significant effect on the organization of locomotor behavior that persisted for several days following the cessation of training under conditions of total darkness (Gritton et al. 2009). In addition, lesions of the basal forebrain cholinergic projections to the SCN prevent the diurnal phenotype associated with daily daytime SAT training (Gritton 2011) and provide evidence that cholinergic mechanisms associated with cognitive task performance contribute to entrainment of circadian rhythms for some stimuli. In this study, we further these findings by demonstrating that animals trained at ZT4 adopt a significantly more diurnal activity pattern than ZT10-trained animals and that training during the dark-phase (ZT16) results in a consolidated activity period, with activity onset occurring later in the dark-phase and activity offset occurring before the transition to the lights-on phase (Figs. 4, 6C). We also demonstrate for the first time that the most significant changes in the diurnal activity profile were correlated with transitions to increasingly difficult stages of training, with peaks in LD ratios corresponding to the periods in which cognitive demands on attention are the highest—when animals advance to the final phase of SAT training (Figs. 4, 6A). Importantly, despite shifting their activity profile to accommodate daytime training, animals trained outside of their endogenous active period were incapable of matching the performance of their dark-phase-trained counterparts.
Our findings for the effects of SAT training on activity were very different from our results for the Morris water maze. We saw almost no effect of daily training on animals' activity rhythms, with only minor increases in daytime activity for ZT4-trained animals characteristic of the effects of handling alone (Gritton et al. 2009; DL Hummer, JBJ Meixner, TM Lee, in prep.). While other experiments have demonstrated that entrainment produced by handling using procedures that are stressful (Hastings et al. 1997) or evoke high levels of activity/arousal (Mrosovsky 1996), our water maze-trained animals showed lower overall levels of timed anticipatory activity than reported in these studies, suggesting water maze training in this study was minimally stressful. The stark difference in activity during the light-phase between animals training in the MWM and animals training on the SAT are notable, although perhaps not unexpected. By selecting two very different cognitive tasks, we were able to test the specificity of the interaction between cognition and activity rhythms. It is tempting to speculate that the amount of time on task may be an important factor in determining the strength of entrainment; however, training on an abbreviated version of the SAT lasting only 8 min was sufficient to maintain measures of anticipatory activity and entrainment to the time of daily training at ZT4 (Figs. 5, 6D,E). These results suggest that the requirement of daily attentional control is the primary driver of continued entrainment and not the length of the training period. It is also consistent with other studies demonstrating minimal or brief periods of time-stimulus interaction can be sufficient to produce significant levels of entrainment under some conditions (Mistlberger and Rusak 1987; Mrosovsky 1988; Hastings et al. 1992, 1997; Amir et al. 1999; Mendoza et al. 2005).
If cholinergic signaling is responsible for daily entrainment associated with SAT training as we propose, it is interesting that water maze training does not more significantly influence daily patterns of activity given the purported role of cholinergic cell signaling in spatial learning and memory (Yamamuro et al. 1995; Fadda et al. 1996; Chang and Gold 2003). While cholinergic activity may complement spatial learning in the water maze, ample evidence exists that cholinergic signaling is not an essential component of task acquisition or performance (Baxter et al. 1995; Frielingsdorf et al. 2006; Wisman et al. 2008); therefore, we suspect that the recruitment of this system, particularly the necessity for sustained demands on attention, may not have been sufficiently large enough in our study to evoke robust changes in activity distribution. The inclusion of a working memory variant of the water maze task to putatively increase cognitive effort may be more successful in producing entrainment, as noted earlier.
Finally, we have demonstrated that, in tasks of cognition that require sustained attentional effort, performance is modulated by the circadian cycle and that such activity may be an important condition of performance during cognition. This is supported by our findings that animals with slower task acquisition rates and lower performance levels produce less anticipatory activity to the time of daily task training (Fig. 6B). Our findings provide evidence that the neural networks mediating cognition have the ability to modulate the underlying activity rhythms and, by doing so, allow some animals to attenuate the deleterious effects of working outside of their endogenous active period. We suggest that these findings argue for a model of bidirectional interactions between cognitive learning and circadian rhythms. Lastly, we have demonstrated, for the first time to our knowledge, a time-of-day training difference in remote memory for the MWM and suggest that the factors that influence memory consolidation could be subject to circadian regulation.
In summary, our results demonstrate an interaction that exists between time of daily training and performance in which time-of-day could represent a factor as important as treatment group or dose, and therefore, circadian influences should be considered in study design. Ongoing experiments are aimed at understanding the interactions between cognitive performance and circadian control and how nonphotic entrainment cues could be used to consolidate or prevent desynchrony in shift-work models. Shift-work, when it includes night work, has detrimental effects on both measures of subjective and physiological sleepiness, cognitive performance, accident risk, immune function, and a variety of health outcomes such as cardiovascular disease and some forms of cancer in humans (Folkard and Akerstedt 2004; Waage et al. 2009; Lange et al. 2010). Although a variety of countermeasures may be used to attenuate the negative impact of shift-work on nighttime sleepiness and daytime insomnia, there are few options to eliminate the majority of the negative effects of shift-work on physiological processes and cognition. Furthermore, our findings suggest that, at least for some tasks in rodents, night-phase performance can never be equaled by light-phase performance regardless of the strength of entrainment to the work schedule. It is interesting to speculate how this finding carries over to human shift-workers and what it means for performance in highly demanding contexts like EMT, police, and emergency care providers where the consequences for mistakes are high and attention to detail is a critical factor in positive outcomes.
Materials and Methods
Subjects
Eighty-eight male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 350 g at the start of the behavioral training were used for these studies. All animals, following arrival from the supplier, were housed individually in standard opaque single cages (27.7 cm × 20.3 cm) maintained on a LD 12:12-h cycle. Cages were lined with corn cob bedding and kept in a humidity- and temperature-controlled environment at 21 ± 1°C. Animals had ad libitum access to food (Purina 5001; Frontier) over the course of the entire experiment. Activity was monitored with intra-abdominal transmitters (pdt 4000 e-mitter-telemetry implants, Minimitter Inc.; n = 18) or via running wheel activity (n = 58). Animals were allowed to acclimate for a minimum of 2 wk before undergoing surgery or having running wheels introduced to their cages. Animals with surgical procedures (see below) were given two additional weeks of recovery before being introduced to running wheels. All procedures were in accordance with protocols approved by the University Committee for the Care and Use of Animals at the University of Michigan.
Data collection
Locomotor activity during baseline and training phases was collected in 10-min bins using Vitalview software (Minimitter). Cages were cleaned during the training phase when animals were absent from their home cage, and animals were weighed twice weekly throughout all water restriction portions of the experiment. At the conclusion of training, all animals were released into constant darkness (DD) for a period of 14 d to assess strength of entrainment. During the initial 48 h following release into DD, animals underwent complete water deprivation. All circadian data were analyzed off-line using Actiview software (Minimitter).
Experimental procedure: SAT Groups I, II, and III
Animals that were designated for operant training were allowed to acclimate for 2 wk after arrival before undergoing surgery to implant telemetry devices. Following recovery (∼2 wk), animals were introduced to running wheels and allowed to acclimate for 2 wk before beginning the baseline portion of the experiment. During the 2-wk baseline period that followed, animals continued to have ad libitum access to food and water while baseline running wheel data were collected, after which animals were mildly water-deprived to ∼95% of their free-feeding weight while having ad libitum access to food. Adjustment to water deprivation occurred over 7 d, beginning with 12-h access on day 1 (starting 6 h prior to their training time; ZT4, ZT10, or ZT16 and continuing for 12 h). Gradually over the next 6 d, animals were titrated down to a single 1-h water-access period, beginning at the intended time of task performance (ZT4 to ZT5, ZT10 to ZT11, or ZT16 to ZT17, respectively). Following the 2-wk acclimation period and gradual water deprivation, rats began operant training procedures to facilitate shaping on a task that measures sustained attention (SAT). All task-performing animals were given free access to water for 20 min following daily training sessions in addition to the quantity of water (∼5 mL) obtained as reward during operant testing. Upon achieving a performance criterion (described below), SAT Group II rats were given additional operant testing in the presence of a visual distracter (i.e., a flashing house-light). Distracter presentation occurred for a total of four sessions. Individual distracter sessions were separated by a minimum of three consecutive days on the standard task version, with animals maintaining performance above criterion performance to ensure recovery before an additional distracter session was administered. Following recovery from the second distracter, SAT Group II animals were removed from daily training until a stable nocturnal pattern of activity re-emerged for all animals (34 d). Animals were then trained with their training times reversed (ZT4 animals trained at ZT16, and ZT16 animals trained at ZT4) (see Fig. 1B) for 30 d. SAT Group II rats had two more distracter sessions during the reversal training phase, once again separated by a minimum of three consecutive days above criterion performance on the standard task, to compare the effects of distracter before and after the reversal. A final group of ZT4 animals underwent training for a period of 115 d—the time necessary for all animals to reach criterion on the final stage of training (SAT) as described below (Group III). These animals were then transitioned to an abbreviated version of the task that was 8 min in duration and trained for 30 consecutive days on the shortened version before release into DD conditions with ad lib water and food.
Sustained attention task
Operant training took place 7 d per week. Behavioral training and testing was conducted in individual operant chambers (MedAssociates Inc.) outfitted with two retractable levers, three red panel lights (2.8 W), and one red house-light (2.8 W). The water dispenser was located on the same wall as the panel lights and levers. Animals were transported from their home cages to a room housing operant chambers in a light-tight shuttle box. The experiment room was maintained in dim red light. Animals were removed from the shuttle box and placed in the unlit operant chamber for 5 min prior to task onset. Operant chambers were housed within individual sound-attenuating cabinets. The shaping protocol for this task has been published in detail previously (Gritton et al. 2009). Briefly, animals were first trained to press a lever for a water reward in accordance with a modified fixed-ratio-1 (FR1) schedule of reinforcement. The FR1 schedule is modified in that it requires animals to respond to both levers (five consecutive responses on one lever requires switching to the opposite lever before the next reward is administered) and deters a selection bias if one exists.
Animals were next trained to discriminate between signal and nonsignal trial types (i.e., illumination of the central panel light for 1 sec vs. nonillumination of the light). During the discrimination-learning (DL) phase, two seconds following a signal or nonsignal event, both levers extended into the chamber and remain active until a lever press occurred or 4 sec had passed. If no response occurred within the allotted period, the trial would be scored as an omission, and the ITI (12 ± 3 sec) would reset. During signal trials, a right-lever press indicated a correct response and was scored as a hit, whereas a left-lever press indicated an incorrect response and was scored as a miss. Conversely, during nonsignal trials, a right-lever press indicated an incorrect response and was scored as a false-alarm and a left-lever press indicated a correct response and was scored as a correct rejection. The lever rules were reversed for half of the animals to account for possibilities of handedness or selection bias. Animals received water rewards only for correct responses (30 µL for each hit and correct rejection), whereas incorrect responses (misses and false alarms) were not rewarded. The house-light was off during this shaping phase. Behavioral sessions consisted of 162 trials per session. Animals progressed to the subsequent step of shaping if they responded correctly to ≥70% of both signal and nonsignal trials for three consecutive days with <20 omitted trials per session.
During the final stage of testing (referred to as the “SAT”), the overhead red house-light was illuminated to increase the requirement for focused attention. The addition of the illuminated house-light requires the animal to constrain their visual focus to the central panel during the ITI to optimize performance. This phase of training also introduced abbreviated signal durations of three different lengths (shortened to 500, 50, or 25 msec; 27 trials of each duration during a daily training session), and the ITI was further reduced to 9 ± 3 sec. Individual sessions were divided into three blocks of 54 trials, each with all signal durations occurring randomly nine times per block. Animals were required to maintain criterion performance (≥70% correct responses to the 500-msec signal trials, ≥70% correct responses to nonsignal trials, and fewer than 20 omissions per session) for three consecutive sessions before task acquisition was considered complete. At the conclusion of this portion of the study, 12 animals (Group III) were trained on an 8-min version of the task so that the resultant changes in activity profiles from the full training session (∼40 min) could be compared with activity records from the abbreviated training session (∼8 min).
SAT Group II animals were additionally presented with visual distracter sessions after reaching criterion (referred to as the “dSAT”). Rats were exposed to a total of four sessions that included the presentation of a visual distracter as a performance challenge (i.e., a house-light flashing at 0.5 Hz) during the second block of 54 trials. Distracter presentation typically results in reduced correct rejection rates for nonsignal trials and a bias for the hit lever on all trials.
SAT data analysis and statistics
SAT performance yielded measures of hits (H), misses (M), false alarms (FA), correct rejections (CR), and omissions. Statistical analyses were carried out on the relative number of hits (%H = H/H + M), the relative number of correct rejections (%CR = CR/CR + FA), and the number of omissions. Additionally, a vigilance index (VI) was also calculated as an overall measure of attentional performance. VI is calculated using the formula VI = (%H-%FA)/[2(%H + %FA) − (%H + %FA)2]. VI values can range from −1 to +1, with +1 indicating that all trials were either hits or correct rejections, 0 being a complete lack of ability to discriminate between signal and nonsignal events, and −1 being all trials scored as misses or false alarms. Statistical analysis of hits (%H), correct rejections (%CR), overall vigilance index (VI), and percent omissions (%O) was tested using a one-way ANOVA with time of daily training as the treatment factor. Significant main effects were further analyzed using Tukey post-hoc analysis. Multiple within-subjects analyses of variance (MANOVAs) were used to determine the effects of distracter on task performance, and mixed analyses tested the main effects and interactions of signal duration (where applicable: 500, 50 and 25 msec) and distracter/trial block (three blocks of 54 trials: blocks 1, 2, and 3) on the relative number of hits, correct rejections, vigilance index, and omissions. During a distracter session, distracter presentation occurred during the second block of trials and is represented in subsequent analyses as the factor “trial-block.” Post-hoc analyses for within-subjects comparisons were carried out using Tukey post-hoc analysis. All analyses were performed using SPSS V16.
Experimental procedure: Water maze Group IV
Following acclimation to their housing environment, rats were introduced to running wheels and allowed to acclimate for 2 wk while undergoing 5 min of random daily handling to introduce the animals to the experimenter before beginning the baseline portion of the experiment. Animals were then left undisturbed for 10 d to record baseline activity. The baseline portion of the experiment was followed by 1 wk of timed daily handling (5 min) at ZT4 or ZT16 consistent with each animal's future MWM training time. Animals had ad libitum access to food and water throughout the course of the experiment. Water maze training took place 7 d per week at either ZT4 or ZT16, based on treatment group. Animals were transported from their home cages to the water maze training room in a light-tight shuttle box while they underwent daily training sessions (1 trial/day) for 28 consecutive days. Animals were given only one trial each day in order to increase task difficulty so that time-of-day effects, if they existed, could be better dissociated. Rats were also given an 8th probe trial 14 d after the last training session to test remote memory for the platform location. Following the last training trial (day 28), rats were released into constant darkness and were maintained in DD until after the remote memory test had been concluded. On the test day, all animals were removed from their home cages, transported under red-light conditions, and administered the remote memory test while under free-running conditions, and thus not tested at a particular time of day per se.
Water maze acquisition and performance
All animals were trained in a 3.2-m2 room illuminated with dim red light (16.2 lux) to prevent ZT16 animals from white-light exposure during daily training. All wall cues consisted of black shapes on white walls to allow for heightened contrast under red-light conditions. Morris water maze training was conducted in a 1.6-m-diameter pool made of black acrylonitrile butadiene styrene (ABS) centered in the middle of the training room. Water was filled to a depth of 38 cm. The platform consisted of black neoprene glued to a 4-cm platform that was placed 1.75 cm below the water surface. Water temperature was maintained at 26°C ± 2.5°C throughout the course of the experiment. Training occurred for 28 d with one trial per day except on probe trial days. Probe trial days were initiated with a probe trial followed by a standard trial to reduce the potential of extinction learning. Standard trials began with the rat being placed on the platform for 15 sec. The rat was then moved to one of five possible start locations and placed into the water facing the wall of the pool. Once the experimenter released the animal, the trial timer was started and ended either when the rat reached the platform location or after 60 sec had elapsed. In the event of an unsuccessful trial, animals were led by the experimenter to the platform location. At the conclusion of each trial, the animal was allowed to remain on the platform for 15 sec before being dried and returned to their opaque transport enclosure. Starting positions for each day were chosen randomly among five start positions; however, every animal was placed into the water from the same location each day. Probe trials were conducted every fourth day. During the probe trial, the escape platform was removed and rats initiated a trial by being placed in the pool at the start location directly opposite of the platform and allowed to swim for 60 sec. At the conclusion of the probe trial, animals were dried off and returned to their transport enclosure long enough for the platform to be re-inserted (∼15 sec). After which a standard trial was initiated. A final probe trial (probe trial 8) was conducted 14 d after the last standard training trial as a measure of remote memory.
Water maze data analysis and statistics
A video camera placed above the pool connected to a DVD recorder was used to record training sessions. Water maze data were analyzed off-line using a motion-tracking software package (Actimetrics). Metrics analyzed included: path length, time to platform, and swim speed. For probe trials, the additional variables of Gallagher proximity and time spent in quadrant were quantified. Multiple within-subjects repeated analyses of variance (MANOVAs) were used to determine the effects of time of daily training on platform acquisition and maze performance. Significant main effects were further analyzed using Tukey post-hoc analysis. Remote memory was assessed by analyzing block performance using paired t-tests, and between-subjects effects were tested using independent t-tests for time-of-day. A probability value of P < 0.05 was used as the criterion to determine statistical significance.
Circadian analysis and statistics
The binning procedure resulted in absolute counts of activity binned into 144 data points over a 24-h period. Light–dark activity ratios were calculated by summing the bins of activity (movement or wheel revolutions) collected in 10-min bins during the light-phase divided by the number of bins of activity during the dark-phase across the 24 h cycle. LD ratios >1 indicate animals with diurnal activity pattern, whereas LD ratios <1 indicated a nocturnal activity pattern. Repeated measures, within-subjects analyses of variance were used to assess the effect of SAT time (ZT4 vs. ZT 10 vs. ZT16) and the ratio of locomotor activity between the light- and dark-phases across different phases of task acquisition. Statistical analysis of LD ratio between groups was tested using a one-way ANOVA with training time-of-day as the factor. The phase of activity onset (anticipation) relative to SAT training time was determined by the presence of three or more consecutive bins of activity >10% above the daily mean activity and was analyzed for the last 10 d of recorded activity from each stage of training. LD ratio and anticipation was assessed in Group III animals using paired t-tests for the long SAT version (40-min) and the abbreviated (8-min) condition. Significant main effects were analyzed using Tukey post-hoc analysis. A probability value of P < 0.05 was used as the criterion to determine statistical significance.
Correlations between circadian measures of entrainment at ZT4, ZT10, and ZT16 and attention performance were performed for hits (%H), correct rejections (%CR), vigilance index (VI), and percent omissions (%O). Significance of correlations was tested using a Pearson correlation with P < 0.01 as the criterion for statistical significance.
Acknowledgments
We thank Dr. Geoff Murphy for his assistance with planning, acquisition, and analysis of the water maze training and testing portion of this experiment. We also thank Nicolai Morris and Yahong Yang for their assistance in training animals and data analysis. This research was supported by PHS Grant RO1 MH079084 (T.M.L., M.S.).
References
- Aguilar-Valles A, Sanchez E, de Gortari P, Balderas I, Ramirez-Amaya V, Bermudez-Rattoni F, Joseph-Bravo P 2005. Analysis of the stress response in rats trained in the water-maze: Differential expression of corticotropin-releasing hormone, CRH-R1, glucocorticoid receptors, and brain-derived neurotrophic factor in limbic regions. Neuroendocrinology 82: 306–319 [DOI] [PubMed] [Google Scholar]
- Allen PA, Grabbe J, McCarthy A, Bush AH, Wallace B 2008. The early bird does not get the worm: Time-of-day effects on college students' basic cognitive processing. Am J Psychol 121: 551–564 [PubMed] [Google Scholar]
- Amir S, Cain S, Sullivan J, Robinson B, Stewart J 1999. Olfactory stimulation enhances light-induced phase shifts in free-running activity rhythms and Fos expression in the suprachiasmatic nucleus. Neuroscience 92: 1165–1170 [DOI] [PubMed] [Google Scholar]
- Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP 2002. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience 114: 451–460 [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL, Goddard GV, Douglas RM, Adamec R 1977. Circadian rhythm of synaptic excitability in rat and monkey central nervous system. Science 197: 91–92 [DOI] [PubMed] [Google Scholar]
- Baxter MG, Gallagher M 1996. Intact spatial learning in both young and aged rats following selective removal of hippocampal cholinergic input. Behav Neurosci 110: 460–467 [DOI] [PubMed] [Google Scholar]
- Baxter MG, Bucci DJ, Gorman LK, Wiley RG, Gallagher M 1995. Selective immunotoxic lesions of basal forebrain cholinergic cells: Effects on learning and memory in rats. Behav Neurosci 109: 714–722 [DOI] [PubMed] [Google Scholar]
- Baxter MG, Bucci DJ, Sobel TJ, Williams MJ, Gorman LK, Gallagher M 1996. Intact spatial learning following lesions of basal forebrain cholinergic neurons. Neuroreport 7: 1417–1420 [DOI] [PubMed] [Google Scholar]
- Brandeis R, Brandys Y, Yehuda S 1989. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci 48: 29–69 [DOI] [PubMed] [Google Scholar]
- Brandner C, Schenk F 1998. Septal lesions impair the acquisition of a cued place navigation task: Attentional or memory deficit? Neurobiol Learn Mem 69: 106–125 [DOI] [PubMed] [Google Scholar]
- Cain SW, Ko CH, Chalmers JA, Ralph MR 2004. Time of day modulation of conditioned place preference in rats depends on the strain of rat used. Neurobiol Learn Mem 81: 217–220 [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE 2003. Switching memory systems during learning: Changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci 23: 3001–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Colwell CS 2002. Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res 133: 95–108 [DOI] [PubMed] [Google Scholar]
- Daan S 2000. Learning and circadian behavior. J Biol Rhythm 15: 296–299 [DOI] [PubMed] [Google Scholar]
- Demeter E, Sarter M, Lustig C 2008. Rats and humans paying attention: Cross-species task development for translational research. Neuropsychology 22: 787–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter E, Hernandez-Garcia L, Sarter M, Lustig C 2011. Challenges to attention: A continuous arterial spin labeling (ASL) study of the effects of distraction on sustained attention. Neuroimage 54: 1518–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devan BD, Goad EH, Petri HL, Antoniadis EA, Hong NS, Ko CH, Leblanc L, Lebovic SS, Lo Q, Ralph MR, et al. 2001. Circadian phase-shifted rats show normal acquisition but impaired long-term retention of place information in the water task. Neurobiol Learn Mem 75: 51–62 [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, Storm DR 2008. Circadian oscillation of hippocampal MAPK activity and cAmp: Implications for memory persistence. Nat Neurosci 11: 1074–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda F, Melis F, Stancampiano R 1996. Increased hippocampal acetylcholine release during a working memory task. Eur J Pharmacol 307: R1–R2 [DOI] [PubMed] [Google Scholar]
- Folkard S, Akerstedt T 2004. Trends in the risk of accidents and injuries and their implications for models of fatigue and performance. Aviat Space Environ Med 75: A161–A167 [PubMed] [Google Scholar]
- Frielingsdorf H, Thal LJ, Pizzo DP 2006. The septohippocampal cholinergic system and spatial working memory in the Morris water maze. Behav Brain Res 168: 37–46 [DOI] [PubMed] [Google Scholar]
- Gerstner JR, Yin JC 2010. Circadian rhythms and memory formation. Nat Rev Neurosci 11: 577–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritton HJ 2011. “Bi-directional interactions between cognition and circadian rhythms.” PhD thesis, University of Michigan, Ann Arbor, Michigan [Google Scholar]
- Gritton HJ, Sutton BC, Martinez V, Sarter M, Lee TM 2009. Interactions between cognition and circadian rhythms: Attentional demands modify circadian entrainment. Behav Neurosci 123: 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP 2009. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res 198: 247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Mead SM, Vindlacheruvu RR, Ebling FJ, Maywood ES, Grosse J 1992. Non-photic phase shifting of the circadian activity rhythm of Syrian hamsters: The relative potency of arousal and melatonin. Brain Res 591: 20–26 [DOI] [PubMed] [Google Scholar]
- Hastings MH, Duffield GE, Ebling FJ, Kidd A, Maywood ES, Schurov I 1997. Non-photic signalling in the suprachiasmatic nucleus. Biol Cell 89: 495–503 [DOI] [PubMed] [Google Scholar]
- Hodges H 1996. Maze procedures: The radial-arm and water maze compared. Brain Res Cogn Brain Res 3: 167–181 [DOI] [PubMed] [Google Scholar]
- Hoffmann HJ, Balschun D 1992. Circadian differences in maze performance of C57bl/6 Ola mice. Behav Process 27: 77–83 [DOI] [PubMed] [Google Scholar]
- Hogan MJ, Kelly CA, Verrier D, Newell J, Hasher L, Robertson IH 2009. Optimal time-of-day and consolidation of learning in younger and older adults. Exp Aging Res 35: 107–128 [DOI] [PubMed] [Google Scholar]
- Holloway FA, Wansley RA 1973a. Multiphasic retention deficits at periodic intervals after passive-avoidance learning. Science 180: 208–210 [DOI] [PubMed] [Google Scholar]
- Holloway FA, Wansley RA 1973b. Multiple retention deficits at periodic intervals after active and passive avoidance learning. Behav Biol 9: 1–14 [DOI] [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M 2006. Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex 16: 9–17 [DOI] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Born J 2010. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci 1193: 48–59 [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M 1995. Behavioral vigilance in rats: Task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 117: 340–357 [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M 1996. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: Selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci 110: 247–265 [DOI] [PubMed] [Google Scholar]
- Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D 2009. New neurons in the adult brain: The role of sleep and consequences of sleep loss. Sleep Med Rev 13: 187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C 2005. A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. Eur J Neurosci 22: 2855–2862 [DOI] [PubMed] [Google Scholar]
- Mistlberger R, Rusak B 1987. Palatable daily meals entrain anticipatory activity rhythms in free-feeding rats: Dependence on meal size and nutrient content. Physiol Behav 41: 219–226 [DOI] [PubMed] [Google Scholar]
- Morris R 1984. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: 47–60 [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J 1982. Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683 [DOI] [PubMed] [Google Scholar]
- Mrosovsky N 1988. Phase response curves for social entrainment. J Comp Physiol A 162: 35–46 [DOI] [PubMed] [Google Scholar]
- Mrosovsky N 1996. Locomotor activity and non-photic influences on circadian clocks. Biol Rev Camb Philos Soc 71: 343–372 [DOI] [PubMed] [Google Scholar]
- Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, Atsumi G, Ohkura N, Azama T, Mesaki M, Yukimasa S, et al. 2003. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem 278: 41519–41527 [DOI] [PubMed] [Google Scholar]
- Parent MB, Baxter MG 2004. Septohippocampal acetylcholine: Involved in but not necessary for learning and memory? Learn Mem 11: 9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M 2007. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron 56: 141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Ko CH, Antoniadis EA, Seco P, Irani F, Presta C, McDonald RJ 2002. The significance of circadian phase for performance on a reward-based learning task in hamsters. Behav Brain Res 136: 179–184 [DOI] [PubMed] [Google Scholar]
- Rutter J, Reick M, McKnight SL 2002. Metabolism and the control of circadian rhythms. Annu Rev Biochem 71: 307–331 [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP 2001. The cognitive neuroscience of sustained attention: Where top-down meets bottom-up. Brain Res Brain Res Rev 35: 146–160 [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B 2005. Unraveling the attentional functions of cortical cholinergic inputs: Interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev 48: 98–111 [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R 2006. More attention must be paid: The neurobiology of attentional effort. Brain Res Rev 51: 145–160 [DOI] [PubMed] [Google Scholar]
- St. Peters M, Demeter E, Lustig C, Bruno JP, Sarter M 2011. Enhanced control of attention by stimulating mesolimbic–corticopetal cholinergic circuitry. J Neurosci 31: 9760–9771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK, Kovacevic NS 1978. Multiple retention deficit in passive avoidance in rats is eliminated by suprachiasmatic lesions. Behav Biol 22: 456–462 [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Ferrari EA 1997. Habituation to sound during morning and night sessions in pigeons (Columba livia). Physiol Behav 62: 1203–1209 [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Buxton OM, Chang AM, Scarbrough K, Ferrari EA, Takahashi JS, Turek FW 2000. Locomotor response to an open field during C57BL/6J active and inactive phases: Differences dependent on conditions of illumination. Physiol Behav 69: 269–275 [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Menna-Barreto L, Xavier GF 2004. Effect of circadian phase on performance of rats in the Morris water maze task. J Biol Rhythms 19: 312–324 [DOI] [PubMed] [Google Scholar]
- Waage S, Moen BE, Pallesen S, Eriksen HR, Ursin H, Akerstedt T, Bjorvatn B 2009. Shift work disorder among oil rig workers in the North Sea. Sleep 32: 558–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansley RA, Holloway FA 1976. Oscillations in retention performance after passive-avoidance training. Learn Motiv 7: 296–302 [Google Scholar]
- Winocur G, Hasher L 2004. Age and time-of-day effects on learning and memory in a non-matching-to-sample test. Neurobiol Aging 25: 1107–1115 [DOI] [PubMed] [Google Scholar]
- Wisman LA, Sahin G, Maingay M, Leanza G, Kirik D 2008. Functional convergence of dopaminergic and cholinergic input is critical for hippocampus-dependent working memory. J Neurosci 28: 7797–7807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamuro Y, Hori K, Tanaka J, Iwano H, Nomura M 1995. Septo-hippocampal cholinergic system under the discrimination learning task in the rat: A microdialysis study with the dual-probe approach. Brain Res 684: 1–7 [DOI] [PubMed] [Google Scholar]
- Yoon C, May CP, Hasher L 1999. Cognition, aging, and self-reports. Psychology Press, Philadelphia, PA [Google Scholar]