Background: PDI is implicated in the intracellular reduction of ricin; other oxidoreductases also have a role in this process.
Results: Overexpression and silencing of TMX affect ricin cytotoxicity.
Conclusion: TMX participates with PDI and/or other reductases in the reduction of ricin and other proteins.
Significance: These findings contribute to the understanding of disulfide reduction in cell intoxication by toxins and virus assembly and entry.
Keywords: Endoplasmic Reticulum (ER), Enzymes, Reductase, Thiol, Toxins, Cytotoxicity, Oxidoreductase, Reduction
Abstract
Members of the type 2 ribosome-inactivating proteins (RIPs) family (e.g. ricin, abrin) are potent cytotoxins showing a strong lethal activity toward eukaryotic cells. Type 2 RIPs contain two polypeptide chains (usually named A, for “activity”, and B, for “binding”) linked by a disulfide bond. The intoxication of the cell is a consequence of a reductive process in which the toxic domain is cleaved from the binding domain by oxidoreductases located in the lumen of the endoplasmic reticulum (ER). The best known example of type 2 RIPs is ricin. Protein disulfide isomerase (PDI) was demonstrated to be involved in the process of ricin reduction; however, when PDI is depleted from cell fraction preparations ricin reduction can still take place, indicating that also other oxidoreductases might be implicated in this process. We have investigated the role of TMX, a transmembrane thioredoxin-related protein member of the PDI family, in the cell intoxication operated by type 2 RIPs ricin and abrin. Overexpressing TMX in A549 cells resulted in a dramatic increase of ricin or abrin cytotoxicity compared with control mock-treated cells. Conversely, no difference in cytotoxicity was observed after treatment of A549 cells or control cells with saporin or Pseudomonas exotoxin A whose intracellular mechanism of activation is not dependent upon reduction (saporin) or only partially dependent upon it (Pseudomonas exotoxin A). Moreover, the silencing of TMX in the prostatic cell line DU145 reduced the sensitivity of the cells to ricin intoxication further confirming a role for this enzyme in intracellular ricin activation.
Introduction
Ricin and abrin are potent cytotoxins found in the seeds of Ricinus communis and Abrus praecatorius, respectively (1). Cell intoxication by ricin has been studied in more detail due to its potential applications in biomedicine as a component of targeted antitumor immunoconjugates (2). Like abrin, ricin belongs to the type 2 family of the ribosome-inactivating proteins (RIPs)2 being composed of two polypeptide chains, an enzymatic A chain (RTA) that damages ribosomes in an irreversible manner by removing one or more adenine residues from rRNA, and a galactose and acetylgalactosamine-specific lectinic B chain (RTB), capable of binding to cell surface structures (3). After endocytosis, ricin travels backward along the secretory pathway to the Golgi apparatus and endoplasmic reticulum (ER) whence RTA is dislocated across the ER membrane to the cytosol. A similar entry mechanism can be hypothesized for abrin.
The holotoxin is not enzymically active (3, 4), and the disulfide bond holding the two chains together must be reduced to release an active A chain. The intervention of intracellular reductases is likely to facilitate separation of the two chains and the subsequent cell intoxication phenomena. Evidence has suggested that the ER must be the site where ricin is reduced, and one of the possible candidates to effect this reductive activation is protein disulfide isomerase (PDI) with a role for thioredoxin reductase as an agent capable of reducing PDI (5, 6).
Other oxidoreductases belonging to the PDI family have been reported to be located within the ER, although no information is presently available as to their possible role in ricin activation. An example of this kind of enzyme is provided by the thioredoxin-like transmembrane (TMX) protein located in the ER membrane (7). TMX belongs to a family of a large number of members catalyzing the oxidation, reduction, and isomerization of disulfide bonds (8) and was identified as a protein encoded by a gene isolated as a TGF-β-responsive gene in retrovirus-mediated gene trap screening (7). PDI was the first characterized protein disulfide isomerase consisting of four thioredoxin-like domains in the configuration abb′a′, where the a and a′ domains contain catalytic CGHC motifs and the b and b′ domains are noncatalytic. The domain layouts and active site sequences of the remaining PDIs show a variety of redox motif sequences. This could reflect separate roles in oxidation, reduction, and isomerization. So far at least 17 putative protein disulfide isomerases have been reported in humans, of which 9 have been shown to catalyze disulfide reactions (9). TMX contains instead a CPAC motif, which is different from the classical CGHC motif found in other members of the PDI family. Interestingly, TMX, despite its unusual catalytic motif, displays reduction activity and can also reactivate scrambled RNase, suggesting that TMX may also function as a disulfide isomerase (8). More recently it was demonstrated that TMX forms disulfide-linked complexes with the MHC class I heavy chain (10). Despite the large body of literature available on the activities of PDI family members in vitro and in vivo, there are still numerous unanswered questions regarding the physiological functions of the individual proteins and their mechanisms of action, especially within the complex environment of the ER. In particular, it is important to determine whether different enzymes either have overlapping or separate and distinct substrate specificities and whether interactions with other intracellular effectors can be envisaged.
Transport of ricin to the ER and dislocation of its A chain to the cytosol entail a complex series of phenomena that may involve numerous interactions with cell membranes and their protein constituents (4), and other members of the type 2 RIPs family likely behave in the same manner as ricin. In this scenario it is not unlikely that the localization of TMX in the ER membrane may provide this oxidoreductase with a privileged role in the reductive activation of ricin and in the subsequent interaction of RTA with components of the ER-associated protein degradation pathway, thereby facilitating the transmembrane passage of RTA and cell intoxication. Here, we investigate the reduction of ricin and abrin operated by TMX under several redox conditions established in vitro and in vivo.
EXPERIMENTAL PROCEDURES
Materials
All chemicals described were of reagent grade. Complete protease inhibitor complex tablets were from Roche; DTT and glutathione (both reduced, GSH and oxidized form, GSSG) were from Sigma-Aldrich. Polyclonal antibody to PDI (rabbit) was from Sigma-Aldrich; polyclonal antibody to TMX (goat) was from AbD Serotec; monoclonal antibody to β-actin was from Santa Cruz Biotechnology; antiserum to RTA was raised by immunizing mice with ricin obtained from seeds (11). Abrin and saporin were kindly supplied by Prof. Andrea Bolognesi (University of Bologna, Italy) and Pseudomonas exotoxin A (PE) was from Sigma-Aldrich.
Cells and Reagents
The following human cell lines were utilized in this work: DU145 (prostate cancer), A549 (respiratory epithelia), PACA44 (pancreatic cancer), T3M4 (pancreatic cancer), GER (pancreatic cancer), MiaPaCa (pancreatic cancer), U251 (human monocytes), PT45 (pancreatic carcinoma), CFPAC-1 (pancreatic cancer), Jurkat (T cell leukemia), and THP-1 (human monocytes). All cell lines were purchased from the American Type Culture Collection and were maintained at 37 °C in a humidified atmosphere of 5% CO2 by serial passages in RPMI 1640 medium containing 2 mm glutamine, 10% fetal bovine serum (FBS), and 50 units/ml both penicillin and streptomycin.
Purification and Preparation of Recombinant Proteins
The vector pGEX-TMX-(27–180), kindly supplied by Dr. J. Yodoi (Kyoto University, Japan), was introduced into Escherichia coli, and the expression of glutathione S-transferase (GST) fusion proteins was induced with 0.1 mm isopropyl 1-thio-β-d-galactopyranoside at 20 °C. GST fusion proteins were bound to glutathione-Sepharose beads in a batch preparation (Amersham Biosciences) and cleaved with Pre-Scission Protease (Amersham Biosciences) at 4 °C in the cleavage buffer containing 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1 mm DTT. The treated beads were transferred to the column, and the flow-through was collected. The solution containing the GST-free protein was concentrated with Microcon filter devices (Millipore).
In Vitro Reductase Assay
The ricin disulfide reduction assay was carried out as follows. DTT-activated TMX was prepared by incubating the enzyme with 10 mm DTT overnight at 4 °C, and the excess DTT was then removed by overnight dialysis against PBS at 4 °C. The role of glutathione was investigated by adding 1 mm reduced GSH, 750 μm GSH:250 μm GSSG, or 750 μm GSSG:250 μl of GSH, respectively, to PBS buffer. Ricin (a 63-kDa protein toxin) was purified from castor beans of R. communis according to Nicolson and Blaustein (11). Ricin was added to each mixture, and after incubation at 37 °C the reaction was terminated by adding SDS-PAGE sample buffer; samples were then analyzed by gel electrophoresis on 10% slab gels.
RNA Silencing
Stably transfected DU145 cells were generated by using shRNA pRS plasmids targeting human TMX1, a no-targeting plasmid, or the empty vector. The TMX1 targeting sequence was CCAAGGACTAAGAAGGACTTCATAAACTT (OriGene). The cells were transfected using the TransIT-LT1 transfection reagent (Mirus). Selection was carried out with puromycin added to the cells at 2 μg/ml 48 h after transfection. The resistant clones were maintained in Complete medium containing 1 μg/ml puromycin. The silencing of TMX1 was confirmed by Western blotting.
TMX-overexpressing Cells
To generate cells stably expressing TMX-myc, pCDNA3.1-TMX-myc kindly supplied by Dr. J. Yodoi, or pCDNA3.1 were transfected into A549 cells by using the TransIT-LT1 transfection reagent. Cells were cultured in selective medium containing 0.5 mg/ml G418 (Roche Applied Science), and resistant colonies were picked and cultured.
Cytotoxicity Assays
In the cytotoxic assays the cells were seeded in 200 μl of RPMI 1640 medium with 10% FBS at a density of 1–5 × 104 cells/well in flat-bottomed 96-well plates (Falcon; BD Biosciences) and allowed to adhere for 24 h at 37 °C and 5% CO2. The toxins ricin, abrin, saporin, and PE, each in a total volume of 200 μl, were added to the wells at 1:10 serial dilutions. The plates were incubated for 48 h at 37 °C and 5% CO2. Cytotoxicity assays were performed using a vital stain XTT assay. Ten μl of 1.5-mg phenazine (Sigma-Aldrich)/ml and PBS were added to 10 ml of a 5-mg XTT (Sigma-Aldrich)/ml RPMI stock solution. This freshly prepared mixture was sterile-filtered, and 40 μl was added to each well containing the cells to be tested. Absorption was measured at 450 nm after 2 h of incubation at 37 °C with 5% CO2.
Statistical Analysis
Nonparametric statistics were performed using SigmaPlot software. p values <0.05 were regarded as significant. All experiments were conducted in triplicate and reproduced at least twice with similar results. Error bars represent S.E.
RESULTS AND DISCUSSION
Overexpression of TMX Enhances Toxicity of Type 2 RIPs
It was shown in previous studies (5, 6) that the PDI can reduce the ricin holotoxin by cleaving the disulfide bridge linking the toxic domain RTA to the binding domain RTB. In contrast, the observation that in PDI-immunodepleted lysates this process was still carried out (6) suggested that the reduction of ricin could be operated also by other oxidoreductases located in the ER.
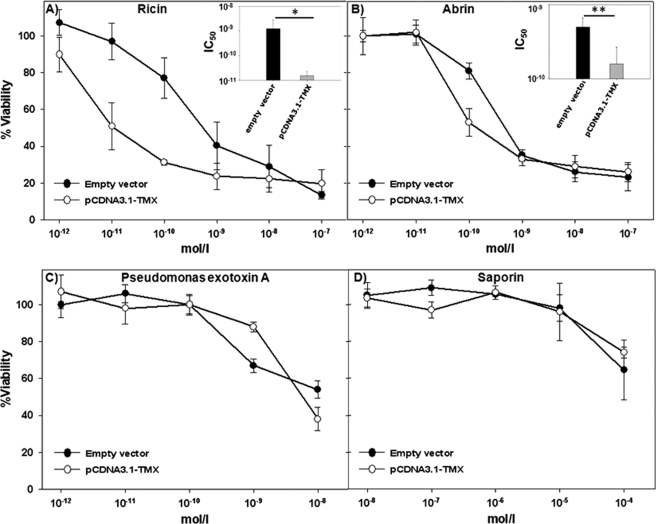
TMX is a transmembrane protein localized in the membrane of the ER with its catalytic site facing the lumen. To evaluate whether TMX could be a candidate in the reductive process of ricin, stable clones were generated by transfecting A549 cells with pCDNA3.1-TMX or with the empty vector. A cytotoxicity assay was then performed. As shown in Fig. 1A, a greater cytotoxic effect was observed with the cells overexpressing TMX with respect to mock-treated (empty vector) control cells.
FIGURE 1.
Sensitivity of A549 transfectants (empty vector and pCDNA3.1-TMX) to toxins. A549 control cells (empty vector) and overexpressing TMX (pCDNA3.1-TMX) were exposed to various concentrations of toxins and their viability assessed 48 h after toxin addition by an XTT assay. The graphs represent, respectively, the intoxication of the target cells after incubation with ricin (A), abrin (B), Pseudomonas exotoxin A (C), and saporin (D). In the upper right corner of A and B are reported the histograms of the IC50 (concentration inhibiting the 50% of the cell viability) value obtained from the cytotoxicity curves after treatment of the cells with ricin or abrin. *, p < 0.01; **, <0.05 (Student's t test).
To confirm that the reductive effects brought about by TMX are directed to A-B type toxins belonging to the type 2 RIP family, cells were treated also in the presence of the toxin abrin. As illustrated in Fig. 1B, also in this case the cytotoxic effects observed were greater in A549 transfected with TMX than in control cells transfected with the empty vector.
To compare cytotoxic effects using different reagents we took into consideration the IC50 value (i.e. the concentration of toxin inhibiting by 50% the cell viability) observed after exposing the cells to different concentrations of ricin or abrin (see insets of Fig. 1, A and B). The IC50 value observed in cells treated with ricin was ∼50-fold higher in the control cells with respect to TMX-overexpressing cells which were therefore 50-fold more sensitive to ricin intoxication (Table 1). TMX transfectants were also more sensitive by 5-fold to abrin intoxication with respect to controls. It should be noticed that even though the cytotoxic effect obtained in the case of abrin was less remarkable compared with ricin, the difference in the cytotoxic activity observed at the concentration of 10−10 m toxin produced a decrease in cell viability of about 40%, suggesting a compelling intervention of TMX also on the intracellular activation of abrin.
TABLE 1.
Comparison of cytotoxicity of different toxins in A459 cells
| Toxin | IC50a |
Increase in cytotoxicityb | |
|---|---|---|---|
| Empty vector | pcDNA3.1-TMX | ||
| mol/liter | mol/liter | -fold | |
| Ricin | 5 × 10−10 | 10−11 | 50 |
| Abrin | 4 × 10−10 | 8 × 10−9 | 5 |
| Pseudomonas exotoxin A | >10−8 | 6 × 10−9 | <1.6 |
| Saporin | >10−4 | >10−4 | <1 |
a IC50 is the concentration of toxin inhibiting 50% of the cell viability.
b Fold increase in cytotoxicity is calculated as follows: IC50 of cells transfected with empty vector/IC50 of cells transfected with pcDNA3.1-TMX.
To make sure that the activity of TMX was indeed specific for the interchain disulfide bridge present in A-B toxins we incubated target cells with two other toxins which intoxicate the cells by exploiting different mechanisms. Saporin is a plant toxin belonging to the type 1 family of RIPs that lack a B chain and bind cell surfaces with difficulty, although in the case of saporin it was described that it can bind the α2 macroglobulin receptor unequally distributed in different types of cells (12). Intracellular trafficking of saporin has not been fully elucidated yet, but it does not require intracellular reduction to intoxicate the target cell. The exotoxin A produced by the bacterium Pseudomonas aeruginosa was also used. PE is formed by a single polypeptide of ∼60 kDa containing three functional domains, an N-terminal domain containing regions able to bind cell surface structures, a C-terminal domain able to catalytically inactivate protein synthesis of the target cell, and a domain involved in transmembrane translocation (13). An intrachain disulfide bond is also present, whose reduction leads to the activation of the toxin inside the cell (possibly in the ER), and it has been reported that PDI (or members of the PDI family) could be involved in this process (14). As shown in Fig. 1 (C and D), these two toxins produced similar intoxication patterns in the TMX-overexpressing cells and in control cells. As demonstrated by the IC50 values calculated from the cytotoxicity curves of PE and saporin (Table 1), TMX overexpression did not increase the sensitivity of the cells to treatment with these two toxins. In fact, in the case of PE and saporin, only slight differences in the percentage of viable cells were found between the TMX-overexpressing cells and the control cells, suggesting that PE and saporin might not be a suitable substrate for TMX under the conditions used in our experiments. Our observations therefore demonstrate that TMX might have a crucial role in reducing the interchain disulfide bridge of A-B type toxins and proteins in general but might be inefficient in reducing intrachain S-S bridges as those found in the PE toxin.
TMX Reduces Ricin in Vitro
Matsuo et al. (7) reported that only prereduced TMX, in that case with DTT, was able to reduce the disulfide interchain bridges of insulin. We therefore performed a similar experiment to establish whether TMX can reduce ricin in vitro following reduction of its catalytic site with 10 mm DTT. In these experiments we tested combinations of enzyme and toxin under different conditions and revealed ricin reduction by staining untreated or treated ricin with an anti-RTA antibody in a Western blot analysis. Due to glycosylation heterogeneity (15, 16) RTA appears as a double band of ∼30 kDa and 32 kDa. TMX was therefore preactivated with DTT or left untreated and incubated with ricin in PBS buffer, pH 7.4, at 37 °C (Fig. 2).
FIGURE 2.
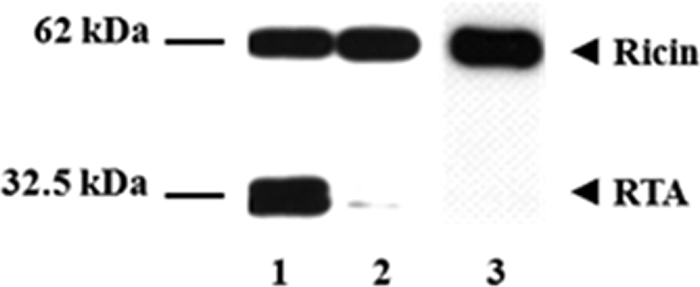
Reduction of ricin with prereduced TMX. Samples with ricin (2 μg) were incubated with 5 μg of DTT-preactivated TMX for 60 min at 37 °C (lane 1) in a final volume of 50 μl. Ricin incubated with TMX without DTT preactivation is represented in lane 2. Ricin alone (lane 3) was included as a control. After 60 min the reaction was stopped by adding loading buffer and running the SDS-PAGE under nonreducing conditions. The appearance of reduced ricin was determined by Western blotting as described under “Results and Discussion.”
In agreement with results reported by Matsuo et al. in the case of insulin, we also observed that the reduction of ricin took place only when TMX was preactivated with DTT (Fig. 2, lane 1). As expected, incubation of ricin with nonpreactivated TMX did not lead to a significant reduction of the molecule (lane 2).
Glutathione Can Activate TMX in Vitro
Most ER oxidoreductases are reduced predominantly in their catalytic site at the steady state. Thus, although the intraluminal pH and protein composition of the ER are optimized for the formation of disulfide bonds, the active site of these oxidoreductases is partially or completely reduced. PDI is known to oxidize substrate proteins and is itself maintained in an oxidized form by Ero1 for which the ultimate electron acceptor can be molecular oxygen (17). However, other oxidoreductases such as ERp57 (18, 19) are thought to act as reductases or isomerases and must therefore be maintained in a reduced state to remain active. Recently, Matsuo et al. provided evidence that also TMX at the steady state is present in its reduced form in two different cell lines (10).
A candidate molecule responsible for the reduction of these enzymes in the ER is the reduced glutathione. We therefore assessed whether this physiological agent could activate in vitro oxidized TMX which may in turn reduce ricin. Thus, we included reduced glutathione in the reaction mixture at a 1 mm concentration, as found in the ER lumen (18). When both TMX and GSH were present in the reaction mixture, a reduction of ricin took place providing evidence that GSH may be required also in vivo to reduce TMX (Fig. 3). Conversely, in the presence of either GSH or TMX alone, only a negligible reducing activity was observed, confirming that an enzyme-assisted mechanism is necessary to effect the reduction of ricin.
FIGURE 3.
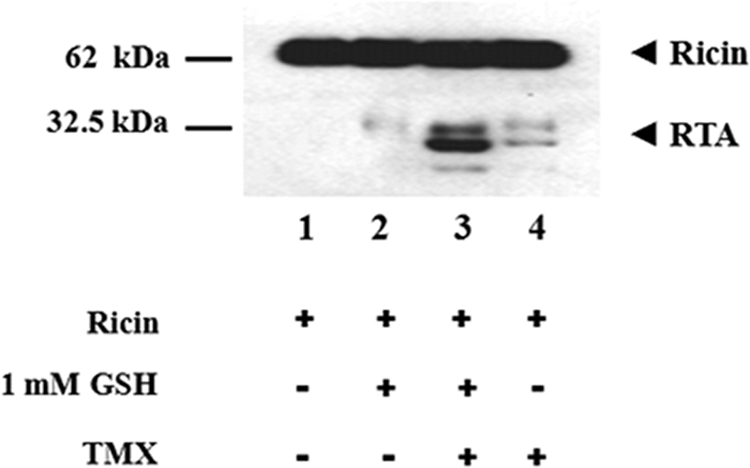
Glutathione can activate TMX in vitro. Samples with ricin (2 μg) were incubated for 60 min at 37 °C in the absence (lanes 1 and 2) or in the presence (lanes 3 and 4) of 5 μg of oxidized TMX (final volume of 50 μl). GSH (1 mm) was present in samples of lanes 2 and 3. Samples with ricin alone (lane 1) or without TMX only (lane 2) were included as controls. The reduction of ricin was evaluated by Western blotting as described.
TMX Can Reduce Ricin in Oxidizing Environment
As reported, the ER lumen is a more oxidizing environment than the cytosol (20). It has been demonstrated that to intoxicate the cell, ricin must be transported retrogradely to the ER lumen where following reduction the toxic domain RTA is translocated to the cytosol (5, 6). We therefore investigated whether TMX can reduce ricin under the redox buffer conditions present in the ER lumen. Ricin and TMX were therefore co-incubated in the presence of an oxidizing or reducing buffer by varying the GSH:GSSG ratio as follows: 1:3 GSH:GSSG (250 μm GSH:750 μm GSSG) for the oxidizing buffer or a 3:1 GSH:GSSG (750 μm GSH:250 μm GSSG) ratio for the reducing buffer.
As shown in Fig. 4 disulfide reductase activity of TMX appears to be evident in the presence of a 3:1 GSH:GSSG ratio (750 mm GSH:250 mm GSSG) in the redox buffer (lanes 2 and 4) both when TMX is oxidized and when it is pretreated with DTT. Conversely, in the presence of a 1:3 GSH:GSSG ratio (250 mm GSH:750 mm GSSG), the disulfide reductase activity of DTT-TMX is dramatically decreased, resulting in a RTA yield similar to that obtained in the presence of a buffer without glutathione (see Fig. 3, lane 4). It cannot be excluded, however, that the tiny band of reduced ricin appearing in lane 3 of Fig. 4, where TMX is used under fully oxidizing conditions, could be due to a minute amount of reduced TMX present in our preparation which has not undergone full oxidation. In conclusion, however, also considering that the GSH:GSSG ratio in the ER is thought to be comprised between 3:1 and 1:1 (20), TMX is capable of reducing ricin in an environment closely resembling that of the ER lumen (Fig. 4, lanes 2 and 4). In the absence of TMX, the incubation of ricin alone with 1:3 and 3:1 GSH:GSSG ratios did not produce a significant reduction of the toxin (data not shown).
FIGURE 4.
Reduction of ricin in an oxidizing environment. To check the ability of TMX to reduce ricin in different redox conditions we included in the buffer (0.1 m phosphate buffer) different GSSG:GSH ratios. To set up the oxidizing environment the samples were incubated with 750 μm GSSG and 250 μm GSH (lanes 1 and 3). To set up the reducing environment the samples were incubated with 250 μm GSSG and 750 μm GSH (lanes 2 and 4). The toxin was added to samples containing oxidized TMX (lanes 1 and 2) or DTT-prereduced TMX (lanes 3 and 4). After a 60-min incubation at 37 °C, the samples were analyzed for the yield of reduced ricin by Western blotting.
Expression and Activation of TMX in Vivo
TMX is a TGF-β up-regulated gene (7). Generally it is not known what modulates the presence of TMX in the ER, but it seems realistic to think that several environmental factors could influence the amount of the enzyme in the ER. Matsuo et al. (7) reported that the amount of enzyme in different tissues is variable. Therefore, the contribution of TMX to the reduction of ricin and of other A-B type molecules can be influenced by the cell type and the environmental conditions.
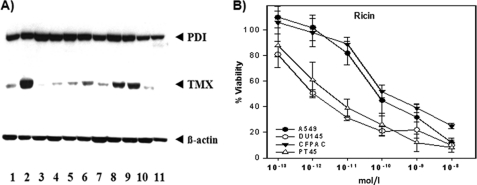
To analyze the relative in vivo relevance of TMX we investigated the expression of TMX and PDI by immunoblotting in different cancer cell lines. In all the cell lines tested we observed a rather constant expression of PDI whereas TMX was expressed at different levels, with a higher expression particularly in DU145 (prostate cancer), CFPAC-1 and PT45 (pancreatic cancer) cells, suggesting that its contribution to the reduction of A-B toxins and possibly of other macromolecular substrates might also be variable depending on the cell histotype (Fig. 5), sometimes possibly prevailing over other ER resident oxidoreductases.
FIGURE 5.
PDI and TMX expression and reduction of ricin in human cell lines. A, immunoblot identification of TMX and PDI in human cell lines extracts. Lane 1, A549 (respiratory epithelia); lane 2, DU145 (prostate cancer); lane 3, LNCaP (prostate cancer); lane 4, T3M4 (pancreatic cancer); lane 5, GER (pancreatic cancer); lane 6, PACA44 (pancreatic cancer); lane 7, MiaPaCa (pancreatic cancer); lane 8, CFPAC-1 (pancreatic cancer); lane 9, PT45 (pancreatic cancer); lane 10, Jurkat (T cell leukemia); lane 11, THP-1 (human monocyte). Human β-actin was used as a control to determine the relative amount of protein for each cell line. B, A549, DU145, PT45, and CFPAC-1 cells were incubated with ricin at various concentrations. The vitality was tested after 48 h by XTT assay as described above.
To test whether the relative amount of TMX influenced the reductive activation of ricin we selected four cell lines in the cytotoxic assay and exposed them to serial dilutions of ricin. As shown in Fig. 5, DU145 and PT45, the cell lines showing in the Western blot in Fig. 5A, the higher amount of TMX was more affected by ricin intoxication with respect to A549 and CFPAC-1. It should be noticed that even though also this last cell line showed a relatively high amount of TMX (Fig. 5, lane 8) its minor sensitivity to ricin was similar to A549 cells which showed a much lower TMX expression. This observation was not surprising; in fact the sensitivity of different cells populations to ricin intoxication is likely to be dependent on several different parameters (e.g. receptor type and abundance, variable presence of other intracellular components) so that a simple correlation of TMX expression with sensitivity to ricin cannot always be expected.
We then focused our attention in particular on DU145 cells. In fact, to test whether the major responsiveness to ricin in DU145 was a consequence, at least partially, of the high relative expression of TMX in this cell line, we tested whether its depletion by silencing might influence the sensitivity of the cell to the intoxication with ricin; TMX expression was drastically reduced by siRNA silencing in DU145 cells. As controls we used the empty vector or the vector containing a no targeting shRNA sequence. After exposure for 48 h to ricin we observed a rightward shift in the curve of cytotoxicity (Fig. 6A), indicating a considerable loss of sensitivity to ricin intoxication. In fact, as reported in the histograms in Fig. 6B the TMX-silenced cells were approximately 10-fold less sensitive to ricin with respect to the control cells.
FIGURE 6.
Reduction of ricin in TMX-silenced DU145 cells. A, DU145 control cells (empty vector), or DU145 cells transfected with a vector carrying a shRNA no targeting or a TMX targeting sequence were exposed to various concentrations of ricin, and their viability was assessed 48 h after toxin addition by XTT assays. B, histogram of the IC50 value obtained from the cytotoxicity plots reported in A after treatment of the cells with ricin. *, p < 0.01; ns, not significant (Student's t test). C, lysates from TMX knockdown cells (lane 1) and empty vector (lane 2) or no targeting shRNA-transfected cells (lane 3) were analyzed by immunoblotting for PDI, TMX, and human β-actin.
CONCLUSIONS
TMX is an oxidoreductase capable of reducing efficiently disulfide bonds. Moreover, TMX is localized in the ER membrane in association with calnexin (10); therefore, it can be hypothesized that this may increase the chances of fortuitously encountering incoming ricin molecules which are retrogradely transported to the ER by a process also entailing binding to calnexin and possibly to other intracellular structures and associated protein components (4, 8). In the present work we found that ricin- and abrin-dependent cytotoxicity are enhanced in TMX-overexpressing cells (50-fold in the presence of ricin and 5-fold in the case of abrin), whereas the toxicity of other toxins such as saporin and PE is not influenced by TMX overexpression. This also ruled out that the augmented amount of TMX found in the ER lumen of TMX transfectants (data not shown) could have generated a nonspecific sensitivity of the treated cells to toxins in general but rather reflects the direct involvement of TMX in the reductive activation of the type 2 RIPs used in this work. Conversely, by silencing TMX in DU145 cells we observed an augmented resistance of these cells to ricin intoxication, further indicating that this enzyme might be effectively involved in the reductive activation of ricin inside the cell.
We observed that TMX can be highly expressed in certain cell types, and the observation that the catalytic site of the enzyme is present in its reduced form under physiological conditions (10) supports the notion that TMX may indeed have a relevant role in the reduction of ricin and possibly of other A-B type substrates in vivo. It must be considered, however, that the reductive activation of ricin taking place along cell intoxication pathways might be the result of the recruitment of several different oxidoreductases that can be sequentially or alternatively involved in this process. Thus, ricin entering the ER lumen could interact with a complex of enzymes, leading to the reduction event operated by a membrane associated oxidoreductase, possibly TMX. Following reduction, ricin may interact with chaperones, such as PDI, recognizing the unfolded subunits arising during the translocation step and assisting further dislocation events exploiting the ER-associated protein degradation pathway that will ultimately lead to the dislocation of RTA to the cell cytosol.
The demonstration that ricin interacts with calnexin but not with calreticulin upon entering the ER (5) and also that TMX binds calnexin during biosynthesis of the MHC class I heavy chain (10) may establish a direct link among TMX, ricin reduction, and RTA dislocation in the cytosol through the intervention of the chaperone molecule calnexin. The in vivo function and catalytic specificity of most cellular oxidoreductases are unknown.
Our study proves that functional redundancy exists in vivo among the several ER oxidoreductases. In fact, for both PDI (5, 6) and TMX (this article) a similar reductive activity was observed in the case of ricin, further suggesting that other oxidoreductases might also be involved in this process. We found that TMX expression is variable in different cancer cell lines, and at least in DU145 cells, the up-regulation of TMX might be responsible for a major increase in sensitivity of the cell to ricin and abrin.
TMX can be up-regulated by the presence in the microenvironment of TGF-β, which becomes available in high concentrations during immune responses and inflammation. Thus, the relative impact of TMX as an intracellular activator of toxins, therapeutic antibody-toxin conjugates, or of virus-associated structures might be greatly enhanced with respect to other intracellular and ER-associated oxidoreductases. Understanding the role and properties of individual components of the oxidoreductases system of the cell may impact also in other areas of cell biology. In fact, the mechanism of type 2 RIPs taking place in the ER may be relevant also for virus assembly and entry, a process that requires the intracellular reduction of crucial protein components of the virus (20). Thus, the results reported in the present paper may have general implications.
Acknowledgments
We thank Dr. J. Yodoi for kindly supplying TMX plasmids, Dr. A. Bolognesi for the toxins abrin and saporin, and Dr. G. Bellisola (Azienda Ospedaliera Universitaria Integrata di Verona) for critical reading of the manuscript and helpful discussions.
This work was supported in part by grants from Fondazione Cariverona (Progetti Bando 2007), by the Associazione delle Biotecnologie in Oncologia (ABO) Foundation, and by Associazione Italiana per la Ricerca sul Cancro (AIRC) Regione Veneto (to M. C.).
- RIP
- ribosome-inactivating protein
- ER
- endoplasmic reticulum
- PDI
- protein disulfide isomerase
- PE
- Pseudomonas endotoxin A
- RTA
- ricin toxin A chain
- RTB
- ricin toxin B chain
- TMX
- thioredoxin-like transmembrane
- XTT
- 2,3-bis-(2-methoxy-4nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide.
REFERENCES
- 1. Barbieri L., Battelli M. G., Stirpe F. (1993) Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta 1154, 237–282 [DOI] [PubMed] [Google Scholar]
- 2. Girbes T., Ferreras J. M., Iglesias R., Citores L., De Torre C., Carbajales M. L., Jimènez P., De Benito F. M., Muñoz R. (1996) Recent advances in the uses and applications of ribosome-inactivating proteins from plants. Cell Mol. Biol. 42, 461–471 [PubMed] [Google Scholar]
- 3. Olsnes S. (2004) The history of ricin, abrin and related toxins. Toxicon 44, 361–370 [DOI] [PubMed] [Google Scholar]
- 4. Sandvig K., Grimmer S., Lauvrak S. U., Torgersen M. L., Skretting G., van Deurs B., Iversen T. G. (2002) Pathways followed by ricin and Shiga toxin into cells. Histochem. Cell Biol. 117, 131–141 [DOI] [PubMed] [Google Scholar]
- 5. Spooner R. A., Watson P. D., Marsden C. J., Smith D. C., Moore K. A., Cook J. P., Lord J. M., Roberts L. M. (2004) Protein disulfide-isomerase reduces ricin to its A and B chains in the endoplasmic reticulum. Biochem. J. 383, 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bellisola G., Fracasso G., Ippoliti R., Menestrina G., Rosén A., Soldà S., Udali S., Tomazzolli R., Tridente G., Colombatti M. (2004) Reductive activation of ricin and ricinA-chain immunotoxins by protein disulfide isomerase and thioredoxin reductase. Biochem. Pharmacol. 67, 1721–1731 [DOI] [PubMed] [Google Scholar]
- 7. Matsuo Y., Akiyama N., Nakamura H., Yodoi J., Noda M., Kizaka-Kondoh S. (2001) Identification of a novel thioredoxin-related transmembrane protein. J. Biol. Chem. 276, 10032–10038 [DOI] [PubMed] [Google Scholar]
- 8. Matsuo Y., Nishinaka Y., Suzuki S., Kojima M., Kizaka-Kondoh S., Kondo N., Son A., Sakakura-Nishiyama J., Yamaguchi Y., Masutani H., Ishii Y., Yodoi J. (2004) TMX, a transmembrane oxido reductase of the thioredoxin family: the possible role in disulfide-linked protein folding in the endoplasmic reticulum. Arch. Biochem. Biophys. 423, 81–87 [DOI] [PubMed] [Google Scholar]
- 9. Ellgaard L., Ruddock L. W. (2005) The human protein disulphide isomerase family: substrate interaction and functional properties. EMBO Rep. 6, 28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsuo Y., Masutani H., Son A., Kizaka-Kondoh S., Yodoi J. (2009) Physical and functional interaction of transmembrane thioredoxin-related protein with major histocompatibility complex class I heavy chain: redox-based protein quality control and its potential relevance to immune responses. Mol. Biol. Cell 20, 4552–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nicolson G. L., Blaustein J. (1972) The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim. Biophys. Acta 266, 543–547 [DOI] [PubMed] [Google Scholar]
- 12. Lombardi A., Bursomanno S., Lopardo T., Traini R., Colombatti M., Ippoliti R., Flavell D. J., Flavell S. U., Ceriotti A., Fabbrini M. S. (2010) Pichia pastoris as a host for secretion of toxic saporin chimeras. FASEB J. 24, 253–265 [DOI] [PubMed] [Google Scholar]
- 13. Morlon-Guyot J., Méré J., Bonhoure A., Beaumelle B. (2009) Processing of Pseudomonas aeruginasa exotoxin A is dispensable for cell intoxication. Infect. Immun. 77, 3090–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKee M. L., FitzGerald D. J. (1999) Reduction of furin-nicked Pseudomonas exotoxin A: an unfolding story. Biochemistry 38, 16507–16513 [DOI] [PubMed] [Google Scholar]
- 15. Rapak A., Falnes P. O., Olsnes S. (1997) Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc. Natl. Acad. Sci. U.S.A. 94, 3783–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis M. S., Youle R. J. (1986) Ricin subunit association: thermodynamics and the role of the disulfide bond in toxicity. J. Biol. Chem. 261, 11571–11577 [PubMed] [Google Scholar]
- 17. Tavender T. J., Bulleid N. J. (2010) Molecular mechanisms regulating oxidative activity of the Ero1 family in the endoplasmic reticulum. Antioxid. Redox Signal. 13, 1177–1187 [DOI] [PubMed] [Google Scholar]
- 18. Jessop C. E., Bulleid N. J. (2004) Glutathione directly reduces an oxidoreductase in the endoplasmic reticulum of mammalian cells. J. Biol. Chem. 279, 55341–55347 [DOI] [PubMed] [Google Scholar]
- 19. Lord J. M., Roberts L. M., Lencer W. I. (2005) Entry of protein toxins into mammalian cells by crossing the endoplasmic reticulum membrane: co-opting basic mechanisms of endoplasmic reticulum-associated degradation. Curr. Top. Microbiol. Immunol. 300, 149–168 [DOI] [PubMed] [Google Scholar]
- 20. Holmgren A. (1979) Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J. Biol. Chem. 254, 9627–9632 [PubMed] [Google Scholar]