Background: RVFV is a cytoplasmic replicating RNA virus that infects both humans and livestock.
Results: DNA damage signaling and cell cycle arrest are induced following RVFV infection.
Conclusion: The ATM signaling pathway and cell cycle arrest are important for RVFV replication.
Significance: Studying virally induced signaling pathways is important for host-based therapeutic design and understanding host-pathogen interactions.
Keywords: Cell cycle, Checkpoint control, DNA damage response, Negative-strand RNA viruses, p53, ATM, Chk.2, Rift Valley fever virus
Abstract
Rift Valley fever virus (RVFV) is a highly pathogenic arthropod-borne virus infecting a wide range of vertebrate hosts. Of particular interest is the nonstructural NSs protein, which forms large filamentous fibril bundles in the nucleus. Past studies have shown NSs to be a multifaceted protein important for virulence through modulation of the interferon response as well acting as a general inhibitor of transcription. Here we investigated the regulation of the DNA damage signaling cascades by RVFV infection and found virally inducted phosphorylation of the classical DNA damage signaling proteins, ataxia-telangiectasia mutated (ATM) (Ser-1981), Chk.2 (Thr-68), H2A.X (Ser-139), and p53 (Ser-15). In contrast, ataxia-telangiectasia mutated and Rad3-related kinase (ATR) (Ser-428) phosphorylation was decreased following RVFV infection. Importantly, both the attenuated vaccine strain MP12 and the fully virulent strain ZH548 showed strong parallels in their up-regulation of the ATM arm of the DNA damage response and in the down-regulation of the ATR pathway. The increase in DNA damage signaling proteins did not result from gross DNA damage as no increase in DNA damage was observed following infection. Rather the DNA damage signaling was found to be dependent on the viral protein NSs, as an NSs mutant virus was not found to induce the equivalent signaling pathways. RVFV MP12-infected cells also displayed an S phase arrest that was found to be dependent on NSs expression. Use of ATM and Chk.2 inhibitors resulted in a marked decrease in S phase arrest as well as viral production. These results indicate that RVFV NSs induces DNA damage signaling pathways that are beneficial for viral replication.
Introduction
Rift Valley fever virus (RVFV)2 (genus Phlebovirus, family Bunyaviridae) is an arthropod-borne virus capable of devastating livestock populations with cyclical epidemics occurring throughout much of sub-Saharan Africa (1). Recent outbreaks have resulted in significant human mortality rates and an increased geographic footprint, escaping continental Africa into Mozambique, Saudi Arabia, and Yemen, demonstrating its capacity to cross significant geographical barriers and emerge into new regions (2). Because of its increasing spread, host susceptibility, vector plasticity, and ease of aerosolization, RVFV has been listed as an emerging infectious disease and a category A select agent by the Centers for Disease Control and Prevention. There is very real concern of its possible spread into Europe and the United States (2). Despite being recognized as an emerging threat, the development of efficacious therapeutics against RVFV is hampered in part by limited knowledge of the mechanisms underlying pathogenesis at the molecular level. Currently there are no United States Food and Drug Administration-licensed vaccines or therapeutics for RVFV, presenting an urgent need to develop a greater understanding of viral replication pathways and host cell-related pathogenesis to develop novel and targeted antiviral therapeutics.
RVFV contains a tripartite single-stranded RNA genome composed of the L, M, and S segments encoding viral polymerase (L), two glycoproteins (M), and the viral nucleocapsid (S). Additionally, two nonstructural proteins, NSm1 and NSm2, are encoded by the (M) segment, and the nonstructural NSs protein is encoded on the (S) segment (3). Of particular interest is the nonstructural protein NSs, which although dispensable for replication is important for virulence (4–6). NSs is known to suppress transcription of host mRNA through interactions with the transcription factor IIH subunit p44 (7). Recently the p62 subunit of transcription factor IIH has been shown to be degraded following RVFV infection through interaction with NSs (8); this could contribute to transcriptional inhibition. NSs is also an interferon antagonist that potently and rapidly inhibits interferon stimulation through alteration of transcription and chromatin remodeling factors (4, 9–13). Le May et al. (12) provided evidence that NSs is able to inhibit IFN-β induction through binding to SAP30 (an mSIN3A-associated protein), and it recruits a number of repressive complexes to the IFN-β promoter, including mSIN3A, nuclear receptor corepressor, and HDAC3. In addition, histone H4K8 and histone H3K18 are known to be deacetylated in the presence of NSs, resulting in a repressed chromatin structure. NSs has also been shown to induce the degradation of protein kinase R (9–11), adding another layer of complexity to its ability to suppress the interferon response. NSs is unique among bunyaviruses in its ability to form filamentous structures in the nucleus. Although NSs filaments appear to be mostly separate from cellular DNA, there is strong evidence showing heterochromatin satellite clusters intimately associated with NSs filaments, likely resulting in a high incidence of nuclear anomalies and known chromosome cohesion and segregation defects (14).
Viruses have naturally evolved elegant strategies to manipulate the host's cellular machinery. Interaction of virus-encoded proteins with host cells plays an important role in viral infection and consequential pathogenesis, often working to bypass traditional defenses such as the interferon response and apoptosis. More recent studies have shown that this antiviral arsenal also includes highly conserved cellular DNA damage response (DDR) mechanisms. Viruses have developed ways to inhibit or circumvent the host's responses as well as methods to hijack cellular DNA repair proteins to aid in their own replication (15, 16). Many DNA viruses and retroviruses induce DNA damage through tethering or integration of their genome into the host DNA (16). In addition, it has been suggested that cellular DNA repair mechanisms are able to recognize nuclear viral genetic material as damage (17). Interestingly, a small number of RNA viruses that replicate exclusively in the cytoplasm have been shown to induce a DDR, including hepatitis C virus (HCV) and La Crosse virus (18, 19). HCV induces the DDR through the viral proteins E6 and E7, producing an environment conducive to viral genomic replication through cell cycle arrest by activation of the DDR (20). HCV has also been shown to cause DNA double strand breaks through its two viral proteins E1 and NS3 through induction of reactive oxygen species (21). The DNA damage response for La Crosse virus is less well understood. It involves the phosphorylation of histone H2A.X and the proteasomal degradation of RNA polymerase II, lending itself to a transcriptional stress response model (19). Interestingly, La Crosse virus is a bunyavirus similar to RVFV, and although its NSs protein is not filamentous in nature, it appears to be involved in the observed response. Elucidating the mechanism by which the DDR is activated for RVFV will allow us to gain greater insight into how DDR pathways are modulated following RNA viral infections.
Our previous studies indicated that p53 was phosphorylated at Ser-15 and Ser-46 following infection with RVFV ZH501 (22). p53 phosphorylation is important for many cellular processes, including apoptosis and DNA damage signaling. In addition, our recently published data indicate that RVFV infection induces oxidative stress (23), which can also influence this signaling pathway (24). Based on these data, we hypothesized that DNA damage signaling was being induced upon RVFV infection. Here we show that multiple DNA damage signaling checkpoint proteins are phosphorylated following RVFV infection, including ataxia-telangiectasia mutated (ATM), Chk.2, H2A.X, and p53 (Ser-15). In addition, S phase arrest was observed following RVFV infection. Both the DNA damage signaling and the concurrent S phase arrest were dependent on the expression of RVFV NSs. Use of specific checkpoint inhibitors (ATM and Chk.2 inhibitors) resulted in a marked decrease in S phase arrest as well viral production. These findings indicate that RVFV NSs induces the ATM signaling arm of the DDR and that this pathway is beneficial for viral replication.
EXPERIMENTAL PROCEDURES
Cell Culture, Viral Infection, and Extract Preparation
Human small airway lung epithelial cells (HSAECs) (Cambrex Inc., Walkersville, MD) from an anonymous donor were grown in Ham's F-12 medium according to the vendor's protocol. Ham's F-12 was supplemented with 1% penicillin/streptomycin, 1% Glutamax, 1% nonessential amino acids, 1% sodium pyruvate, 0.001% β-mercaptoethanol (1000×), and 10% FBS. Vero cells were grown in DMEM supplemented with 10% FBS, 1% l-glutamine, and 1% penicillin/streptomycin. For experiments using RVFV MP12 strain, 106 HSAECs were cultured in 6-well plates. Cells were infected with MP12 or the MP12 NSs mutants (ΔNSs) at an m.o.i. of 3.0. For infection, the growth medium was removed, and cells were washed with phosphate-buffered saline (PBS without calcium and magnesium), overlaid with a 400-μl suspension of virus in medium, and incubated for an hour at 37 °C at 5% CO2. Following 1-h incubation at 37 °C, infectious supernatant was removed, cells were washed with PBS, 2 ml of supplemented Ham's F-12 culture medium were added to each well, and the cells were maintained at 37 °C. Experiments involving Clone 13 and recombinant ZH548 (rZH548) virus were performed in Vero or A549 cells. Cells were grown in 6-well plates and seeded at 2.5 × 105. Lysis buffer used for Western blot collection and analysis consisted of a 1:1 mixture of T-PER reagent (Pierce) and 2× Tris-glycine SDS sample buffer (Novex, Invitrogen), 33 mm DTT, and protease and phosphatase inhibitor mixture (1× Halt mixture, Pierce). Cells were collected directly in lysis buffer and boiled for 10 min.
Drug Treatments and Plaque Assay
HSAECs were seeded at 5 × 104 in a 96-well plate and pretreated for 2 h with DMSO, ATM, or Chk.2 kinase inhibitors (10 μm) in growth medium. ATM inhibitor 2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one and Chk.2 inhibitor 2-(4-(4-chlorophenoxy)phenyl)-1H-benzimidazole-5-carboxamide were purchased from EMD4Biosciences. Cultured cells were infected at an m.o.i. of 1 as previously described and then reintroduced to supplemented growth medium with 10 μm concentrations. Supernatants were collected at 24 h postinfection and analyzed by plaque assays. For plaque assays, Vero cells were plated in 6-well plates at 106 to achieve 90–100% confluence (two wells per sample). Samples were diluted in growth medium from 101 to 108, and infections were then carried for each dilution in duplicate as described. After a 1-h infection, an overlay of 3 ml of a 1:1 solution of 0.6% agarose in distilled H2O with 2× Eagle's minimal essential medium containing 2.5% FBS, 1% l-glutamine, 2% penicillin/streptomycin, 1% nonessential amino acids, and 1% sodium pyruvate was added to each well, allowed to solidify, and incubated at 37 °C at 5% CO2 for 72 h. After 72 h, cells were fixed using 10% formaldehyde for 1 h at room temperature, agar plugs were discarded, and fixed cellular monolayers were stained with a 1% crystal violet, 20% methanol solution for 30 min to allow visualization of plaques. Averages were taken from duplicates with dilutions containing fewer than five or more than 100 plaques being discounted.
Western Blot Analysis
Twenty-five to 30 μl of cell lysates were separated on 10-well 4–12% Bis-Tris gels and transferred either to nitrocellulose membranes using an iBlot gel transfer apparatus (Invitrogen) or to PVDF by overnight wet transfer. The membranes were blocked with a boiled 3% dry milk solution in PBS-Tween 20 for an hour at room temperature. Membranes were probed with antibodies from a DNA Damage Antibody Sampler kit (catalog number 9947, Cell Signaling Technology), anti-RVFV nucleoprotein (N), anti-RVFV Gn, or HRP-conjugated actin (catalog number ab49900-100, Abcam) diluted in 3% milk solution at 1:1000 and incubated overnight at 4 °C. The blots were then washed three times with PBS-Tween 20 and incubated with secondary HRP-coupled goat anti-rabbit and anti-mouse antibodies diluted 1:10,000 in 3% milk. The blots were visualized by chemiluminescence using a SuperSignal West Femto Maximum Sensitivity Substrate kit (ThermoScientific) and the Molecular Imager ChemiDoc XRS system (Bio-Rad).
Immunofluorescent Staining
HeLa cells were grown and treated on coverslips in a 6-well plate, fixed with 4% formaldehyde, permeabilized with 0.5% Triton X-100 in 1× PBS (without calcium and magnesium), blocked in 3% BSA, and incubated with primary antibody in blocking buffer for 1 h at 37 °C using antibodies as described above. After incubation, cells were washed three times in PBS plus 300 mm NaCl and 0.1% Triton X-100 for 3 min. Secondary antibody Alexa Fluor 568 donkey anti-rabbit 1:200 (Invitrogen) was incubated using as described above, then washed, and stained with DAPI to visualize nuclei. Comet assays were performed using a comet assay kit according to the manufacturer's directions (catalog number 4250-050-K, Trevigen). Fluorescence microscopy was carried out using a Nikon Eclipse 90i microscope.
Flow Cytometry
Cells were infected, prepared, and treated in 6- or 12-well plates. For collection, the cells were washed in 1× PBS (without calcium and magnesium) and trypsinized. The trypsin was neutralized by adding back cold medium, cells were collected, spun down, washed twice in 1× PBS (without calcium and magnesium), and resuspended in 70% ice-cold ethanol. The cells were then rehydrated using 1× PBS (without calcium and magnesium) for at least 15 min and spun down as described. The cells were stained with 1 ml of propidium iodide staining solution, and cell cycle analysis was performed on an Accuri C6 flow cytometer using CFlow Plus from Accuri Cytometers Inc. Data analysis was performed with Multicycle AV and FCS Express. Bromodeoxyuridine (BrdU) staining was performed using a BD Pharmingen FITC BrdU Flow kit according to the manufacturer's instructions. In cell cycle experiments, A549 cells were serum-starved for 48 h, treated, and infected as described, released into full growth medium postinfection, and collected 24 h postinfection. Cells were stained as described. Experiments using A549 cells were analyzed using a Guava EasyCyte MINI flow cytometer.
Statistical Analyses
All quantifications are based on data obtained from triplicate samples unless indicated otherwise. Error bars in all figures indicate standard deviations (S.D.). p values were calculated using unpaired Student's t test.
RESULTS
DNA Damage Signaling Is Induced following RVFV MP12 Infection
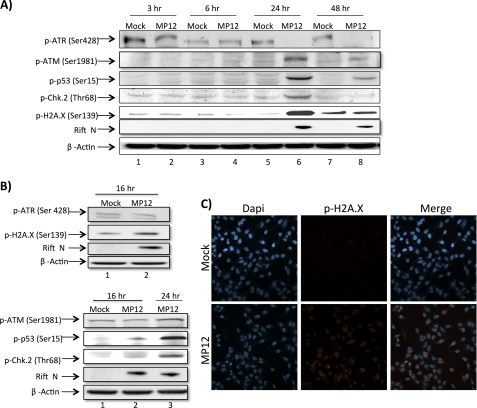
Many protein-protein interactions are regulated through phosphorylation events as phosphorylation plays a significant role in a wide range of cellular processes, including the antiviral response. Recently we conducted a high throughput proteomics study following RVFV infection using reverse phase protein microarrays (22). In this study, we found that p53 phosphorylation (both Ser-15 and Ser-46) is highly up-regulated following infection. p53-dependent transcriptional events are wide ranging and numerous in their role as cell regulators and have been implicated in numerous DNA damage signaling pathways (25). In addition, our recently published data indicate that RVFV infection induces oxidative stress (23). Reactive oxygen species are well known cellular messengers that play a key role in antimicrobial and antitumor defense and have also been shown to influence the DNA damage signaling pathways (24). Based on these data along with the well established role NSs plays in nuclear filament formation, we hypothesized that DNA damage signaling was being induced upon RVFV infection. To further explore this hypothesis, we initially analyzed a number of classical DNA damage signaling components following RVFV infection through immunoblot analysis (Fig. 1, A and B). Beginning at 16 h postinfection, we observed phosphorylation of ATM, p53, Chk.2, and H2A.X. However, the phosphorylation was much more pronounced at 24 h postinfection. In contrast, ataxia-telangiectasia mutated and Rad3-related kinase (ATR) was not phosphorylated following RVFV infection; but rather its phosphorylation decreased in an antiparallel sense. These data suggest that the ATM pathway is activated as p53 (Ser-15), Chk.2 (Thr-68), and H2A.X (Ser-139) are all substrates of the ATM arm in the classical DNA damage signaling pathway. We further confirmed the phosphorylation of H2A.X following RVFV infection through confocal fluorescence microscopy. After phosphorylation, H2A.X is known to form distinct nuclear puncta (DNA damage foci), which we detected at 24 h postinfection (Fig. 1C). Collectively, these results indicate that DNA damage signaling, specifically the ATM pathway, is induced following RVFV infection.
FIGURE 1.
DNA damage signaling is induced following RVFV MP12 infection. A, HSAECs were mock-infected or infected at an m.o.i. of 3.0 with MP12 and collected at 3, 6, 24, and 48 h postinfection. Whole cell protein lysates were separated by SDS-PAGE and examined by Western blot analysis utilizing anti-p-ATR (Ser-428), anti-p-ATM (Ser-1981), anti-p-p53 (Ser-15), anti-p-Chk.2 (Thr-68), anti-p-H2A.X (Ser-139), anti-RVFV N protein, and anti-β-actin antibodies. B, HSAECs were treated as in A and collected at 16 h postinfection. C, HeLa cells were mock-infected or infected at an m.o.i. of 3.0 with MP12 and collected 24 h postinfection for immunofluorescent staining with anti-p-H2A.X (Ser-139) primary antibody and an Alexa Fluor 568 secondary antibody. Nuclear staining was detected utilizing DAPI.
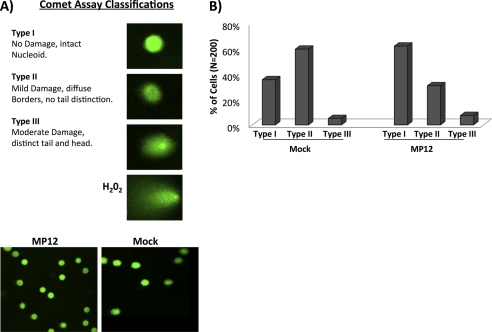
DNA Damage Is Not Increased in RVFV MP12-infected Cells
As activation of the DNA damage response was observed, we sought to determine whether DNA damage was occurring following RVFV infection. It is plausible that host DNA is not directly damaged but rather that the cell is under exogenous stress, which leads to the activation of the DDR pathway. Comet assays were performed to determine whether DNA damage occurred following RVFV infection. The comet assay is a sensitive technique that allows the detection of DNA damage induced by double strand breaks, single strand breaks, alkali-labile sites, oxidative base damage, and DNA cross-linking with DNA or protein. The “head” of the comet is intact DNA, whereas the “tail” is the damaged DNA. Cells were classified as being Type I (minimal/no damage, intact nucleoid) Type II (mild damage, diffuse borders, no tail distinction), or Type III (moderate damage, distinct tail and head) (Fig. 2A). Interestingly, RVFV-infected cells did not display an increase in DNA damage and actually appear to have less Type II damage than mock-infected cells (Fig. 2B). One potential explanation is that the DDR pathway is being specifically targeted and hijacked irrespective of actual DNA damage with activation actually helping to repair any minor DNA damage that is naturally occurring, whereas the mock-infected cells lacking viral DDR activation show typical and minor endogenous and exogenous damage. These results demonstrate that no gross alterations in chromosomal DNA damage were observed in RVFV-infected cells; however, it may be possible that small nicks/breaks on transcriptionally active sites are present.
FIGURE 2.
DNA damage is not increased in RVFV MP12-infected cells. A, HSAECs were infected at an m.o.i. of 4 with MP12 and collected 24 h postinfection. Comet assays were performed according to the manufacturer's instructions (Trevigen comet assay kit). H2O2 was utilized as a positive control for DNA damage. Examples of the different classifications of comets are shown as well as representative frames of mock- and MP12-infected cells. B, over 200 comets were randomly selected for each condition from various slides, avoiding the edges and damaged parts of the gel as well as dead cells (comets without a distinct “comet head”) and the superimposed comets. The averages from two separate experiments were combined and classified as Type I (no damage, intact nucleoid) Type II (mild damage, diffuse borders, no tail distinction), and Type III (moderate damage, distinct tail and head).
Cells Are Arrested in S Phase following RVFV MP12 Infection
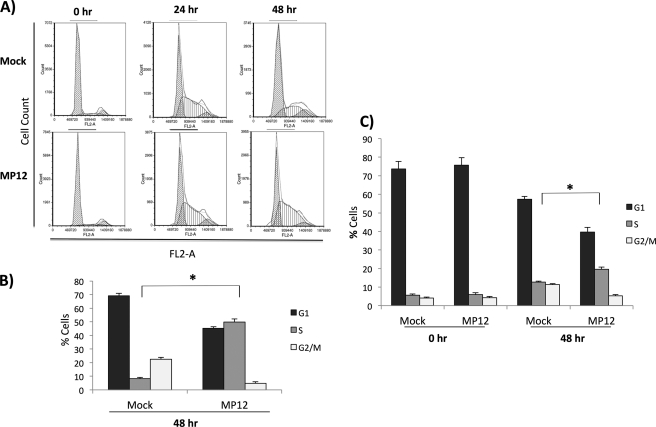
Cell cycle checkpoint arrest is one of the major downstream events that occur following induction of the DDR pathway with numerous viruses known to exploit the cell cycle to promote a favorable pathogen environment. We were interested in determining whether RVFV infection resulted in cell cycle arrest due to activation of the ATM pathway. To this end, cells were serum-starved for 3 days and mock-infected or infected with MP12. Following infection, cells were released into complete medium and collected at 0, 24, and 48 h postinfection. Cells were stained with propidium iodine (PI) and processed for cell cycle analysis. Fig. 3A displays representative histograms with collected events representing cell numbers displayed on the y axis (Count) and fluorescence from the PI stain representing DNA content displayed on the x axis (FL2-A). Results indicate that at 0 h the serum-starved cells have arrested as the majority of the cells remain in the G1/G0 peak (greater than 80%). At 24 h, both mock- and MP12-infected cells have a large S phase population. However, at 48 h, mock-infected cells have recovered and have a classical cell cycle distribution, whereas MP12-infected cells display a significant S phase arrest that is quantitated in Fig. 3B. BrdU analysis was performed to further confirm the S phase arrest. BrdU is a thymidine analog that is incorporated into DNA during replication. Indeed, BrdU analysis confirmed that RVFV MP12-infected cells were arrested in S phase (Fig. 3C). We also observed S phase arrest following MP12 infection in A549 cells (data not shown). These results indicate that RVFV MP12 infection induces an S phase arrest.
FIGURE 3.
Cells are arrested in S phase following RVFV MP12 infection. A, HSAECs were serum-starved for 3 days and infected with MP12 (m.o.i., 3.0). Following infection, cells were released into full medium (containing 10% FBS). Cells were collected at 0, 24, and 48 h postrelease. Cell cycle analysis was performed with PI staining on an Accuri C6 flow cytometer using CFlow Plus from Accuri Cytometers Inc. Shown are representative histograms with collected events displayed on the y axis (Count) and fluorescence from the PI stain displayed on the x axis (FL2-A). Data analysis was performed with Multicycle AV and FCS Express. B, quantitation of 0- and 48-h data displayed in A. *, p value <0.01. C, HSAECs were serum-starved and infected as in A and collected at 0 and 48 h postinfection for further cell cycle analysis using a BD Pharmingen FITC BrdU Flow kit according to the manufacturer's instructions, and cell cycle analysis was performed as described. Quantitation of 0- and 48-h data is displayed. *, p value <0.01. Error bars indicate S.D.
DNA Damage Response Is Dependent on NSs
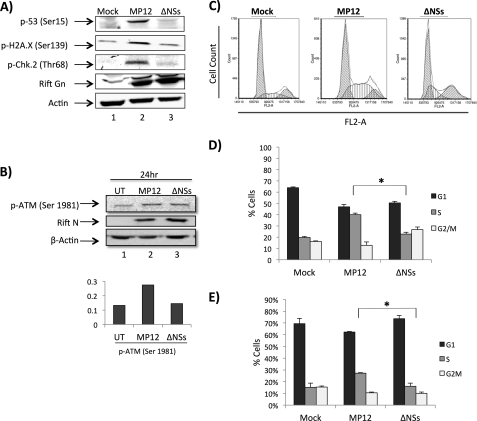
NSs is a major RVFV virulence factor that forms distinct filaments in the nucleus. Although NSs filaments appear to be mostly separate from cellular DNA, there is strong evidence that heterochromatin satellite clusters are intimately associated with NSs filaments, likely resulting in a high incidence of nuclear anomalies and known chromosome cohesion and segregation defects (14). Thus, the influence of NSs on the activation of the major DNA damage signaling checkpoints was assessed. Based on our data from Fig. 1 suggesting an ATM mode of activation, we examined phosphorylation of ATM (Ser-1981), p53 (Ser-15), Chk.2 (Thr-68), and H2A.X (Ser-139) at 24 h postinfection using a ΔNSs strain in parallel with mock and MP12 infections. Western blot analysis demonstrated a severe reduction of phosphorylation at all sites following infection with the ΔNSs strain in comparison with the observed increase in phosphorylation for the MP12 infection (Fig. 4, A and B). These results demonstrate a specific and significant NSs dependence in the viral activation of the DNA damage response.
FIGURE 4.
DNA damage response and cell cycle arrest are dependent on NSs. A, Vero cells were mock-infected or infected at an m.o.i. of 3 with MP12 or MP12 ΔNSs and collected 24 h postinfection. Whole cell protein lysates were examined by Western blotting for changes in p-p53 (Ser-15), p-H2A.X (Ser-139), p-Chk.2 (Thr-68), RVFV Gn, and β-actin. B, Vero cells were treated as in A and collected for Western blotting using p-ATM (Ser-1981), RVFV N protein, and β-actin 24 h postinfection. Quantitation of p-ATM (Ser-1981) was performed and normalized to actin in UT, untreated. C, HSAECs were serum-starved for 3 days and infected with MP12 (m.o.i., 1.0). Following infection, cells were released into full medium (containing 10% FBS) and collected at 48 h post release. Cell cycle analysis was performed with PI staining on an Accuri C6 flow cytometer using CFlow Plus from Accuri Cytometers Inc. Data analysis was performed with Multicycle AV and FCS Express. D, quantitation of data displayed in C. *, p value <0.01. E, HSAECs were serum-starved and infected as in C and collected at 48 h postinfection for further cell cycle analysis using a BD Pharmingen FITC BrdU Flow kit according to the manufacturer's instructions, and cell cycle analysis was performed as described. Quantitation of 48-h data is displayed. *, p value <0.01. Error bars indicate S.D.
Having determined that the DDR pathway is being activated by MP12 in an NSs-dependent manner, we wanted to see whether S phase arrest was also NSs-dependent. To this end, cells were serum-starved cells for 3 days and mock-infected or infected either with MP12 or ΔNSs at an m.o.i. of 1.0. Following infection, cells were released into complete medium and collected at 48 h postinfection. Cells under similar conditions were either stained with PI or BrdU-treated and processed for cell cycle analysis. At 48 h postinfection, the S phase population was markedly increased in MP12-infected cells as compared with both mock- and ΔNSs-infected cells (Fig. 4, C, D, and E). These experiments indicate that S phase arrest is specifically activated by RVFV MP12 in an NSs-dependent manner.
ATM and Chk.2 Inhibitors Rescue MP12-infected Cells from S Phase Arrest
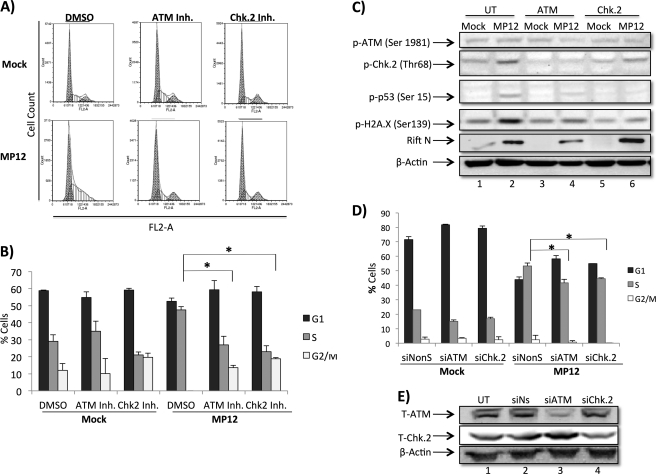
We next sought to determine whether ATM and Chk.2 activity directly contributed to the observed MP12-induced S phase arrest. HSAECs were serum-starved for 2 days, treated with chemical inhibitors, infected with MP12 (m.o.i., 1.0), serum-starved for an additional 24 h, then released into complete medium for an additional 20 h, and collected. Following collection, cells were stained with PI, and cell cycle analysis was performed. RVFV infection resulted in the expected S phase arrest in untreated cells (Fig. 5A), whereas cells that were treated with ATM or Chk.2 inhibitors did not display S phase arrest but rather displayed a cell cycle distribution similar to that in mock-infected cells. The reversal of the S phase arrest by both ATM and Chk.2 inhibitors was shown to be statistically significant (Fig. 5B). As a control, phosphorylation of Chk.2, p53, and H2A.X was evaluated following ATM and Chk.2 inhibitor treatment. Results in Fig. 5C indicate that ATM and Chk.2 inhibitor treatment efficiently decreased the levels of Chk.2, p53, and H2A.X phosphorylation. Experiments were also performed with siRNA against ATM and Chk.2 to confirm the involvement of ATM and Chk.2 in the MP12-induced S phase arrest. Both ATM and Chk.2 siRNA knockdown resulted in a statistically significant reduction of MP12-induced S phase arrest (Fig. 5D). Confirmatory Western blots of ATM and Chk.2 levels following siRNA transfection are shown in Fig. 5E where total ATM and Chk.2 were reduced by at least 50%. It is possible that there is a pool of phosphorylated ATM and Chk.2 remaining that could contribute to the modest decrease in S phase arrest observed following knockdown. Collectively, these results suggest that ATM and Chk.2 activity are important for RVFV DDR-induced S phase arrest.
FIGURE 5.
ATM and Chk.2 inhibitors rescue RVFV MP12-infected cells from S phase arrest. A, HSAECs were serum-starved for 2 days, infected with MP12 (m.o.i., 1.0), serum-starved for an additional 24 h, released into full medium for an additional 20 h, and then collected. Cells were treated with DMSO, ATM kinase inhibitor (10 μm), or Chk.2 inhibitor (Inh.) (10 μm) 2 h prior to infection and postinfection. Cell cycle analysis was performed with PI staining on an Accuri C6 flow cytometer, and data were acquired using CFlow Plus from Accuri Cytometers Inc. Data analysis was performed with Multicycle AV and FCS Express software. B, quantitation of data displayed in A. *, p value <0.01. C, HSAECs were plated, either left untreated or pre- and post-treated for 2 h with 10 μm concentrations of ATM kinase inhibitor or Chk.2 inhibitor, infected, then collected as described in Fig. 1A, and then probed by Western blot for anti-p-p53 (Ser-15), anti-p-Chk.2 (Thr-68), anti-p-H2A.X (Ser-139), anti-RVFV N protein, and anti-β-actin antibodies. D, HSAECs were plated and serum-starved for 72 h and then either mock- or MP12 (m.o.i., 1)-infected for 1 h followed by transfection using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions using either Ambion Silencer Negative Control siRNA2 (NonS), Qiagen FlexiTube siRNA Hs_ATM_5, or Hs_CHEK2_10 at a final concentration of 80 nm. 20 h postinfection/transfection, cells were released into full medium and then collected at 24 h. PI staining and FACS analysis were performed as described. E, HSAECs were plated, left untreated (UT) or transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions using either Ambion Silencer Negative Control siRNA2, Qiagen FlexiTube siRNA Hs_ATM_5, or Hs_CHEK2_10 at a final concentration of 80 nm, and then collected as described for Western blot analysis 24 h post-transfection using antibodies for total ATM (T-ATM) and total Chk.2 (T-Chk.2). Error bars indicate S.D. si, siRNA.
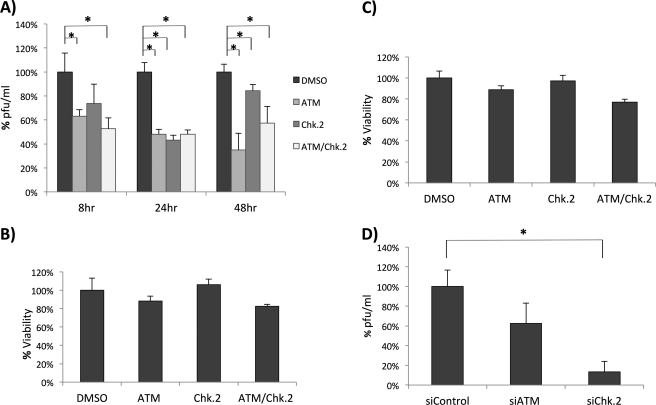
Inhibition of ATM and Chk.2 Decreases RVFV Production
Next, we aimed to determine the influence of ATM and Chk.2 on RVFV replication. To this end, we infected cells at an m.o.i. of 1.0 followed by treatment with ATM inhibitor, Chk.2 inhibitor, or a combination of the two. Viral replication was examined by plaque assays at 8, 24, and 48 h postinfection. Interestingly, we consistently observed a decrease in viral replication in ATM, Chk.2, and ATM/Chk.2 inhibitor-treated cells at all three time points (Fig. 6A). These results suggest that lack of ATM and Chk.2 activity leads to decreased viral replication. As a control, we examined the effect of ATM and Chk.2 inhibitors on cellular viability and metabolic profiles through CellTiter-Glo (Fig. 6B) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Fig. 6C) assays. Both assays demonstrated little to no toxicity or change associated with inhibitor treatment. siRNA knockdowns of ATM and Chk.2 were also performed to confirm their influence on RVFV MP12 replication. Plaque assays indicated that Chk.2 knockdown significantly inhibited RVFV replication (Fig. 6D). These results suggest that NSs activation of ATM and Chk.2 enhances RVFV replication.
FIGURE 6.
Inhibition of ATM and Chk.2 decreases RVFV MP12 production. A, HSAECs were infected at an m.o.i. of 1.0 followed by treatment with DMSO, ATM inhibitor (10 μm), Chk.2 inhibitor (10 μm), or a combination of both inhibitors (10 μm). Supernatants were then collected at 8, 24, and 48 h postinfection, and released virus was analyzed by plaque assay and plotted as a percentage of the DMSO control. *, p value <0.01. B, HSAECS were treated with DMSO, ATM inhibitor (10 μm), Chk.2 inhibitor (10 μm), or a combination of both inhibitors (10 μm), and cell viability was determined 48 h later by a CellTiter-Glo luminescence assay (Promega). Percent viability is expressed as the percentage of the DMSO control. C, HSAECs were treated as in B, and cell viability was determined by thiazolyl blue tetrazolium bromide (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. At 48 h post-treatment, 20 μl of 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide were added to each well on a 96-well plate and incubated for 3.5 h. Formazan crystals were dissolved using 4 mm HCl, 0.1% Nonidet P-40 in isopropanol. Absorbance was read at 590 nm with a reference filter of 620 nm. Percent viability is expressed as the percentage of the DMSO control. D, HSAECs were transfected in triplicate using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions using either Ambion Silencer Negative Control siRNA2, Qiagen FlexiTube siRNA Hs_ATM_5, or Hs_CHEK2_10 at a final concentration of 80 nm. 24 h post-transfection, cells were infected for 48 h at an m.o.i. of 1, supernatants were then collected, and plaque assays were performed. *, unpaired Student's t -test. Error bars indicate S.D.
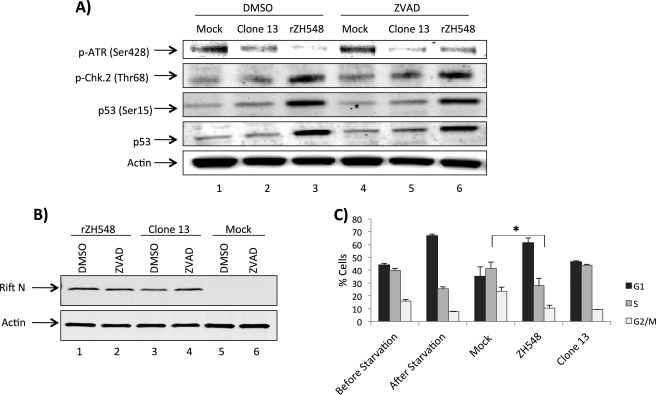
DNA Damage Signaling and Cell Cycle Arrest in rZH548-infected Samples
To determine whether the DDR response observed in MP12 and ΔNSs was replicated in a fully virulent virus, we tested the DDR response in ZH548-infected cells. For these studies, rZH548 and Clone 13, which is a naturally attenuated isolate that is missing 69% of the NSs open reading frame, were utilized (26). Cells were mock-infected or infected with rZH548 or Clone 13, post-treated with DMSO or Z-VAD-fmk, and then collected 24 h postinfection. Whole cell protein lysates were separated by SDS-PAGE and examined by Western blot analysis utilizing anti-p-ATR (Ser-428), anti-p53, anti-p-p53 (Ser-15), anti-p-Chk.2 (Thr-68), anti-RVFV nucleoprotein, and anti-β-actin antibodies (Fig. 7, A and B). Results with rZH548- and Clone 13 (Fig. 7A)-infected cells parallel those shown seen with MP12 and ΔNSs (Figs. 1 and 4). The results from Clone 13 further demonstrate and strengthen the case for NSs-dependent activation of the DDR pathway. In addition, Z-VAD-fmk-treated cells displayed DDR signaling similar to that in DMSO-treated samples, suggesting that RVFV-induced apoptosis is not inducing the DDR response as a by-product of infection.
FIGURE 7.
DNA damage signaling and cell cycle arrest in ZH548-infected samples. A, Vero cells were mock-infected or infected at an m.o.i. of 1.0 with either Clone 13 or rZH548, post-treated with DMSO (0.5%) or Z-VAD (100 μm), and then collected 24 h postinfection. Whole cell protein lysates were separated by SDS-PAGE and examined by Western blotting utilizing anti-p-ATR (Ser-428), anti-p53, anti-p-p53 (Ser-15), and anti-p-Chk.2 (Thr-68) (A) and anti-RVFV N protein and anti-β-actin antibodies (B). C, A549 cells were plated (one sample collected the following day as an asynchronous control), serum-starved for 48 h, then either mock-infected or infected with Clone 13 or rZH548, collected 24 h postinfection, PI-treated, and analyzed. Error bars indicate S.D.
Next, cell cycle analysis was performed following rZH548 and Clone 13 infection to determine the influence of the DDR pathway activation. Interestingly, rZH548-infected cells did not display the marked S phase arrest observed following MP12 infection but rather a G0/G1 arrest (Fig. 7C). These results point to clear differences between the vaccine strain and the fully virulent strain. The cell cycle distribution following Clone 13 infection mirrored that in the mock-infected cells, further supporting the role of NSs in altered cell cycle progression. Collectively, these results indicate that DDR signaling and a G0/G1 arrest are induced following infection with the rZH548 RVFV strain.
DISCUSSION
Cellular DNA is constantly under assault from both exogenous and endogenous sources, resulting in highly conserved and elaborate cellular machinery to monitor damage and ensure replication fidelity. After DNA damage occurs, major signaling checkpoints in the DDR are activated, resulting in the initiation of cell cycle checkpoints (27). Checkpoint activation freezes the cell cycle either at G1/S, intra-S phase, or the G2/M boundaries, allowing time for cellular repair mechanisms to either repair the damage or push the cell into apoptosis. The DDR is traditionally controlled by two master kinases, ATM and ATR, responding to double-stranded and single-stranded breaks, respectively (27). ATM and ATR are phosphatidylinositol 3-kinase-related kinase family members that phosphorylate multiple substrates on serine or threonine residues that are followed by a glutamine in response to DNA damage or replication blocks (28–30). Our data indicate that ATM and a number of its substrates, Chk.2, H2A.X, and p53, were robustly phosphorylated following RVFV infection. Interestingly, we observed ATR phosphorylation in both our mock- and RVFV-infected cells at early time points postinfection and a dramatic decrease in ATR phosphorylation at later time points after infection. Studies have shown that transient ATR activation may be necessary during S phase possibly to regulate the firing of replication origins or in response to DNA replication problems resulting from endogenous DNA damage (31). Some viruses such as HSV-1 have been shown to activate antibodies for ATM arm of the DDR to promote viral replication while specifically degrading the ATR pathway, which has been implicated in antiviral responses (32). These data suggest that RVFV specifically activates the ATM arm of the DDR while inactivating the ATR pathway.
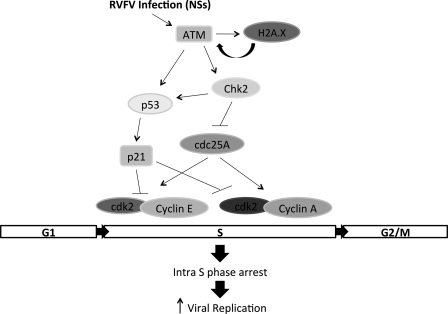
Fig. 8 displays our current working model of signaling events occurring following RVFV MP12 infection resulting in subsequent S phase arrest and increased viral replication. Following RVFV infection and specifically NSs expression, ATM is activated, and it in turn is able to phosphorylate p53 and Chk.2 (33). RVFV infection also induced phosphorylation of H2A.X at Ser-139 and formation of H2A.X foci in the nucleus, which can result in a positive feedback loop further enhancing ATM phosphorylation and is considered a well established hallmark of DNA damage (34, 35). p53 is also a substrate of Chk.2 and is phosphorylated on Ser-20 in response to DNA damage, resulting in dissociation of p53 with MDM2 and increasing p53 stability (36, 37). p53 phosphorylation not only increases its stability but also its DNA binding ability, resulting in the transcriptional activation of numerous downstream targets, including the cyclin/cdk inhibitor p21 and proapoptotic genes (38, 39). Another well characterized substrate of Chk.2 is cdc25A, a phosphatase that must remove the inhibitory phosphorylation from cdk2 complexes (40, 41). Cyclin E-cdk2 and cyclin A-cdk2 complexes are critical for the transition into S phase and for progression through S phase (42, 43). Chk.2 phosphorylation of cdc25A induces cdc25A proteasomal degradation, thus preventing activation of cyclin E-cdk2 or cyclin A-cdk2 complexes (41). In addition, cdk2 must be phosphorylated on residue Thr-160 by the cyclin-activating kinase, which is composed of cyclin H-cdk7 (44). Cyclin-activating kinase is a component of the basal transcription factor IIH (45). Interestingly, RVFV infection results in down-regulation of a number of transcription factor IIH components, including p44 and p62 (7, 8, 46). We have observed a decrease in cdk7 expression following RVFV infection (data not shown). Therefore, we suspect that cdk2 activity will be inhibited due to loss of Thr-160 phosphorylation. The net result of the above described events is induction of cell cycle arrest and in the case of RVFV MP12 infection, an intra-S phase arrest. Although we have not addressed the involvement of cdc25A, cdk2 complexes, or p21 in the current study, the modulation of these proteins and their contribution to RVFV-induced S phase arrest will be characterized in future studies.
FIGURE 8.
Model of DNA damage response occurring after RVFV MP12 infection. Following RVFV MP12 infection and NSs expression, ATM is activated, and it in turn phosphorylates p53 and Chk.2. cdc25A (a substrate of Chk.2) is a phosphatase that must remove the inhibitory phosphorylation from cdk2 complexes. Chk.2 phosphorylation of cdc25A induces cdc25A proteasomal degradation, thus preventing activation of cyclin E-cdk2 or cyclin A-cdk2 complexes. p53 phosphorylation results in activation of numerous downstream targets, including the cyclin-cdk inhibitor p21/waf1. The net result is an intra-S phase arrest that facilitates viral replication.
Interestingly, induction of the DDR was also observed with the virulent ZH548 strain of RVFV. However, the downstream effects differed with ZH548-infected cells arresting at G1, whereas MP12-infected cells arrested at S phase. Both ATM and p53 signaling pathways are involved in cell cycle arrest at both the G1 and S phase checkpoints. Virally induced G1 arrest is a well established strategy for a number of viruses that allows increased viral protein expression and subsequent viral progeny (47, 48). Alternatively, ZH548-infected cells may be unable to reenter the cell cycle completely and are retained in G0. In contrast, the S phase arrest observed in MP12-infected cells suggests that there are additional mechanisms present allowing cells to progress through the G1 checkpoint but be retained in S phase. Future studies will be focused on exploring the differences between the MP12 and ZH548 cell cycle responses as these studies will highlight factors that may contribute to pathogenesis.
The induction of the DDR does not appear to be dependent on increased gross DNA damage but rather due to the viral protein NSs. NSs filament formation typically begins 3–4 h postinfection with distinct puncta forming and coalescing into large filamentous fibril bundles, often averaging two to four filaments per cell (7). Despite their large size, NSs filaments do not interact with the majority of cellular DNA; interacting only with specific γ satellite clusters and the SAP30-Sin3A complex with no known interaction with the nucleolus (14). It is possible that the large size of the filaments could interfere with DNA replication, thus inducing a DNA replication stress response (49). In addition, NSs filaments form suppressive structures on specific promoters such as IFN-β, restricting access and potentially further contributing to a DNA replication stress response model. Finally, RVFV infection induces a high number of nuclear anomalies (formation of micronuclei, lobulated nuclei, and intranuclear bridges) as well as chromosome cohesion and segregation defects, all of which are dependent on NSs (14). Characterizing the interplay between RVFV-induced cell cycle arrest and unique nuclear anomalies is of great interest and will be essential to understanding the multifaceted role of NSs in viral replication and pathogenicity.
Importantly, ATM and Chk.2 inhibition resulted in decreased viral replication, suggesting that the induction of these pathways and the resultant cell cycle arrest are important for viral replication. A number of DNA and RNA viruses induce cell cycle arrest to produce a favorable environment for viral replication. Specifically, a few cytoplasmically replicating RNA viruses induce S phase arrest, including HCV and severe acute respiratory syndrome coronavirus. S phase arrest is induced by the NS2 protein of HCV through down-regulation of cyclin A expression (50). The severe acute respiratory syndrome coronavirus N induces S phase arrest by decreasing the levels of cdk2, cyclin E, and cyclin A proteins as well as through direct binding and inhibition of cyclin E-cdk2 and cyclin A-cdk2 activity (51). Although our studies were able to directly link ATM and Chk.2 to viral replication and to S phase arrest, the direct benefit to RVFV is not entirely clear. It is possible that S phase arrest may aid viral replication through an increase in critical cellular materials (nucleotide, amino acid, or lipid). For example, phospholipids accumulate to higher levels during S phase in mammalian cells (52, 53), a potential contributor to increased viral assembly.
In summary, our research has shown a novel function of the NSs protein in its ability to activate the ATM arm of the DDR pathway, resulting in a cell cycle arrest and directly impacting viral replication. The ultimate goal of our research is to identify critical viral and host protein interactions, which could serve as potential therapeutic targets to inhibit viral replication and limit the acute and rapid pathogenesis of RVFV. The induction of the DDR pathway by NSs has been shown to be important for viral replication and warrants further investigation.
Acknowledgments
We thank Dr. Sina Bavari (United States Army Medical Research Institute for Infectious Diseases) for providing the MP12 strain, Dr. Shinji Makino (University of Texas Medical Branch) for the MP12 ΔNSs mutant strain of RVFV, and Dr. Connie Schmaljohn (United States Army Medical Research Institute for Infectious Diseases) for the RVFV N protein antibody.
This work was supported by United States Department of Energy Grant DE-FC52-04NA25455 (to C. B.) and by Grants We2616/5-2 and SFB 593 (to F. W.) from the Deutsche Forschungsgemeinschaft.
- RVFV
- Rift Valley fever virus
- ATM
- ataxia-telangiectasia mutated
- ATR
- ataxia-telangiectasia mutated and Rad3-related kinase
- DDR
- DNA damage response
- HCV
- hepatitis C virus
- HSAEC
- human small airway lung epithelial cell
- m.o.i.
- multiplicity of infection
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- N
- nucleoprotein
- PI
- propidium iodine
- rZH548
- recombinant ZH548
- Z
- benzyloxycarbonyl
- fmk
- fluoromethyl ketone
- NS
- nonstructural.
REFERENCES
- 1. Weber F., Elliott R. M. (2002) Antigenic drift, antigenic shift and interferon antagonists: how bunyaviruses counteract the immune system. Virus Res. 88, 129–136 [DOI] [PubMed] [Google Scholar]
- 2. Pepin M., Bouloy M., Bird B. H., Kemp A., Paweska J. (2010) Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet. Res. 41, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bouloy M., Weber F. (2010) Molecular biology of Rift Valley fever virus. Open Virol. J. 4, 8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouloy M., Janzen C., Vialat P., Khun H., Pavlovic J., Huerre M., Haller O. (2001) Genetic evidence for an interferon-antagonistic function of Rift Valley fever virus nonstructural protein NSs. J. Virol. 75, 1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bird B. H., Albariño C. G., Hartman A. L., Erickson B. R., Ksiazek T. G., Nichol S. T. (2008) Rift Valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J. Virol. 82, 2681–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vialat P., Billecocq A., Kohl A., Bouloy M. (2000) The S segment of Rift Valley fever Phlebovirus (Bunyaviridae) carries determinants for attenuation and virulence in mice. J. Virol. 74, 1538–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le May N., Dubaele S., Proietti De Santis L., Billecocq A., Bouloy M., Egly J. M. (2004) TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell 116, 541–550 [DOI] [PubMed] [Google Scholar]
- 8. Kalveram B., Lihoradova O., Ikegami T. (2011) NSs protein of Rift Valley fever virus promotes posttranslational downregulation of the TFIIH subunit p62. J. Virol. 85, 6234–6243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikegami T., Narayanan K., Won S., Kamitani W., Peters C. J., Makino S. (2009) Dual functions of Rift Valley fever virus NSs protein: inhibition of host mRNA transcription and post-transcriptional downregulation of protein kinase PKR. Ann. N.Y. Acad. Sci. 1171, Suppl. 1, E75–E85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Habjan M., Pichlmair A., Elliott R. M., Overby A. K., Glatter T., Gstaiger M., Superti-Furga G., Unger H., Weber F. (2009) NSs protein of Rift Valley fever virus induces the specific degradation of the double-stranded RNA-dependent protein kinase. J. Virol. 83, 4365–4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ikegami T., Narayanan K., Won S., Kamitani W., Peters C. J., Makino S. (2009) Rift Valley fever virus NSs protein promotes post-transcriptional downregulation of protein kinase PKR and inhibits eIF2α phosphorylation. PLoS Pathog. 5, e1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le May N., Mansuroglu Z., Léger P., Josse T., Blot G., Billecocq A., Flick R., Jacob Y., Bonnefoy E., Bouloy M. (2008) A SAP30 complex inhibits IFN-β expression in Rift Valley fever virus infected cells. PLoS Pathog. 4, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Billecocq A., Spiegel M., Vialat P., Kohl A., Weber F., Bouloy M., Haller O. (2004) NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J. Virol. 78, 9798–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mansuroglu Z., Josse T., Gilleron J., Billecocq A., Leger P., Bouloy M., Bonnefoy E. (2010) Nonstructural NSs protein of Rift Valley fever virus interacts with pericentromeric DNA sequences of the host cell, inducing chromosome cohesion and segregation defects. J. Virol. 84, 928–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li R., Hayward S. D. (2011) The Ying-Yang of the virus-host interaction: control of the DNA damage response. Future Microbiol. 6, 379–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lilley C. E., Schwartz R. A., Weitzman M. D. (2007) Using or abusing: viruses and the cellular DNA damage response. Trends Microbiol. 15, 119–126 [DOI] [PubMed] [Google Scholar]
- 17. Nikitin P. A., Yan C. M., Forte E., Bocedi A., Tourigny J. P., White R. E., Allday M. J., Patel A., Dave S. S., Kim W., Hu K., Guo J., Tainter D., Rusyn E., Luftig M. A. (2010) An ATM/Chk2-mediated DNA damage-responsive signaling pathway suppresses Epstein-Barr virus transformation of primary human B cells. Cell Host Microbe 8, 510–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Machida K., Cheng K. T., Sung V. M., Lee K. J., Levine A. M., Lai M. M. (2004) Hepatitis C virus infection activates the immunologic (type II) isoform of nitric oxide synthase and thereby enhances DNA damage and mutations of cellular genes. J. Virol. 78, 8835–8843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verbruggen P., Ruf M., Blakqori G., Överby A. K., Heidemann M., Eick D., Weber F. (2011) Interferon antagonist NSs of La Crosse virus triggers a DNA damage response-like degradation of transcribing RNA polymerase II. J. Biol. Chem. 286, 3681–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munakata T., Liang Y., Kim S., McGivern D. R., Huibregtse J., Nomoto A., Lemon S. M. (2007) Hepatitis C virus induces E6AP-dependent degradation of the retinoblastoma protein. PLoS Pathog. 3, 1335–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Machida K., Cheng K. T., Lai C. K., Jeng K. S., Sung V. M., Lai M. M. (2006) Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation. J. Virol. 80, 7199–7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Popova T. G., Turell M. J., Espina V., Kehn-Hall K., Kidd J., Narayanan A., Liotta L., Petricoin E. F., 3rd, Kashanchi F., Bailey C., Popov S. G. (2010) Reverse-phase phosphoproteome analysis of signaling pathways induced by Rift Valley fever virus in human small airway epithelial cells. PLoS One 5, e13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Narayanan A., Popova T., Turell M., Kidd J., Chertow J., Popov S. G., Bailey C., Kashanchi F., Kehn-Hall K. (2011) Alteration in superoxide dismutase 1 causes oxidative stress and p38 MAPK activation following RVFV infection. PLoS One 6, e20354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barzilai A., Yamamoto K. (2004) DNA damage responses to oxidative stress. DNA Repair 3, 1109–1115 [DOI] [PubMed] [Google Scholar]
- 25. Yoshida K., Miki Y. (2010) The cell death machinery governed by the p53 tumor suppressor in response to DNA damage. Cancer Sci. 101, 831–835 [DOI] [PubMed] [Google Scholar]
- 26. Muller R., Saluzzo J. F., Lopez N., Dreier T., Turell M., Smith J., Bouloy M. (1995) Characterization of clone 13, a naturally attenuated avirulent isolate of Rift Valley fever virus, which is altered in the small segment. Am. J. Trop. Med. Hyg. 53, 405–411 [DOI] [PubMed] [Google Scholar]
- 27. Ljungman M. (2005) Activation of DNA damage signaling. Mutat. Res. 577, 203–216 [DOI] [PubMed] [Google Scholar]
- 28. Kastan M. B., Lim D. S. (2000) The many substrates and functions of ATM. Nat. Rev. Mol. Cell Biol. 1, 179–186 [DOI] [PubMed] [Google Scholar]
- 29. Abraham R. T. (2004) PI 3-kinase related kinases: 'big' players in stress-induced signaling pathways. DNA Repair 3, 883–887 [DOI] [PubMed] [Google Scholar]
- 30. Shechter D., Costanzo V., Gautier J. (2004) Regulation of DNA replication by ATR: signaling in response to DNA intermediates. DNA Repair 3, 901–908 [DOI] [PubMed] [Google Scholar]
- 31. Mordes D. A., Cortez D. (2008) Activation of ATR and related PIKKs. Cell Cycle 7, 2809–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lilley C. E., Chaurushiya M. S., Boutell C., Everett R. D., Weitzman M. D. (2011) The intrinsic antiviral defense to incoming HSV-1 genomes includes specific DNA repair proteins and is counteracted by the viral protein ICP0. PLoS Pathog. 7, e1002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuoka S., Huang M., Elledge S. J. (1998) Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282, 1893–1897 [DOI] [PubMed] [Google Scholar]
- 34. Rogakou E. P., Pilch D. R., Orr A. H., Ivanova V. S., Bonner W. M. (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 [DOI] [PubMed] [Google Scholar]
- 35. Burma S., Chen B. P., Murphy M., Kurimasa A., Chen D. J. (2001) ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276, 42462–42467 [DOI] [PubMed] [Google Scholar]
- 36. Hirao A., Kong Y. Y., Matsuoka S., Wakeham A., Ruland J., Yoshida H., Liu D., Elledge S. J., Mak T. W. (2000) DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287, 1824–1827 [DOI] [PubMed] [Google Scholar]
- 37. Chehab N. H., Malikzay A., Appel M., Halazonetis T. D. (2000) Chk2/hCds1 functions as a DNA damage checkpoint in G1 by stabilizing p53. Genes Dev. 14, 278–288 [PMC free article] [PubMed] [Google Scholar]
- 38. Giono L. E., Manfredi J. J. (2006) The p53 tumor suppressor participates in multiple cell cycle checkpoints. J. Cell. Physiol. 209, 13–20 [DOI] [PubMed] [Google Scholar]
- 39. Olsson A., Manzl C., Strasser A., Villunger A. (2007) How important are post-translational modifications in p53 for selectivity in target-gene transcription and tumour suppression? Cell Death Differ. 14, 1561–1575 [DOI] [PubMed] [Google Scholar]
- 40. Hoffmann I., Draetta G., Karsenti E. (1994) Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J. 13, 4302–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jinno S., Suto K., Nagata A., Igarashi M., Kanaoka Y., Nojima H., Okayama H. (1994) Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J. 13, 1549–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wohlbold L., Fisher R. P. (2009) Behind the wheel and under the hood: functions of cyclin-dependent kinases in response to DNA damage. DNA Repair 8, 1018–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Woo R. A., Poon R. Y. (2003) Cyclin-dependent kinases and S phase control in mammalian cells. Cell Cycle 2, 316–324 [PubMed] [Google Scholar]
- 44. Gu Y., Rosenblatt J., Morgan D. O. (1992) Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 11, 3995–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zurita M., Merino C. (2003) The transcriptional complexity of the TFIIH complex. Trends Genet. 19, 578–584 [DOI] [PubMed] [Google Scholar]
- 46. Dasgupta A. (2004) Targeting TFIIH to inhibit host cell transcription by Rift Valley fever virus. Mol. Cell 13, 456–458 [DOI] [PubMed] [Google Scholar]
- 47. He Y., Xu K., Keiner B., Zhou J., Czudai V., Li T., Chen Z., Liu J., Klenk H. D., Shu Y. L., Sun B. (2010) Influenza A virus replication induces cell cycle arrest in G0/G1 phase. J. Virol. 84, 12832–12840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang T., Zhao R., Wu Y., Kong D., Zhang L., Wu D., Li C., Zhang C., Yu Z., Jin X. (2011) Hepatitis B virus induces G1 phase arrest by regulating cell cycle genes in HepG2.2.15 cells. Virol. J. 8, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Osborn A. J., Elledge S. J., Zou L. (2002) Checking on the fork: the DNA-replication stress-response pathway. Trends Cell Biol. 12, 509–516 [DOI] [PubMed] [Google Scholar]
- 50. Yang X. J., Liu J., Ye L., Liao Q. J., Wu J. G., Gao J. R., She Y. L., Wu Z. H., Ye L. B. (2006) HCV NS2 protein inhibits cell proliferation and induces cell cycle arrest in the S-phase in mammalian cells through down-regulation of cyclin A expression. Virus Res. 121, 134–143 [DOI] [PubMed] [Google Scholar]
- 51. Surjit M., Liu B., Chow V. T., Lal S. K. (2006) The nucleocapsid protein of severe acute respiratory syndrome-coronavirus inhibits the activity of cyclin-cyclin-dependent kinase complex and blocks S phase progression in mammalian cells. J. Biol. Chem. 281, 10669–10681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Habenicht A. J., Glomset J. A., Goerig M., Gronwald R., Grulich J., Loth U., Schettler G. (1985) Cell cycle-dependent changes in arachidonic acid and glycerol metabolism in Swiss 3T3 cells stimulated by platelet-derived growth factor. J. Biol. Chem. 260, 1370–1373 [PubMed] [Google Scholar]
- 53. Jackowski S. (1994) Coordination of membrane phospholipid synthesis with the cell cycle. J. Biol. Chem. 269, 3858–3867 [PubMed] [Google Scholar]