Background: MAPK signaling is an important mechanism controlling keratinocyte differentiation.
Results: PRMT5 and p38δ interact as part of a multiprotein signaling complex, and PRMT5 and p38δ produce opposing actions in regulating differentiation.
Conclusion: PRMT5 modulates p38δ MAPK kinase phosphorylation and signaling.
Significance: This is a novel mechanism that links p38δ MAPK signaling and PRMT5 signaling.
Keywords: Cell Differentiation, Gene Expression, Keratinocytes, p38 MAPK, Signal Transduction, PRMT5, Protein Kinase Cδ, Skin Cancer
Abstract
PKCδ is a key regulator of keratinocyte differentiation that activates p38δ phosphorylation leading to increased differentiation as measured by an increased expression of the structural protein involucrin. Our previous studies suggest that p38δ exists in association with protein partners. A major goal is to identify these partners and understand their role in regulating keratinocyte differentiation. In this study we use affinity purification and mass spectrometry to identify protein arginine methyltransferase 5 (PRMT5) as part of the p38δ signaling complex. PRMT5 is an arginine methyltransferase that symmetrically dimethylates arginine residues on target proteins to alter target protein function. We show that PRMT5 knockdown is associated with increased p38δ phosphorylation, suggesting that PRMT5 impacts the p38δ signaling complex. At a functional level we show that PRMT5 inhibits the PKCδ- or 12-O-tetradecanoylphorbol-13-acetate-dependent increase in human involucrin expression, and PRMT5 dimethylates proteins in the p38δ complex. Moreover, PKCδ expression reduces the PRMT5 level, suggesting that PKCδ activates differentiation in part by reducing PRMT5 level. These studies indicate antagonism between the PKCδ and PRMT5 signaling in control of keratinocyte differentiation.
Introduction
Mitogen-activated protein kinases (MAPK) are dual-specificity serine/threonine kinases that drive intracellular signal transduction (1). MAPK family kinases share sequence similarity and conserved structural domains and include the extracellular signal-regulated kinases (ERK), Jun N-terminal kinases (JNK), and p38 MAPK. ERK is activated by mitogens and growth factors (2), whereas JNK and p38 kinases are typically activated in response to cellular stress (3). MAPKs play a central role in control of keratinocyte cell fate, and the balance between ERK and p38δ activity is a key determiner of keratinocyte survival status. Enhanced ERK activity is associated with survival, whereas enhanced p38δ activity is associated with differentiation and apoptosis (4–10). Three p38 isoforms, p38α, p38β, and p38δ, are expressed in keratinocytes (5, 8). Among these, p38δ has a key role as a positive regulator of keratinocyte differentiation (7). p38δ mediates the response to differentiating agents including phorbol ester, calcium, okadaic acid, and green tea polyphenol (7,10–15).
It is clear that MAPK signaling is regulated by cross-talk from other signaling cascades; however, this regulation is not well understood. This is because the key cascades that cross-talk with MAPK signaling are not well defined, are likely cell type- and context-specific, and the impact of this cross-talk on biological outcome is not well understood. In this study we demonstrate a role for protein arginine methyltransferase five (PRMT5)2 in modulating MAPK signaling in keratinocytes. PRMT5 is an enzyme that dimethylates protein-bound arginine residues (16). Protein methylation is receiving increasing attention as an important post-translational modification. Protein arginine methyl transferases (PRMTs) are evolutionarily conserved enzymes that catalyze transfer of methyl groups from S-adenosyl methionine to the guanidino nitrogen of protein-bound arginine. Eight functional PRMT proteins are encoded in the mammalian genome (17). These enzymes mono- and dimethylate arginine residues in proteins and are classified as type I (PRMT1, 2, 3, 4, 6, and 8) and type II (PRMT5 and 7) enzymes. Type I enzymes catalyze formation of asymmetrically dimethylated arginine (16, 18).
PRMT5 is the sole type II member of the PRMT family that catalyzes formation of symmetrically dimethylated arginine (SDMA) (16). PRMT5 was discovered by yeast two-hybrid screening as Janus kinase-interacting protein 1 (16). PRMT5 dimethylates a variety of histone and non-histone proteins. Histone targets include histones H3 and H4 (19, 20), whereas non-histone targets include small heterodimer partner (21), myelin basic protein (22), and a host of others. PRMT5 interacts in a number of protein complexes that regulate RNA processing, signal transduction, and transcription (19, 23–29). PRMT5 is a critical determinant of circadian period in Arabidopsis (30), and as a component of the androgen receptor cofactor complex, PRMT5 positively modulates androgen receptor-driven transcription independent of its methyltransferase activity (31, 32). PRMT5 modulates enhanced GFR-mediated ERK activation (33) and is required for p53 expression and induction of p53 targets (34). PRMT5 also binds to death receptor 4 (35). An important study shows that PRMT1 modulates p38 MAPK regulation of differentiation in megakaryocytes (36). In addition to these functions in signal transduction, PRMT5 also participates in the assembly of the transcriptional repressor complex on various eukaryotic promoters (37). Thus, PRMT5 and protein arginine dimethylation are emerging as important regulators of cell function.
Involucrin is a keratinocyte structural protein that functions as a precursor of the cornified envelope and is expressed in the suprabasal layers of epidermis (38, 39). Regulation of involucrin gene expression has been extensively studied as a model for understanding regulation of differentiation-dependent gene expression in epidermis (5). A PKC, Ras, MEKK1, MEK3/MEK6 signaling cascade has been implicated as a key control pathway in regulating involucrin expression (5). In this study we use this system to study cross-talk between PRMT5 and MAPK signaling in regulating keratinocyte differentiation. We show that PRMT5 reduces involucrin expression in normal human keratinocytes (KERn). These studies further show that PRMT5 is part of a p38δ-ERK signaling complex and that PRMT5 modification of proteins in this complex is associated with reduced p38δ phosphorylation. The net impact is that PRMT5 antagonizes p38δ-dependent keratinocyte differentiation.
EXPERIMENTAL PROCEDURES
Reagents
Keratinocyte serum-free medium (KSFM), trypsin and Hanks' balanced salt solution were purchased from Invitrogen. Rabbit polyclonal antibodies specific for ERK1/2 (sc-94), protein kinase Cδ (PKCδ, sc-213), PKCα (sc-208), p38δ (sc-7587), p38α (sc-7572), p38β (sc-6176), PRMT5 (sc-22132), PRMT1 (sc-59648), and peroxidase-conjugated anti-goat IgG (sc-2033) were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin (A5441), anti-FLAG M2 mouse monoclonal antibody (F-3165), anti-FLAG M2 affinity gel (A2220), and 12-O-tetradecanoylphorbol-13-acetate (TPA) were obtained from Sigma. Peroxidase-conjugated anti-mouse IgG (NXA931) and peroxidase-conjugated anti-rabbit IgG (NA934V) were purchased from GE Healthcare. The antibody for detection of symmetric dimethyl arginine (07-412) was from Millipore (Bedford, MA). Knockdown of PKCδ and PRMT5 was achieved using siRNA. Pooled PKCδ-siRNA was from Santa Cruz (sc-36253) and from Dharmacon (J-003524-08). We also used individual siRNAs that target PKCδ mRNA sequences 5′-AGAAGAAGCCGACCAUGUAU (PKCδ-1) and 5′-GUUGAUGUCUGUUCAGUAUUU (PKCδ-2) (40). All were equally effective at reducing PKCδ level. Pooled PRMT5-siRNA (NM_001039619) was from Santa Cruz (sc-41073) and Origene (SR307063). In addition, we used individual siRNA that target the PRMT5 mRNA sequences 5′-CAGCCACUGAUGGACAAUCUGGAAU (PRMT5-1) and 5′-CCGGCUACUUUGAGACUGUGCUUUA (PRMT5-2) (35). All were equally effective at reducing PRMT5 levels. Rabbit anti-human involucrin was previously described (42, 43). Rabbit anti-phospho-p38 (9211S) was from Cell Signaling (Danvers, MA), and mouse monoclonal anti-phospho-ERK1/2 (M8159) was from Sigma. The involucrin promoter luciferase reporter construct, pINV-2473, was previously described (44).
Primary Keratinocyte Culture and Adenovirus Production
Newborn foreskin epidermis was separated from dermis with dispase, and the keratinocytes were dispersed with trypsin and cultured in KSFM supplemented with epidermal growth factor and pituitary extract (44, 45). Adenoviruses encoding wild-type FLAG-p38δ, PKCδ, and empty control adenovirus (Ad5-FLAG-p38δ, Ad5-PKCδ, Ad5-EV) were prepared as previously described by propagation in 293 cells and purification by cesium chloride centrifugation (9, 46). For infection, keratinocytes were treated with multiplicity of infection 10 of adenovirus in the presence of 2.5 μg/ml Polybrene for 4 h before the addition of fresh KSFM.
Affinity Purification of FLAG-p38δ and Mass Spectrometry
Keratinocytes were infected with Ad5-FLAG-p38δ and after 24 h extracts were prepared in lysate buffer (20 mm Tris-HCl, pH 7.4, containing 150 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 1% Triton, 2.5 mm sodium pyrophosphate, 1 mm β-mercaptoethanol, 1 mm Na3VO4, 1 μg/ml leupeptin, 1 mm phenylmethylsulfonyl fluoride, 1 mm dithiothreitol, and proteinase inhibitor mixture). Anti-FLAG M2 affinity gel was washed with a large volume of lysis buffer followed by two bed volumes of 20 mm glycine-HCl, pH 2.8, and then re-equilibrated with lysis buffer. Cell lysate (120 mg of total protein) was incubated with M2 gel for 12 h at 4 °C with shaking and then packed in to PD10 column and washed with lysis buffer until A280 < 0.02 to remove unbound protein. FLAG-p38δ-associated proteins were eluted with 20 mm glycine-HCl, pH 2.8, and the collected fractions (200 μl) were neutralized by the addition of 1 m Tris before storage at −20 C.
Affinity-purified FLAG-p38δ-associated proteins, prepared as outlined above, were concentrated by trichloroacetic acid precipitation, and samples were separated by SDS-PAGE followed by Coomassie Blue staining. Gel bands were excised and digested with trypsin, and the tryptic peptides were analyzed by liquid chromatography-tandem mass spectroscopy using a Bruker Omniflex benchtop MALDI-TOF MS/MS. For protein identification, the monoisotopic mass maps of tryptic peptides were searched in databases using the MASCOT search engine.
Immunoprecipitation and Immunoblot
Total extracts in lysis buffer were incubated with appropriate primary antibody and protein A/G-agarose overnight at 4 ºC and washed 4 times with Tris-buffered saline, pH 7.4. After the final wash the beads were collected, boiled with sample buffer, and centrifuged, and supernatant was electrophoresed and transferred to nitrocellulose membrane. The blots were blocked with 5% skimmed milk in Tris-buffered saline containing 0.1% Tween 20 and incubated with appropriate primary and horseradish peroxidase-conjugated secondary antibody, and antibody binding was visualized by chemiluminescent detection (Amersham Biosciences).
Keratinocyte Electroporation
Keratinocytes were electroporated with plasmids using an Amaxa electroporator and the VPD-1002 nucleofection kit (Amaxa, Cologne, Germany). Keratinocytes were harvested with trypsin and replated 1 day before use. On the day of electroporation, 1.2 million replated cells were harvested with trypsin and resuspended in KSFM. The cells were collected at 2000 × g, washed with 1 ml of sterile phosphate-buffered saline, and suspended in 100 μl of keratinocyte nucleofection solution. The cell suspension, which included 3 μg of plasmid or siRNA, was mixed by gentle up and down pipetting and electroporated using the T-018 program. KSFM (500 μl) was added, and the suspension was transferred to a 60-mm cell culture dish.
Construction of pcDNA3-myc-hPRMT5
The pOTB7-hPRMT5 (GenBankTM BC025979) plasmid (MHS1011-7508890) was obtained from Open Biosystems (Huntsville, AL). hPRMT5 was amplified to create a BamHI/NotI fragment using forward (5′-GATCGAATTCGGATCCATGGAACAAAAACTTATTTCTGAAGAAGATCTGATGGCGGCGATGGCGGTCGGG3) and reverse (5′-GATCTCTAGAGCGGCCGCCTAGAGGCCAATGGTATATGAGCGGCCTGT3)primers. The ATG start codon (double underline) and myc epitope (underlined) are indicated. The insert was then cloned into pcDNA3 to create pcDNA3-myc-hPRMT5.
Quantitative PCR
RNA was isolated using RNAspin Mini kit (25-0500-71) (GE Healthcare). RNA (1 μg) was used for cDNA synthesis with Moloney murine leukemia virus transcriptase (Invitrogen). Gene expression was measured by real time PCR using Light Cycler 480 SYBR Green I Master mix (04-707 516 001) from Roche Diagnostics and specific primers for hPRMT5 (forward, 5′-TGAGGCCCAGTTTGAGATGCCTTA; reverse, 5′-AGTAGCCGGCAAAGCCATGTAGTA3), involucrin (forward, 5′-CCTCAGCCTTACTGTGAG; reverse, 5′-GGGAGGCAGTGGAGTTGG), PKCδ (forward, 5′-GGCCACATCAAGATTGCCGACTTT; reverse, 5′-ACTGGCCAATGAGCATCTCGTACA), and GAPDH (forward, 5′-TCCACTGGCGTCTTCACC; reverse, 5′-GGCAGAGATGATGACCCTTT).
RESULTS
Identification of FLAG-p38δ-associated Proteins
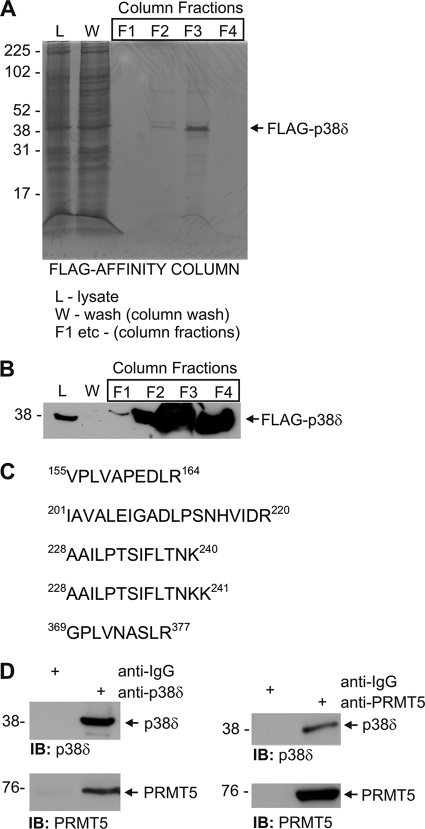
Our previous studies describe a p38δ-ERK signaling complex in keratinocytes that regulates keratinocyte differentiation (6). Although these studies show that p38δ interacts with ERK1/2, it is likely that p38δ also interacts with other proteins and that these proteins influence function. To identify additional interaction partners, extracts were prepared from FLAG-p38δ-expressing keratinocytes, and p38δ-associated proteins were purified by FLAG affinity column chromatography. The column was loaded with 120 mg of total cell lysate and washed with loading buffer, and affinity-bound proteins were eluted with glycine buffer. Fig. 1A shows a Coomassie-stained gel illustrating the affinity purification of FLAG-p38δ. The fractions show the lysate (L) that was loaded onto the column, the column wash (W), and column elution fractions (F1, etc.). The Coomassie-stained gel shows the presence of FLAG-p38δ in elution fractions F2, F3, and F4 but most prominently in F3 (Fig. 1A). Anti-FLAG immunoblot of proteins derived from the total lysate and elution fractions reveals FLAG-p38δ in elution fractions F2, F3, and F4 (Fig. 1B). These fractions were pooled and analyzed by matrix-assisted laser desorption ionization mass spectrometry, which revealed the presence of several proteins. Among these was PRMT5. Fig. 1C indicates that PRMT5-derived tryptic peptides identified by mass spectrometry. PRMT5 is a protein arginine methyltransferase that modifies arginine in target proteins to form SDMA (16). This covalent post-translational modification acts to alter target protein function (16). These findings suggest that PRMT5 is a binding partner for p38δ. To confirm this, we prepared total cell extracts from human keratinocytes and immunoprecipitated endogenous p38δ with anti-p38δ. The precipitate was electrophoresed, and the gel was stained with anti-PRMT5. Fig. 1D shows that PRMT5 co-precipitates with p38δ whether the precipitation is performed with anti-p38δ or anti-PRMT5, thereby providing additional evidence for p38δ/PRMT5 intracellular interaction.
FIGURE 1.
FLAG-p38δ complex includes PRMT5. A, KERn were infected with multiplicity of infection 10 of Ad5-FLAG-p38δ, and after 24 h extracts were prepared for FLAG affinity chromatography. Total cell lysate (L), column wash (W), and fractions eluted from the FLAG affinity column (F1, etc.) were electrophoresed, and the gel was Coomassie-stained. Molecular mass markers are indicated. B, shown is detection of FLAG-p38δ. Samples were electrophoresed and immunoblotted with anti-FLAG. FLAG-p38δ was absent from the wash fraction. F3 contains the highest concentration of FLAG-p38δ. C, amino acid sequence of PRMT5 tryptic fragments were identified by mass spectrum analysis. D, co-precipitation of p38δ and PRMT5 is shown. Total cell extracts were prepared from KERn and then immunoprecipitated (IB) with anti-IgG (control), anti-p38δ, or anti-PRMT5. The precipitates were then electrophoresed for immunoblot detection of p38δ and PRMT5. Similar results were observed in each of three experiments.
Role of PRMT5 in Regulating Keratinocyte Differentiation
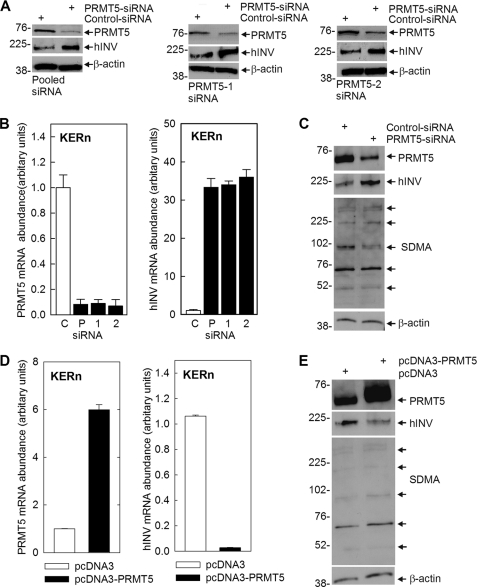
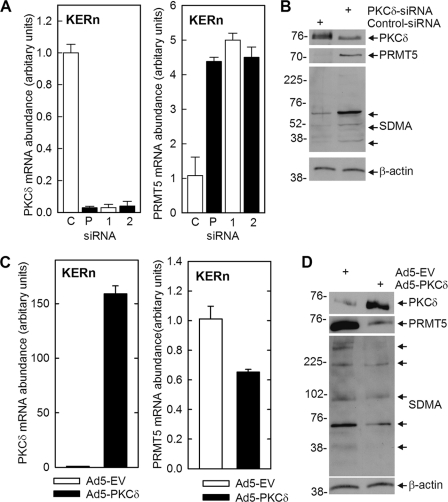
Our previous studies demonstrate that a PKCδ signaling cascade increases p38δ activity, which leads to increased cell differentiation (5, 6, 10, 47, 48). Because PRMT5 interacts with p38δ, we hypothesized that it may regulate differentiation-associated responses that depend upon p38δ activity. To assess this we monitored the impact of PRMT5 on involucrin (hINV) expression. hINV is a marker protein that is increased during keratinocyte differentiation and in response to differentiation stimuli (5, 6, 10, 47, 48). We began by assessing the impact of PRMT5 knockdown and overexpression on hINV mRNA and protein levels. Fig. 2A shows that treatment with PRMT5-pooled (Santa Cruz, sc-41073) or individual (PRMT5-1 or PRMT5-2) siRNA (35) reduces PRMT5 levels, and this is associated with a marked increase in hINV protein levels. Fig. 2B shows this is due to changes in mRNA levels. Treatment with PRMT5-pooled (P), PRMT5-1 (1) or PRMT5-2 (2) siRNA reduces PRMT5 mRNA, and this is associated with increased hINV mRNA. Consistent with these findings, the converse is also observed, as overexpression of PRMT5 reduces hINV mRNA and protein level (Fig. 2, D and E).
FIGURE 2.
PRMT5 regulates hINV mRNA and protein level. A, KERns were electroporated with 3 μg of control, PRMT5-pooled (Santa Cruz), PRMT5-1, or PRMT5-2 siRNA (35), and after 48 h, PRMT5 and hINV protein levels were assessed by immunoblot. B, KERns were electroporated with 3 μg of the control (C), PRMT5-pooled (P, Santa Cruz), or individual (PRMT5-1, 1) or PRMT5-2, 2) siRNA and after 48 h PRMT5 and hINV mRNA was measured, by qPCR. Bars indicate relative levels of PRMT5 and hINV RNA normalized relative to GAPDH RNA levels and are the mean ± S.D. for triplicate samples in a single experiment. Similar results were obtained in three independent experiments. C, KERns were electroporated with 3 μg of control or PRMT5-pooled (Santa Cruz) siRNA. hINV, PRMT5, and SDMA were measured in total cell extracts prepared at 48 h post-electroporation. The arrows in the SDMA blot indicate the major SDMA-modified proteins. β-Actin is the control to normalize protein loading. D and E, KERn were electroporated with 3 μg of pcDNA3 or pcDNA3-hPRMT5, PRMT5 and hINV mRNA levels were measured after 24 h, and protein levels were measured at 48 h post-exposure. The values are the mean ± S.D. of triplicate samples in a single experiment. Similar results were observed in each of three experiments. The arrows in the SDMA blot indicate the major SDMA-modified proteins.
PRMT5 is a protein arginine methyltransferase that catalyzes modification of arginine residues in target proteins to form SDMA, a modification that modulates target protein function (16, 18). We, therefore, examined the impact of PRMT5 knockdown and overexpression on SDMA formation. Extracts from PRMT5-siRNA-treated and PRMT5-overexpressing cells were prepared for anti-SDMA immunoblot. Proteins ranging in molecular mass from 50 to 300 kDa are detected (Fig. 2, C and E). Immunostaining of a limited number of proteins covering a broad range of molecular weights is typical of anti-SDMA reagents (49). Using this as a crude assay of PRMT5 activity, we observe a 10–15% reduction in average SDMA intensity in PRMT5-siRNA-treated cells and a similar 10–15% increase in extracts derived from PRMT5-overexpressing cells. We do not presently know which proteins are identified in this assay, but these findings suggest that increased PRMT5-dependent SDMA formation is associated with suppression of involucrin gene expression.
Regulation of hINV Expression by PKCδ and PRMT5
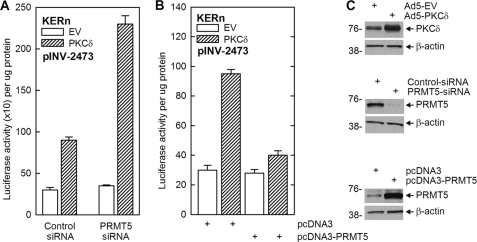
The above studies indicate that PRMT5 suppresses hINV mRNA and protein levels. We next compared the action of PRMT5 against two treatments that increase differentiation, PKCδ overexpression and TPA treatment. PKCδ promotes keratinocyte differentiation and increases involucrin gene expression via activation of p38δ (4, 5, 47). pINV-2473 encodes the full-length hINV promoter linked to a luciferase reporter gene such that increased promoter activity is reflected in increased luciferase activity (44). Keratinocytes were transfected with pINV-2473 in the presence of control-siRNA or PRMT5-siRNA and empty or PKCδ-encoding expression vector. In the presence of control-siRNA, PKCδ increases hINV promoter activity 3-fold (Fig. 3A). In contrast, when PMRT5-siRNA is present and PRMT5 is reduced (Fig. 3C), the induction is 6.5-fold (Fig. 3A). Moreover, the PKCδ induction of hINV promoter activity is suppressed (Fig. 3B) in PRMT5 overexpressing (Fig. 3C) cells.
FIGURE 3.
Impact of PRMT5 on PKCδ-dependent hINV promoter activity. A, KERn were electroporated with 3 μg of control- or PRMT5-pooled (Santa Cruz) siRNA, and after 48 h the cells were trypsinized and electroporated with 1.5 μg of pINV-2473 (involucrin promoter luciferase reporter) in the presence of 1.5 μg of empty vector (pEGFN1) or PKCδ-encoding plasmid (pPKCδ-EGFN1). After an additional 18 h, cell extract was prepared for assay of luciferase activity. Values are the mean ± S.E., n = 3. The control-siRNA EV and PKCδ values are significantly different, p < 0.001; the PRMT5-siRNA EV and PKCδ values are significantly different, p < 0.001; the Control-siRNA PKCδ and PRMT5-siRNA PKCδ values are significantly different, p < 0.001. B, KERn were electroporated with 1.5 μg of pcDNA3 or pcDNA3-PRMT5 expression vector, and after 48 h cells were re-electroporated with 1.5 μg of pINV-2473 and 0.5 μg of pEGFN1 or pPKCδ-EGFN1. After an additional 18 h, extracts were prepared for luciferase assay. The values are the mean ± S.E., n = 3. The pcDNA3 EV and PKCδ values are significantly different, p < 0.001. C, KERn were incubated with the indicated expression vectors or siRNA, and after 24 h extracts were prepared for detection of the indicated proteins by immunoblot. Similar results were observed at 48 and 72 h (not shown).
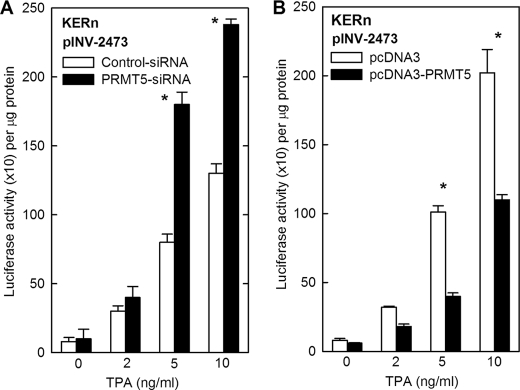
TPA is a pharmacological agent that increases PKC activity and stimulates differentiation (44). We used TPA treatment as a second method of PKCδ activation. Keratinocytes were transfected with pINV-2473 and treated with TPA in the presence of control- or PRMT5-siRNA. TPA at 5 and 10 ng/ml increases hINV promoter activity, and a further 2-fold increase in promoter activity is observed in PRMT5 knockdown cells (Fig. 4A). In the converse experiment in which PRMT5 is overexpressed, the TPA-dependent increase in hINV promoter activity is suppressed by 2-fold (Fig. 4B). These studies suggest that PRMT5 opposes the pro-differentiation action of TPA and PKCδ.
FIGURE 4.
Impact of PRMT5 on TPA-dependent hINV promoter activity. A, KERn were electroporated with 3 μg of control- or PRMT5-pooled (Santa Cruz) siRNA. After 48 h cells were re-electroporated with 3 μg of pINV-2473 and involucrin promoter luciferase reporter construct. After an additional 6 h, TPA was added, and after an additional 18 h cells were lysed for luciferase activity assay. The values are the mean ± S.E., n = 3. The asterisks indicate a significant difference as determined by Student's t test, p < 0.001, between control-siRNA and PRMT5-siRNA at 5 and 10 ng/ml TPA. B, KERn were electroporated with 3 μg of pcDNA3 or pcDNA-PRMT5 and after 48 h re-electroporated with 3 μg of pINV-2473. After an additional 6 h the cells were treated with TPA for 18 h, and then extracts were prepared for luciferase activity assay. The results are presented as the mean ± S.E., n = 3. The asterisks indicate a significant difference as determined by Student's t test, p < 0.001, between pcDNA3 and pcDNA3-PRMT5 at 5 and 10 ng/ml TPA.
It is well established that PKCδ enhances keratinocyte differentiation (4–6, 47). We wondered whether part of this mechanism may involve PKCδ suppression of PRMT5 expression. To test this we manipulated PKCδ level and monitored the impact on PRMT5 expression. Fig. 5A shows that PRMT5 RNA level is increased 4-fold when PKCδ mRNA levels is reduced by treating KERn with PKCδ-pooled (Santa Cruz) or individual (PKCδ-1 or PKCδ-2) (40) siRNA. Moreover, the reduction in PKCδ level is associated with increased PRMT5 protein expression and SDMA formation (Fig. 5B). In the inverse experiment, PKCδ overexpression reduces PRMT5 RNA and protein level (Fig. 5, C and D), and this is associated with reduced SDMA formation (Fig. 5D). As noted in Fig. 2, the anti-SDMA blots shown in Fig. 5, B and D, provide a generally index of PRMT5 activity (49).
FIGURE 5.
PKCδ regulates PRMT5 level. A, KERns were electroporated with 3 μg of control- and PKCδ-pooled (P, Santa Cruz) or individual (PKCδ-1, PKCδ-2) (40) siRNA, and after 24 h, PKCδ and PRMT5 mRNA levels were monitored as outlined in Fig. 2. B KERns were electroporated with 3 μg of control- or PKCδ-pooled (Santa Cruz) siRNA, and after 24 h extracts were prepared for detection of PKCδ, PRMT5, and SDMA by immunoblot. The values are the mean ± S.D. of three replicates, and similar results were observed in each of three experiments. C and D, KERn were infected with the indicated adenovirus, and after 24 h mRNA and protein extracts were prepared for detection of PKCδ and PMRT5 RNA and protein. SDMA was also monitored by immunoblot. The arrows in the SDMA blot indicate the major SDMA-modified proteins. The bar graph values are the mean ± S.D. of three replicated samples. Similar results were observed in each of three experiments.
PRMT5 Knockdown Enhances Total p38 Phosphorylation
PKCδ or TPA stimulation of differentiation is associated with increased p38 MAPK activity as evidenced by increased p38 phosphorylation (4, 6, 10, 15). We hypothesized that PRMT5 may regulate differentiation by modulating the extent of this activation. Because no antibody is available that specifically detects phosphorylation of the individual p38α, -β, and -δ isoforms, we began by monitoring total p38 phosphorylation (i.e. phosphorylation of p38α, -β, and -δ) (9, 46). Keratinocytes were electroporated with control-siRNA or PRMT5-siRNA, and the level of total phosphorylated p38 was measured. Fig. 6 shows that PRMT5 knockdown increases total p38 phosphorylation (p38T-P). We also monitored the impact of PRMT5 knockdown on the level of other key regulators. p38α, -β, and -δ are expressed in keratinocytes (9, 46), but p38δ is the major form phosphorylated by stimuli that drive differentiation (4, 10, 47). ERK1/2 is also an important regulator, and ERK1/2 phosphorylation enhances keratinocyte survival (4). Fig. 6 shows that PKCδ, p38δ, and ERK1/2 levels and ERK1/2 phosphorylation are not altered by PRMT5 knockdown. p38α and β levels were also not altered by PRMT5 knockdown (not shown). Thus, a major impact of PRMT5 knockdown is to enhance total p38 phosphorylation.
FIGURE 6.
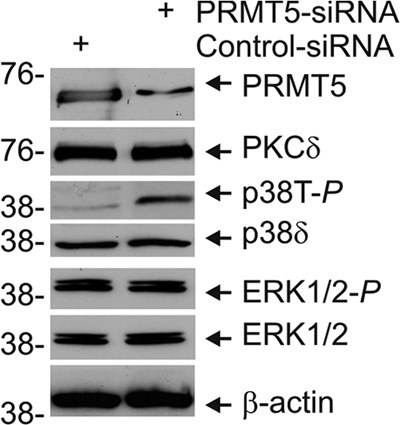
PRMT5 suppresses total p38 phosphorylation. Keratinocytes were electroporated with 3 μg of control- or PRMT5-siRNA, and after 48 h cell extracts were prepared and electrophoresed for immunoblot detection of the indicated proteins. Similar results were observed in each of two experiments.
PRMT5-dependent Covalent Modification of Proteins in p38δ Complex Is Associated with Increased p38δ Phosphorylation
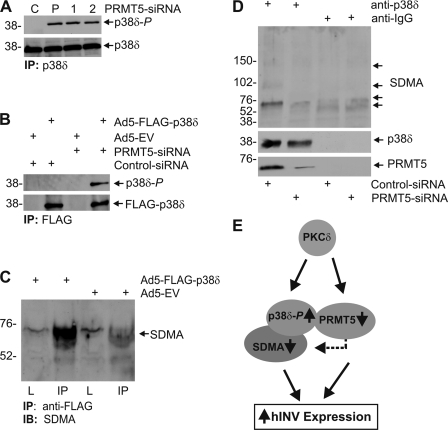
Given that PRMT5 knockdown enhances total p38 phosphorylation, a key question is whether PRMT5 specifically modulates phosphorylation of the p38δ isoform. This could be expected, as previous studies indicate that p38δ is the key p38 isoform involved in regulation of differentiation (6, 7, 9). A second key issue is whether any such ability of PRMT5 to regulate p38δ phosphorylation is associated with altered SDMA modification of proteins in the p38δ signaling complex. To assess this, we performed two types of experiments. We first treated cells with control or PRMT5-pooled PRMT5-1 or PRMT5-2 siRNA for 48 h and then immunoprecipitated p38δ and assessed phosphorylation status. In Fig. 7A shows that p38δ is phosphorylated in PRMT5 knockdown but not control cells. As a second method to confirm this result, we monitored the phosphorylation state of vector-expressed FLAG-p38δ. Cells were treated with control- or PRMT5-siRNA and then infected with Ad5-EV or Ad5-FLAG-p38δ. At 24 h post-infection, FLAG-p38δ was immunoprecipitated with anti-FLAG, and the p38δ phosphorylation state was measured. Fig. 7B confirms that PRMT5 knockdown is associated with increased p38δ phosphorylation.
FIGURE 7.
PRMT5 regulates p38δ phosphorylation and SDMA modification of p38δ-associated proteins. A, KERn were electroporated with 3 μg of control, PRMT5-pooled, PRMT5-1, or PRMT5-2 siRNA, and after 48 h extracts were prepared for immunoprecipitation (IP) with anti-p38δ. The precipitated material was then monitored by immunoblot to detect p38δ and phosphorylated p38δ. B, KERns were electroporated with 3 μg of control- or PRMT5-pooled (Santa Cruz) siRNA and after 24 h infected with multiplicity of infection 10 of Ad5-EV or Ad5-p38δ and after an additional 24 h extracts were prepared for immunoprecipitation with anti-FLAG. The precipitated proteins were electrophoresed, and the level of FLAG-p38δ (anti-FLAG) and phosphorylated p38δ was monitored by immunoblot. C, KERn (70% confluent) was infected with multiplicity of infection 10 Ad5-EV or Ad5-FLAG-p38δ, and after 24 h lysate was prepared, and 200 μg of protein was immunoprecipitated with anti-FLAG antibody. The resulting pellet was electrophoresed for immunoblot (IB) with anti-SDMA. 20 μg of total lysate (L) was electrophoresed in a parallel lane. The blot was then incubated with anti-SDMA. D, KERn were electroporated with 3 μg of control- or PRMT5-pooled (Santa Cruz) siRNA, and after 48 h extracts were prepared for immunoprecipitation with anti-IgG or anti-p38δ. The precipitates were then electrophoresed for immunodetection of p38δ, PRMT5, and SDMA. The arrows indicate the major SDMA modified bands. E, PKCδ and PRMT5 regulation of p38δ activity; a working model. The model is explained in the “Discussion.” We realize that multiple complexes may exist and that some may lack one or more of the components, and that it is likely that other proteins are also present in this complex. We also note that we do not know whether there is direct interaction between the indicated components. The arrows indicate relative change in level. The dashed arrow indicates PRMT5 SDMA modification of target proteins.
We next monitored whether the p38δ complex contains SDMA modified proteins. We infected cells with Ad5-EV or Ad5-FLAG-p38δ and after 24 h immunoprecipitated with anti-FLAG and monitored the level of SDMA in the precipitate. Fig. 7C shows that SDMA modified proteins are present in the p38δ complex. We next assessed whether this SDMA modification requires PRMT5. We treated cells with control- or PRMT5-siRNA and after 48 h prepared extracts for immunoprecipitation with anti-p38δ. Fig. 7D shows that a SDMA-modified 60-kDa protein is associated with the p38δ complex. Moreover, PRMT5 knockdown reduced the level of this protein or the extent of its SDMA modification. This protein has a molecular mass of 60 kDa, suggesting that it is a protein other than p38δ. As a control for assay integrity, we confirm appropriate immunoprecipitation of p38δ and PRMT5. Taken together, these findings suggest that PRMT5 suppresses p38δ phosphorylation and that this is associated with increased SDMA modification of a 60-kDa protein present in the p38δ complex.
DISCUSSION
Epidermal Differentiation
The epidermal keratinocyte, the major cell type present in the epidermis, undergoes a complex and choreographed program of differentiation. This process requires a balance among keratinocyte proliferation, differentiation, and apoptosis. Ultimately, this process leads to the formation of a multilayered epidermis that contains a proliferative basal zone beneath several layers of cells that are at various stages in the differentiation process (50). Involucrin is a marker of this process that is increased in differentiated cells (5) and is an important component of the scaffolding of the cornified envelope (43, 51–53). Understanding how this process is regulated to produce a multilayered tissue is a major goal in epidermal biology, as disorders in this process are observed in a host of epidermal disease states and also in cancer.
p38δ-ERK Signaling Complex
Previous studies show that a p38δ-ERK complex regulates expression of key apoptosis, differentiation, and proliferation-associated genes in keratinocytes (4–6, 10, 11). p38δ activity is increased, and ERK1/2 activity is reduced in differentiated keratinocytes (6). The importance of interaction within this complex is illustrated by the role of p38δ and ERK1/2. p38δ and ERK1/2 are associated either directly or indirectly in this complex, and increased p38δ phosphorylation and reduced ERK1/2 activity is observed in response to treatment with differentiation stimuli (4–7). We propose that this complex serves to integrate incoming signals and that the net output then determines cell fate. For example, activation of PKCδ increases p38δ activity and cell differentiation and death, whereas activation of ERK1/2 by delivery of constitutively active Raf antagonizes this response and promotes cell survival (5). The fact that a p38δ-ERK complex is involved in this regulation suggests that other proteins may also be partners in this regulatory complex.
An important goal is identification of additional partners that are part of this complex and influence the signaling activity. To achieve this we expressed FLAG-p38δ in keratinocytes and identified proteins associated with p38δ by FLAG-affinity chromatography and mass spectrometry. One of the proteins that co-purified with p38δ was PRMT5. This interaction was confirmed by co-immunoprecipitation of PRMT5 and p38δ from keratinocyte extracts using a p38δ-specific antibody. This confirmation is important, as it has been reported in one study that PRMT5 can interact with anti-FLAG (16). Protein arginine methyltransferases are a family of proteins that dimethylate arginine residues in target proteins (16). Arginine modification in this context influences target protein structure, function, and activity (16). PRMTs have a role in numerous cellular processes including regulation of cell signaling and gene expression (17, 32, 33, 54–57). However, additional studies are needed to explore the physiological mechanisms whereby PRMTs regulate cells survival, differentiation, and apoptosis. Despite the recognized importance of PRMT-dependent modification in a host of cell types, the role of PRMT in regulating keratinocyte function has not been studied.
PRMT5 Regulates Basal Involucrin Expression
Our previous studies show that keratinocyte differentiation requires activity in a PKCδ, Ras, MEKK1, MEK3 cascade that triggers changes in activity in a p38δ-ERK complex to increase p38δ activity relative to ERK activity (4–7, 9–11, 15, 47, 48, 58). Activation of this cascade is associated with cessation of cell proliferation and increased morphological differentiation (8, 10, 15), and prolonged stimulation can cause apoptosis (4, 8). Stimulation of MAPK activity by this cascade is not associated with changes in expression of p38δ or ERK1/2 level but is associated with increased p38δ activity and reduced ERK1/2 activity (6). Involucrin is a marker of differentiation that is increased in differentiated cells and is expressed at a fixed basal level in resting keratinocytes (5). An interesting and unexpected finding is that PRMT5 knockdown increases basal involucrin expression including increasing hINV mRNA and protein levels and promoter activity. Moreover, the converse is also observed in that PRMT5 overexpression reduces basal involucrin mRNA and protein levels.
PRMT5 Influences Stimulus-dependent Involucrin Expression
We also monitored the impact of PRMT5 on response to agents that activate p38δ signaling to increase differentiation. Overexpression of PKCδ and treatment with TPA enhance p38δ phosphorylation and increase involucrin expression (4, 6). TPA is a diacylglycerol analog that enhances PKCδ activity and induces differentiation (44, 48). We show that PRMT5 knockdown enhances the differentiation promoting ability of these agents and the PRMT5 overexpression suppresses the differentiation promoting response. These findings suggest that PRMT5 is a negative regulator of keratinocyte differentiation. Thus, these studies identify an important new PRMT5-mediated regulatory circuit in keratinocytes that functions to suppress differentiation. In addition to an important role in normal cells, these findings suggest a role for PRMT5 in skin cancer. PRMT5 levels are increased (29), and PKCδ levels are decreased in cancer (59, 60). Thus, the balance between these signaling inputs is likely to be important in disease development, and we anticipate that PRMT5 may serve to enhance skin cancer cell survival. The possibility that PRMT5-related regulation is increased is an important area for further investigation in skin cancer. PRMT5 may also link the p38δ complex to other signaling cascades, as PRMT5 associates with Janus kinases and STAT (54, 56). Thus, the knowledge that PRMT5 is part of the p38δ complex helps us in our effort to assembly the signaling network that controls keratinocyte differentiation.
Suppression of PRMT5 Level by PKCδ
There are numerous situations where increased pro-differentiation signaling is associated with reduced pro-survival signaling and vice versa. An example in keratinocytes is the coupling of increased p38δ and decreased ERK1/2 activity in differentiation agent-stimulated cells (5). PKCδ and PRMT5 appear to share such a relationship, as PKCδ overexpression is associated with reduced PRMT5, and PKCδ knockdown is associated with increased PRMT5. Thus, it is possible that suppression of the PRMT5 level and activity may be a mechanism whereby PKCδ drives keratinocyte differentiation. We know that PKCδ-dependent reduction in PRMT5 is associated with reduced PRMT5 mRNA and protein levels. This suggests a potential impact of PKCδ-associated signaling on transcription of the PRMT5 gene or an impact on PRMT5 RNA stability; however, further studies will be necessary to fully understand this regulation.
Impact of PRMT5 on p38δ Signaling Complex
To gain insight regarding the role of PRMT5 in p38δ MAPK signaling, we examined the impact of PRMT5 on the complex. These studies are extremely interesting, as they suggest that PRMT5 influences the p38δ phosphorylation state. First, we show that PRMT5 is a partner in the p38δ complex. PRMT5 is found associated with FLAG-p38δ when analyzed by mass spectrometry and also co-immunoprecipitates with p38δ present in total cell extracts. That this interaction is real is supported by functional studies. At a functional level, PRMT5 influences molecular events in this complex. Overexpression of PRMT5 results in reduced p38δ phosphorylation of p38δ, and this is associated with increased SDMA modification of a 60-kDa protein that also co-precipitates with p38δ. Moreover, this regulation is reversed in PRMT5 knockdown cells.
The identity of the 60-kDa protein is not presently known, but it appears to be a bona fide target of PRMT5, as PRMT5 knockdown results in reduced anti-SDMA detection of this band. This is likely due to decreased SDMA labeling of this protein but could also be due to a reduction in protein level. It is interesting that this 60-kDa band is also a major SDMA-modified protein in total keratinocyte cell extracts, suggesting that it may be a major PRMT5 target that is involved in a range of processes. It should also be noted that we do not intend to imply that all p38δ complexes included all of these partners (ERK1/2, PRMT5, 60-kDa protein, etc.), as it is likely that multiple subcomplexes of varying composition exist and that these complexes may have unique intracellular functions. It is also worth noting that no previous reports described an impact of PRMT5 on MAPK signaling. The only available report relates an impact of PRMT1, a type I PRMT that asymmetrically dimethylates arginine (16, 18), on p38α function in megakaryocyte differentiation (36).
Working Model
Our previous studies show that PKCδ activates involucrin gene expression (keratinocyte differentiation) by stimulating a cascade that regulates a p38δ and ERK1/2-containing complex to increase p38δ and decrease ERK1/2 phosphorylation (4–6, 10, 48). This change in the balance of p38δ and ERK1/2 activity ultimately leads to increased nuclear AP1 factor levels, increased AP1 factor interaction with specific sites on the hINV promoter, and increased transcription (41, 44, 61, 62).
Based on the studies presented in this proposal, we propose that PRMT5 is a new component of this signaling complex. The role of PRMT5 in this complex is to inhibit p38δ activity and suppress differentiation by catalyzing SDMA modification at the arginine residue(s) of a 60-kDa protein (and perhaps other proteins) that are present in this complex. We propose that SDMA modification of these targets alters the complex in a way that leads to reduced p38δ phosphorylation. Thus, when PRMT5 levels are high and PRMT5 is active, differentiation-dependent gene (hINV) expression is suppressed. Under conditions where cells are exposed to a differentiation stimulus, PKCδ activity triggers signaling events that drive differentiation. We propose that PKCδ acts to increase p38δ phosphorylation by two potential mechanisms (Fig. 7E). One mechanism is stimulating signaling events that lead to increased p38δ phosphorylation (6). Our present studies suggest that a second mechanism is suppression of PRMT5 mRNA and protein levels, thereby reducing PRMT5 modification of proteins in the p38δ complex. Our studies show that reduced PRMT5 levels are associated with increased p38δ phosphorylation. Thus, the present studies identify a new signaling protein present in the p38δ complex that suppresses differentiation-associated p38δ activity.
Acknowledgment
We thank Dr. Austin Yang, Director of University of Maryland School of Medicine Proteomics Facility, for assistance with mass spectrometry and informatics analysis.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AR046494 (to R. L. E.).
- PRMT
- protein arginine methyltransferase
- TPA
- 12-O-tetradecanoylphorbol-13-acetate
- KERn
- normal human keratinocyte
- SDMA
- symmetric dimethylarginine
- hINV
- involucrin
- KSFM
- keratinocyte serum-free medium.
REFERENCES
- 1. Schaeffer H. J., Weber M. J. (1999) Mitogen-activated protein kinases. Specific messages from ubiquitous messengers. Mol. Cell. Biol. 19, 2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roux P. P., Blenis J. (2004) ERK and p38 MAPK-activated protein kinases. A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68, 320–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Enslen H., Brancho D. M., Davis R. J. (2000) Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. EMBO J. 19, 1301–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Efimova T., Broome A. M., Eckert R. L. (2004) Protein kinase Cδ regulates keratinocyte death and survival by regulating activity and subcellular localization of a p38δ extracellular signal-regulated kinase 1/2 complex. Mol. Cell. Biol. 24, 8167–8183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eckert R. L., Crish J. F., Efimova T., Dashti S. R., Deucher A., Bone F., Adhikary G., Huang G., Gopalakrishnan R., Balasubramanian S. (2004) Regulation of involucrin gene expression. J. Invest. Dermatol. 123, 13–22 [DOI] [PubMed] [Google Scholar]
- 6. Efimova T., Broome A. M., Eckert R. L. (2003) A regulatory role for p38δ MAPK in keratinocyte differentiation. Evidence for p38δ-ERK1/2 complex formation. J. Biol. Chem. 278, 34277–34285 [DOI] [PubMed] [Google Scholar]
- 7. Eckert R. L., Efimova T., Balasubramanian S., Crish J. F., Bone F., Dashti S. (2003) p38 mitogen-activated protein kinases on the body surface. A function for p38δ. J. Invest. Dermatol. 120, 823–828 [DOI] [PubMed] [Google Scholar]
- 8. Eckert R. L., Efimova T., Dashti S. R., Balasubramanian S., Deucher A., Crish J. F., Sturniolo M., Bone F. (2002) Keratinocyte survival, differentiation, and death. Many roads lead to mitogen-activated protein kinase. J. Investig. Dermatol. Symp. Proc. 7, 36–40 [DOI] [PubMed] [Google Scholar]
- 9. Dashti S. R., Efimova T., Eckert R. L. (2001) MEK7-dependent activation of p38 MAP kinase in keratinocytes. J. Biol. Chem. 276, 8059–8063 [DOI] [PubMed] [Google Scholar]
- 10. Efimova T., LaCelle P., Welter J. F., Eckert R. L. (1998) Regulation of human involucrin promoter activity by a protein kinase C, Ras, MEKK1, MEK3, p38/RK, AP1 signal transduction pathway. J. Biol. Chem. 273, 24387–24395 [DOI] [PubMed] [Google Scholar]
- 11. Kraft C. A., Efimova T., Eckert R. L. (2007) Activation of PKCδ and p38δ MAPK during okadaic acid-dependent keratinocyte apoptosis. Arch. Dermatol. Res. 299, 71–83 [DOI] [PubMed] [Google Scholar]
- 12. Eckert R. L., Crish J. F., Efimova T., Balasubramanian S. (2006) Opposing action of curcumin and green tea polyphenol in human keratinocytes. Mol. Nutr. Food Res. 50, 123–129 [DOI] [PubMed] [Google Scholar]
- 13. Balasubramanian S., Eckert R. L. (2007) Keratinocyte proliferation, differentiation, and apoptosis. Differential mechanisms of regulation by curcumin, EGCG, and apigenin. Toxicol. Appl. Pharmacol. 224, 214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eckert R. L., Crish J. F., Efimova T., Balasubramanian S. (2004) Antioxidants regulate normal human keratinocyte differentiation. Biochem. Pharmacol. 68, 1125–1131 [DOI] [PubMed] [Google Scholar]
- 15. Balasubramanian S., Efimova T., Eckert R. L. (2002) Green tea polyphenol stimulates a Ras, MEKK1, MEK3, and p38 cascade to increase activator protein 1 factor-dependent involucrin gene expression in normal human keratinocytes. J. Biol. Chem. 277, 1828–1836 [DOI] [PubMed] [Google Scholar]
- 16. Bedford M. T., Clarke S. G. (2009) Protein arginine methylation in mammals. Who, what, and why. Mol. Cell 33, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pahlich S., Zakaryan R. P., Gehring H. (2006) Protein arginine methylation. Cellular functions and methods of analysis. Biochim. Biophys. Acta 1764, 1890–1903 [DOI] [PubMed] [Google Scholar]
- 18. Bachand F. (2007) Protein arginine methyltransferases. From unicellular eukaryotes to humans. Eukaryot. Cell 6, 889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pal S., Vishwanath S. N., Erdjument-Bromage H., Tempst P., Sif S. (2004) Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol. Cell. Biol. 24, 9630–9645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dacwag C. S., Ohkawa Y., Pal S., Sif S., Imbalzano A. N. (2007) The protein arginine methyltransferase Prmt5 is required for myogenesis because it facilitates ATP-dependent chromatin remodeling. Mol. Cell. Biol. 27, 384–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanamaluru D., Xiao Z., Fang S., Choi S. E., Kim D. H., Veenstra T. D., Kemper J. K. (2011) Arginine methylation by PRMT5 at a naturally occurring mutation site is critical for liver metabolic regulation by small heterodimer partner. Mol. Cell. Biol. 31, 1540–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lim Y., Kwon Y. H., Won N. H., Min B. H., Park I. S., Paik W. K., Kim S. (2005) Multimerization of expressed protein-arginine methyltransferases during the growth and differentiation of rat liver. Biochim. Biophys. Acta 1723, 240–247 [DOI] [PubMed] [Google Scholar]
- 23. Pollack B. P., Kotenko S. V., He W., Izotova L. S., Barnoski B. L., Pestka S. (1999) The human homologue of the yeast proteins Skb1 and Hsl7p interacts with Jak kinases and contains protein methyltransferase activity. J. Biol. Chem. 274, 31531–31542 [DOI] [PubMed] [Google Scholar]
- 24. Friesen W. J., Paushkin S., Wyce A., Massenet S., Pesiridis G. S., Van Duyne G., Rappsilber J., Mann M., Dreyfuss G. (2001) The methylosome, a 20 S complex containing JBP1 and pICln, produces dimethylarginine-modified Sm proteins. Mol. Cell. Biol. 21, 8289–8300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fabbrizio E., El Messaoudi S., Polanowska J., Paul C., Cook J. R., Lee J. H., Negre V., Rousset M., Pestka S., Le Cam A., Sardet C. (2002) Negative regulation of transcription by the type II arginine methyltransferase PRMT5. EMBO Rep. 3, 641–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pal S., Yun R., Datta A., Lacomis L., Erdjument-Bromage H., Kumar J., Tempst P., Sif S. (2003) mSin3A/histone deacetylase 2- and PRMT5-containing Brg1 complex is involved in transcriptional repression of the Myc target gene cad. Mol. Cell. Biol. 23, 7475–7487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ancelin K., Lange U. C., Hajkova P., Schneider R., Bannister A. J., Kouzarides T., Surani M. A. (2006) Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat. Cell Biol. 8, 623–630 [DOI] [PubMed] [Google Scholar]
- 28. Tee W. W., Pardo M., Theunissen T. W., Yu L., Choudhary J. S., Hajkova P., Surani M. A. (2010) Prmt5 is essential for early mouse development and acts in the cytoplasm to maintain ES cell pluripotency. Genes Dev. 24, 2772–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pal S., Baiocchi R. A., Byrd J. C., Grever M. R., Jacob S. T., Sif S. (2007) Low levels of miR-92b/96 induce PRMT5 translation and H3R8/H4R3 methylation in mantle cell lymphoma. EMBO J. 26, 3558–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hong S., Song H. R., Lutz K., Kerstetter R. A., Michael T. P., McClung C. R. (2010) Type II protein arginine methyltransferase 5 (PRMT5) is required for circadian period determination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 107, 21211–21216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hosohata K., Li P., Hosohata Y., Qin J., Roeder R. G., Wang Z. (2003) Purification and identification of a novel complex that is involved in androgen receptor-dependent transcription. Mol. Cell. Biol. 23, 7019–7029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paik W. K., Paik D. C., Kim S. (2007) Historical review. The field of protein methylation. Trends Biochem. Sci. 32, 146–152 [DOI] [PubMed] [Google Scholar]
- 33. Hsu J. M., Chen C. T., Chou C. K., Kuo H. P., Li L. Y., Lin C. Y., Lee H. J., Wang Y. N., Liu M., Liao H. W., Shi B., Lai C. C., Bedford M. T., Tsai C. H., Hung M. C. (2011) Cross-talk between Arg-1175 methylation and Tyr-1173 phosphorylation negatively modulates EGFR-mediated ERK activation. Nat. Cell Biol. 13, 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scoumanne A., Zhang J., Chen X. (2009) PRMT5 is required for cell cycle progression and p53 tumor suppressor function. Nucleic Acids Res. 37, 4965–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tanaka H., Hoshikawa Y., Oh-hara T., Koike S., Naito M., Noda T., Arai H., Tsuruo T., Fujita N. (2009) PRMT5, a novel TRAIL receptor-binding protein, inhibits TRAIL-induced apoptosis via nuclear factor-κB activation. Mol. Cancer Res. 7, 557–569 [DOI] [PubMed] [Google Scholar]
- 36. Chang Y. I., Hua W. K., Yao C. L., Hwang S. M., Hung Y. C., Kuan C. J., Leou J. S., Lin W. J. (2010) Protein-arginine methyltransferase 1 suppresses megakaryocytic differentiation via modulation of the p38 MAPK pathway in K562 cells. J. Biol. Chem. 285, 20595–20606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cesaro E., De Cegli R., Medugno L., Florio F., Grosso M., Lupo A., Izzo P., Costanzo P. (2009) The Kruppel-like zinc finger protein ZNF224 recruits the arginine methyltransferase PRMT5 on the transcriptional repressor complex of the aldolase A gene. J. Biol. Chem. 284, 32321–32330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rice R. H., Green H. (1979) Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope. Activation of the cross-linking by calcium ions. Cell 18, 681–694 [DOI] [PubMed] [Google Scholar]
- 39. Rice R. H., Green H. (1977) The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell 11, 417–422 [DOI] [PubMed] [Google Scholar]
- 40. Cai Q., Li J., Gao T., Xie J., Evers B. M. (2009) Protein kinase Cδ negatively regulates hedgehog signaling by inhibition of Gli1 activity. J. Biol. Chem. 284, 2150–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crish J. F., Eckert R. L. (2008) Synergistic activation of human involucrin gene expression by Fra-1 and p300. Evidence for the presence of a multiprotein complex. J. Invest. Dermatol. 128, 530–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. LaCelle P. T., Lambert A., Ekambaram M. C., Robinson N. A., Eckert R. L. (1998) In vitro cross-linking of recombinant human involucrin. Skin Pharmacol. Appl. Skin Physiol. 11, 214–226 [DOI] [PubMed] [Google Scholar]
- 43. Robinson N. A., LaCelle P. T., Eckert R. L. (1996) Involucrin is a covalently cross-linked constituent of highly purified epidermal corneocytes. Evidence for a common pattern of involucrin cross-linking in vivo and in vitro. J. Invest. Dermatol. 107, 101–107 [DOI] [PubMed] [Google Scholar]
- 44. Welter J. F., Crish J. F., Agarwal C., Eckert R. L. (1995) Fos-related antigen (Fra-1), junB, and junD activate human involucrin promoter transcription by binding to proximal and distal AP1 sites to mediate phorbol ester effects on promoter activity. J. Biol. Chem. 270, 12614–12622 [DOI] [PubMed] [Google Scholar]
- 45. Banks E. B., Crish J. F., Welter J. F., Eckert R. L. (1998) Characterization of human involucrin promoter distal regulatory region transcriptional activator elements. A role for Sp1 and AP1 binding sites. Biochem. J. 331, 61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dashti S. R., Efimova T., Eckert R. L. (2001) MEK6 regulates human involucrin gene expression via a p38α- and p38δ-dependent mechanism. J. Biol. Chem. 276, 27214–27220 [DOI] [PubMed] [Google Scholar]
- 47. Efimova T., Deucher A., Kuroki T., Ohba M., Eckert R. L. (2002) Novel protein kinase C isoforms regulate human keratinocyte differentiation by activating a p38δ mitogen-activated protein kinase cascade that targets CCAAT/enhancer-binding protein α. J. Biol. Chem. 277, 31753–31760 [DOI] [PubMed] [Google Scholar]
- 48. Efimova T., Eckert R. L. (2000) Regulation of human involucrin promoter activity by novel protein kinase C isoforms. J. Biol. Chem. 275, 1601–1607 [DOI] [PubMed] [Google Scholar]
- 49. Boisvert F. M., Côté J., Boulanger M. C., Richard S. (2003) A proteomic analysis of arginine-methylated protein complexes. Mol. Cell. Proteomics 2, 1319–1330 [DOI] [PubMed] [Google Scholar]
- 50. Eckert R. L., Crish J. F., Robinson N. A. (1997) The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol. Rev. 77, 397–424 [DOI] [PubMed] [Google Scholar]
- 51. Robinson N. A., Lapic S., Welter J. F., Eckert R. L. (1997) S100A11, S100A10, annexin I, desmosomal proteins, small proline-rich proteins, plasminogen activator inhibitor-2, and involucrin are components of the cornified envelope of cultured human epidermal keratinocytes. J. Biol. Chem. 272, 12035–12046 [DOI] [PubMed] [Google Scholar]
- 52. Nemes Z., Marekov L. N., Steinert P. M. (1999) Involucrin cross-linking by transglutaminase 1. Binding to membranes directs residue specificity. J. Biol. Chem. 274, 11013–11021 [DOI] [PubMed] [Google Scholar]
- 53. Steinert P. M., Marekov L. N. (1999) Initiation of assembly of the cell envelope barrier structure of stratified squamous epithelia. Mol. Biol. Cell 10, 4247–4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu F., Zhao X., Perna F., Wang L., Koppikar P., Abdel-Wahab O., Harr M. W., Levine R. L., Xu H., Tefferi A., Deblasio A., Hatlen M., Menendez S., Nimer S. D. (2011) JAK2V617F-mediated phosphorylation of PRMT5 down-regulates its methyltransferase activity and promotes myeloproliferation. Cancer Cell 19, 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rank G., Cerruti L., Simpson R. J., Moritz R. L., Jane S. M., Zhao Q. (2010) Identification of a PRMT5-dependent repressor complex linked to silencing of human fetal globin gene expression. Blood 116, 1585–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Igarashi H., Kuwahara K., Yoshida M., Xing Y., Maeda K., Nakajima K., Sakaguchi N. (2009) GANP suppresses the arginine methyltransferase PRMT5 regulating IL-4-mediated STAT6-signaling to IgE production in B cells. Mol. Immunol. 46, 1031–1041 [DOI] [PubMed] [Google Scholar]
- 57. Guderian G., Peter C., Wiesner J., Sickmann A., Schulze-Osthoff K., Fischer U., Grimmler M. (2011) RioK1, a new interactor of protein arginine methyltransferase 5 (PRMT5), competes with pICln for binding and modulates PRMT5 complex composition and substrate specificity. J. Biol. Chem. 286, 1976–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Deucher A., Efimova T., Eckert R. L. (2002) Calcium-dependent involucrin expression is inversely regulated by protein kinase Cα (PKCα)α and PKCδ. J. Biol. Chem. 277, 17032–17040 [DOI] [PubMed] [Google Scholar]
- 59. Reno E. M., Haughian J. M., Dimitrova I. K., Jackson T. A., Shroyer K. R., Bradford A. P. (2008) Analysis of protein kinase Cδ (PKCδ) expression in endometrial tumors. Hum. Pathol. 39, 21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yadav V., Yanez N. C., Fenton S. E., Denning M. F. (2010) Loss of protein kinase C δ gene expression in human squamous cell carcinomas. A laser capture microdissection study. Am. J. Pathol. 176, 1091–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Crish J. F., Zaim T. M., Eckert R. L. (1998) The distal regulatory region of the human involucrin promoter is required for expression in epidermis. J. Biol. Chem. 273, 30460–30465 [DOI] [PubMed] [Google Scholar]
- 62. Crish J. F., Gopalakrishnan R., Bone F., Gilliam A. C., Eckert R. L. (2006) The distal and proximal regulatory regions of the involucrin gene promoter have distinct functions and are required for in vivo involucrin expression. J. Invest. Dermatol. 126, 305–314 [DOI] [PubMed] [Google Scholar]